 Found 105 hits with Last Name = 'auger' and Initial = 'f'
Found 105 hits with Last Name = 'auger' and Initial = 'f' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21283

(3-[(2-iodo-5-nitrophenyl)carbonyl]-1-[(1-methylpip...)Show SMILES CN1CCCCC1Cn1cc(C(=O)c2cc(ccc2I)[N+]([O-])=O)c2ccccc12 Show InChI InChI=1S/C22H22IN3O3/c1-24-11-5-4-6-16(24)13-25-14-19(17-7-2-3-8-21(17)25)22(27)18-12-15(26(28)29)9-10-20(18)23/h2-3,7-10,12,14,16H,4-6,11,13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50089965
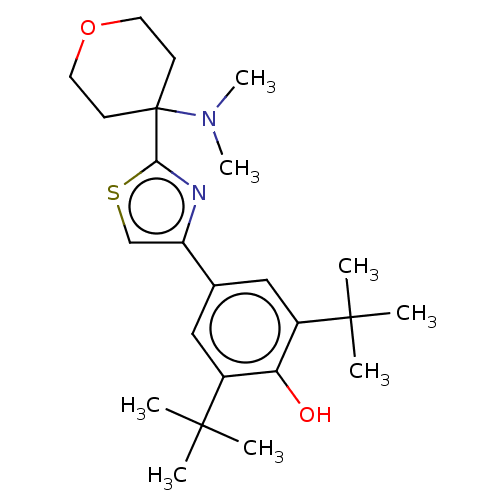
(CHEMBL3581222)Show SMILES CN(C)C1(CCOCC1)c1nc(cs1)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C24H36N2O2S/c1-22(2,3)17-13-16(14-18(20(17)27)23(4,5)6)19-15-29-21(25-19)24(26(7)8)9-11-28-12-10-24/h13-15,27H,9-12H2,1-8H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50089958
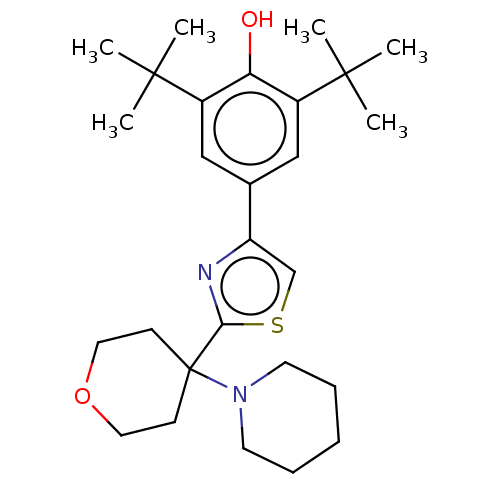
(CHEMBL3581226)Show SMILES CC(C)(C)c1cc(cc(c1O)C(C)(C)C)-c1csc(n1)C1(CCOCC1)N1CCCCC1 Show InChI InChI=1S/C27H40N2O2S/c1-25(2,3)20-16-19(17-21(23(20)30)26(4,5)6)22-18-32-24(28-22)27(10-14-31-15-11-27)29-12-8-7-9-13-29/h16-18,30H,7-15H2,1-6H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50089956
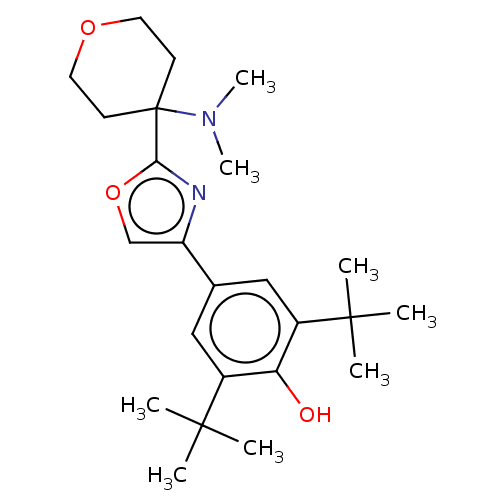
(CHEMBL3581228)Show SMILES CN(C)C1(CCOCC1)c1nc(co1)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C24H36N2O3/c1-22(2,3)17-13-16(14-18(20(17)27)23(4,5)6)19-15-29-21(25-19)24(26(7)8)9-11-28-12-10-24/h13-15,27H,9-12H2,1-8H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50089959
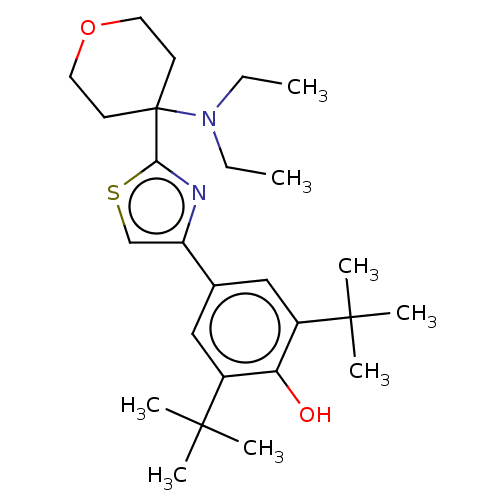
