

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50394718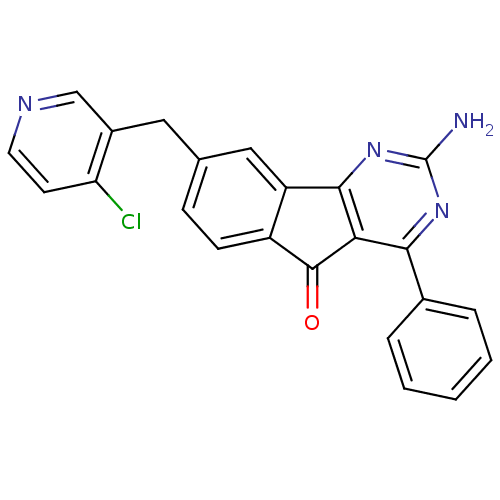 (CHEMBL2165801) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50394722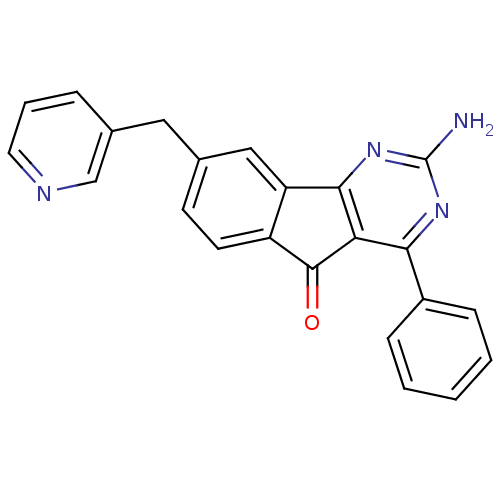 (CHEMBL2165807) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50394718 (CHEMBL2165801) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A1 assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50394717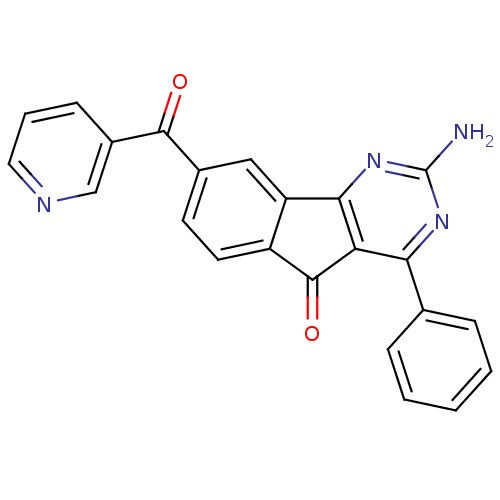 (CHEMBL2165802) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50308131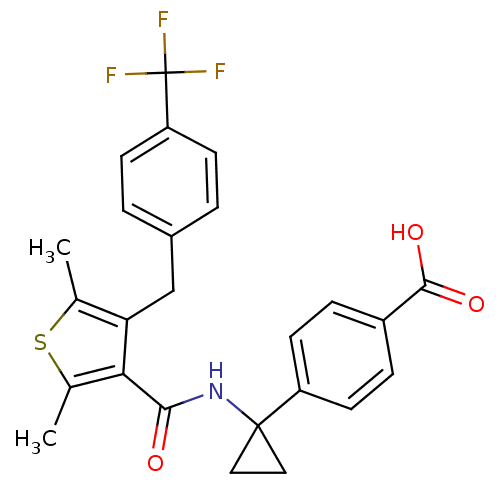 (4-{1-[({2,5-Dimethyl-4-[4-(trifluoromethyl)benzyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK293-EBNA cells by scintillation counting | J Med Chem 53: 2227-38 (2010) Article DOI: 10.1021/jm901771h BindingDB Entry DOI: 10.7270/Q29W0FMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50308132 (2,5-Dimethyl-N-{1-[4-(2H-tetrazol-5-yl)phenyl]cycl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK293-EBNA cells by scintillation counting | J Med Chem 53: 2227-38 (2010) Article DOI: 10.1021/jm901771h BindingDB Entry DOI: 10.7270/Q29W0FMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Rattus norvegicus) | BDBM50308131 (4-{1-[({2,5-Dimethyl-4-[4-(trifluoromethyl)benzyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from rat EP4 receptor expressed in HEK293-EBNA cells by scintillation counting | J Med Chem 53: 2227-38 (2010) Article DOI: 10.1021/jm901771h BindingDB Entry DOI: 10.7270/Q29W0FMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50394719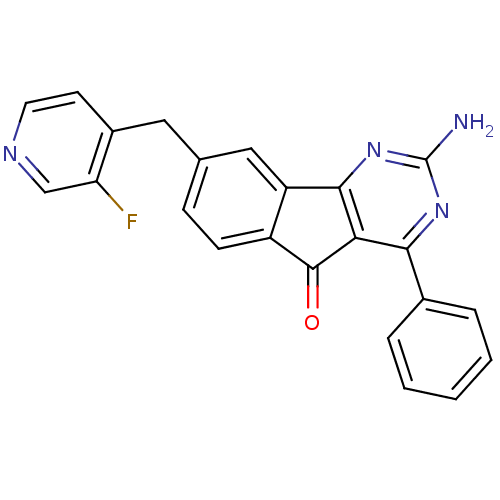 (CHEMBL2165800) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50308134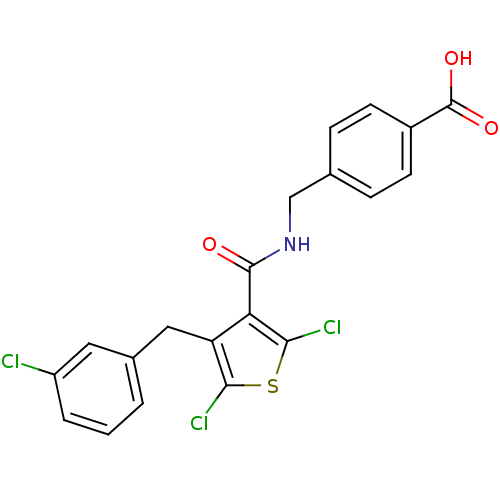 (4-[({[2,5-Dichloro-4-(3-chlorobenzyl)-3-thienyl]ca...