 Found 1216 hits with Last Name = 'bach' and Initial = 'p'
Found 1216 hits with Last Name = 'bach' and Initial = 'p' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Aryl hydrocarbon receptor
(Mus musculus (Mouse)) | BDBM50506039
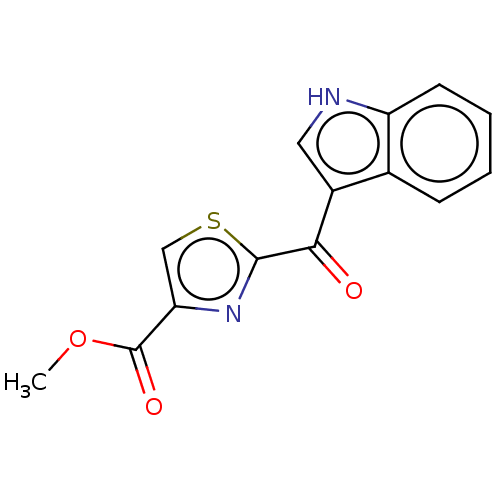
(CHEMBL1324296)Show InChI InChI=1S/C14H10N2O3S/c1-19-14(18)11-7-20-13(16-11)12(17)9-6-15-10-5-3-2-4-8(9)10/h2-7,15H,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00208
BindingDB Entry DOI: 10.7270/Q27D3076 |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447515
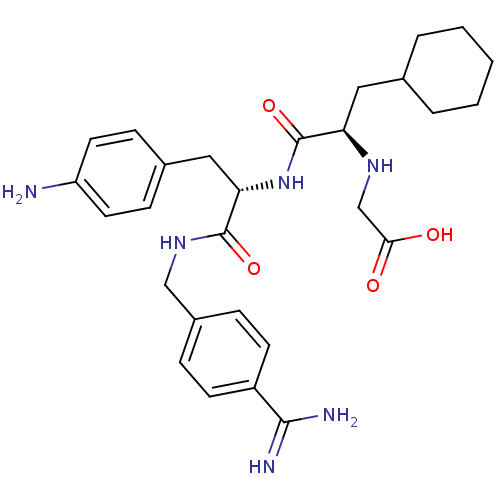
(CHEMBL3115901)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](Cc2ccc(N)cc2)NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)cc1 |r| Show InChI InChI=1S/C28H38N6O4/c29-22-12-8-19(9-13-22)15-24(27(37)33-16-20-6-10-21(11-7-20)26(30)31)34-28(38)23(32-17-25(35)36)14-18-4-2-1-3-5-18/h6-13,18,23-24,32H,1-5,14-17,29H2,(H3,30,31)(H,33,37)(H,34,38)(H,35,36)/t23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Aryl hydrocarbon receptor
(Mus musculus (Mouse)) | BDBM50605060
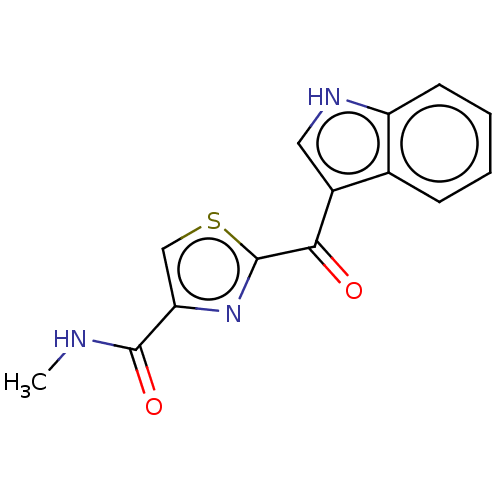
(CHEMBL5198290) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00208
BindingDB Entry DOI: 10.7270/Q27D3076 |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447516
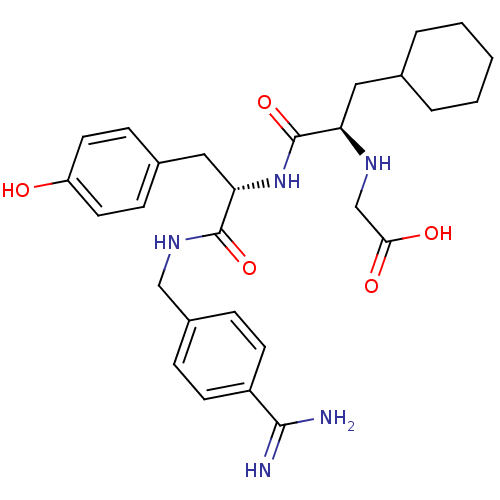
(CHEMBL3115900)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)cc1 |r| Show InChI InChI=1S/C28H37N5O5/c29-26(30)21-10-6-20(7-11-21)16-32-27(37)24(15-19-8-12-22(34)13-9-19)33-28(38)23(31-17-25(35)36)14-18-4-2-1-3-5-18/h6-13,18,23-24,31,34H,1-5,14-17H2,(H3,29,30)(H,32,37)(H,33,38)(H,35,36)/t23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447528
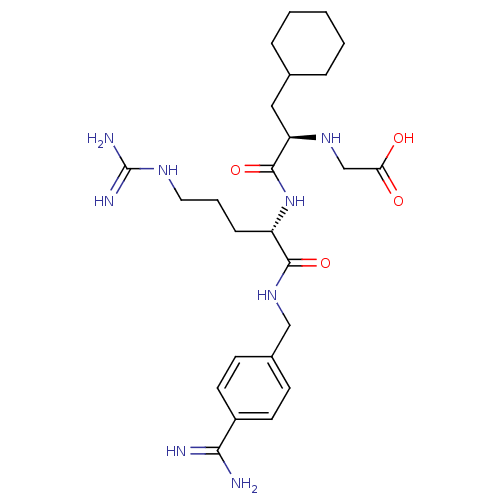
(CHEMBL3115904)Show SMILES NC(=N)NCCC[C@H](NC(=O)[C@@H](CC1CCCCC1)NCC(O)=O)C(=O)NCc1ccc(cc1)C(N)=N |r| Show InChI InChI=1S/C25H40N8O4/c26-22(27)18-10-8-17(9-11-18)14-32-23(36)19(7-4-12-30-25(28)29)33-24(37)20(31-15-21(34)35)13-16-5-2-1-3-6-16/h8-11,16,19-20,31H,1-7,12-15H2,(H3,26,27)(H,32,36)(H,33,37)(H,34,35)(H4,28,29,30)/t19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447523