(CHEMBL3581225)Show SMILES CCN(CC)C1(CCOCC1)c1nc(cs1)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C26H40N2O2S/c1-9-28(10-2)26(11-13-30-14-12-26)23-27-21(17-31-23)18-15-19(24(3,4)5)22(29)20(16-18)25(6,7)8/h15-17,29H,9-14H2,1-8H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50089968
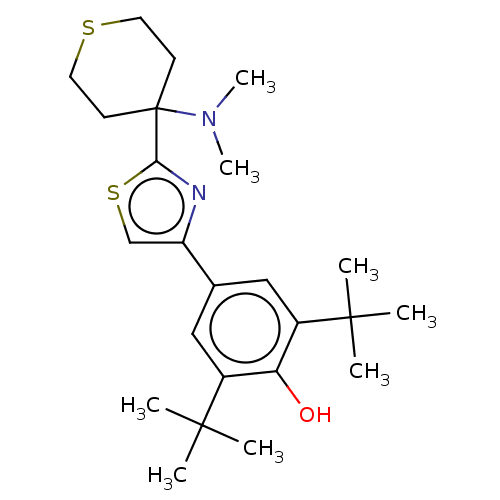
(CHEMBL3581220)Show SMILES CN(C)C1(CCSCC1)c1nc(cs1)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C24H36N2OS2/c1-22(2,3)17-13-16(14-18(20(17)27)23(4,5)6)19-15-29-21(25-19)24(26(7)8)9-11-28-12-10-24/h13-15,27H,9-12H2,1-8H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50089970
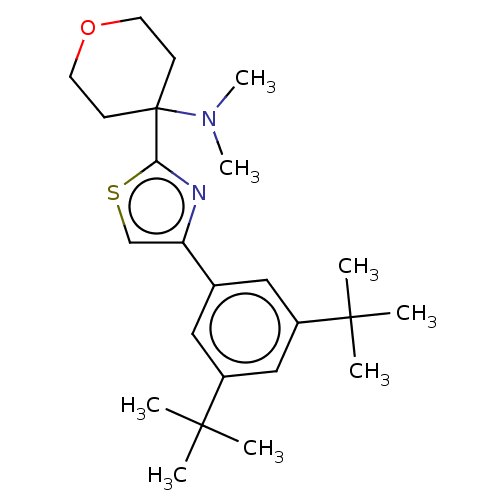
(CHEMBL3581229)Show SMILES CN(C)C1(CCOCC1)c1nc(cs1)-c1cc(cc(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C24H36N2OS/c1-22(2,3)18-13-17(14-19(15-18)23(4,5)6)20-16-28-21(25-20)24(26(7)8)9-11-27-12-10-24/h13-16H,9-12H2,1-8H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 127 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50089963
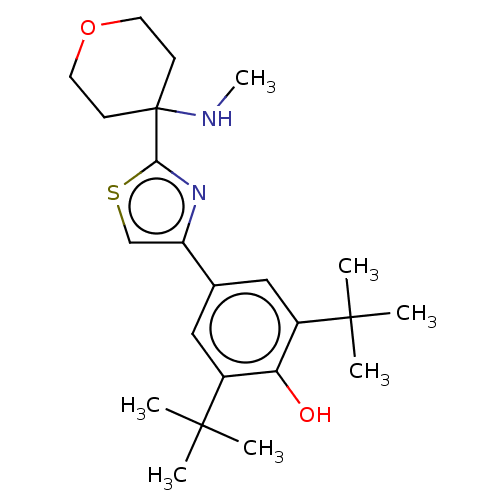
(CHEMBL3581224)Show SMILES CNC1(CCOCC1)c1nc(cs1)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C23H34N2O2S/c1-21(2,3)16-12-15(13-17(19(16)26)22(4,5)6)18-14-28-20(25-18)23(24-7)8-10-27-11-9-23/h12-14,24,26H,8-11H2,1-7H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 144 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50089957
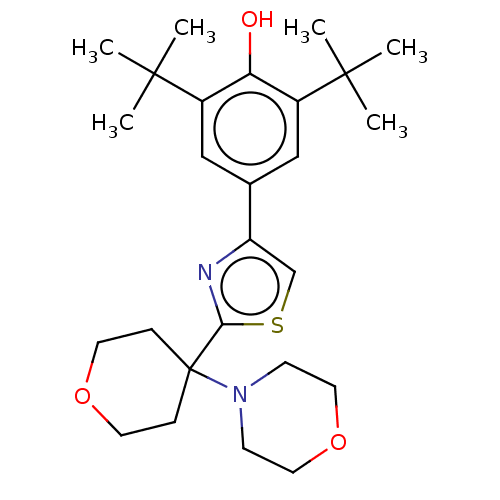
(CHEMBL3581227)Show SMILES CC(C)(C)c1cc(cc(c1O)C(C)(C)C)-c1csc(n1)C1(CCOCC1)N1CCOCC1 Show InChI InChI=1S/C26H38N2O3S/c1-24(2,3)19-15-18(16-20(22(19)29)25(4,5)6)21-17-32-23(27-21)26(7-11-30-12-8-26)28-9-13-31-14-10-28/h15-17,29H,7-14H2,1-6H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 177 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50089956

