

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50050525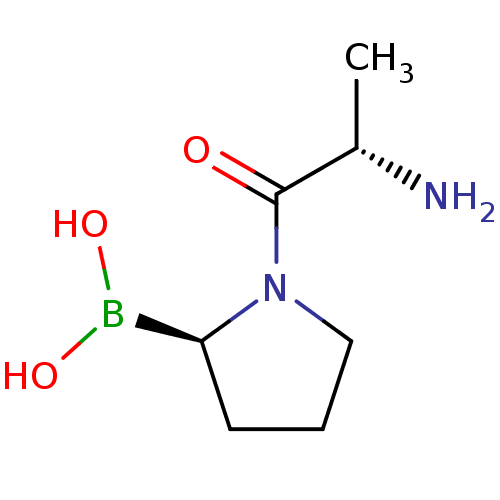 ((R)-1-((S)-2-aminopropanoyl)pyrrolidin-2-ylboronic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human placental DPP4 | J Med Chem 50: 2391-8 (2007) Article DOI: 10.1021/jm061321+ BindingDB Entry DOI: 10.7270/Q2NC6407 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50050513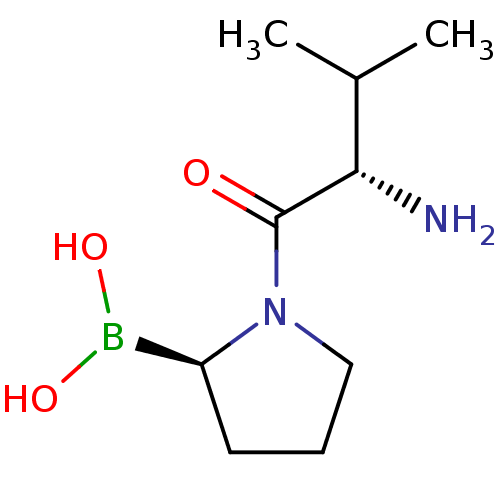 ((R)-1-((S)-2-amino-3-methylbutanoyl)pyrrolidin-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human placental DPP4 | J Med Chem 50: 2391-8 (2007) Article DOI: 10.1021/jm061321+ BindingDB Entry DOI: 10.7270/Q2NC6407 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50050513 ((R)-1-((S)-2-amino-3-methylbutanoyl)pyrrolidin-2-y...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human placental DPP4 | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50050525 ((R)-1-((S)-2-aminopropanoyl)pyrrolidin-2-ylboronic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human placental DPP4 | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50050511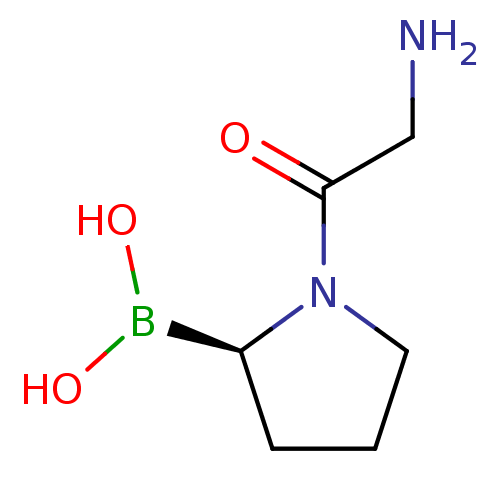 ((R)-1-(2-aminoacetyl)pyrrolidin-2-ylboronic acid |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human placental DPP4 | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 9 (Homo sapiens (Human)) | BDBM50050511 ((R)-1-(2-aminoacetyl)pyrrolidin-2-ylboronic acid |...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP9 expressed in HEK293T cells | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 9 (Homo sapiens (Human)) | BDBM50050525 ((R)-1-((S)-2-aminopropanoyl)pyrrolidin-2-ylboronic...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP9 expressed in HEK293T cells | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50431227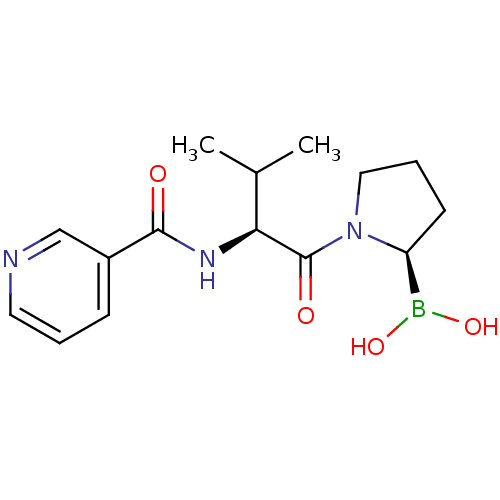 (CHEMBL2333024) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Sackler School of Biomedical Sciences Curated by ChEMBL | Assay Description Competitive inhibition of human PREP using Suc-GP-AMC as substrate by Lineweaver-Burk plot analysis | J Med Chem 56: 3467-77 (2013) Article DOI: 10.1021/jm400351a BindingDB Entry DOI: 10.7270/Q2C53N7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 9 (Homo sapiens (Human)) | BDBM50050513 ((R)-1-((S)-2-amino-3-methylbutanoyl)pyrrolidin-2-y...