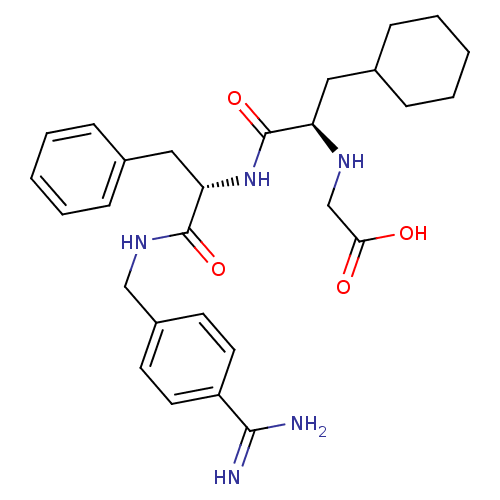
(CHEMBL3115897)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)cc1 |r| Show InChI InChI=1S/C28H37N5O4/c29-26(30)22-13-11-21(12-14-22)17-32-27(36)24(16-20-9-5-2-6-10-20)33-28(37)23(31-18-25(34)35)15-19-7-3-1-4-8-19/h2,5-6,9-14,19,23-24,31H,1,3-4,7-8,15-18H2,(H3,29,30)(H,32,36)(H,33,37)(H,34,35)/t23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447517
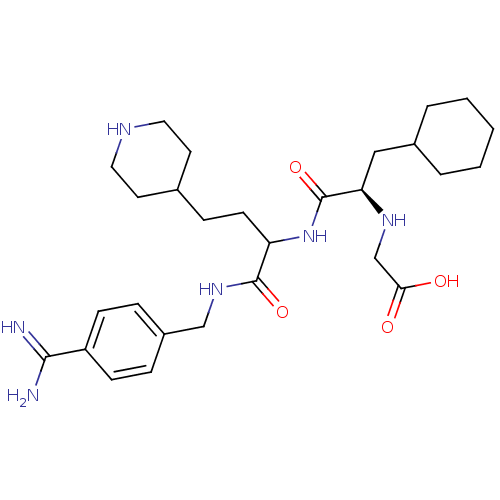
(CHEMBL3115894)Show SMILES NC(=N)c1ccc(CNC(=O)C(CCC2CCNCC2)NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)cc1 |r| Show InChI InChI=1S/C28H44N6O4/c29-26(30)22-9-6-21(7-10-22)17-33-27(37)23(11-8-19-12-14-31-15-13-19)34-28(38)24(32-18-25(35)36)16-20-4-2-1-3-5-20/h6-7,9-10,19-20,23-24,31-32H,1-5,8,11-18H2,(H3,29,30)(H,33,37)(H,34,38)(H,35,36)/t23?,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447521
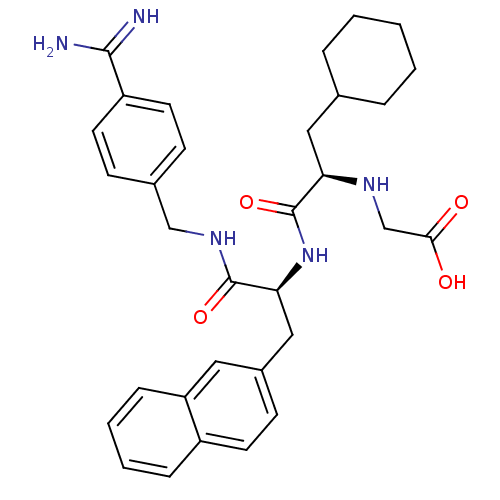
(CHEMBL3115899)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](Cc2ccc3ccccc3c2)NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)cc1 |r| Show InChI InChI=1S/C32H39N5O4/c33-30(34)25-14-10-22(11-15-25)19-36-31(40)28(18-23-12-13-24-8-4-5-9-26(24)16-23)37-32(41)27(35-20-29(38)39)17-21-6-2-1-3-7-21/h4-5,8-16,21,27-28,35H,1-3,6-7,17-20H2,(H3,33,34)(H,36,40)(H,37,41)(H,38,39)/t27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447514
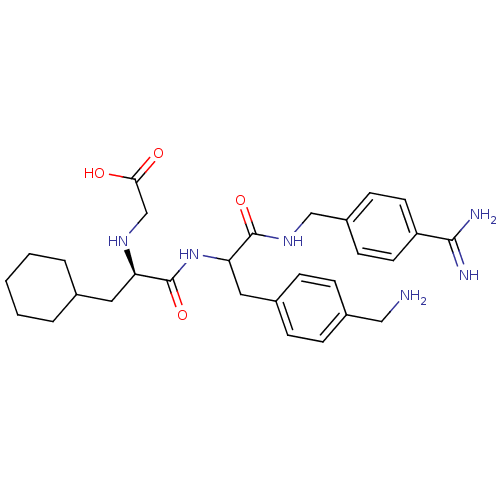
(CHEMBL3115903)Show SMILES NCc1ccc(CC(NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)C(=O)NCc2ccc(cc2)C(N)=N)cc1 |r| Show InChI InChI=1S/C29H40N6O4/c30-16-21-8-6-20(7-9-21)15-25(28(38)34-17-22-10-12-23(13-11-22)27(31)32)35-29(39)24(33-18-26(36)37)14-19-4-2-1-3-5-19/h6-13,19,24-25,33H,1-5,14-18,30H2,(H3,31,32)(H,34,38)(H,35,39)(H,36,37)/t24-,25?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447522