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK293-EBNA cells by scintillation counting in presence of 10% human serum | J Med Chem 53: 2227-38 (2010) Article DOI: 10.1021/jm901771h BindingDB Entry DOI: 10.7270/Q29W0FMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50308130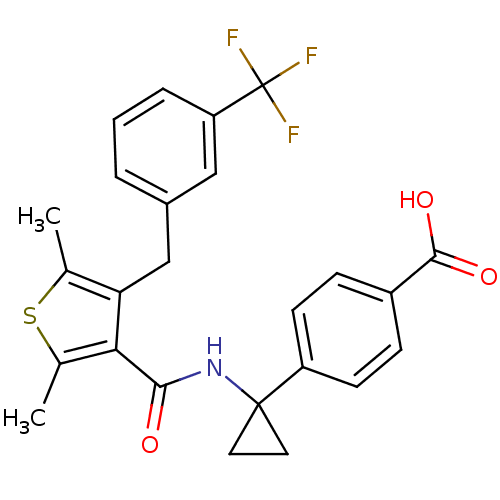 (4-{1-[({2,5-Dimethyl-4-[3-(trifluoromethyl)benzyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK293-EBNA cells by scintillation counting in presence of 10% human serum | J Med Chem 53: 2227-38 (2010) Article DOI: 10.1021/jm901771h BindingDB Entry DOI: 10.7270/Q29W0FMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50308133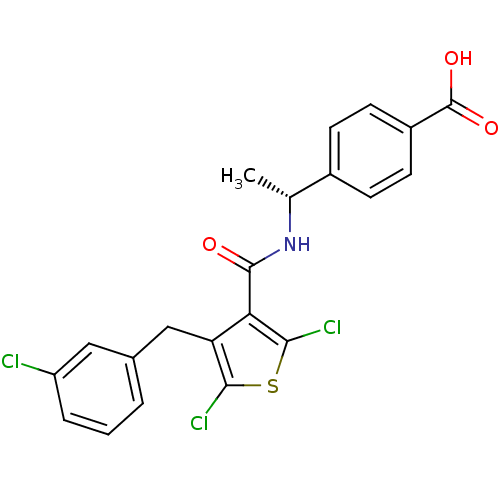 (4-[(1R)-1-({[2,5-Dichloro-4-(3-chlorobenzyl)-3-thi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK293-EBNA cells by scintillation counting | J Med Chem 53: 2227-38 (2010) Article DOI: 10.1021/jm901771h BindingDB Entry DOI: 10.7270/Q29W0FMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50316892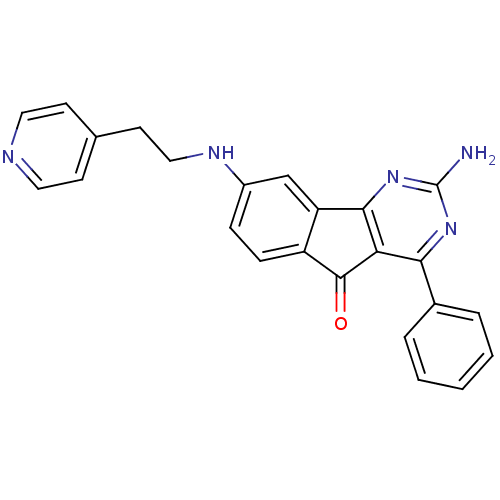 (2-amino-4-phenyl-8-(2-(pyridin-4-yl)ethylamino)-5H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonistic activity at adenosine A2A receptor | Bioorg Med Chem Lett 20: 2868-71 (2010) Article DOI: 10.1016/j.bmcl.2010.03.024 BindingDB Entry DOI: 10.7270/Q27S7NXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50316893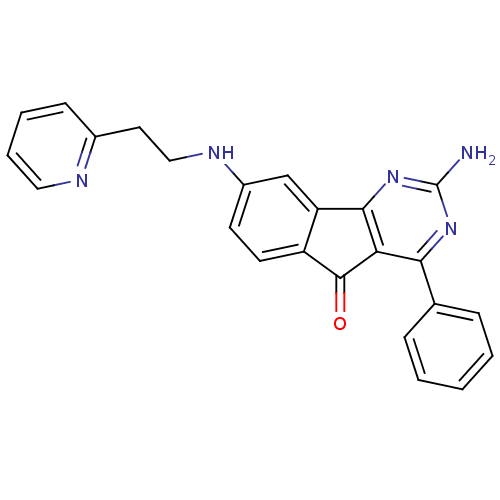 (2-amino-4-phenyl-8-(2-(pyridin-2-yl)ethylamino)-5H...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonistic activity at adenosine A2A receptor | Bioorg Med Chem Lett 20: 2868-71 (2010) Article DOI: 10.1016/j.bmcl.2010.03.024 BindingDB Entry DOI: 10.7270/Q27S7NXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50039026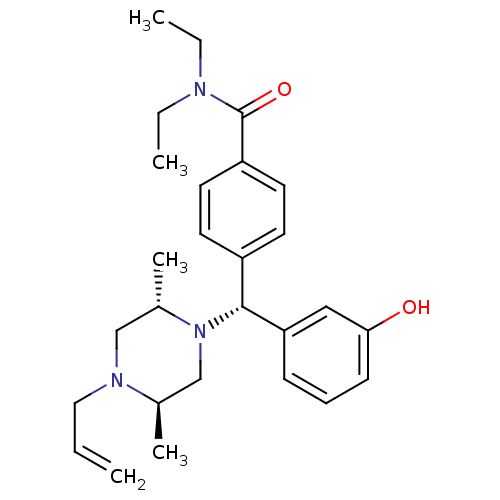 (4-((R)-((2S,5R)-4-allyl-2,5-dimethylpiperazin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity towards opioid Delta receptor using [3H]DADLE as radioligand | Bioorg