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP9 expressed in HEK293T cells | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM50050513 ((R)-1-((S)-2-amino-3-methylbutanoyl)pyrrolidin-2-y...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP8 expressed in HEK293T cells | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50253621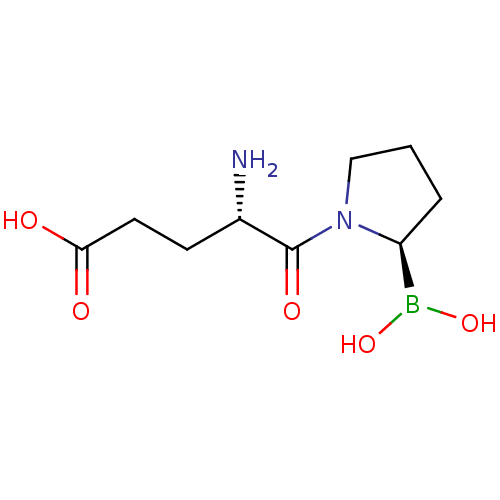 ((S)-4-amino-5-((R)-2-boronopyrrolidin-1-yl)-5-oxop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human placental DPP4 | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM50050525 ((R)-1-((S)-2-aminopropanoyl)pyrrolidin-2-ylboronic...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP8 expressed in HEK293T cells | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50476305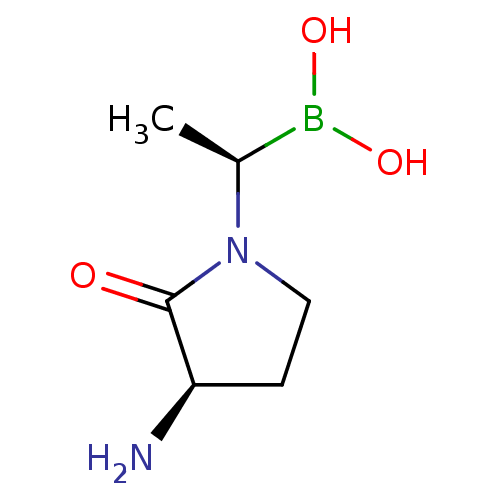 (CHEMBL2068511) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human placental DPP4 | J Med Chem 50: 2391-8 (2007) Article DOI: 10.1021/jm061321+ BindingDB Entry DOI: 10.7270/Q2NC6407 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 9 (Homo sapiens (Human)) | BDBM50253621 ((S)-4-amino-5-((R)-2-boronopyrrolidin-1-yl)-5-oxop...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP9 expressed in HEK293T cells | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM50050511 ((R)-1-(2-aminoacetyl)pyrrolidin-2-ylboronic acid |...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP8 expressed in HEK293T cells | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50171556 ((R)-1-((S)-2-amino-3-methylbutanamido)ethylboronic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human placental DPP4 | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50253639 ((S)-4-amino-5-((R)-1-boronoethylamino)-5-oxopentan...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human placental DPP4 | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Homo sapiens (Human)) | BDBM50431242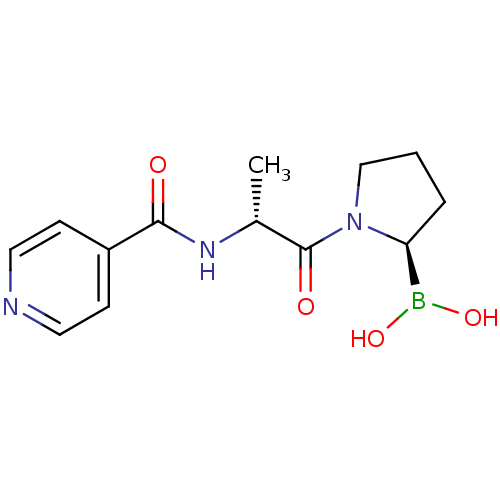 (CHEMBL2333026) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Sackler School of Biomedical Sciences Curated by ChEMBL | Assay Description Competitive inhibition of human FAP using Suc-GP-AMC as substrate by Lineweaver-Burk plot analysis | J Med Chem 56: 3467-77 (2013) Article DOI: 10.1021/jm400351a BindingDB Entry DOI: 10.7270/Q2C53N7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM50253621 ((S)-4-amino-5-((R)-2-boronopyrrolidin-1-yl)-5-oxop...