

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21283 (3-[(2-iodo-5-nitrophenyl)carbonyl]-1-[(1-methylpip...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO-K1 cell membrane by competitive displacement assay | Bioorg Med Chem Lett 25: 88-91 (2015) Article DOI: 10.1016/j.bmcl.2014.11.003 BindingDB Entry DOI: 10.7270/Q2736SN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50089965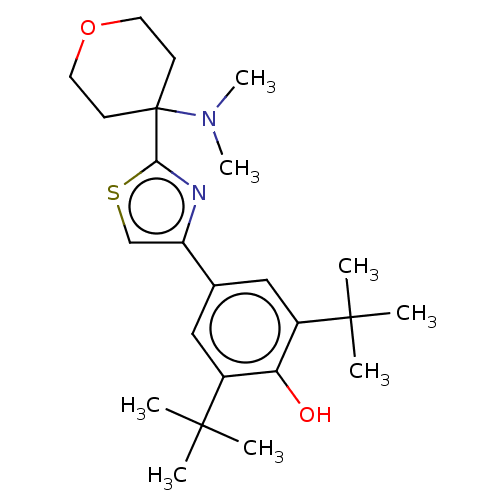 (CHEMBL3581222) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO-K1 cell membrane by competitive displacement assay | Bioorg Med Chem Lett 25: 88-91 (2015) Article DOI: 10.1016/j.bmcl.2014.11.003 BindingDB Entry DOI: 10.7270/Q2736SN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50089958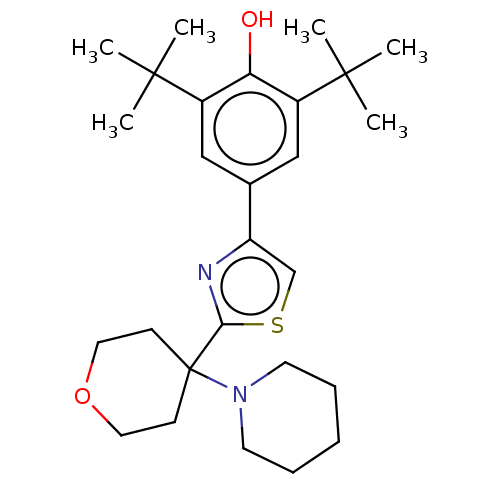 (CHEMBL3581226) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO-K1 cell membrane by competitive displacement assay | Bioorg Med Chem Lett 25: 88-91 (2015) Article DOI: 10.1016/j.bmcl.2014.11.003 BindingDB Entry DOI: 10.7270/Q2736SN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50089956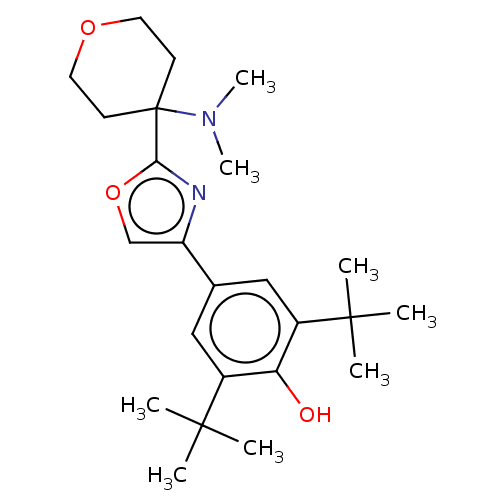 (CHEMBL3581228) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO-K1 cell membrane by competitive displacement assay | Bioorg Med Chem Lett 25: 88-91 (2015) Article DOI: 10.1016/j.bmcl.2014.11.003 BindingDB Entry DOI: 10.7270/Q2736SN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50089959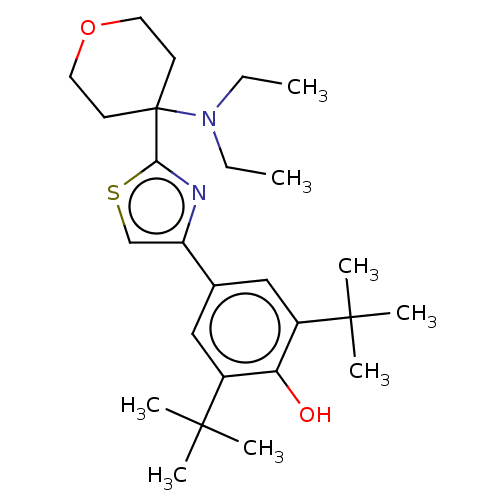 (CHEMBL3581225) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO-K1 cell membrane by competitive displacement assay | Bioorg Med Chem Lett 25: 88-91 (2015) Article DOI: 10.1016/j.bmcl.2014.11.003 BindingDB Entry DOI: 10.7270/Q2736SN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50089968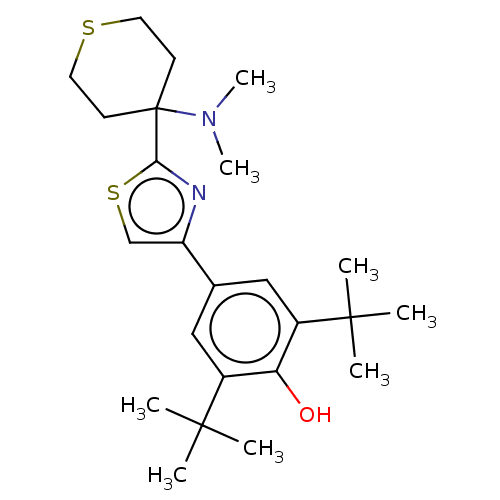 (CHEMBL3581220) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO-K1 cell membrane by competitive displacement assay | Bioorg Med Chem Lett 25: 88-91 (2015) Article DOI: 10.1016/j.bmcl.2014.11.003 BindingDB Entry DOI: 10.7270/Q2736SN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50089970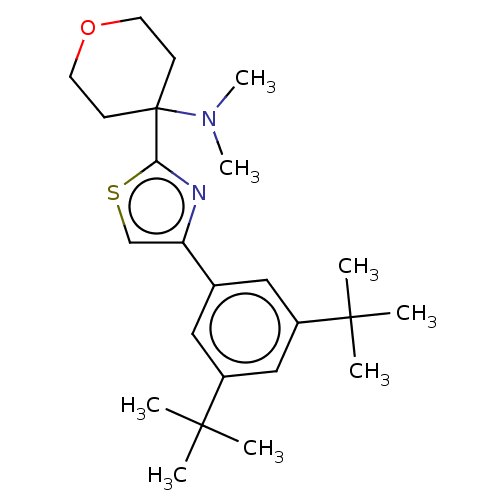 (CHEMBL3581229) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 127 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO-K1 cell membrane by competitive displacement assay | Bioorg Med Chem Lett 25: 88-91 (2015) Article DOI: 10.1016/j.bmcl.2014.11.003 BindingDB Entry DOI: 