

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50243125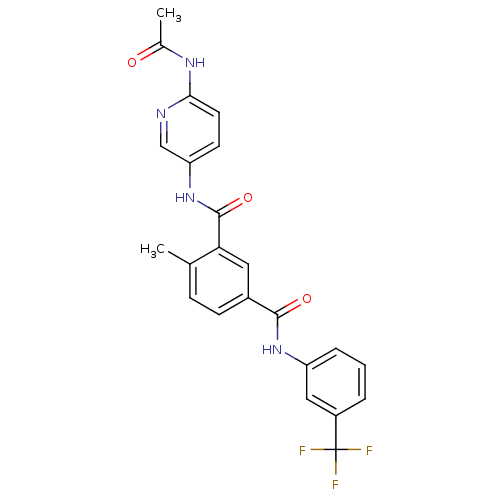 (CHEMBL487864 | N3-(6-acetamidopyridin-3-yl)-4-meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of c-kit (unknown origin) | Bioorg Med Chem Lett 18: 4137-41 (2008) Article DOI: 10.1016/j.bmcl.2008.05.089 BindingDB Entry DOI: 10.7270/Q20001WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM50251959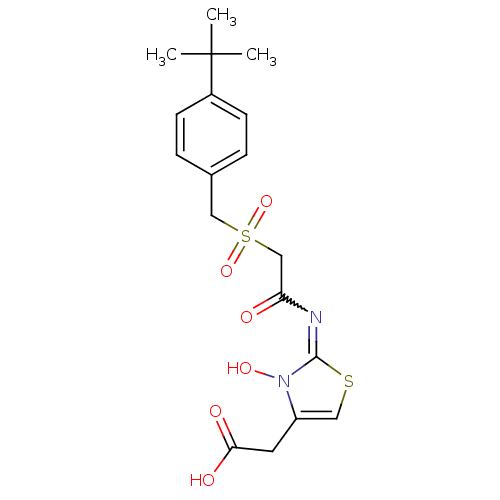 (2-(2-(2-(4-tert-butylbenzylsulfonyl)acetamido)-3-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of human PHD2 assesssed as hydroxylation of Pro564 in human HIF-1alpha by TR-FRET assay | Bioorg Med Chem Lett 18: 3925-8 (2008) Article DOI: 10.1016/j.bmcl.2008.06.031 BindingDB Entry DOI: 10.7270/Q2XW4JM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM50264220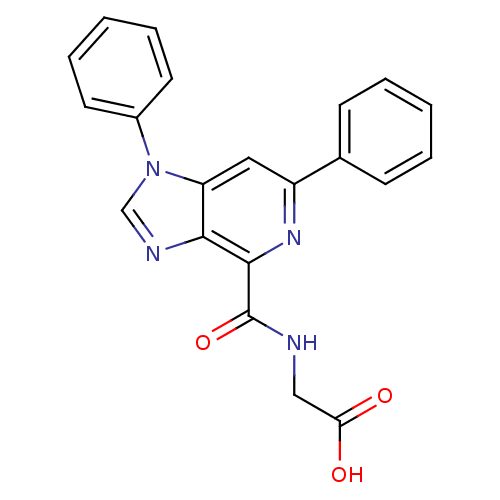 (2-(1,6-diphenyl-1H-imidazo[4,5-c]pyridine-4-carbox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of PHD2 (unknown origin) by fluorescence energy transfer analysis | Bioorg Med Chem Lett 18: 5023-6 (2008) Article DOI: 10.1016/j.bmcl.2008.08.012 BindingDB Entry DOI: 10.7270/Q2ZS2WB7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM26365 (3-N-(2-aminopyrimidin-5-yl)-4-methyl-1-N-[3-(trifl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of c-kit (unknown origin) | Bioorg Med Chem Lett 18: 4137-41 (2008) Article DOI: 10.1016/j.bmcl.2008.05.089 BindingDB Entry DOI: 10.7270/Q20001WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50243076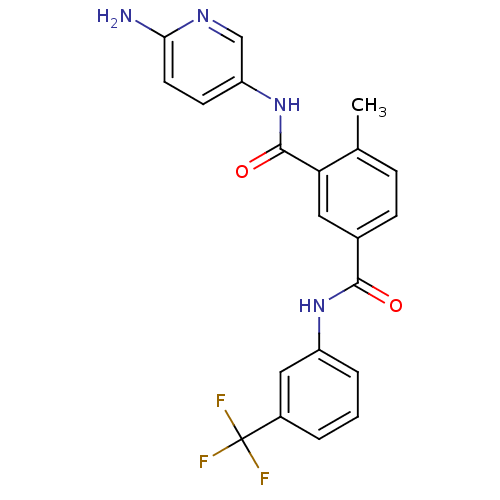 (CHEMBL470483 | N3-(6-aminopyridin-3-yl)-4-methyl-N...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of c-kit (unknown origin) | Bioorg Med Chem Lett 18: 4137-41 (2008) Article DOI: 10.1016/j.bmcl.2008.05.089 BindingDB Entry DOI: 10.7270/Q20001WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50243181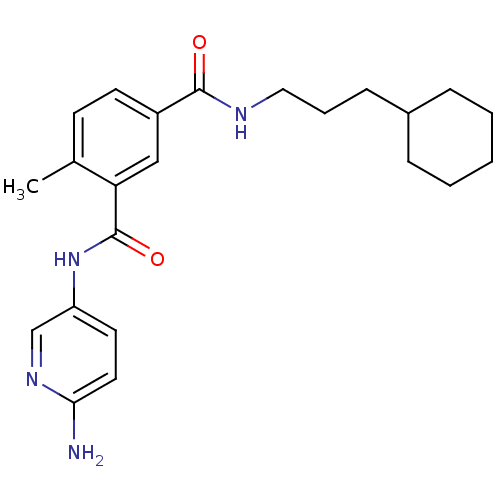 (CHEMBL520718 | N3-(6-aminopyridin-3-yl)-N1-(3-cycl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of c-kit (unknown origin) | Bioorg Med Chem Lett 18: 4137-41 (2008) Article DOI: 10.1016/j.bmcl.2008.05.089 BindingDB Entry DOI: 10.7270/Q20001WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM293911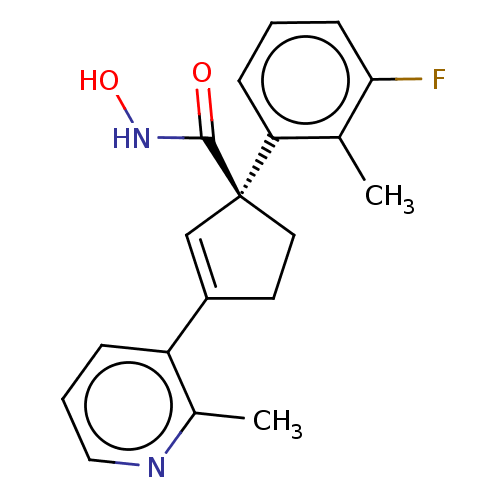 ((S)-1-(3-Fluoro-2- methylphenyl)-N- hydroxy-3-(2- ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description 2 μl (200×) of