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP8 expressed in HEK293T cells | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50253640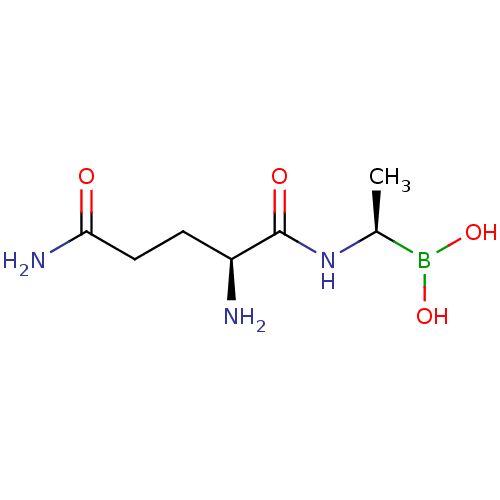 ((R)-1-((S)-2,5-diamino-5-oxopentanamido)ethylboron...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human placental DPP4 | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 9 (Homo sapiens (Human)) | BDBM50171556 ((R)-1-((S)-2-amino-3-methylbutanamido)ethylboronic...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP9 expressed in HEK293T cells | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM50171556 ((R)-1-((S)-2-amino-3-methylbutanamido)ethylboronic...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 96 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP8 expressed in HEK293T cells | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM50253640 ((R)-1-((S)-2,5-diamino-5-oxopentanamido)ethylboron...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP8 expressed in HEK293T cells | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 9 (Homo sapiens (Human)) | BDBM50253640 ((R)-1-((S)-2,5-diamino-5-oxopentanamido)ethylboron...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP9 expressed in HEK293T cells | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM50253639 ((S)-4-amino-5-((R)-1-boronoethylamino)-5-oxopentan...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP8 expressed in HEK293T cells | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50476304 (CHEMBL2068512) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human placental DPP4 | J Med Chem 50: 2391-8 (2007) Article DOI: 10.1021/jm061321+ BindingDB Entry DOI: 10.7270/Q2NC6407 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 9 (Homo sapiens (Human)) | BDBM50253639 ((S)-4-amino-5-((R)-1-boronoethylamino)-5-oxopentan...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP9 expressed in HEK293T cells | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase (Homo sapiens (Human)) | BDBM50431242 (CHEMBL2333026) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Sackler School of Biomedical Sciences Curated by ChEMBL | Assay Description Competitive inhibition of human PREP using Suc-GP-AMC as substrate by Lineweaver-Burk plot analysis | J Med Chem 56: 3467-77 (2013) Article DOI: 10.1021/jm400351a BindingDB Entry DOI: 10.7270/Q2C53N7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50253641 ((R)-1-((S)-2-acetamido-3-methylbutanoyl)pyrrolidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human placental DPP4 | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 9 (Homo sapiens (Human)) | BDBM50253641 ((R)-1-((S)-2-acetamido-3-methylbutanoyl)pyrrolidin...