(CHEMBL3581228)Show SMILES CN(C)C1(CCOCC1)c1nc(co1)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C24H36N2O3/c1-22(2,3)17-13-16(14-18(20(17)27)23(4,5)6)19-15-29-21(25-19)24(26(7)8)9-11-28-12-10-24/h13-15,27H,9-12H2,1-8H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 817 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50089965

(CHEMBL3581222)Show SMILES CN(C)C1(CCOCC1)c1nc(cs1)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C24H36N2O2S/c1-22(2,3)17-13-16(14-18(20(17)27)23(4,5)6)19-15-29-21(25-19)24(26(7)8)9-11-28-12-10-24/h13-15,27H,9-12H2,1-8H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 857 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21283

(3-[(2-iodo-5-nitrophenyl)carbonyl]-1-[(1-methylpip...)Show SMILES CN1CCCCC1Cn1cc(C(=O)c2cc(ccc2I)[N+]([O-])=O)c2ccccc12 Show InChI InChI=1S/C22H22IN3O3/c1-24-11-5-4-6-16(24)13-25-14-19(17-7-2-3-8-21(17)25)22(27)18-12-15(26(28)29)9-10-20(18)23/h2-3,7-10,12,14,16H,4-6,11,13H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50089958

(CHEMBL3581226)Show SMILES CC(C)(C)c1cc(cc(c1O)C(C)(C)C)-c1csc(n1)C1(CCOCC1)N1CCCCC1 Show InChI InChI=1S/C27H40N2O2S/c1-25(2,3)20-16-19(17-21(23(20)30)26(4,5)6)22-18-32-24(28-22)27(10-14-31-15-11-27)29-12-8-7-9-13-29/h16-18,30H,7-15H2,1-6H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 2.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50089963

(CHEMBL3581224)Show SMILES CNC1(CCOCC1)c1nc(cs1)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C23H34N2O2S/c1-21(2,3)16-12-15(13-17(19(16)26)22(4,5)6)18-14-28-20(25-18)23(24-7)8-10-27-11-9-23/h12-14,24,26H,8-11H2,1-7H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50089968

(CHEMBL3581220)Show SMILES CN(C)C1(CCSCC1)c1nc(cs1)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C24H36N2OS2/c1-22(2,3)17-13-16(14-18(20(17)27)23(4,5)6)19-15-29-21(25-19)24(26(7)8)9-11-28-12-10-24/h13-15,27H,9-12H2,1-8H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50089964
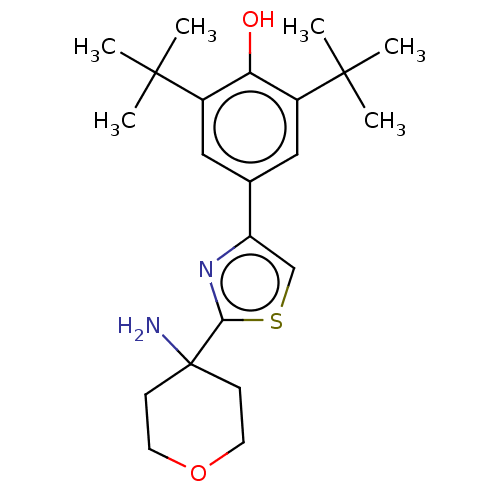
(CHEMBL3581223)Show SMILES CC(C)(C)c1cc(cc(c1O)C(C)(C)C)-c1csc(n1)C1(N)CCOCC1 Show InChI InChI=1S/C22H32N2O2S/c1-20(2,3)15-11-14(12-16(18(15)25)21(4,5)6)17-13-27-19(24-17)22(23)7-9-26-10-8-22/h11-13,25H,7-10,23H2,1-6H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50089959

(CHEMBL3581225)Show SMILES CCN(CC)C1(CCOCC1)c1nc(cs1)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C26H40N2O2S/c1-9-28(10-2)26(11-13-30-14-12-26)23-27-21(17-31-23)18-15-19(24(3,4)5)22(29)20(16-18)25(6,7)8/h15-17,29H,9-14H2,1-8H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50089970