each diluted solution and each control (full activity: 100% DMSO alone or full inhibition 1 mM) is stamped into V-bottomed polypr... | US Patent US9617259 (2017) BindingDB Entry DOI: 10.7270/Q2DN4743 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM293914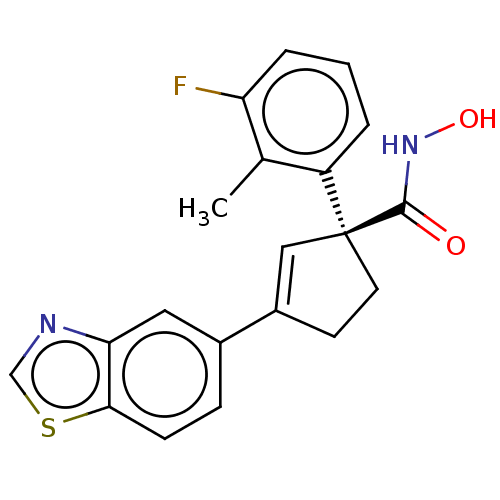 ((S)-3-(Benzo[d]thiazol-5- yl)-1-(3-fluoro-2- methy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI FOUNDATION, INC. US Patent | Assay Description 5 μl of each solution of 1:20 diluted compound from above is transferred to a clear bottomed, black, 384-well assay plate using the Bravo or the... | US Patent US10106535 (2018) BindingDB Entry DOI: 10.7270/Q2F47R6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM293914 ((S)-3-(Benzo[d]thiazol-5- yl)-1-(3-fluoro-2- methy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description 2 μl (200×) of each diluted solution and each control (full activity: 100% DMSO alone or full inhibition 1 mM) is stamped into V-bottomed polypr... | US Patent US9617259 (2017) BindingDB Entry DOI: 10.7270/Q2DN4743 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM293916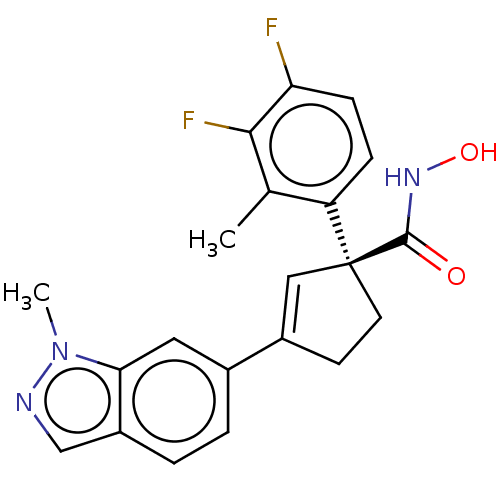 ((S)-1-(3,4-Difluoro-2- methylphenyl)-N- hydroxy-3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description 2 μl (200×) of each diluted solution and each control (full activity: 100% DMSO alone or full inhibition 1 mM) is stamped into V-bottomed polypr... | US Patent US9617259 (2017) BindingDB Entry DOI: 10.7270/Q2DN4743 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM293916 ((S)-1-(3,4-Difluoro-2- methylphenyl)-N- hydroxy-3-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI FOUNDATION, INC. US Patent | Assay Description 5 μl of each solution of 1:20 diluted compound from above is transferred to a clear bottomed, black, 384-well assay plate using the Bravo or the... | US Patent US10106535 (2018) BindingDB Entry DOI: 10.7270/Q2F47R6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM293880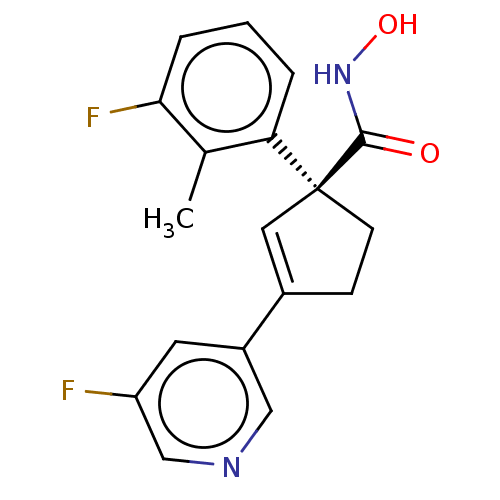 ((S)-1-(3-Fluoro-2- methylphenyl)-3-(5- fluoropyrid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description 2 μl (200×) of each diluted solution and each control (full activity: 100% DMSO alone or full inhibition 1 mM) is stamped into V-bottomed polypr... | US Patent US9617259 (2017) BindingDB Entry DOI: 10.7270/Q2DN4743 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM293886 ((S)-1-(3-Fluoro-2- methylphenyl)-N- hydroxy-3-(qui...