10.7270/Q2736SN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50089963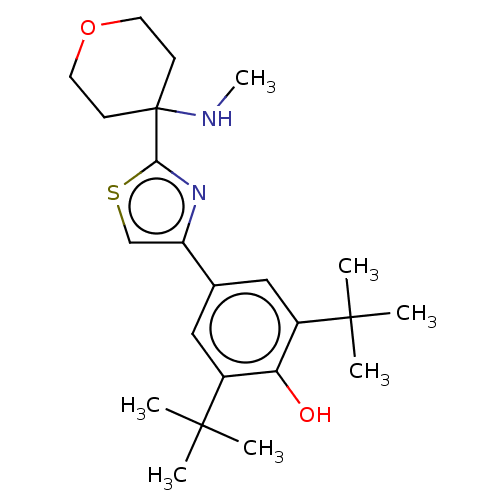 (CHEMBL3581224) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 144 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO-K1 cell membrane by competitive displacement assay | Bioorg Med Chem Lett 25: 88-91 (2015) Article DOI: 10.1016/j.bmcl.2014.11.003 BindingDB Entry DOI: 10.7270/Q2736SN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50089957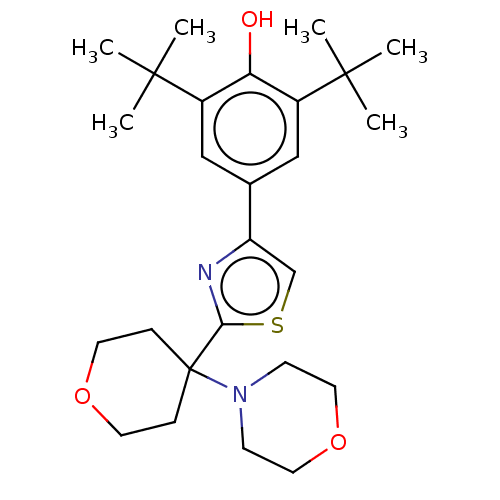 (CHEMBL3581227) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 177 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO-K1 cell membrane by competitive displacement assay | Bioorg Med Chem Lett 25: 88-91 (2015) Article DOI: 10.1016/j.bmcl.2014.11.003 BindingDB Entry DOI: 10.7270/Q2736SN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50089956 (CHEMBL3581228) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 817 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cell membrane by competitive displacement assay | Bioorg Med Chem Lett 25: 88-91 (2015) Article DOI: 10.1016/j.bmcl.2014.11.003 BindingDB Entry DOI: 10.7270/Q2736SN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50089965 (CHEMBL3581222) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 857 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cell membrane by competitive displacement assay | Bioorg Med Chem Lett 25: 88-91 (2015) Article DOI: 10.1016/j.bmcl.2014.11.003 BindingDB Entry DOI: 10.7270/Q2736SN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21283 (3-[(2-iodo-5-nitrophenyl)carbonyl]-1-[(1-methylpip...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | 2.08E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cell membrane by competitive displacement assay | Bioorg Med Chem Lett 25: 88-91 (2015) Article DOI: 10.1016/j.bmcl.2014.11.003 BindingDB Entry DOI: 10.7270/Q2736SN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50089958 (CHEMBL3581226) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents | Article PubMed | 2.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cell membrane by competitive displacement assay | Bioorg Med Chem Lett 25: 88-91 (2015) Article DOI: 10.1016/j.bmcl.2014.11.003 BindingDB Entry DOI: 10.7270/Q2736SN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50089963 (CHEMBL3581224) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.27E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cell membrane by competitive displacement assay | Bioorg Med Chem