(CHEMBL3581229)Show SMILES CN(C)C1(CCOCC1)c1nc(cs1)-c1cc(cc(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C24H36N2OS/c1-22(2,3)18-13-17(14-19(15-18)23(4,5)6)20-16-28-21(25-20)24(26(7)8)9-11-27-12-10-24/h13-16H,9-12H2,1-8H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50089966
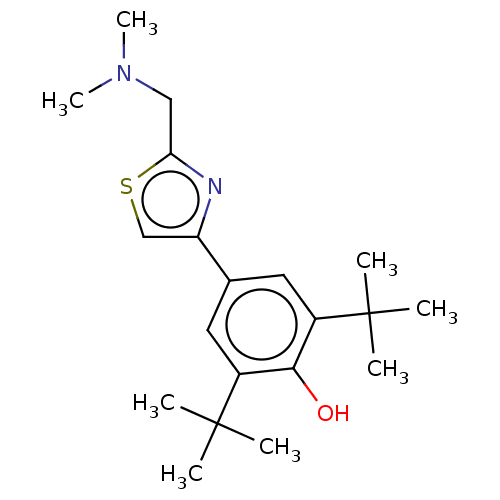
(CHEMBL3581221)Show SMILES CN(C)Cc1nc(cs1)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C20H30N2OS/c1-19(2,3)14-9-13(10-15(18(14)23)20(4,5)6)16-12-24-17(21-16)11-22(7)8/h9-10,12,23H,11H2,1-8H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50089964

(CHEMBL3581223)Show SMILES CC(C)(C)c1cc(cc(c1O)C(C)(C)C)-c1csc(n1)C1(N)CCOCC1 Show InChI InChI=1S/C22H32N2O2S/c1-20(2,3)15-11-14(12-16(18(15)25)21(4,5)6)17-13-27-19(24-17)22(23)7-9-26-10-8-22/h11-13,25H,7-10,23H2,1-6H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cell membrane by competitive displacement assay |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50517832
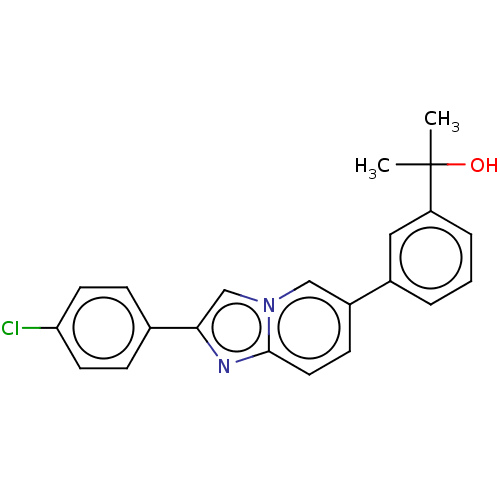
(CHEMBL4564985)Show SMILES CC(C)(O)c1cccc(c1)-c1ccc2nc(cn2c1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C22H19ClN2O/c1-22(2,26)18-5-3-4-16(12-18)17-8-11-21-24-20(14-25(21)13-17)15-6-9-19(23)10-7-15/h3-14,26H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 29: 929-932 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.024
BindingDB Entry DOI: 10.7270/Q2BG2SB9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50517833
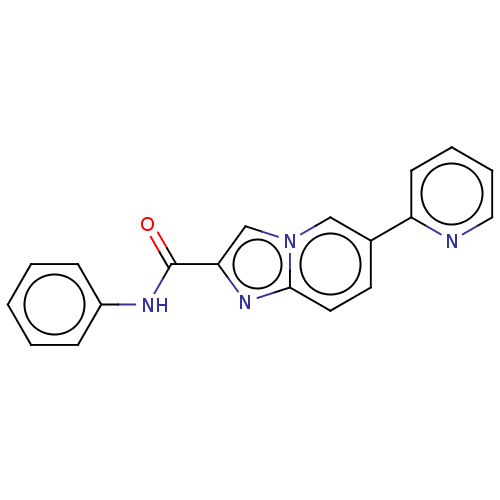
(CHEMBL4472621)Show InChI InChI=1S/C19H14N4O/c24-19(21-15-6-2-1-3-7-15)17-13-23-12-14(9-10-18(23)22-17)16-8-4-5-11-20-16/h1-13H,(H,21,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 29: 929-932 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.024
BindingDB Entry DOI: 10.7270/Q2BG2SB9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50517811

(CHEMBL4576867)Show InChI InChI=1S/C20H15ClN2O/c21-18-7-4-15(5-8-18)19-12-23-11-17(6-9-20(23)22-19)16-3-1-2-14(10-16)13-24/h1-12,24H,13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 29: 929-932 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.024
BindingDB Entry DOI: 10.7270/Q2BG2SB9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50517811