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description 2 μl (200×) of each diluted solution and each control (full activity: 100% DMSO alone or full inhibition 1 mM) is stamped into V-bottomed polypr... | US Patent US9617259 (2017) BindingDB Entry DOI: 10.7270/Q2DN4743 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM293895 ((S)-1-(3-Fluoro-2- methylphenyl)-N- hydroxy-3-(imi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description 2 μl (200×) of each diluted solution and each control (full activity: 100% DMSO alone or full inhibition 1 mM) is stamped into V-bottomed polypr... | US Patent US9617259 (2017) BindingDB Entry DOI: 10.7270/Q2DN4743 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM293899 ((S)-1-(3-Fluoro-2- methylphenyl)-N- hydroxy-3-(6- ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description 2 μl (200×) of each diluted solution and each control (full activity: 100% DMSO alone or full inhibition 1 mM) is stamped into V-bottomed polypr... | US Patent US9617259 (2017) BindingDB Entry DOI: 10.7270/Q2DN4743 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM293900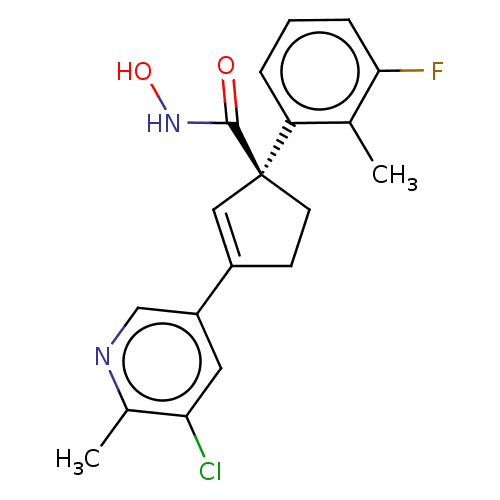 ((S)-3-(5-Chloro-6- methylpyridin-3-yl)-1-(3- fluor...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description 2 μl (200×) of each diluted solution and each control (full activity: 100% DMSO alone or full inhibition 1 mM) is stamped into V-bottomed polypr... | US Patent US9617259 (2017) BindingDB Entry DOI: 10.7270/Q2DN4743 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 [648-729,745-1057] (Homo sapiens (Human)) | BDBM50160879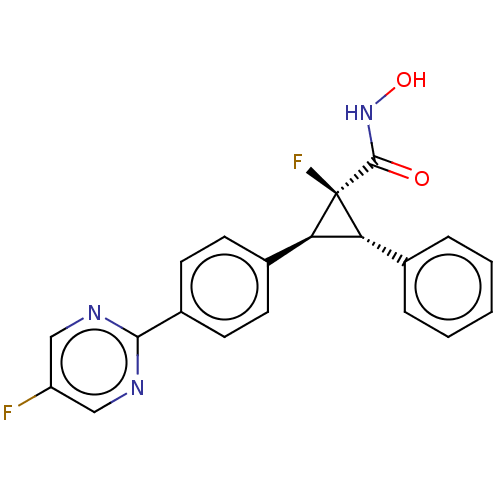 (CHEMBL3793392 | US9505736, (1S,2S,3S)-1-Fluoro-2-(...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents | PDB US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 8.0 | 25 |
CHDI Foundation, Inc. US Patent | Assay Description The potency of Class IIa Histone Deacetylase (HDAC) inhibitors is quantified by measuring the Histone Deacetylase 4 (HDAC4) catalytic domain enzymati... | US Patent US9505736 (2016) BindingDB Entry DOI: 10.7270/Q2T152KR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM293880 ((S)-1-(3-Fluoro-2- methylphenyl)-3-(5- fluoropyrid...