Lett 25: 88-91 (2015) Article DOI: 10.1016/j.bmcl.2014.11.003 BindingDB Entry DOI: 10.7270/Q2736SN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50089968 (CHEMBL3581220) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cell membrane by competitive displacement assay | Bioorg Med Chem Lett 25: 88-91 (2015) Article DOI: 10.1016/j.bmcl.2014.11.003 BindingDB Entry DOI: 10.7270/Q2736SN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50089964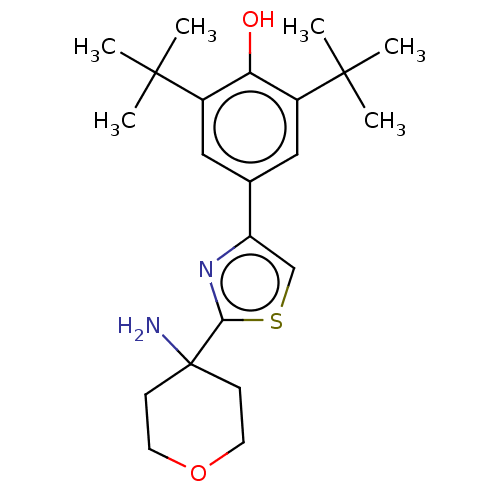 (CHEMBL3581223) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO-K1 cell membrane by competitive displacement assay | Bioorg Med Chem Lett 25: 88-91 (2015) Article DOI: 10.1016/j.bmcl.2014.11.003 BindingDB Entry DOI: 10.7270/Q2736SN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50089959 (CHEMBL3581225) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cell membrane by competitive displacement assay | Bioorg Med Chem Lett 25: 88-91 (2015) Article DOI: 10.1016/j.bmcl.2014.11.003 BindingDB Entry DOI: 10.7270/Q2736SN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50089970 (CHEMBL3581229) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cell membrane by competitive displacement assay | Bioorg Med Chem Lett 25: 88-91 (2015) Article DOI: 10.1016/j.bmcl.2014.11.003 BindingDB Entry DOI: 10.7270/Q2736SN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50089966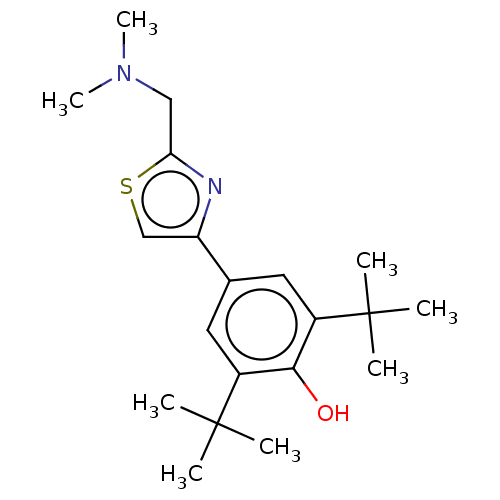 (CHEMBL3581221) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB2 receptor expressed in CHO-K1 cell membrane by competitive displacement assay | Bioorg Med Chem Lett 25: 88-91 (2015) Article DOI: 10.1016/j.bmcl.2014.11.003 BindingDB Entry DOI: 10.7270/Q2736SN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50089964 (CHEMBL3581223) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Ipsen Innovation Curated by ChEMBL | Assay Description Displacement of [3H]CP55940 from human CB1 receptor expressed in CHO-K1 cell membrane by competitive displacement assay | Bioorg Med Chem Lett 25: 88-91 (2015) Article DOI: 10.1016/j.bmcl.2014.11.003 BindingDB Entry DOI: 10.7270/Q2736SN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50065843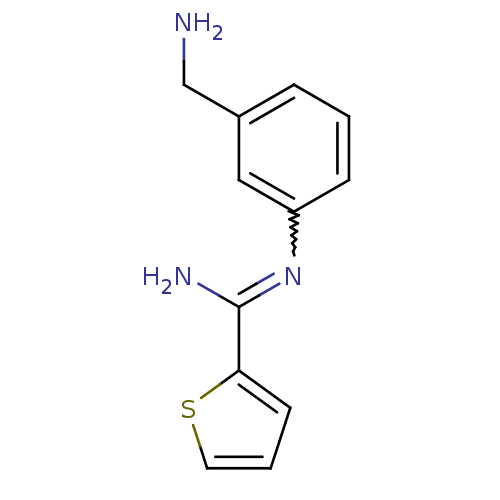 (CHEMBL553081 | CHEMBL555715 | N-(3-Aminomethyl-phe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour Curated by ChEMBL | Assay Description Inhibitory concentration tested against neuronal nitric oxide synthase (nNOS ) from rat cerebellum | Bioorg Med Chem