(CHEMBL4576867)Show InChI InChI=1S/C20H15ClN2O/c21-18-7-4-15(5-8-18)19-12-23-11-17(6-9-20(23)22-19)16-3-1-2-14(10-16)13-24/h1-12,24H,13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 29: 929-932 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.024
BindingDB Entry DOI: 10.7270/Q2BG2SB9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50517811

(CHEMBL4576867)Show InChI InChI=1S/C20H15ClN2O/c21-18-7-4-15(5-8-18)19-12-23-11-17(6-9-20(23)22-19)16-3-1-2-14(10-16)13-24/h1-12,24H,13H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 29: 929-932 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.024
BindingDB Entry DOI: 10.7270/Q2BG2SB9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50517833

(CHEMBL4472621)Show InChI InChI=1S/C19H14N4O/c24-19(21-15-6-2-1-3-7-15)17-13-23-12-14(9-10-18(23)22-17)16-8-4-5-11-20-16/h1-13H,(H,21,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) |
Bioorg Med Chem Lett 29: 929-932 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.024
BindingDB Entry DOI: 10.7270/Q2BG2SB9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50517833

(CHEMBL4472621)Show InChI InChI=1S/C19H14N4O/c24-19(21-15-6-2-1-3-7-15)17-13-23-12-14(9-10-18(23)22-17)16-8-4-5-11-20-16/h1-13H,(H,21,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 29: 929-932 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.024
BindingDB Entry DOI: 10.7270/Q2BG2SB9 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50089968

(CHEMBL3581220)Show SMILES CN(C)C1(CCSCC1)c1nc(cs1)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C24H36N2OS2/c1-22(2,3)17-13-16(14-18(20(17)27)23(4,5)6)19-15-29-21(25-19)24(26(7)8)9-11-28-12-10-24/h13-15,27H,9-12H2,1-8H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 59 | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50089965

(CHEMBL3581222)Show SMILES CN(C)C1(CCOCC1)c1nc(cs1)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C24H36N2O2S/c1-22(2,3)17-13-16(14-18(20(17)27)23(4,5)6)19-15-29-21(25-19)24(26(7)8)9-11-28-12-10-24/h13-15,27H,9-12H2,1-8H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 42 | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50089959

(CHEMBL3581225)Show SMILES CCN(CC)C1(CCOCC1)c1nc(cs1)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C26H40N2O2S/c1-9-28(10-2)26(11-13-30-14-12-26)23-27-21(17-31-23)18-15-19(24(3,4)5)22(29)20(16-18)25(6,7)8/h15-17,29H,9-14H2,1-8H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 72 | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50089958

(CHEMBL3581226)Show SMILES CC(C)(C)c1cc(cc(c1O)C(C)(C)C)-c1csc(n1)C1(CCOCC1)N1CCCCC1 Show InChI InChI=1S/C27H40N2O2S/c1-25(2,3)20-16-19(17-21(23(20)30)26(4,5)6)22-18-32-24(28-22)27(10-14-31-15-11-27)29-12-8-7-9-13-29/h16-18,30H,7-15H2,1-6H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50089956

(CHEMBL3581228)Show SMILES CN(C)C1(CCOCC1)c1nc(co1)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C24H36N2O3/c1-22(2,3)17-13-16(14-18(20(17)27)23(4,5)6)19-15-29-21(25-19)24(26(7)8)9-11-28-12-10-24/h13-15,27H,9-12H2,1-8H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 44 | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50089968

(CHEMBL3581220)Show SMILES CN(C)C1(CCSCC1)c1nc(cs1)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C24H36N2OS2/c1-22(2,3)17-13-16(14-18(20(17)27)23(4,5)6)19-15-29-21(25-19)24(26(7)8)9-11-28-12-10-24/h13-15,27H,9-12H2,1-8H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.38E+3 | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Agonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50089965

(CHEMBL3581222)Show SMILES CN(C)C1(CCOCC1)c1nc(cs1)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C24H36N2O2S/c1-22(2,3)17-13-16(14-18(20(17)27)23(4,5)6)19-15-29-21(25-19)24(26(7)8)9-11-28-12-10-24/h13-15,27H,9-12H2,1-8H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.67E+3 | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Agonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50089959

(CHEMBL3581225)Show SMILES CCN(CC)C1(CCOCC1)c1nc(cs1)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C26H40N2O2S/c1-9-28(10-2)26(11-13-30-14-12-26)23-27-21(17-31-23)18-15-19(24(3,4)5)22(29)20(16-18)25(6,7)8/h15-17,29H,9-14H2,1-8H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 4.04E+3 | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Agonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50089958

(CHEMBL3581226)Show SMILES CC(C)(C)c1cc(cc(c1O)C(C)(C)C)-c1csc(n1)C1(CCOCC1)N1CCCCC1 Show InChI InChI=1S/C27H40N2O2S/c1-25(2,3)20-16-19(17-21(23(20)30)26(4,5)6)22-18-32-24(28-22)27(10-14-31-15-11-27)29-12-8-7-9-13-29/h16-18,30H,7-15H2,1-6H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.26E+3 | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Agonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50089956