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI FOUNDATION, INC. US Patent | Assay Description 5 μl of each solution of 1:20 diluted compound from above is transferred to a clear bottomed, black, 384-well assay plate using the Bravo or the... | US Patent US10106535 (2018) BindingDB Entry DOI: 10.7270/Q2F47R6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM293886 ((S)-1-(3-Fluoro-2- methylphenyl)-N- hydroxy-3-(qui...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI FOUNDATION, INC. US Patent | Assay Description 5 μl of each solution of 1:20 diluted compound from above is transferred to a clear bottomed, black, 384-well assay plate using the Bravo or the... | US Patent US10106535 (2018) BindingDB Entry DOI: 10.7270/Q2F47R6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM293895 ((S)-1-(3-Fluoro-2- methylphenyl)-N- hydroxy-3-(imi...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI FOUNDATION, INC. US Patent | Assay Description 5 μl of each solution of 1:20 diluted compound from above is transferred to a clear bottomed, black, 384-well assay plate using the Bravo or the... | US Patent US10106535 (2018) BindingDB Entry DOI: 10.7270/Q2F47R6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM293899 ((S)-1-(3-Fluoro-2- methylphenyl)-N- hydroxy-3-(6- ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI FOUNDATION, INC. US Patent | Assay Description 5 μl of each solution of 1:20 diluted compound from above is transferred to a clear bottomed, black, 384-well assay plate using the Bravo or the... | US Patent US10106535 (2018) BindingDB Entry DOI: 10.7270/Q2F47R6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM293900 ((S)-3-(5-Chloro-6- methylpyridin-3-yl)-1-(3- fluor...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI FOUNDATION, INC. US Patent | Assay Description 5 μl of each solution of 1:20 diluted compound from above is transferred to a clear bottomed, black, 384-well assay plate using the Bravo or the... | US Patent US10106535 (2018) BindingDB Entry DOI: 10.7270/Q2F47R6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM293911 ((S)-1-(3-Fluoro-2- methylphenyl)-N- hydroxy-3-(2- ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI FOUNDATION, INC. US Patent | Assay Description 5 μl of each solution of 1:20 diluted compound from above is transferred to a clear bottomed, black, 384-well assay plate using the Bravo or the... | US Patent US10106535 (2018) BindingDB Entry DOI: 10.7270/Q2F47R6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM293912 ((S)-1-(3-Fluoro-2- methylphenyl)-N- hydroxy-3-(1-m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI FOUNDATION, INC. US Patent | Assay Description 5 μl of each solution of 1:20 diluted compound from above is transferred to a clear bottomed, black, 384-well assay plate using the Bravo or the... | US Patent US10106535 (2018) BindingDB Entry DOI: 10.7270/Q2F47R6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM293912 ((S)-1-(3-Fluoro-2- methylphenyl)-N- hydroxy-3-(1-m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description 2 μl (200×) of each diluted solution and each control (full activity: 100% DMSO alone or full inhibition 1 mM) is stamped into V-bottomed polypr... | US Patent US9617259 (2017) BindingDB Entry DOI: 10.7270/Q2DN4743 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Egl nine homolog 1 (Homo sapiens (Human)) | BDBM50251958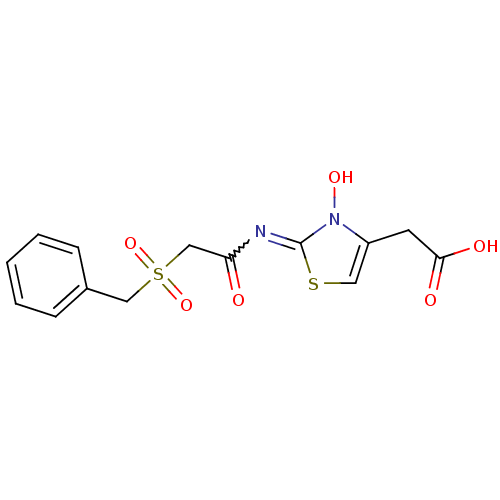 (2-(2-(2-(benzylsulfonyl)acetamido)-3-hydroxy-2,3-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description Inhibition of human PHD2 assesssed as hydroxylation of Pro564 in human HIF-1alpha by TR-FRET assay | Bioorg Med Chem Lett 18: 3925-8 (2008) Article DOI: 10.1016/j.bmcl.2008.06.031 BindingDB Entry DOI: 10.7270/Q2XW4JM5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM272075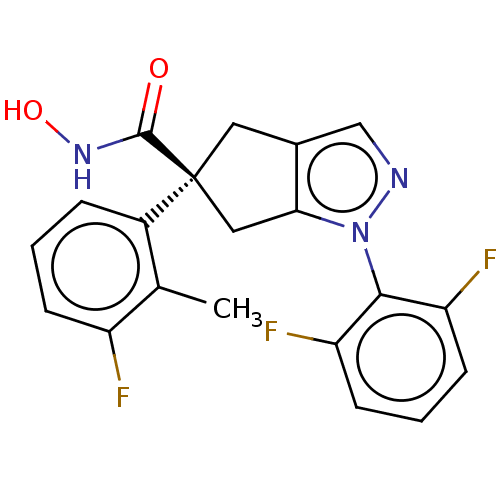 ((S)-1-(2,6-Difluorophenyl)-5-(3-fluoro-2- methylph...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys... | US Patent US10065948 (2018) BindingDB Entry DOI: 10.7270/Q2154K23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM272075 ((S)-1-(2,6-Difluorophenyl)-5-(3-fluoro-2- methylph...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys... | US Patent US10457675 (2019) BindingDB Entry DOI: 10.7270/Q22J6F7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM26373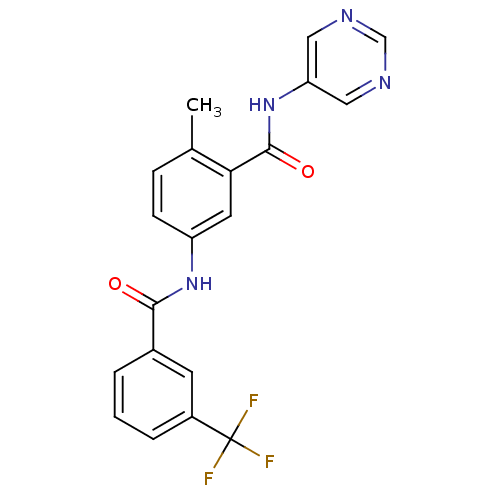 (4-methyl-3-N-(pyrimidin-5-yl)-1-N-[3-(trifluoromet...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of c-kit (unknown origin) | Bioorg Med Chem Lett 18: 4137-41 (2008) Article DOI: 10.1016/j.bmcl.2008.05.089 BindingDB Entry DOI: 10.7270/Q20001WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM272071 ((S)-1-(2-Chlorophenyl)-5-(3-fluoro-2-methylphenyl)...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys... | US Patent US10457675 (2019) BindingDB Entry DOI: 10.7270/Q22J6F7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM272078 ((S)-1-(2-Chloro-6-fluorophenyl)-5-(3-fluoro-2- met...