Lett 13: 209-12 (2002) BindingDB Entry DOI: 10.7270/Q2K9382B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50122299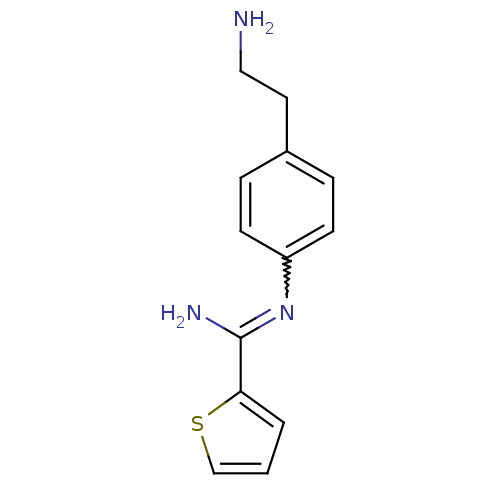 (CHEMBL538500 | N-[4-(2-Amino-ethyl)-phenyl]-thioph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour Curated by ChEMBL | Assay Description Inhibitory concentration tested against neuronal nitric oxide synthase (nNOS ) from rat cerebellum | Bioorg Med Chem Lett 13: 209-12 (2002) BindingDB Entry DOI: 10.7270/Q2K9382B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50122300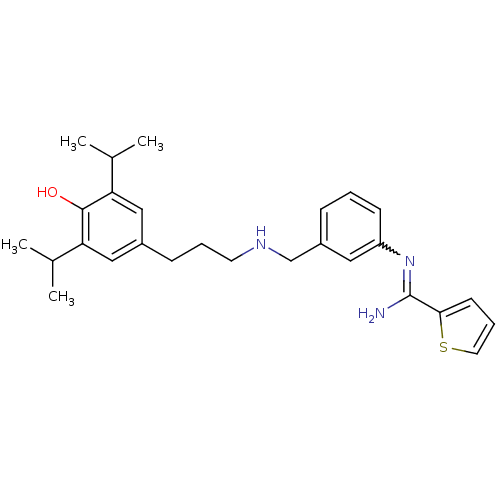 (CHEMBL541300 | N-(3-{[3-(4-Hydroxy-3,5-diisopropyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour Curated by ChEMBL | Assay Description Inhibitory concentration tested against neuronal nitric oxide synthase (nNOS ) from rat cerebellum | Bioorg Med Chem Lett 13: 209-12 (2002) BindingDB Entry DOI: 10.7270/Q2K9382B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM196649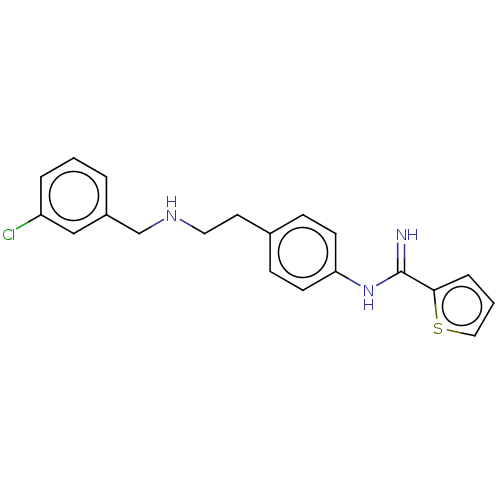 (US9212144, 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank MCE PC cid PC sid PDB UniChem | DrugBank PDB PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour Curated by ChEMBL | Assay Description Inhibitory concentration tested against neuronal nitric oxide synthase (nNOS ) from rat cerebellum | Bioorg Med Chem Lett 13: 209-12 (2002) BindingDB Entry DOI: 10.7270/Q2K9382B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50122301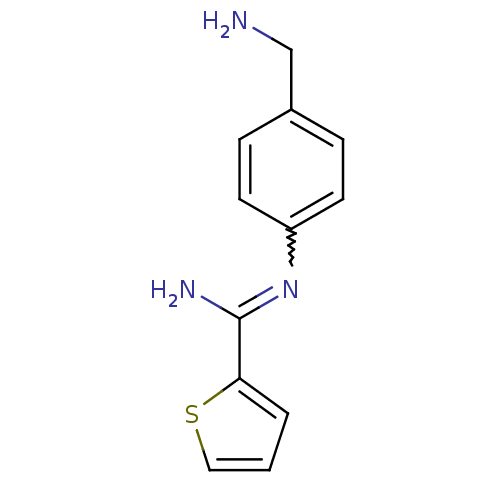 (CHEMBL542633 | N-(4-Aminomethyl-phenyl)-thiophene-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour Curated by ChEMBL | Assay Description Inhibitory concentration tested against neuronal nitric oxide synthase (nNOS ) from rat cerebellum | Bioorg Med Chem Lett 13: 209-12 (2002) BindingDB Entry DOI: 10.7270/Q2K9382B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50122305 (CHEMBL552629 | N-(3-{[3-(3,5-Di-tert-butyl-4-hydro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour Curated by ChEMBL | Assay Description Inhibitory concentration tested against neuronal nitric oxide synthase (nNOS ) from rat cerebellum | Bioorg Med Chem Lett 13: 209-12 (2002) BindingDB Entry DOI: 10.7270/Q2K9382B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50113345 (5-[1,2]Dithiolan-3-yl-pentanoic acid (2-{4-[(thiop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Henri Beaufour Curated by ChEMBL | Assay Description In vitro inhibition concentration of the