(CHEMBL3581228)Show SMILES CN(C)C1(CCOCC1)c1nc(co1)-c1cc(c(O)c(c1)C(C)(C)C)C(C)(C)C Show InChI InChI=1S/C24H36N2O3/c1-22(2,3)17-13-16(14-18(20(17)27)23(4,5)6)19-15-29-21(25-19)24(26(7)8)9-11-28-12-10-24/h13-15,27H,9-12H2,1-8H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Agonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM21283

(3-[(2-iodo-5-nitrophenyl)carbonyl]-1-[(1-methylpip...)Show SMILES CN1CCCCC1Cn1cc(C(=O)c2cc(ccc2I)[N+]([O-])=O)c2ccccc12 Show InChI InChI=1S/C22H22IN3O3/c1-24-11-5-4-6-16(24)13-25-14-19(17-7-2-3-8-21(17)25)22(27)18-12-15(26(28)29)9-10-20(18)23/h2-3,7-10,12,14,16H,4-6,11,13H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 16 | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Agonist activity at human CB2 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM21283

(3-[(2-iodo-5-nitrophenyl)carbonyl]-1-[(1-methylpip...)Show SMILES CN1CCCCC1Cn1cc(C(=O)c2cc(ccc2I)[N+]([O-])=O)c2ccccc12 Show InChI InChI=1S/C22H22IN3O3/c1-24-11-5-4-6-16(24)13-25-14-19(17-7-2-3-8-21(17)25)22(27)18-12-15(26(28)29)9-10-20(18)23/h2-3,7-10,12,14,16H,4-6,11,13H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 1.04E+3 | n/a | n/a | n/a | n/a |
Ipsen Innovation
Curated by ChEMBL
| Assay Description
Agonist activity at human CB1 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production |
Bioorg Med Chem Lett 25: 88-91 (2015)
Article DOI: 10.1016/j.bmcl.2014.11.003
BindingDB Entry DOI: 10.7270/Q2736SN4 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Homo sapiens (Human)) | BDBM50517808
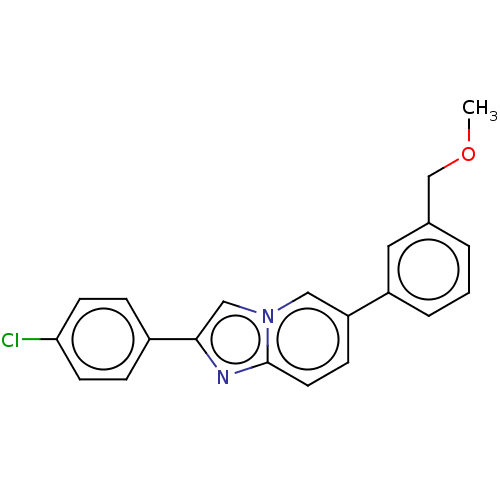
(CHEMBL4465741)Show InChI InChI=1S/C21H17ClN2O/c1-25-14-15-3-2-4-17(11-15)18-7-10-21-23-20(13-24(21)12-18)16-5-8-19(22)9-6-16/h2-13H,14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >3.00E+3 | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Agonist activity at full-length Gal4-fused NOT (unknown origin) expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 29: 929-932 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.024
BindingDB Entry DOI: 10.7270/Q2BG2SB9 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Homo sapiens (Human)) | BDBM50517809
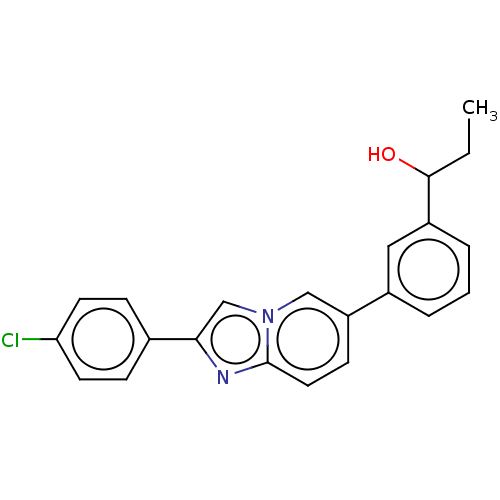
(CHEMBL4587411)Show SMILES CCC(O)c1cccc(c1)-c1ccc2nc(cn2c1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C22H19ClN2O/c1-2-21(26)17-5-3-4-16(12-17)18-8-11-22-24-20(14-25(22)13-18)15-6-9-19(23)10-7-15/h3-14,21,26H,2H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Agonist activity at full-length Gal4-fused NOT (unknown origin) expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 29: 929-932 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.024
BindingDB Entry DOI: 10.7270/Q2BG2SB9 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Mus musculus) | BDBM50517810
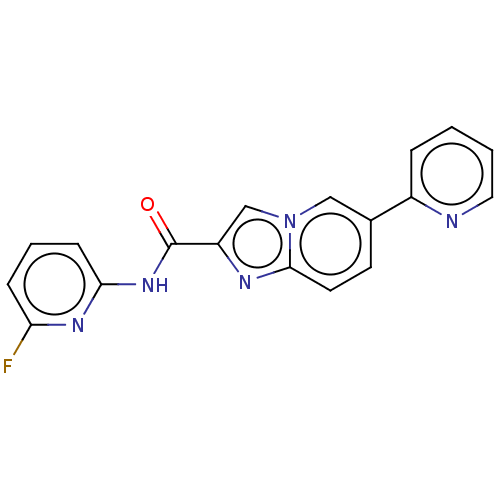
(CHEMBL4579076)Show InChI InChI=1S/C18H12FN5O/c19-15-5-3-6-16(22-15)23-18(25)14-11-24-10-12(7-8-17(24)21-14)13-4-1-2-9-20-13/h1-11H,(H,22,23,25) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Agonist activity at Nurr1 in mouse N2A cells harboring NBRE by luciferase reporter gene assay |
Bioorg Med Chem Lett 29: 929-932 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.024
BindingDB Entry DOI: 10.7270/Q2BG2SB9 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Mus musculus) | BDBM50517811