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys... | US Patent US10065948 (2018) BindingDB Entry DOI: 10.7270/Q2154K23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM272071 ((S)-1-(2-Chlorophenyl)-5-(3-fluoro-2-methylphenyl)...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys... | US Patent US10065948 (2018) BindingDB Entry DOI: 10.7270/Q2154K23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM272078 ((S)-1-(2-Chloro-6-fluorophenyl)-5-(3-fluoro-2- met...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys... | US Patent US10457675 (2019) BindingDB Entry DOI: 10.7270/Q22J6F7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM272067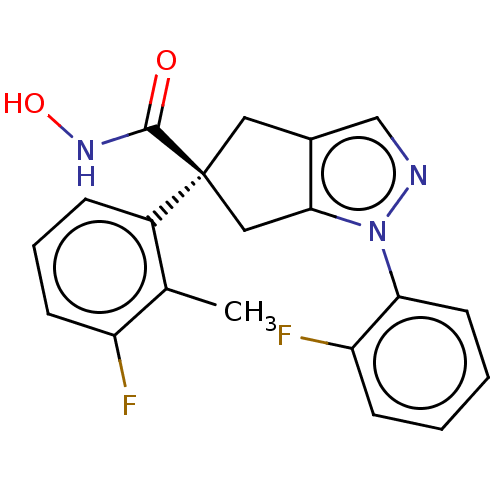 ((S)-5-(3-Fluoro-2-methylphenyl)-l-(2-fluorophenyl)...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys... | US Patent US10457675 (2019) BindingDB Entry DOI: 10.7270/Q22J6F7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM272074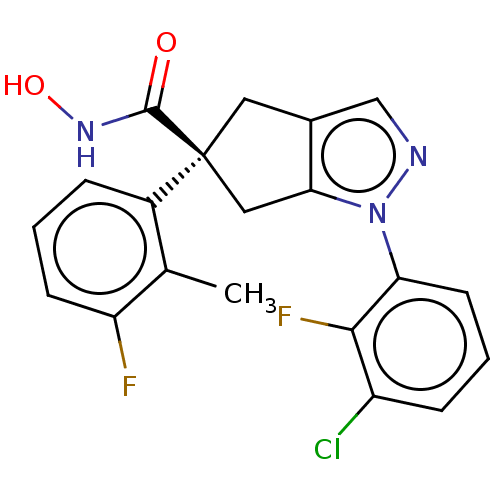 ((S)-1-(3-Chloro-2-fluorophenyl)-5-(3-fluoro-2- met...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys... | US Patent US10065948 (2018) BindingDB Entry DOI: 10.7270/Q2154K23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM272067 ((S)-5-(3-Fluoro-2-methylphenyl)-l-(2-fluorophenyl)...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys... | US Patent US10065948 (2018) BindingDB Entry DOI: 10.7270/Q2154K23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM272074 ((S)-1-(3-Chloro-2-fluorophenyl)-5-(3-fluoro-2- met...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys... | US Patent US10457675 (2019) BindingDB Entry DOI: 10.7270/Q22J6F7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mast/stem cell growth factor receptor Kit (Homo sapiens (Human)) | BDBM50243075 (4-methyl-N3-(pyridin-3-yl)-N1-(3-(trifluoromethyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of c-kit (unknown origin) | Bioorg Med Chem Lett 18: 4137-41 (2008) Article DOI: 10.1016/j.bmcl.2008.05.089 BindingDB Entry DOI: 10.7270/Q20001WP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM272079 ((S)-5-(3-Fluoro-2-methylphenyl)-1-(2-fluoro-6- met...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys... | US Patent US10065948 (2018) BindingDB Entry DOI: 10.7270/Q2154K23 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 9 (Homo sapiens (Human)) | BDBM272079 ((S)-5-(3-Fluoro-2-methylphenyl)-1-(2-fluoro-6- met...