(CHEMBL3115898)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](Cc2cccc3ccccc23)NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)cc1 |r| Show InChI InChI=1S/C32H39N5O4/c33-30(34)24-15-13-22(14-16-24)19-36-31(40)28(18-25-11-6-10-23-9-4-5-12-26(23)25)37-32(41)27(35-20-29(38)39)17-21-7-2-1-3-8-21/h4-6,9-16,21,27-28,35H,1-3,7-8,17-20H2,(H3,33,34)(H,36,40)(H,37,41)(H,38,39)/t27-,28+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50447515

(CHEMBL3115901)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](Cc2ccc(N)cc2)NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)cc1 |r| Show InChI InChI=1S/C28H38N6O4/c29-22-12-8-19(9-13-22)15-24(27(37)33-16-20-6-10-21(11-7-20)26(30)31)34-28(38)23(32-17-25(35)36)14-18-4-2-1-3-5-18/h6-13,18,23-24,32H,1-5,14-17,29H2,(H3,30,31)(H,33,37)(H,34,38)(H,35,36)/t23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human coagulation factor 11a using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447525
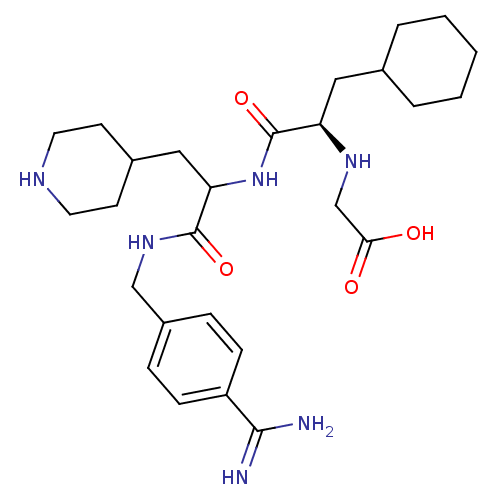
(CHEMBL3115893)Show SMILES NC(=N)c1ccc(CNC(=O)C(CC2CCNCC2)NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)cc1 |r| Show InChI InChI=1S/C27H42N6O4/c28-25(29)21-8-6-20(7-9-21)16-32-26(36)23(15-19-10-12-30-13-11-19)33-27(37)22(31-17-24(34)35)14-18-4-2-1-3-5-18/h6-9,18-19,22-23,30-31H,1-5,10-17H2,(H3,28,29)(H,32,36)(H,33,37)(H,34,35)/t22-,23?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50193243
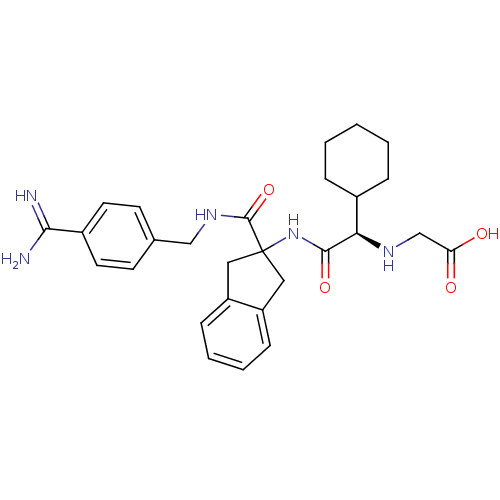
(({(R)-[2-(4-carbamimidoyl-benzylcarbamoyl)-indan-2...)Show SMILES NC(=N)c1ccc(CNC(=O)C2(Cc3ccccc3C2)NC(=O)[C@H](NCC(O)=O)C2CCCCC2)cc1 Show InChI InChI=1S/C28H35N5O4/c29-25(30)20-12-10-18(11-13-20)16-32-27(37)28(14-21-8-4-5-9-22(21)15-28)33-26(36)24(31-17-23(34)35)19-6-2-1-3-7-19/h4-5,8-13,19,24,31H,1-3,6-7,14-17H2,(H3,29,30)(H,32,37)(H,33,36)(H,34,35)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447527
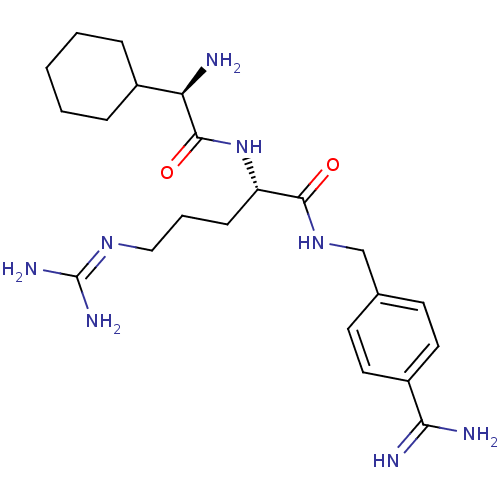
(CHEMBL3115905)Show SMILES [#7]-[#6@H](-[#6]-1-[#6]-[#6]-[#6]-[#6]-[#6]-1)-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](=O)-[#7]-[#6]-c1ccc(cc1)-[#6](-[#7])=[#7] |r| Show InChI InChI=1S/C22H36N8O2/c23-18(15-5-2-1-3-6-15)21(32)30-17(7-4-12-28-22(26)27)20(31)29-13-14-8-10-16(11-9-14)19(24)25/h8-11,15,17-18H,1-7,12-13,23H2,(H3,24,25)(H,29,31)(H,30,32)(H4,26,27,28)/t17-,18+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447519
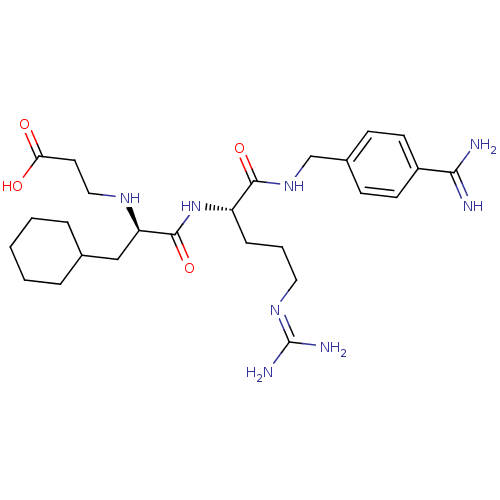
(CHEMBL3115906)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-1-[#6]-[#6]-[#6]-[#6]-[#6]-1)-[#7]-[#6]-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-c1ccc(cc1)-[#6](-[#7])=[#7] |r| Show InChI InChI=1S/C26H42N8O4/c27-23(28)19-10-8-18(9-11-19)16-33-24(37)20(7-4-13-32-26(29)30)34-25(38)21(31-14-12-22(35)36)15-17-5-2-1-3-6-17/h8-11,17,20-21,31H,1-7,12-16H2,(H3,27,28)(H,33,37)(H,34,38)(H,35,36)(H4,29,30,32)/t20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Aryl hydrocarbon receptor
(Mus musculus (Mouse)) | BDBM50605061
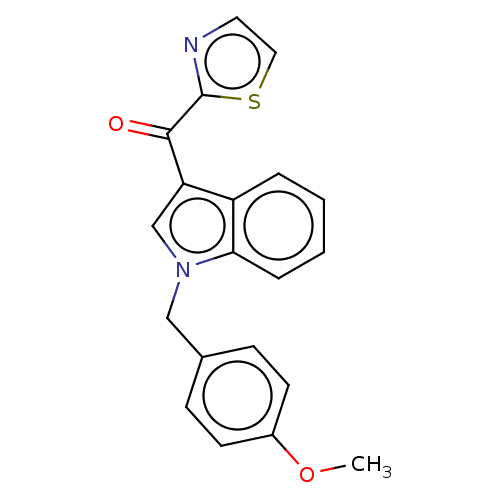
(CHEMBL5179114) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00208
BindingDB Entry DOI: 10.7270/Q27D3076 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50447516