compound was evaluated on the effect of conversion by Nitric Oxide synthase of [3H]-L-arginine into [3H]-L-c... | Bioorg Med Chem Lett 12: 1439-42 (2002) BindingDB Entry DOI: 10.7270/Q26972WQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50113344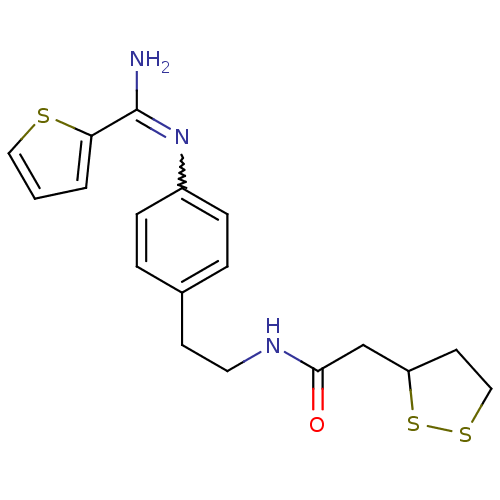 (2-[1,2]Dithiolan-3-yl-N-(2-{4-[(thiophene-2-carbox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Henri Beaufour Curated by ChEMBL | Assay Description In vitro inhibition concentration of the compound was evaluated on the effect of conversion by Nitric Oxide synthase of [3H]-L-arginine into [3H]-L-c... | Bioorg Med Chem Lett 12: 1439-42 (2002) BindingDB Entry DOI: 10.7270/Q26972WQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50122302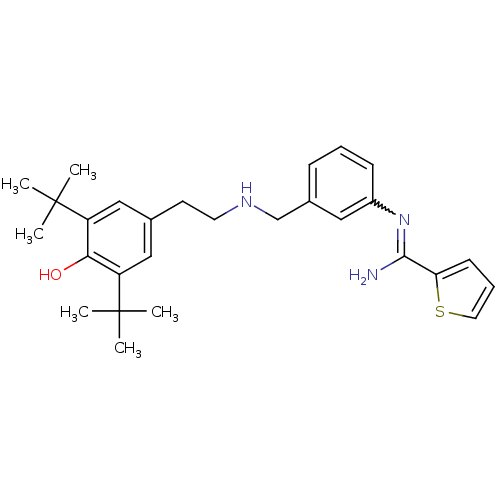 (CHEMBL66682 | N-(3-{[2-(3,5-Di-tert-butyl-4-hydrox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour Curated by ChEMBL | Assay Description Inhibitory concentration tested against neuronal nitric oxide synthase (nNOS ) from rat cerebellum | Bioorg Med Chem Lett 13: 209-12 (2002) BindingDB Entry DOI: 10.7270/Q2K9382B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50122306 (CHEMBL559220 | N-{3-[(3,5-Di-tert-butyl-4-hydroxy-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour Curated by ChEMBL | Assay Description Inhibitory concentration tested against neuronal nitric oxide synthase (nNOS ) from rat cerebellum | Bioorg Med Chem Lett 13: 209-12 (2002) BindingDB Entry DOI: 10.7270/Q2K9382B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50113348 (5-[1,2]Dithiolan-3-yl-pentanoic acid | CHEMBL33240...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour Curated by ChEMBL | Assay Description Inhibitory concentration tested against neuronal nitric oxide synthase (nNOS ) from rat cerebellum | Bioorg Med Chem Lett 13: 209-12 (2002) BindingDB Entry DOI: 10.7270/Q2K9382B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50113347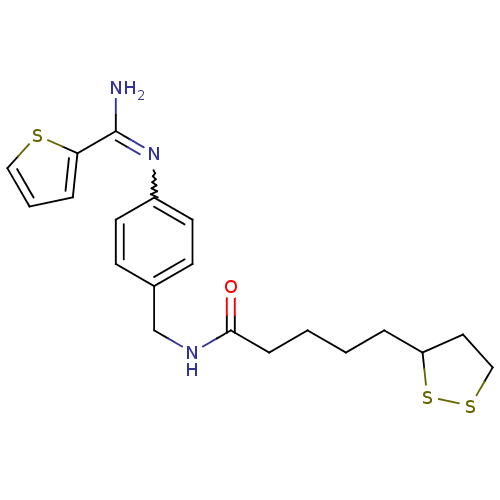 (5-[1,2]Dithiolan-3-yl-pentanoic acid 4-[(thiophene...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Henri Beaufour Curated by ChEMBL | Assay Description In vitro inhibition concentration of the compound was evaluated on the effect of conversion by Nitric Oxide synthase of [3H]-L-arginine into [3H]-L-c... | Bioorg Med Chem Lett 12: 1439-42 (2002) BindingDB Entry DOI: 10.7270/Q26972WQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50113346 (5-[1,2]Dithiolan-3-yl-pentanoic acid {4-[(thiophen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Henri Beaufour Curated by ChEMBL | Assay Description In vitro inhibition concentration of the compound was evaluated on the effect of conversion by Nitric Oxide synthase of [3H]-L-arginine into [3H]-L-c... | Bioorg Med Chem Lett 12: 1439-42 (2002) BindingDB Entry DOI: 10.7270/Q26972WQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50113348 (5-[1,2]Dithiolan-3-yl-pentanoic acid | CHEMBL33240...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Henri Beaufour Curated by ChEMBL | Assay Description In vitro inhibition concentration of the compound was evaluated on the effect of conversion by Nitric oxide synthase of [3H]-L-arginine into [3H]-L-c... | Bioorg Med Chem Lett 12: 1439-42 (2002) BindingDB Entry DOI: 10.7270/Q26972WQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Homo sapiens (Human)) | BDBM50113343 (2-[1,2]Dithiolan-3-yl-N-{4-[(thiophene-2-carboximi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute Henri Beaufour Curated by ChEMBL | Assay Description In vitro inhibition concentration of the compound was evaluated on the effect of conversion by Nitric Oxide synthase of [3H]-L-arginine into [3H]-L-c... | Bioorg Med Chem Lett 12: 1439-42 (2002) BindingDB Entry DOI: 10.7270/Q26972WQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Bos taurus (bovine)) | BDBM50122305 (CHEMBL552629 | N-(3-{[3-(3,5-Di-tert-butyl-4-hydro...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour Curated by ChEMBL | Assay Description Inhibitory concentration tested against bovine endothelial nitric oxide synthase (eNOS) | Bioorg Med Chem Lett 13: 209-12 (2002) BindingDB Entry DOI: 10.7270/Q2K9382B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Bos taurus (bovine)) | BDBM196649 (US9212144, 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | DrugBank MCE PC cid PC sid PDB UniChem | PubMed | n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour Curated by ChEMBL | Assay Description Inhibitory concentration tested against bovine endothelial nitric oxide synthase (eNOS) | Bioorg Med Chem Lett 13: 209-12 (2002) BindingDB Entry DOI: 10.7270/Q2K9382B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Bos taurus (bovine)) | BDBM50122300 (CHEMBL541300 | N-(3-{[3-(4-Hydroxy-3,5-diisopropyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour Curated by ChEMBL | Assay Description Inhibitory concentration tested against bovine endothelial nitric oxide synthase (eNOS) | Bioorg Med Chem Lett 13: 209-12 (2002) BindingDB Entry DOI: 10.7270/Q2K9382B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Bos taurus (bovine)) | BDBM50113348 (5-[1,2]Dithiolan-3-yl-pentanoic acid | CHEMBL33240...