(CHEMBL4576867)Show InChI InChI=1S/C20H15ClN2O/c21-18-7-4-15(5-8-18)19-12-23-11-17(6-9-20(23)22-19)16-3-1-2-14(10-16)13-24/h1-12,24H,13H2 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Agonist activity at Nurr1 in mouse N2A cells harboring NBRE by luciferase reporter gene assay |
Bioorg Med Chem Lett 29: 929-932 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.024
BindingDB Entry DOI: 10.7270/Q2BG2SB9 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Homo sapiens (Human)) | BDBM50517812
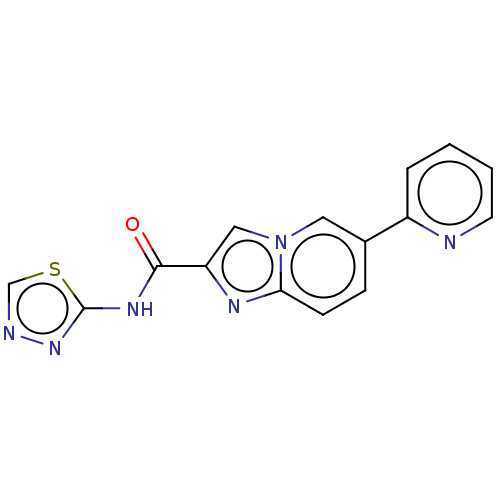
(CHEMBL4454933)Show InChI InChI=1S/C15H10N6OS/c22-14(19-15-20-17-9-23-15)12-8-21-7-10(4-5-13(21)18-12)11-3-1-2-6-16-11/h1-9H,(H,19,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Agonist activity at full-length Gal4-fused NOT (unknown origin) expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 29: 929-932 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.024
BindingDB Entry DOI: 10.7270/Q2BG2SB9 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Homo sapiens (Human)) | BDBM50517813
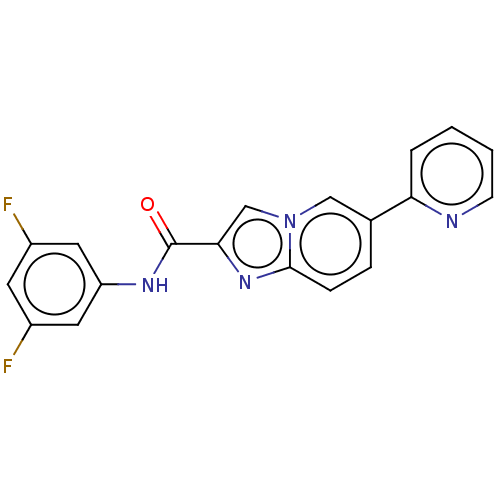
(CHEMBL4464547)Show SMILES Fc1cc(F)cc(NC(=O)c2cn3cc(ccc3n2)-c2ccccn2)c1 Show InChI InChI=1S/C19H12F2N4O/c20-13-7-14(21)9-15(8-13)23-19(26)17-11-25-10-12(4-5-18(25)24-17)16-3-1-2-6-22-16/h1-11H,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Agonist activity at full-length Gal4-fused NOT (unknown origin) expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 29: 929-932 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.024
BindingDB Entry DOI: 10.7270/Q2BG2SB9 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Homo sapiens (Human)) | BDBM50517814
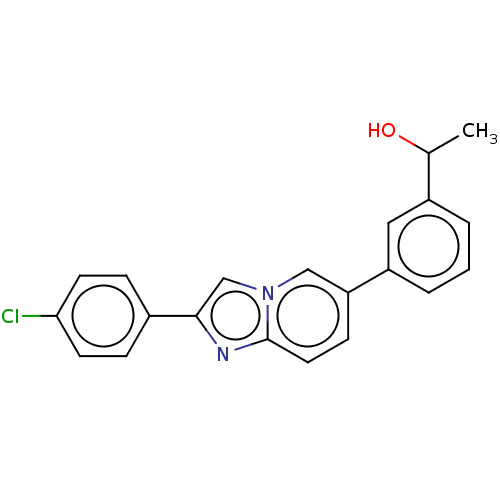
(CHEMBL4461137)Show SMILES CC(O)c1cccc(c1)-c1ccc2nc(cn2c1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C21H17ClN2O/c1-14(25)16-3-2-4-17(11-16)18-7-10-21-23-20(13-24(21)12-18)15-5-8-19(22)9-6-15/h2-14,25H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 219 | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Agonist activity at full-length Gal4-fused NOT (unknown origin) expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 29: 929-932 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.024
BindingDB Entry DOI: 10.7270/Q2BG2SB9 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Homo sapiens (Human)) | BDBM50517815