(CHEMBL3115900)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)cc1 |r| Show InChI InChI=1S/C28H37N5O5/c29-26(30)21-10-6-20(7-11-21)16-32-27(37)24(15-19-8-12-22(34)13-9-19)33-28(38)23(31-17-25(35)36)14-18-4-2-1-3-5-18/h6-13,18,23-24,31,34H,1-5,14-17H2,(H3,29,30)(H,32,37)(H,33,38)(H,35,36)/t23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human coagulation factor 11a using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447524
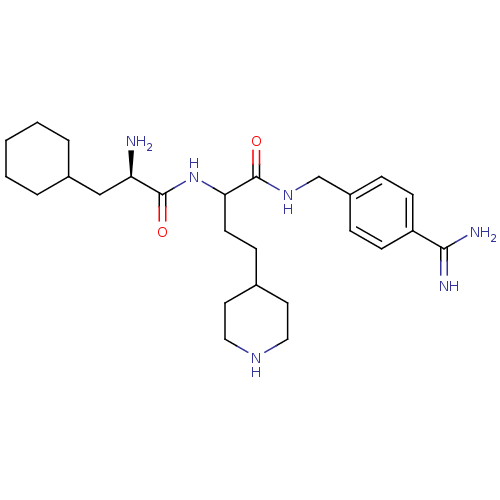
(CHEMBL3115896)Show SMILES N[C@H](CC1CCCCC1)C(=O)NC(CCC1CCNCC1)C(=O)NCc1ccc(cc1)C(N)=N |r| Show InChI InChI=1S/C26H42N6O2/c27-22(16-19-4-2-1-3-5-19)25(33)32-23(11-8-18-12-14-30-15-13-18)26(34)31-17-20-6-9-21(10-7-20)24(28)29/h6-7,9-10,18-19,22-23,30H,1-5,8,11-17,27H2,(H3,28,29)(H,31,34)(H,32,33)/t22-,23?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447518
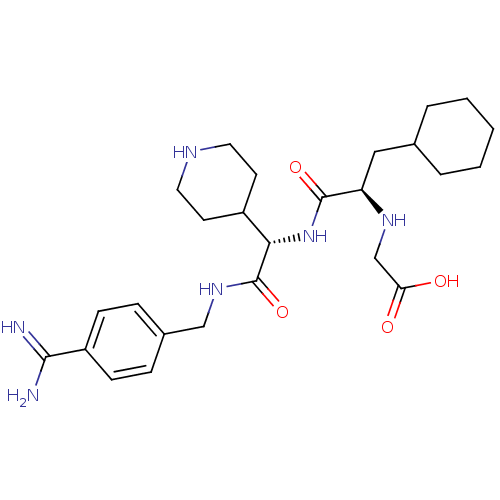
(CHEMBL3115892)Show SMILES NC(=N)c1ccc(CNC(=O)[C@@H](NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)C2CCNCC2)cc1 |r| Show InChI InChI=1S/C26H40N6O4/c27-24(28)20-8-6-18(7-9-20)15-31-26(36)23(19-10-12-29-13-11-19)32-25(35)21(30-16-22(33)34)14-17-4-2-1-3-5-17/h6-9,17,19,21,23,29-30H,1-5,10-16H2,(H3,27,28)(H,31,36)(H,32,35)(H,33,34)/t21-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447520
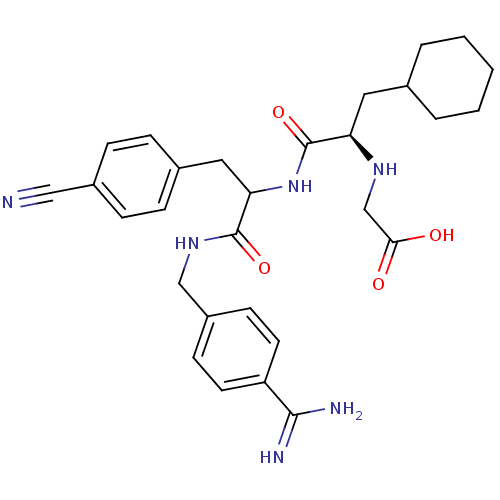
(CHEMBL3115902)Show SMILES NC(=N)c1ccc(CNC(=O)C(Cc2ccc(cc2)C#N)NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)cc1 |r| Show InChI InChI=1S/C29H36N6O4/c30-16-21-8-6-20(7-9-21)15-25(28(38)34-17-22-10-12-23(13-11-22)27(31)32)35-29(39)24(33-18-26(36)37)14-19-4-2-1-3-5-19/h6-13,19,24-25,33H,1-5,14-15,17-18H2,(H3,31,32)(H,34,38)(H,35,39)(H,36,37)/t24-,25?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Vitamin K-dependent protein C
(Homo sapiens (Human)) | BDBM50447526
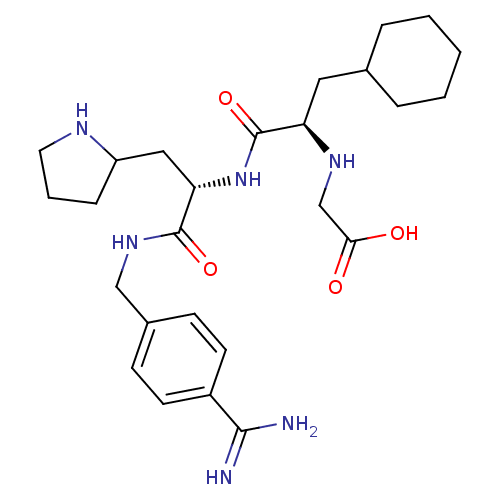
(CHEMBL3115907)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](CC2CCCN2)NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)cc1 |r| Show InChI InChI=1S/C26H40N6O4/c27-24(28)19-10-8-18(9-11-19)15-31-25(35)22(14-20-7-4-12-29-20)32-26(36)21(30-16-23(33)34)13-17-5-2-1-3-6-17/h8-11,17,20-22,29-30H,1-7,12-16H2,(H3,27,28)(H,31,35)(H,32,36)(H,33,34)/t20?,21-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human APC using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50447516

(CHEMBL3115900)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)cc1 |r| Show InChI InChI=1S/C28H37N5O5/c29-26(30)21-10-6-20(7-11-21)16-32-27(37)24(15-19-8-12-22(34)13-9-19)33-28(38)23(31-17-25(35)36)14-18-4-2-1-3-5-18/h6-13,18,23-24,31,34H,1-5,14-17H2,(H3,29,30)(H,32,37)(H,33,38)(H,35,36)/t23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human coagulation factor 10a using S-2765 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50447514

(CHEMBL3115903)Show SMILES NCc1ccc(CC(NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)C(=O)NCc2ccc(cc2)C(N)=N)cc1 |r| Show InChI InChI=1S/C29H40N6O4/c30-16-21-8-6-20(7-9-21)15-25(28(38)34-17-22-10-12-23(13-11-22)27(31)32)35-29(39)24(33-18-26(36)37)14-19-4-2-1-3-5-19/h6-13,19,24-25,33H,1-5,14-18,30H2,(H3,31,32)(H,34,38)(H,35,39)(H,36,37)/t24-,25?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human coagulation factor 10a using S-2765 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50447515

(CHEMBL3115901)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](Cc2ccc(N)cc2)NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)cc1 |r| Show InChI InChI=1S/C28H38N6O4/c29-22-12-8-19(9-13-22)15-24(27(37)33-16-20-6-10-21(11-7-20)26(30)31)34-28(38)23(32-17-25(35)36)14-18-4-2-1-3-5-18/h6-13,18,23-24,32H,1-5,14-17,29H2,(H3,30,31)(H,33,37)(H,34,38)(H,35,36)/t23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human coagulation factor 10a using S-2765 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50193243