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description The potency of the compounds is quantified by measuring the Histone Deacetylase 9 (HDAC9) enzymatic activity using the fluorogenic substrate, Boc-Lys... | US Patent US10457675 (2019) BindingDB Entry DOI: 10.7270/Q22J6F7J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM293915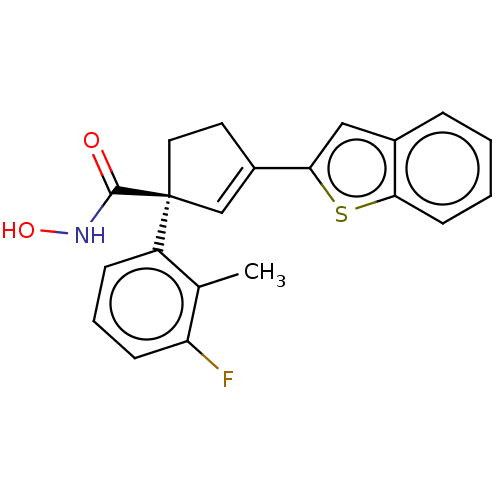 ((S)-3-(Benzo[b]thiophen- 2-yl)-1-(3-fluoro-2- meth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description 2 μl (200×) of each diluted solution and each control (full activity: 100% DMSO alone or full inhibition 1 mM) is stamped into V-bottomed polypr... | US Patent US9617259 (2017) BindingDB Entry DOI: 10.7270/Q2DN4743 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM293910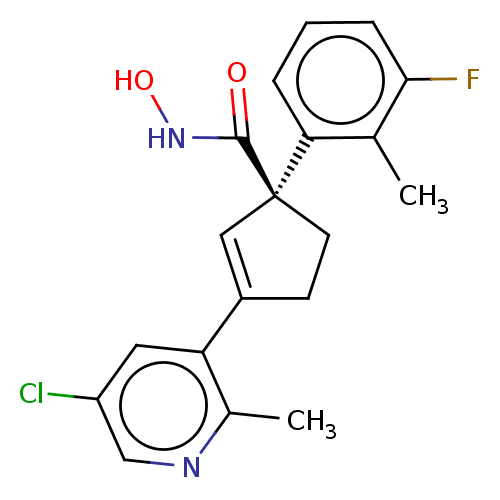 ((S)-3-(5-Chloro-2- methylpyridin-3-yl)-1-(3- fluor...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI FOUNDATION, INC. US Patent | Assay Description 5 μl of each solution of 1:20 diluted compound from above is transferred to a clear bottomed, black, 384-well assay plate using the Bravo or the... | US Patent US10106535 (2018) BindingDB Entry DOI: 10.7270/Q2F47R6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM340863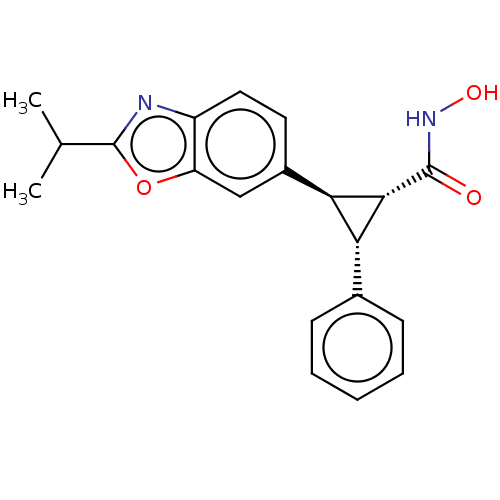 (US9765054, Compound 39A) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description 5 μl of each solution of 1:20 diluted compound from above is transferred to a clear bottomed, black, 384-well assay plate using the Bravo or the... | US Patent US9765054 (2017) BindingDB Entry DOI: 10.7270/Q2XW4MZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50446476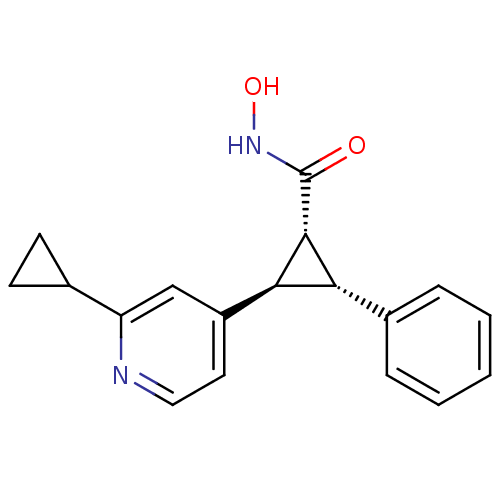 (CHEMBL3109993 | US9765054, Compound 28B) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description 5 μl of each solution of 1:20 diluted compound from above is transferred to a clear bottomed, black, 384-well assay plate using the Bravo or the... | US Patent US9765054 (2017) BindingDB Entry DOI: 10.7270/Q2XW4MZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM50446474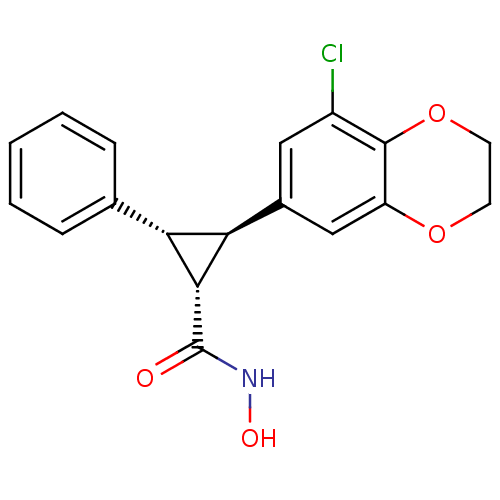 (CHEMBL3109980 | US9765054, Compound 25e) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description 5 μl of each solution of 1:20 diluted compound