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour Curated by ChEMBL | Assay Description Inhibitory concentration tested against bovine endothelial nitric oxide synthase (eNOS) | Bioorg Med Chem Lett 13: 209-12 (2002) BindingDB Entry DOI: 10.7270/Q2K9382B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Bos taurus (bovine)) | BDBM50122302 (CHEMBL66682 | N-(3-{[2-(3,5-Di-tert-butyl-4-hydrox...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour Curated by ChEMBL | Assay Description Inhibitory concentration tested against bovine endothelial nitric oxide synthase (eNOS) | Bioorg Med Chem Lett 13: 209-12 (2002) BindingDB Entry DOI: 10.7270/Q2K9382B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Bos taurus (bovine)) | BDBM50122306 (CHEMBL559220 | N-{3-[(3,5-Di-tert-butyl-4-hydroxy-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour Curated by ChEMBL | Assay Description Inhibitory concentration tested against bovine endothelial nitric oxide synthase (eNOS) | Bioorg Med Chem Lett 13: 209-12 (2002) BindingDB Entry DOI: 10.7270/Q2K9382B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, brain (Rattus norvegicus (rat)) | BDBM50122304 (CHEMBL539004 | N-(4-Amino-phenyl)-thiophene-2-carb...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour Curated by ChEMBL | Assay Description Inhibitory concentration tested against neuronal nitric oxide synthase (nNOS ) from rat cerebellum | Bioorg Med Chem Lett 13: 209-12 (2002) BindingDB Entry DOI: 10.7270/Q2K9382B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Bos taurus (bovine)) | BDBM50065843 (CHEMBL553081 | CHEMBL555715 | N-(3-Aminomethyl-phe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour Curated by ChEMBL | Assay Description Inhibitory concentration tested against bovine endothelial nitric oxide synthase (eNOS) | Bioorg Med Chem Lett 13: 209-12 (2002) BindingDB Entry DOI: 10.7270/Q2K9382B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Bos taurus (bovine)) | BDBM50122299 (CHEMBL538500 | N-[4-(2-Amino-ethyl)-phenyl]-thioph...