(({(R)-[2-(4-carbamimidoyl-benzylcarbamoyl)-indan-2...)Show SMILES NC(=N)c1ccc(CNC(=O)C2(Cc3ccccc3C2)NC(=O)[C@H](NCC(O)=O)C2CCCCC2)cc1 Show InChI InChI=1S/C28H35N5O4/c29-25(30)20-12-10-18(11-13-20)16-32-27(37)28(14-21-8-4-5-9-22(21)15-28)33-26(36)24(31-17-23(34)35)19-6-2-1-3-7-19/h4-5,8-13,19,24,31H,1-3,6-7,14-17H2,(H3,29,30)(H,32,37)(H,33,36)(H,34,35)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human coagulation factor 10a using S-2765 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50193243

(({(R)-[2-(4-carbamimidoyl-benzylcarbamoyl)-indan-2...)Show SMILES NC(=N)c1ccc(CNC(=O)C2(Cc3ccccc3C2)NC(=O)[C@H](NCC(O)=O)C2CCCCC2)cc1 Show InChI InChI=1S/C28H35N5O4/c29-25(30)20-12-10-18(11-13-20)16-32-27(37)28(14-21-8-4-5-9-22(21)15-28)33-26(36)24(31-17-23(34)35)19-6-2-1-3-7-19/h4-5,8-13,19,24,31H,1-3,6-7,14-17H2,(H3,29,30)(H,32,37)(H,33,36)(H,34,35)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human coagulation factor 11a using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50447517

(CHEMBL3115894)Show SMILES NC(=N)c1ccc(CNC(=O)C(CCC2CCNCC2)NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)cc1 |r| Show InChI InChI=1S/C28H44N6O4/c29-26(30)22-9-6-21(7-10-22)17-33-27(37)23(11-8-19-12-14-31-15-13-19)34-28(38)24(32-18-25(35)36)16-20-4-2-1-3-5-20/h6-7,9-10,19-20,23-24,31-32H,1-5,8,11-18H2,(H3,29,30)(H,33,37)(H,34,38)(H,35,36)/t23?,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human coagulation factor 11a using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50447519

(CHEMBL3115906)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#6]-1-[#6]-[#6]-[#6]-[#6]-[#6]-1)-[#7]-[#6]-[#6]-[#6](-[#8])=O)-[#6](=O)-[#7]-[#6]-c1ccc(cc1)-[#6](-[#7])=[#7] |r| Show InChI InChI=1S/C26H42N8O4/c27-23(28)19-10-8-18(9-11-19)16-33-24(37)20(7-4-13-32-26(29)30)34-25(38)21(31-14-12-22(35)36)15-17-5-2-1-3-6-17/h8-11,17,20-21,31H,1-7,12-16H2,(H3,27,28)(H,33,37)(H,34,38)(H,35,36)(H4,29,30,32)/t20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human coagulation factor 11a using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50447514

(CHEMBL3115903)Show SMILES NCc1ccc(CC(NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)C(=O)NCc2ccc(cc2)C(N)=N)cc1 |r| Show InChI InChI=1S/C29H40N6O4/c30-16-21-8-6-20(7-9-21)15-25(28(38)34-17-22-10-12-23(13-11-22)27(31)32)35-29(39)24(33-18-26(36)37)14-19-4-2-1-3-5-19/h6-13,19,24-25,33H,1-5,14-18,30H2,(H3,31,32)(H,34,38)(H,35,39)(H,36,37)/t24-,25?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human coagulation factor 11a using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50447518

(CHEMBL3115892)Show SMILES NC(=N)c1ccc(CNC(=O)[C@@H](NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)C2CCNCC2)cc1 |r| Show InChI InChI=1S/C26H40N6O4/c27-24(28)20-8-6-18(7-9-20)15-31-26(36)23(19-10-12-29-13-11-19)32-25(35)21(30-16-22(33)34)14-17-4-2-1-3-5-17/h6-9,17,19,21,23,29-30H,1-5,10-16H2,(H3,27,28)(H,31,36)(H,32,35)(H,33,34)/t21-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human coagulation factor 11a using S-2366 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50447517

(CHEMBL3115894)Show SMILES NC(=N)c1ccc(CNC(=O)C(CCC2CCNCC2)NC(=O)[C@@H](CC2CCCCC2)NCC(O)=O)cc1 |r| Show InChI InChI=1S/C28H44N6O4/c29-26(30)22-9-6-21(7-10-22)17-33-27(37)23(11-8-19-12-14-31-15-13-19)34-28(38)24(32-18-25(35)36)16-20-4-2-1-3-5-20/h6-7,9-10,19-20,23-24,31-32H,1-5,8,11-18H2,(H3,29,30)(H,33,37)(H,34,38)(H,35,36)/t23?,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Competitive inhibition of human coagulation factor 10a using S-2765 as substrate |
Bioorg Med Chem Lett 24: 821-7 (2014)
Article DOI: 10.1016/j.bmcl.2013.12.094
BindingDB Entry DOI: 10.7270/Q2542Q3B |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50339190
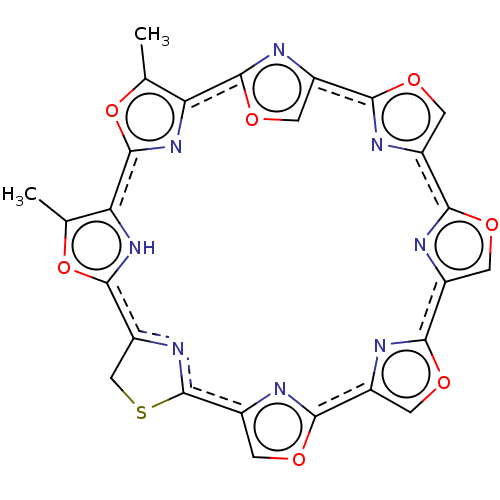
(CHEMBL443683 | telomestatin)Show SMILES Cc1oc2nc1c1nc(co1)c1nc(co1)c1nc(co1)c1nc(co1)c1nc(co1)c1SCc(n1)c1[nH]c2c(C)o1 Show InChI InChI=1S/C26H14N8O7S/c1-9-17-24-30-14(6-39-24)21-28-12(4-37-21)19-27-11(3-35-19)20-29-13(5-36-20)22-31-15(7-38-22)26-32-16(8-42-26)23-33-18(10(2)40-23)25(34-17)41-9/h3-7,33H,8H2,1-2H3/b20-11+,21-14+,26-15+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut National de la Sant£ et de la Recherche M£dicale
Curated by ChEMBL
| Assay Description
Inhibition of human telomerase expressed in HEK293T cells treated before telomerase elongation by telomeric repeat amplification protocol assay |
Proc Natl Acad Sci USA 104: 17347-52 (2007)
Article DOI: 10.1073/pnas.0707365104
BindingDB Entry DOI: 10.7270/Q26974FQ |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50339190