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP9 expressed in HEK293T cells | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50005111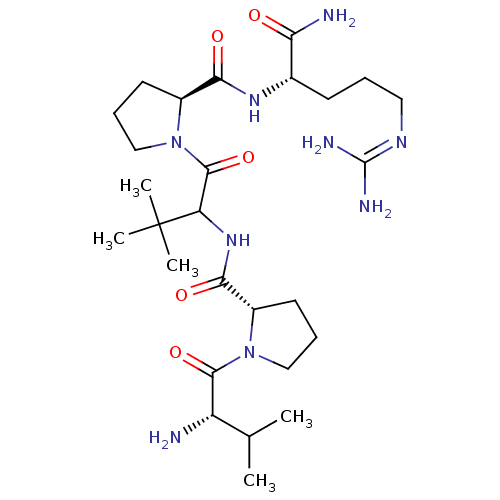 (CHEMBL3086656) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant DPP4 using AP-pNA as substrate preincubated for 30 mins followed by substrate addition measured after 30 ... | J Med Chem 56: 8339-51 (2013) Article DOI: 10.1021/jm400423p BindingDB Entry DOI: 10.7270/Q2PK0HMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50005109 (CHEMBL3086660) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant DPP4 using AP-pNA as substrate preincubated for 30 mins followed by substrate addition measured after 30 ... | J Med Chem 56: 8339-51 (2013) Article DOI: 10.1021/jm400423p BindingDB Entry DOI: 10.7270/Q2PK0HMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50005107 (CHEMBL3086661) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant DPP4 using AP-pNA as substrate preincubated for 30 mins followed by substrate addition measured after 30 ... | J Med Chem 56: 8339-51 (2013) Article DOI: 10.1021/jm400423p BindingDB Entry DOI: 10.7270/Q2PK0HMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 8 (Homo sapiens (Human)) | BDBM50253641 ((R)-1-((S)-2-acetamido-3-methylbutanoyl)pyrrolidin...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Inhibition of human recombinant DPP8 expressed in HEK293T cells | J Med Chem 51: 6005-13 (2008) Article DOI: 10.1021/jm800390n BindingDB Entry DOI: 10.7270/Q2GM874W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50005112 (CHEMBL3086658) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 6.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant DPP4 using AP-pNA as substrate preincubated for 30 mins followed by substrate addition measured after 30 ... | J Med Chem 56: 8339-51 (2013) Article DOI: 10.1021/jm400423p BindingDB Entry DOI: 10.7270/Q2PK0HMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50005110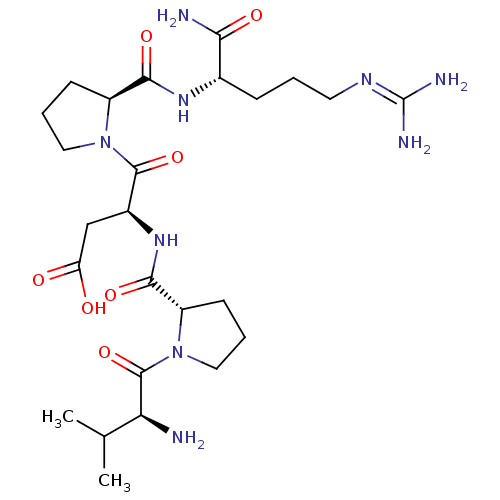 (CHEMBL3086657) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.42E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Curated