from above is transferred to a clear bottomed, black, 384-well assay plate using the Bravo or the... | US Patent US9765054 (2017) BindingDB Entry DOI: 10.7270/Q2XW4MZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM340962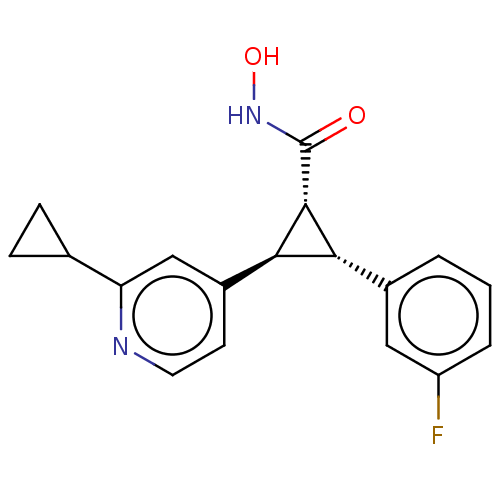 (US9765054, Compound 100b) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description 5 μl of each solution of 1:20 diluted compound from above is transferred to a clear bottomed, black, 384-well assay plate using the Bravo or the... | US Patent US9765054 (2017) BindingDB Entry DOI: 10.7270/Q2XW4MZG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM293915 ((S)-3-(Benzo[b]thiophen- 2-yl)-1-(3-fluoro-2- meth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI FOUNDATION, INC. US Patent | Assay Description 5 μl of each solution of 1:20 diluted compound from above is transferred to a clear bottomed, black, 384-well assay plate using the Bravo or the... | US Patent US10106535 (2018) BindingDB Entry DOI: 10.7270/Q2F47R6G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM293876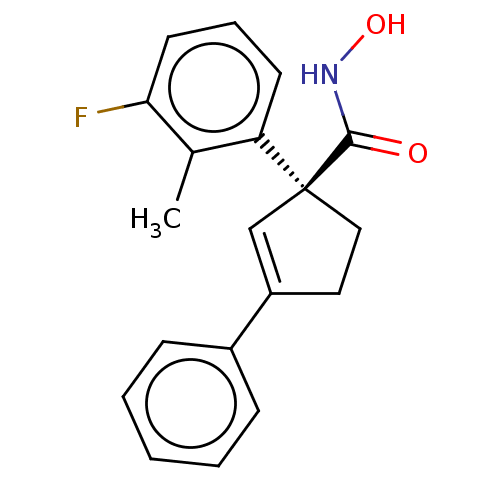 ((S)-1-(3-Fluoro-2- methylphenyl)-N- hydroxy-3- phe...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description 2 μl (200×) of each diluted solution and each control (full activity: 100% DMSO alone or full inhibition 1 mM) is stamped into V-bottomed polypr... | US Patent US9617259 (2017) BindingDB Entry DOI: 10.7270/Q2DN4743 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM293878 ((S)-1-(3-Fluoro-2- methylphenyl)-N- hydroxy-3-(o- ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description 2 μl (200×) of each diluted solution and each control (full activity: 100% DMSO alone or full inhibition 1 mM) is stamped into V-bottomed polypr... | US Patent US9617259 (2017) BindingDB Entry DOI: 10.7270/Q2DN4743 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Histone deacetylase 4 (Homo sapiens (Human)) | BDBM293885 ((S)-3-(5-Chloropyridin-3- yl)-1-(3-fluoro-2- methy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
CHDI Foundation, Inc. US Patent | Assay Description 2 μl (200×) of each diluted solution and each control (full activity: 100% DMSO alone or full inhibition 1 mM) is stamped into V-bottomed polypr... | US Patent US9617259 (2017) BindingDB Entry DOI: 10.7270/Q2DN4743 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 624 total ) | Next | Last >> |