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour Curated by ChEMBL | Assay Description Inhibitory concentration tested against bovine endothelial nitric oxide synthase (eNOS) | Bioorg Med Chem Lett 13: 209-12 (2002) BindingDB Entry DOI: 10.7270/Q2K9382B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Bos taurus (bovine)) | BDBM50122301 (CHEMBL542633 | N-(4-Aminomethyl-phenyl)-thiophene-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.71E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour Curated by ChEMBL | Assay Description Inhibitory concentration tested against bovine endothelial nitric oxide synthase (eNOS) | Bioorg Med Chem Lett 13: 209-12 (2002) BindingDB Entry DOI: 10.7270/Q2K9382B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, endothelial (Bos taurus (bovine)) | BDBM50122304 (CHEMBL539004 | N-(4-Amino-phenyl)-thiophene-2-carb...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Henri Beaufour Curated by ChEMBL | Assay Description Inhibitory concentration tested against bovine endothelial nitric oxide synthase (eNOS) | Bioorg Med Chem Lett 13: 209-12 (2002) BindingDB Entry DOI: 10.7270/Q2K9382B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50089965 (CHEMBL3581222) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 42 | n/a | n/a | n/a | n/a |
Ipsen Innovation Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 25: 88-91 (2015) Article DOI: 10.1016/j.bmcl.2014.11.003 BindingDB Entry DOI: 10.7270/Q2736SN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50089968 (CHEMBL3581220) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 59 | n/a | n/a | n/a | n/a |
Ipsen Innovation Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 25: 88-91 (2015) Article DOI: 10.1016/j.bmcl.2014.11.003 BindingDB Entry DOI: 10.7270/Q2736SN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50089959 (CHEMBL3581225) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 72 | n/a | n/a | n/a | n/a |
Ipsen Innovation Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 25: 88-91 (2015) Article DOI: 10.1016/j.bmcl.2014.11.003 BindingDB Entry DOI: 10.7270/Q2736SN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50089958 (CHEMBL3581226) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | n/a | n/a | 40 | n/a | n/a | n/a | n/a |
Ipsen Innovation Curated by ChEMBL | Assay Description Agonist activity at human CB2 receptor expressed in CHO-K1 cells assessed as inhibition of forskolin-stimulated cAMP production | Bioorg Med Chem Lett 25: 88-91 (2015) Article DOI: 10.1016/j.bmcl.2014.11.003 BindingDB Entry DOI: 10.7270/Q2736SN4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 58 total ) | Next | Last >> |