(CHEMBL443683 | telomestatin)Show SMILES Cc1oc2nc1c1nc(co1)c1nc(co1)c1nc(co1)c1nc(co1)c1nc(co1)c1SCc(n1)c1[nH]c2c(C)o1 Show InChI InChI=1S/C26H14N8O7S/c1-9-17-24-30-14(6-39-24)21-28-12(4-37-21)19-27-11(3-35-19)20-29-13(5-36-20)22-31-15(7-38-22)26-32-16(8-42-26)23-33-18(10(2)40-23)25(34-17)41-9/h3-7,33H,8H2,1-2H3/b20-11+,21-14+,26-15+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut National de la Sant£ et de la Recherche M£dicale
Curated by ChEMBL
| Assay Description
Inhibition of human telomerase expressed in HEK293T cells treated after telomerase elongation by telomeric repeat amplification protocol assay |
Proc Natl Acad Sci USA 104: 17347-52 (2007)
Article DOI: 10.1073/pnas.0707365104
BindingDB Entry DOI: 10.7270/Q26974FQ |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50019017
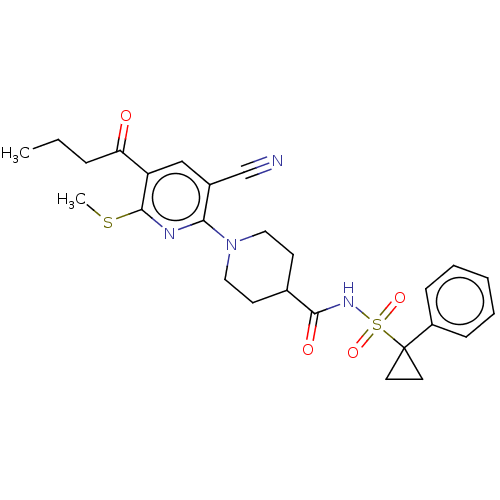
(CHEMBL3288123)Show SMILES CCCC(=O)c1cc(C#N)c(nc1SC)N1CCC(CC1)C(=O)NS(=O)(=O)C1(CC1)c1ccccc1 Show InChI InChI=1S/C26H30N4O4S2/c1-3-7-22(31)21-16-19(17-27)23(28-25(21)35-2)30-14-10-18(11-15-30)24(32)29-36(33,34)26(12-13-26)20-8-5-4-6-9-20/h4-6,8-9,16,18H,3,7,10-15H2,1-2H3,(H,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Antagonist activity P2Y12 receptor in human washed platelets assessed as inhibition of ADP-induced platelet aggregation after 5 to 90 mins by spectro... |
Bioorg Med Chem Lett 24: 2963-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.001
BindingDB Entry DOI: 10.7270/Q24X59CQ |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50376748

(CHEMBL259739 | Phen-DC3)Show SMILES C[n+]1cc(NC(=O)c2ccc3ccc4ccc(nc4c3n2)C(=O)Nc2c[n+](C)c3ccccc3c2)cc2ccccc12 Show InChI InChI=1S/C34H24N6O2/c1-39-19-25(17-23-7-3-5-9-29(23)39)35-33(41)27-15-13-21-11-12-22-14-16-28(38-32(22)31(21)37-27)34(42)36-26-18-24-8-4-6-10-30(24)40(2)20-26/h3-20H,1-2H3/p+2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut National de la Sant£ et de la Recherche M£dicale
Curated by ChEMBL
| Assay Description
Inhibition of human telomerase expressed in HEK293T cells treated before telomerase elongation by telomeric repeat amplification protocol assay |
Proc Natl Acad Sci USA 104: 17347-52 (2007)
Article DOI: 10.1073/pnas.0707365104
BindingDB Entry DOI: 10.7270/Q26974FQ |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM92862

(US9284315, BEZ-235 | mTOR Inhibitor, BEZ235)Show SMILES Cn1c2cnc3ccc(cc3c2n(-c2ccc(cc2)C(C)(C)C#N)c1=O)-c1cnc2ccccc2c1 Show InChI InChI=1S/C30H23N5O/c1-30(2,18-31)22-9-11-23(12-10-22)35-28-24-15-19(21-14-20-6-4-5-7-25(20)32-16-21)8-13-26(24)33-17-27(28)34(3)29(35)36/h4-17H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00267
BindingDB Entry DOI: 10.7270/Q28P64KJ |
More data for this
Ligand-Target Pair | |
Telomerase reverse transcriptase
(Homo sapiens (Human)) | BDBM50376748

(CHEMBL259739 | Phen-DC3)Show SMILES C[n+]1cc(NC(=O)c2ccc3ccc4ccc(nc4c3n2)C(=O)Nc2c[n+](C)c3ccccc3c2)cc2ccccc12 Show InChI InChI=1S/C34H24N6O2/c1-39-19-25(17-23-7-3-5-9-29(23)39)35-33(41)27-15-13-21-11-12-22-14-16-28(38-32(22)31(21)37-27)34(42)36-26-18-24-8-4-6-10-30(24)40(2)20-26/h3-20H,1-2H3/p+2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut National de la Sant£ et de la Recherche M£dicale
Curated by ChEMBL
| Assay Description
Inhibition of human telomerase expressed in HEK293T cells treated after telomerase elongation by telomeric repeat amplification protocol assay |
Proc Natl Acad Sci USA 104: 17347-52 (2007)
Article DOI: 10.1073/pnas.0707365104
BindingDB Entry DOI: 10.7270/Q26974FQ |
More data for this
Ligand-Target Pair | |
P2Y purinoceptor 12
(Homo sapiens (Human)) | BDBM50019017

(CHEMBL3288123)Show SMILES CCCC(=O)c1cc(C#N)c(nc1SC)N1CCC(CC1)C(=O)NS(=O)(=O)C1(CC1)c1ccccc1 Show InChI InChI=1S/C26H30N4O4S2/c1-3-7-22(31)21-16-19(17-27)23(28-25(21)35-2)30-14-10-18(11-15-30)24(32)29-36(33,34)26(12-13-26)20-8-5-4-6-9-20/h4-6,8-9,16,18H,3,7,10-15H2,1-2H3,(H,29,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [125I]AZ11931285 from human P2Y12 receptor expressed in CHOK1 cell membrane after 60 mins |
Bioorg Med Chem Lett 24: 2963-8 (2014)
Article DOI: 10.1016/j.bmcl.2014.04.001
BindingDB Entry DOI: 10.7270/Q24X59CQ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50112819