by ChEMBL | Assay Description Competitive inhibition of human recombinant DPP4 using AP-pNA as substrate preincubated for 30 mins followed by substrate addition measured after 30 ... | J Med Chem 56: 8339-51 (2013) Article DOI: 10.1021/jm400423p BindingDB Entry DOI: 10.7270/Q2PK0HMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM142088 (US11096924, DASH-inhibitors 2243 | US11559537, Com...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description This assay is described in Bachovchin et al. Nature Chemical Biology 10, 656-663 (2014). Briefly, purified enzymes are coupled to Luminex microsphere... | Citation and Details BindingDB Entry DOI: 10.7270/Q28W3J7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 2 (Homo sapiens (Human)) | BDBM50050516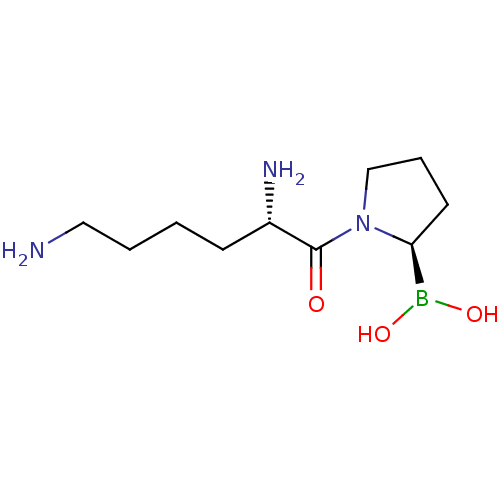 (Boronic acid derivative | CHEMBL63726 | US11096924...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description This assay is described in Bachovchin et al. Nature Chemical Biology 10, 656-663 (2014). Briefly, purified enzymes are coupled to Luminex microsphere... | Citation and Details BindingDB Entry DOI: 10.7270/Q28W3J7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM142088 (US11096924, DASH-inhibitors 2243 | US11559537, Com...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description This assay is described in Bachovchin et al. Nature Chemical Biology 10, 656-663 (2014). Briefly, purified enzymes are coupled to Luminex microsphere... | Citation and Details BindingDB Entry DOI: 10.7270/Q28W3J7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50050525 ((R)-1-((S)-2-aminopropanoyl)pyrrolidin-2-ylboronic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | 2.0 | n/a |
Trustees of Tufts College US Patent | Assay Description The inhibitor solution is prepared by dissolving 3-5 mg of inhibitor in pH 2 solution (0.01 N HCl), such that the concentration of the solution is eq... | US Patent US8933056 (2015) BindingDB Entry DOI: 10.7270/Q2ST7NJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50389480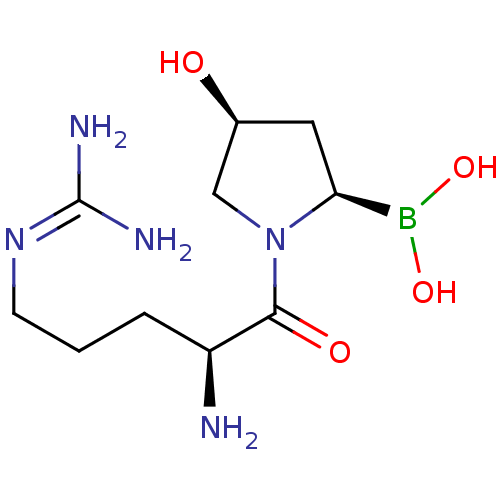 (CHEMBL2063040) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human DPP4 expressed in HEK293 cells using Ala-Pro-p-nitroanilide hydrochloride salt as substrate preincubated for 10 mins prior to add... | Bioorg Med Chem Lett 22: 