Med Chem Lett 9: 3053-6 (1999) BindingDB Entry DOI: 10.7270/Q28916BJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50039026 (4-((R)-((2S,5R)-4-allyl-2,5-dimethylpiperazin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity towards opioid Delta receptor using [3H]DADLE as radioligand | Bioorg Med Chem Lett 9: 3347-50 (2000) BindingDB Entry DOI: 10.7270/Q2MK6DDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50039026 (4-((R)-((2S,5R)-4-allyl-2,5-dimethylpiperazin-1-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Affinity of [H]DADLE to the delta opioid receptor from rat brain | J Med Chem 44: 972-87 (2001) BindingDB Entry DOI: 10.7270/Q2V69K8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50308131 (4-{1-[({2,5-Dimethyl-4-[4-(trifluoromethyl)benzyl]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK293-EBNA cells by scintillation counting in presence of 10% human serum | J Med Chem 53: 2227-38 (2010) Article DOI: 10.1021/jm901771h BindingDB Entry DOI: 10.7270/Q29W0FMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50308125 (4-[(1S)-1-({[2,5-Dichloro-4-(3-chlorobenzyl)-3-thi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK293-EBNA cells by scintillation counting | J Med Chem 53: 2227-38 (2010) Article DOI: 10.1021/jm901771h BindingDB Entry DOI: 10.7270/Q29W0FMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50394720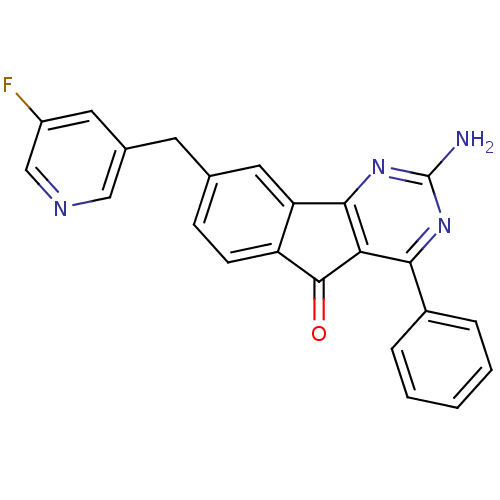 (CHEMBL2165799) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50394721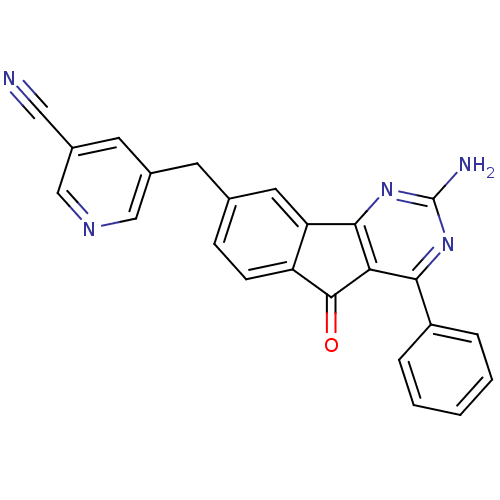 (CHEMBL2165808) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50308129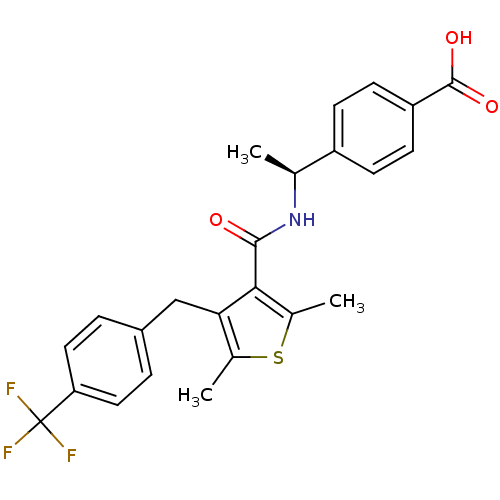 (4-{(1S)-1-[({2,5-Dimethyl-4-[4-(trifluoromethyl)be...