(CHEMBL3609522)Show SMILES CC(C)(C)c1ncc2CCc3nc(NC(=O)N4CCC[C@H]4C(N)=O)sc3-c2n1 |r| Show InChI InChI=1S/C19H24N6O2S/c1-19(2,3)16-21-9-10-6-7-11-14(13(10)23-16)28-17(22-11)24-18(27)25-8-4-5-12(25)15(20)26/h9,12H,4-8H2,1-3H3,(H2,20,26)(H,22,24,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using phosphatidylinositol as substrate preincubated for 15 mins followed by ATP addition measured after 1 h... |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50112822
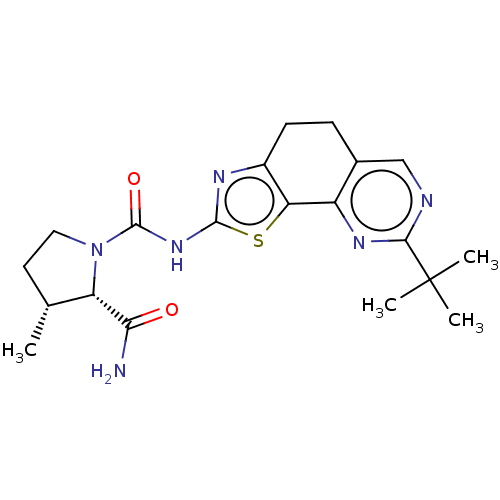
(CHEMBL3609525)Show SMILES C[C@@H]1CCN([C@@H]1C(N)=O)C(=O)Nc1nc2CCc3cnc(nc3-c2s1)C(C)(C)C |r| Show InChI InChI=1S/C20H26N6O2S/c1-10-7-8-26(14(10)16(21)27)19(28)25-18-23-12-6-5-11-9-22-17(20(2,3)4)24-13(11)15(12)29-18/h9-10,14H,5-8H2,1-4H3,(H2,21,27)(H,23,25,28)/t10-,14+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated human PI3Kalpha expressed in Rat1 cells assessed as inhibition of Akt phosphorylatuion at Ser473 by ELISA |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50112823

(CHEMBL3609526)Show SMILES C[C@@H]1CCN([C@@H]1C(N)=O)C(=O)Nc1nc2CCc3cnc(nc3-c2s1)C(C)(C)C(F)(F)F |r| Show InChI InChI=1S/C20H23F3N6O2S/c1-9-6-7-29(13(9)15(24)30)18(31)28-17-26-11-5-4-10-8-25-16(19(2,3)20(21,22)23)27-12(10)14(11)32-17/h8-9,13H,4-7H2,1-3H3,(H2,24,30)(H,26,28,31)/t9-,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated human PI3Kalpha expressed in Rat1 cells assessed as inhibition of Akt phosphorylatuion at Ser473 by ELISA |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50112820
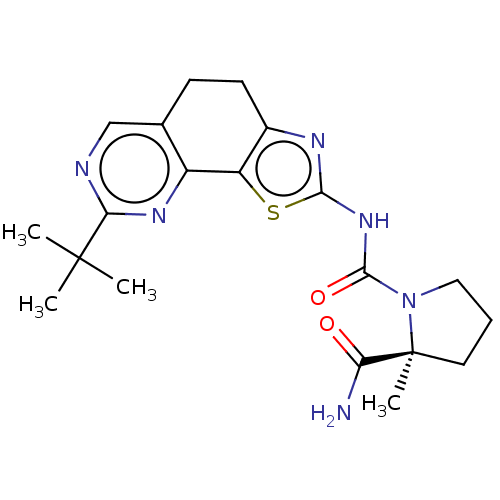
(CHEMBL3609523)Show SMILES CC(C)(C)c1ncc2CCc3nc(NC(=O)N4CCC[C@@]4(C)C(N)=O)sc3-c2n1 |r| Show InChI InChI=1S/C20H26N6O2S/c1-19(2,3)16-22-10-11-6-7-12-14(13(11)24-16)29-17(23-12)25-18(28)26-9-5-8-20(26,4)15(21)27/h10H,5-9H2,1-4H3,(H2,21,27)(H,23,25,28)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal myristoylated human PI3Kalpha expressed in Rat1 cells assessed as inhibition of Akt phosphorylatuion at Ser473 by ELISA |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50112826
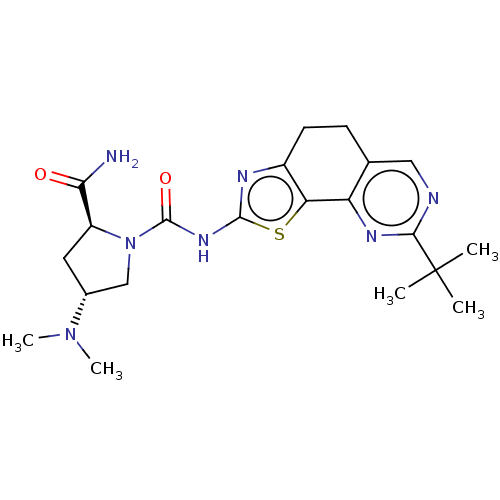
(CHEMBL3609529)Show SMILES CN(C)[C@@H]1C[C@H](N(C1)C(=O)Nc1nc2CCc3cnc(nc3-c2s1)C(C)(C)C)C(N)=O |r| Show InChI InChI=1S/C21H29N7O2S/c1-21(2,3)18-23-9-11-6-7-13-16(15(11)25-18)31-19(24-13)26-20(30)28-10-12(27(4)5)8-14(28)17(22)29/h9,12,14H,6-8,10H2,1-5H3,(H2,22,29)(H,24,26,30)/t12-,14+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using phosphatidylinositol as substrate preincubated for 15 mins followed by ATP addition measured after 1 h... |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM143236
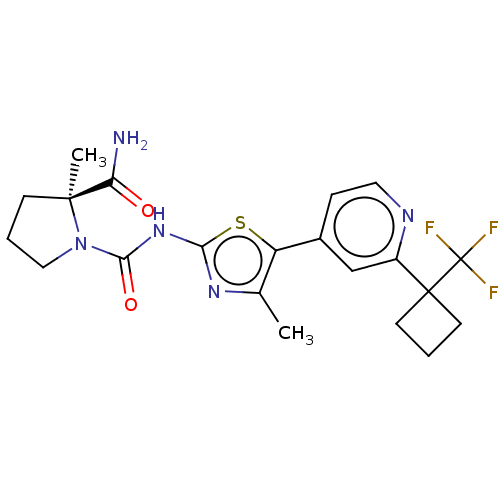
(US8940771, 48)Show SMILES Cc1nc(NC(=O)N2CCC[C@@]2(C)C(N)=O)sc1-c1ccnc(c1)C1(CCC1)C(F)(F)F |r| Show InChI InChI=1S/C21H24F3N5O2S/c1-12-15(13-5-9-26-14(11-13)20(7-3-8-20)21(22,23)24)32-17(27-12)28-18(31)29-10-4-6-19(29,2)16(25)30/h5,9,11H,3-4,6-8,10H2,1-2H3,(H2,25,30)(H,27,28,31)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Novartis AG
US Patent
| Assay Description
Typically frozen cells from 10 L of Tn5 cell culture were resuspended in Lysis Buffer 20 mM Tris-Cl, pH 7.5, 500 mM NaCl, 5% glycerol, 5 mM imidazole... |
US Patent US8940771 (2015)
BindingDB Entry DOI: 10.7270/Q2TM78VF |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50191127
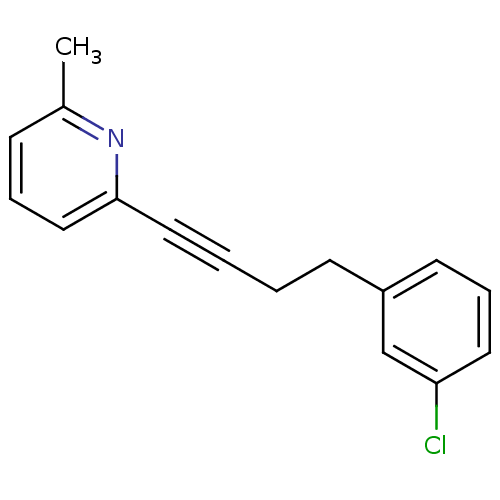
(2-(4-(3-chlorophenyl)but-1-ynyl)-6-methylpyridine ...)Show InChI InChI=1S/C16H14ClN/c1-13-6-4-11-16(18-13)10-3-2-7-14-8-5-9-15(17)12-14/h4-6,8-9,11-12H,2,7H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Mölndal
Curated by ChEMBL
| Assay Description
Activity at human mGluR5d assessed as inhibition of glutamate-induced calcium influx by FLIPR assay |
Bioorg Med Chem Lett 16: 4788-91 (2006)
Article DOI: 10.1016/j.bmcl.2006.06.078
BindingDB Entry DOI: 10.7270/Q2VX0G48 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50112820