(CHEMBL4559712)Show SMILES CC(O)(CO)c1cccc(c1)-c1ccc2nc(cn2c1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C22H19ClN2O2/c1-22(27,14-26)18-4-2-3-16(11-18)17-7-10-21-24-20(13-25(21)12-17)15-5-8-19(23)9-6-15/h2-13,26-27H,14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Agonist activity at full-length Gal4-fused NOT (unknown origin) expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 29: 929-932 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.024
BindingDB Entry DOI: 10.7270/Q2BG2SB9 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Homo sapiens (Human)) | BDBM50517816
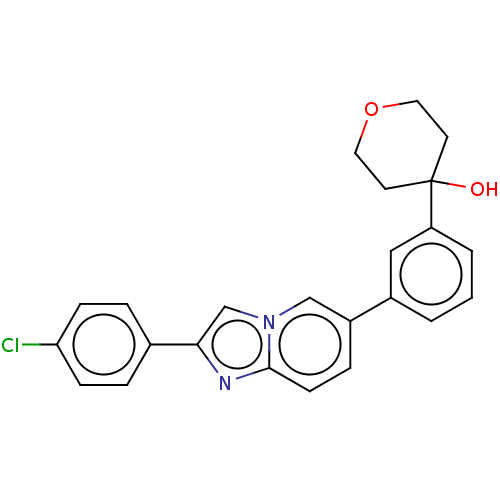
(CHEMBL4440984)Show SMILES OC1(CCOCC1)c1cccc(c1)-c1ccc2nc(cn2c1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C24H21ClN2O2/c25-21-7-4-17(5-8-21)22-16-27-15-19(6-9-23(27)26-22)18-2-1-3-20(14-18)24(28)10-12-29-13-11-24/h1-9,14-16,28H,10-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Agonist activity at full-length Gal4-fused NOT (unknown origin) expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 29: 929-932 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.024
BindingDB Entry DOI: 10.7270/Q2BG2SB9 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Homo sapiens (Human)) | BDBM50517817

(CHEMBL4446736)Show SMILES OC1(COC1)c1cccc(c1)-c1ccc2nc(cn2c1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C22H17ClN2O2/c23-19-7-4-15(5-8-19)20-12-25-11-17(6-9-21(25)24-20)16-2-1-3-18(10-16)22(26)13-27-14-22/h1-12,26H,13-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 323 | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Agonist activity at full-length Gal4-fused NOT (unknown origin) expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 29: 929-932 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.024
BindingDB Entry DOI: 10.7270/Q2BG2SB9 |
More data for this
Ligand-Target Pair | |
Nuclear receptor subfamily 4 group A member 2
(Homo sapiens (Human)) | BDBM50517814

(CHEMBL4461137)Show SMILES CC(O)c1cccc(c1)-c1ccc2nc(cn2c1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C21H17ClN2O/c1-14(25)16-3-2-4-17(11-16)18-7-10-21-23-20(13-24(21)12-18)15-5-8-19(22)9-6-15/h2-14,25H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | n/a | 75 | n/a | n/a | n/a | n/a |
Sanofi
Curated by ChEMBL
| Assay Description
Agonist activity at full-length Gal4-fused NOT (unknown origin) expressed in CHO cells by luciferase reporter gene assay |
Bioorg Med Chem Lett 29: 929-932 (2019)
Article DOI: 10.1016/j.bmcl.2019.01.024
BindingDB Entry DOI: 10.7270/Q2BG2SB9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data