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK293-EBNA cells by scintillation counting | J Med Chem 53: 2227-38 (2010) Article DOI: 10.1021/jm901771h BindingDB Entry DOI: 10.7270/Q29W0FMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50491094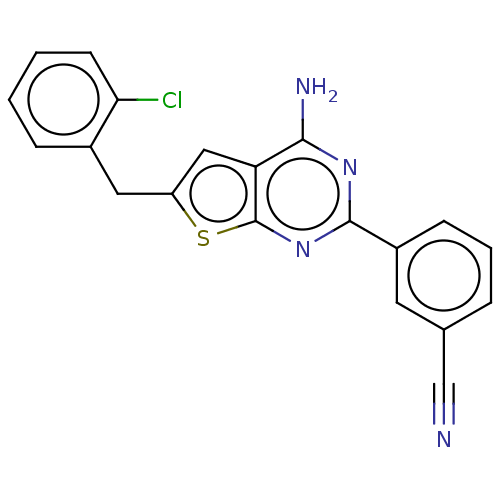 (CHEMBL2377092) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development, LLC Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2a receptor expressed in CHOK1 cells assessed as inhibition of NECA/forskolin-induced cAMP accumulation incub... | Bioorg Med Chem Lett 23: 2688-91 (2013) Article DOI: 10.1016/j.bmcl.2013.02.078 BindingDB Entry DOI: 10.7270/Q2222XPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50039029 ((+)-4-((alpha R)-((2S,5R)-4-allyl-2,5-dimethylpipe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Affinity of [H]DADLE to the delta opioid receptor from rat brain | J Med Chem 44: 972-87 (2001) BindingDB Entry DOI: 10.7270/Q2V69K8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50394716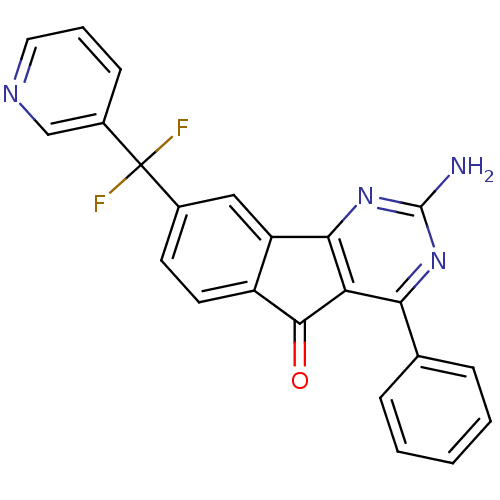 (CHEMBL2165803) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50039029 ((+)-4-((alpha R)-((2S,5R)-4-allyl-2,5-dimethylpipe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity towards opioid Delta receptor using [3H]DADLE as radioligand | Bioorg Med Chem Lett 9: 3347-50 (2000) BindingDB Entry DOI: 10.7270/Q2MK6DDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50039029 ((+)-4-((alpha R)-((2S,5R)-4-allyl-2,5-dimethylpipe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity towards opioid Delta receptor using [3H]DADLE as radioligand | Bioorg Med Chem Lett 9: 3053-6 (1999) BindingDB Entry DOI: 10.7270/Q28916BJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50394717 (CHEMBL2165802) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A1 assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50394715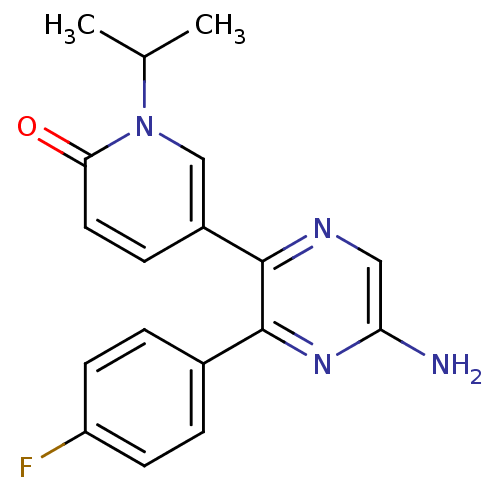 (CHEMBL2165804) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50098462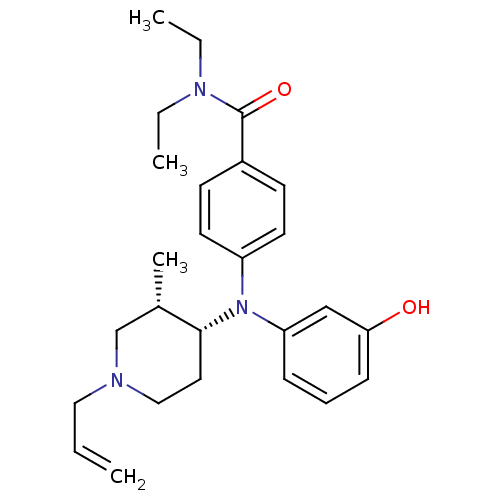 (4-[(1-Allyl-3-methyl-piperidin-4-yl)-(3-hydroxy-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Affinity of [H]DADLE to the delta opioid receptor from rat brain | J Med Chem 44: 972-87 (2001) BindingDB Entry DOI: 10.7270/Q2V69K8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50491101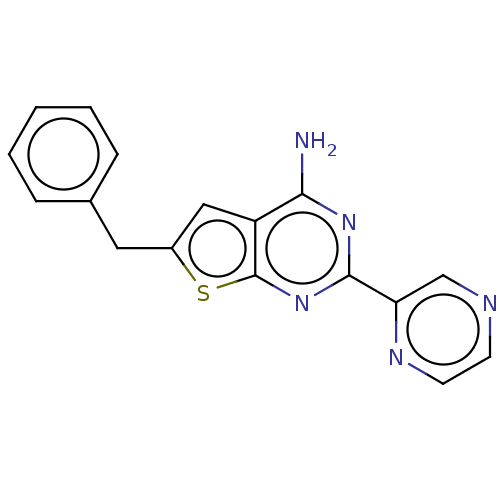 (CHEMBL2377103) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development, LLC Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2a receptor expressed in CHOK1 cells assessed as inhibition of NECA/forskolin-induced cAMP accumulation incub... | Bioorg Med Chem Lett 23: 2688-91 (2013) Article DOI: 10.1016/j.bmcl.2013.02.078 BindingDB Entry DOI: 10.7270/Q2222XPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50394722 (CHEMBL2165807) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A1 assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50308127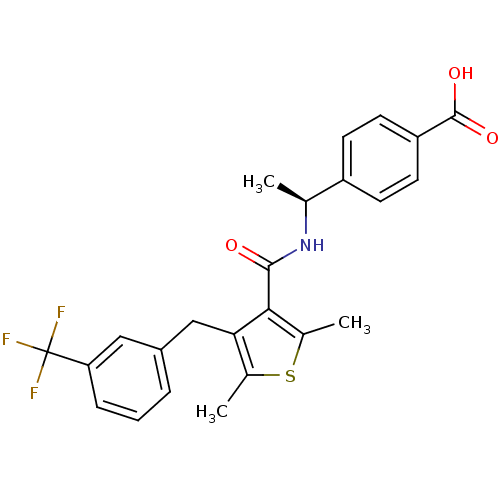 (4-{(1S)-1-[({2,5-Dimethyl-4-[3-(trifluoromethyl)be...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK293-EBNA cells by scintillation counting | J Med Chem 53: 2227-38 (2010) Article DOI: 10.1021/jm901771h BindingDB Entry DOI: 10.7270/Q29W0FMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50394719 (CHEMBL2165800) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A1 assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50316899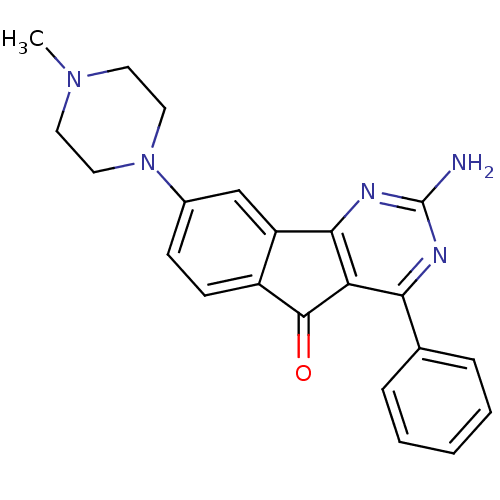 (2-amino-8-(4-methylpiperazin-1-yl)-4-phenyl-5H-ind...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonistic activity at adenosine A2A receptor | Bioorg Med Chem Lett 20: 2868-71 (2010) Article DOI: 10.1016/j.bmcl.2010.03.024 BindingDB Entry DOI: 10.7270/Q27S7NXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50491105 (CHEMBL2377110) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development, LLC Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2a