(CHEMBL3609523)Show SMILES CC(C)(C)c1ncc2CCc3nc(NC(=O)N4CCC[C@@]4(C)C(N)=O)sc3-c2n1 |r| Show InChI InChI=1S/C20H26N6O2S/c1-19(2,3)16-22-10-11-6-7-12-14(13(11)24-16)29-17(23-12)25-18(28)26-9-5-8-20(26,4)15(21)27/h10H,5-9H2,1-4H3,(H2,21,27)(H,23,25,28)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using phosphatidylinositol as substrate preincubated for 15 mins followed by ATP addition measured after 1 h... |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50112823

(CHEMBL3609526)Show SMILES C[C@@H]1CCN([C@@H]1C(N)=O)C(=O)Nc1nc2CCc3cnc(nc3-c2s1)C(C)(C)C(F)(F)F |r| Show InChI InChI=1S/C20H23F3N6O2S/c1-9-6-7-29(13(9)15(24)30)18(31)28-17-26-11-5-4-10-8-25-16(19(2,3)20(21,22)23)27-12(10)14(11)32-17/h8-9,13H,4-7H2,1-3H3,(H2,24,30)(H,26,28,31)/t9-,13+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using phosphatidylinositol as substrate preincubated for 15 mins followed by ATP addition measured after 1 h... |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | |
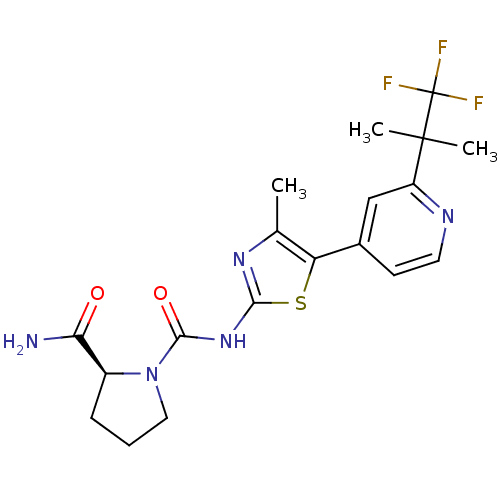
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50436459
(CHEMBL2396661)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(c1)C(C)(C)C(F)(F)F |r| Show InChI InChI=1S/C19H22F3N5O2S/c1-10-14(11-6-7-24-13(9-11)18(2,3)19(20,21)22)30-16(25-10)26-17(29)27-8-4-5-12(27)15(23)28/h6-7,9,12H,4-5,8H2,1-3H3,(H2,23,28)(H,25,26,29)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) |
Bioorg Med Chem Lett 25: 3569-74 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.078
BindingDB Entry DOI: 10.7270/Q2W098XH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50436459

(CHEMBL2396661)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccnc(c1)C(C)(C)C(F)(F)F |r| Show InChI InChI=1S/C19H22F3N5O2S/c1-10-14(11-6-7-24-13(9-11)18(2,3)19(20,21)22)30-16(25-10)26-17(29)27-8-4-5-12(27)15(23)28/h6-7,9,12H,4-5,8H2,1-3H3,(H2,23,28)(H,25,26,29)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of P110alpha (unknown origin) using L-a-phosphatidylinositol as substrate by luminescence assay |
Bioorg Med Chem Lett 23: 3741-8 (2013)
Article DOI: 10.1016/j.bmcl.2013.05.007
BindingDB Entry DOI: 10.7270/Q2M046VD |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50112824
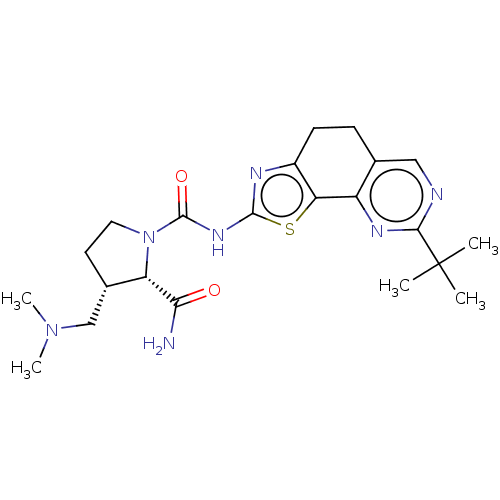
(CHEMBL3609527)Show SMILES CN(C)C[C@@H]1CCN([C@@H]1C(N)=O)C(=O)Nc1nc2CCc3cnc(nc3-c2s1)C(C)(C)C |r| Show InChI InChI=1S/C22H31N7O2S/c1-22(2,3)19-24-10-12-6-7-14-17(15(12)26-19)32-20(25-14)27-21(31)29-9-8-13(11-28(4)5)16(29)18(23)30/h10,13,16H,6-9,11H2,1-5H3,(H2,23,30)(H,25,27,31)/t13-,16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using phosphatidylinositol as substrate preincubated for 15 mins followed by ATP addition measured after 1 h... |
Bioorg Med Chem Lett 25: 3575-81 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.067
BindingDB Entry DOI: 10.7270/Q2DF6SZ5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data