receptor expressed in CHOK1 cells assessed as inhibition of NECA/forskolin-induced cAMP accumulation incub... | Bioorg Med Chem Lett 23: 2688-91 (2013) Article DOI: 10.1016/j.bmcl.2013.02.078 BindingDB Entry DOI: 10.7270/Q2222XPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50316898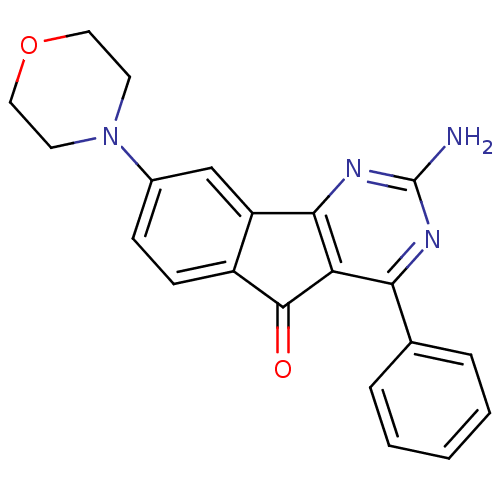 (2-amino-8-morpholino-4-phenyl-5H-indeno[1,2-d]pyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonistic activity at adenosine A2A receptor | Bioorg Med Chem Lett 20: 2868-71 (2010) Article DOI: 10.1016/j.bmcl.2010.03.024 BindingDB Entry DOI: 10.7270/Q27S7NXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50308128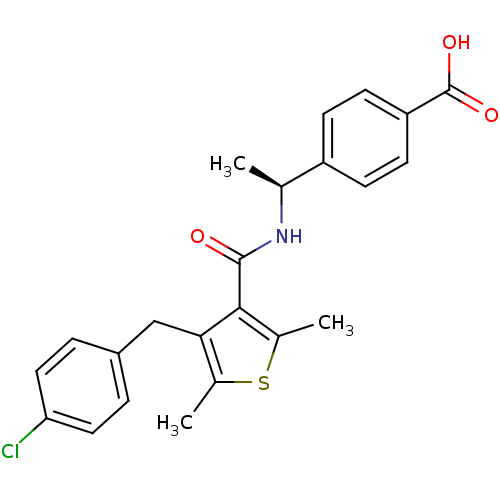 (4-[(1S)-1-({[4-(4-Chlorobenzyl)-2,5-dimethyl-3-thi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK293-EBNA cells by scintillation counting | J Med Chem 53: 2227-38 (2010) Article DOI: 10.1021/jm901771h BindingDB Entry DOI: 10.7270/Q29W0FMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50316895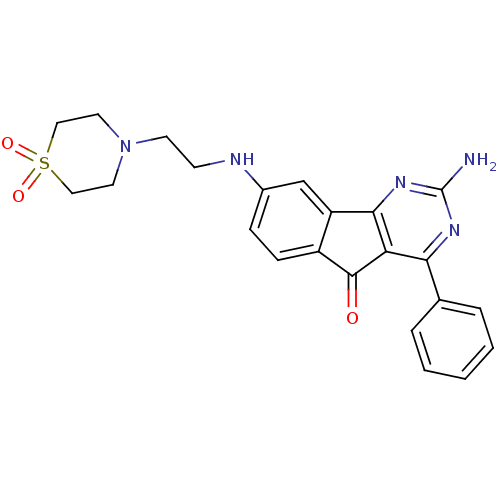 (2-Amino-8-[2-(1,1-dioxo-1lambda*6*-thiomorpholin-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonistic activity at adenosine A2A receptor | Bioorg Med Chem Lett 20: 2868-71 (2010) Article DOI: 10.1016/j.bmcl.2010.03.024 BindingDB Entry DOI: 10.7270/Q27S7NXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50394723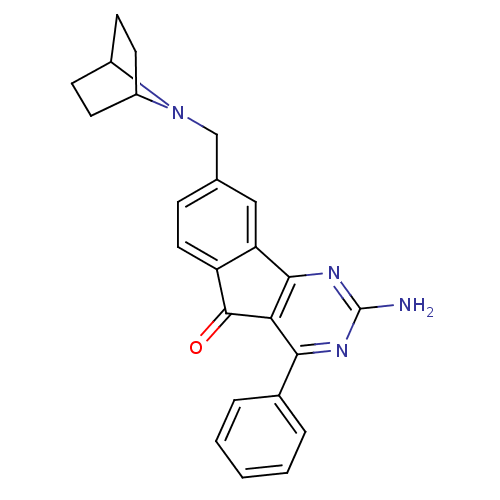 (CHEMBL2165806) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50308126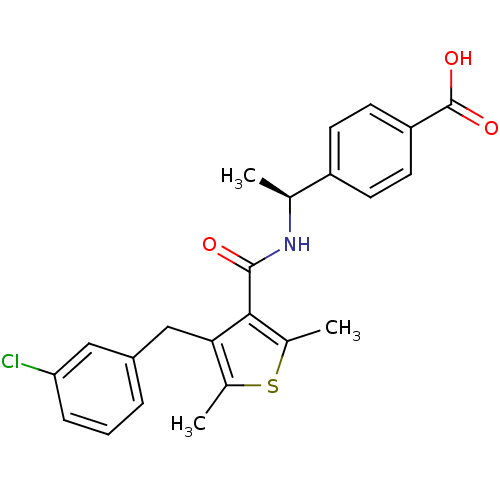 (4-[(1S)-1-({[4-(3-Chlorobenzyl)-2,5-dimethyl-3-thi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK293-EBNA cells by scintillation counting | J Med Chem 53: 2227-38 (2010) Article DOI: 10.1021/jm901771h BindingDB Entry DOI: 10.7270/Q29W0FMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50491075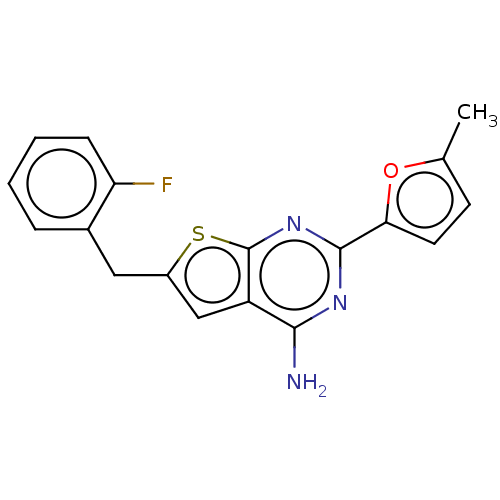 (CHEMBL2377111) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development, LLC Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2a receptor expressed in CHOK1 cells assessed as inhibition of NECA/forskolin-induced cAMP accumulation incub... | Bioorg Med Chem Lett 23: 2688-91 (2013) Article DOI: 10.1016/j.bmcl.2013.02.078 BindingDB Entry DOI: 10.7270/Q2222XPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50330987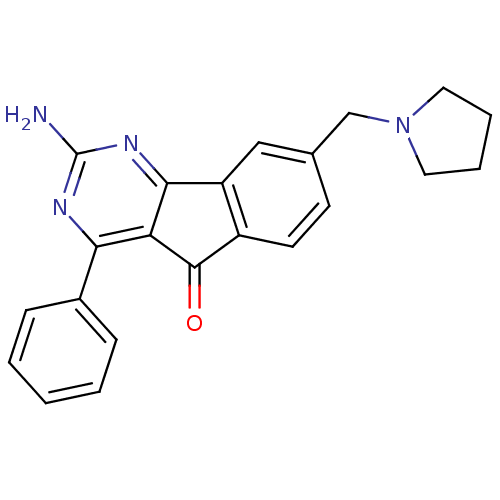 (2-amino-4-phenyl-8-(pyrrolidin-1-ylmethyl)-5H-inde...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A2a assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50316894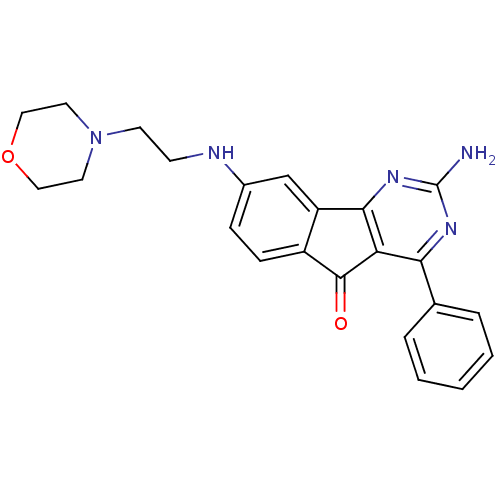 (2-amino-8-(2-morpholinoethylamino)-4-phenyl-5H-ind...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonistic activity at adenosine A2A receptor | Bioorg Med Chem Lett 20: 2868-71 (2010) Article DOI: 10.1016/j.bmcl.2010.03.024 BindingDB Entry DOI: 10.7270/Q27S7NXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50316891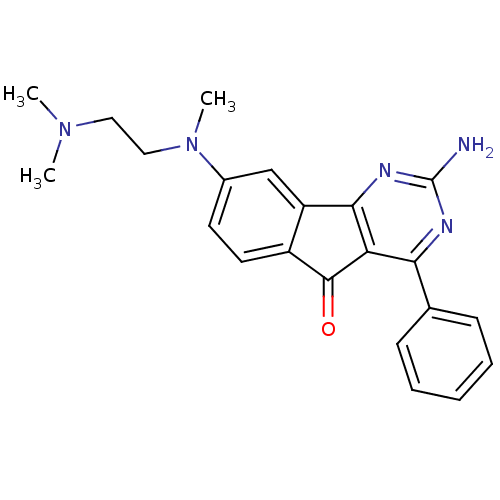 (2-amino-8-((2-(dimethylamino)ethyl)(methyl)amino)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonistic activity at adenosine A2A receptor | Bioorg Med Chem Lett 20: 2868-71 (2010) Article DOI: 10.1016/j.bmcl.2010.03.024 BindingDB Entry DOI: 10.7270/Q27S7NXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50491100 (CHEMBL2377228) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research & Development, LLC Curated by ChEMBL | Assay Description Antagonist activity at human adenosine A2a receptor expressed in CHOK1 cells assessed as inhibition of NECA/forskolin-induced cAMP accumulation incub... | Bioorg Med Chem Lett 23: 2688-91 (2013) Article DOI: 10.1016/j.bmcl.2013.02.078 BindingDB Entry DOI: 10.7270/Q2222XPT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2a (Homo sapiens (Human)) | BDBM50316897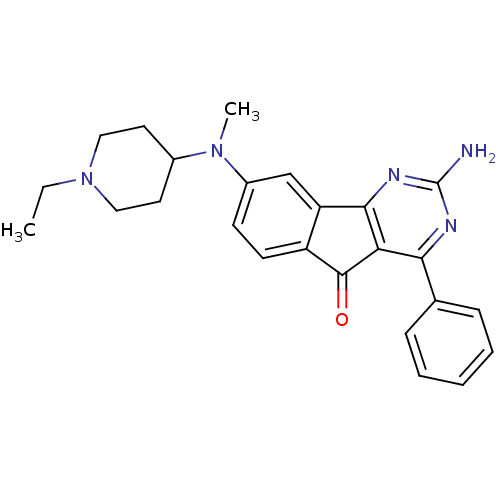 (2-amino-8-((1-ethylpiperidin-4-yl)(methyl)amino)-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Antagonistic activity at adenosine A2A receptor | Bioorg Med Chem Lett 20: 2868-71 (2010) Article DOI: 10.1016/j.bmcl.2010.03.024 BindingDB Entry DOI: 10.7270/Q27S7NXB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50394721 (CHEMBL2165808) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen Research and Development, L.L.C. Curated by ChEMBL | Assay Description Antagonist activity at human Adenosine receptor A1 assessed as inhibition of cAMP production | J Med Chem 55: 1402-17 (2012) Article DOI: 10.1021/jm201640m BindingDB Entry DOI: 10.7270/Q2CZ388Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50083637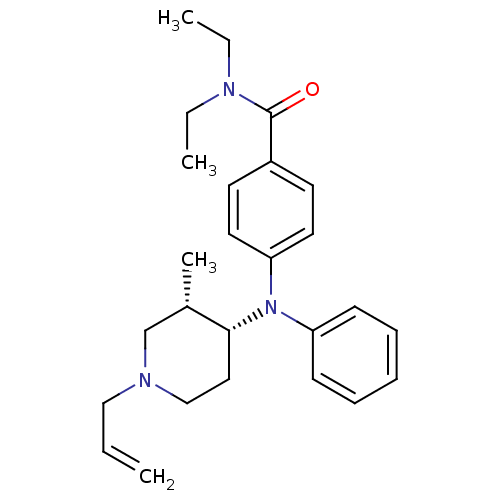 (4-(((3S,4R)-1-allyl-3-methylpiperidin-4-yl)(phenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Affinity of [H]DADLE to the delta opioid receptor from rat brain | J Med Chem 44: 972-87 (2001) BindingDB Entry DOI: 10.7270/Q2V69K8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50083637 (4-(((3S,4R)-1-allyl-3-methylpiperidin-4-yl)(phenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Affinity of [H]DADLE to the delta opioid receptor from rat brain | J Med Chem 44: 972-87 (2001) BindingDB Entry DOI: 10.7270/Q2V69K8G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (MOUSE) | BDBM50082341 (4-[((3R,4S)-1-Allyl-3-methyl-piperidin-4-yl)-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research Triangle Institute Curated by ChEMBL | Assay Description Binding affinity towards opioid Delta receptor using [3H]DADLE as radioligand | Bioorg Med Chem Lett 9: 3347-50 (2000) BindingDB Entry DOI: 10.7270/Q2MK6DDP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 874 total ) | Next | Last >> |