5536-40 (2012) Article DOI: 10.1016/j.bmcl.2012.07.033 BindingDB Entry DOI: 10.7270/Q2M909QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 9 (Homo sapiens (Human)) | BDBM50389480 (CHEMBL2063040) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human DPP9 expressed in HEK293 cells using Ala-Pro-p-nitroanilide hydrochloride salt as substrate preincubated for 10 mins prior to add... | Bioorg Med Chem Lett 22: 5536-40 (2012) Article DOI: 10.1016/j.bmcl.2012.07.033 BindingDB Entry DOI: 10.7270/Q2M909QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM142088 (US11096924, DASH-inhibitors 2243 | US11559537, Com...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | 2.0 | n/a |
Trustees of Tufts College US Patent | Assay Description The inhibitor solution is prepared by dissolving 3-5 mg of inhibitor in pH 2 solution (0.01 N HCl), such that the concentration of the solution is eq... | US Patent US8933056 (2015) BindingDB Entry DOI: 10.7270/Q2ST7NJQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Homo sapiens (Human)) | BDBM609784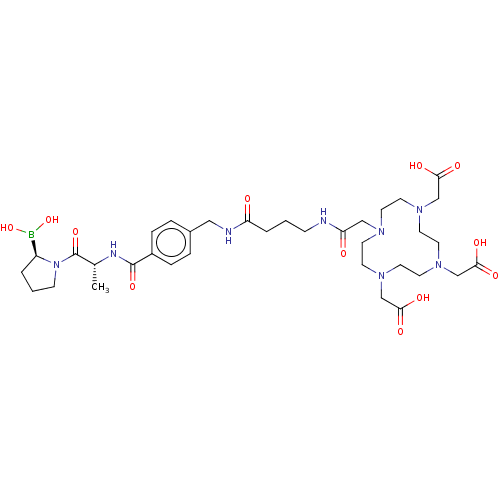 (US11707539, Compound 6591) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q26D5Z4W | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Homo sapiens (Human)) | BDBM609783 (US11707539, Compound 6590LU) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q26D5Z4W | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50050525 ((R)-1-((S)-2-aminopropanoyl)pyrrolidin-2-ylboronic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University School of Medicine Curated by ChEMBL | Assay Description Inhibition of human DPP4 expressed in HEK293 cells using Ala-Pro-p-nitroanilide hydrochloride salt as substrate preincubated for 10 mins prior to add... | Bioorg Med Chem Lett 22: 5536-40 (2012) Article DOI: 10.1016/j.bmcl.2012.07.033 BindingDB Entry DOI: 10.7270/Q2M909QF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prolyl endopeptidase FAP (Homo sapiens (Human)) | BDBM50431243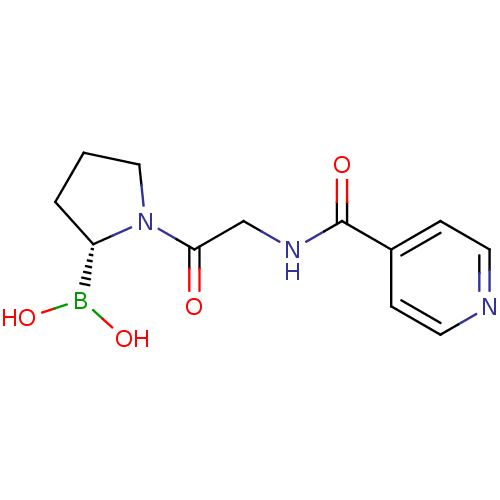 (CHEMBL2333025) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Tufts University Sackler School of Biomedical Sciences Curated by ChEMBL | Assay Description Inhibition of human FAP using Z-Gly-Pro-AMC as substrate preincubated for 10 mins prior to substrate addition by fluorescence assay | J Med Chem 56: 3467-77 (2013) Article DOI: 10.1021/jm400351a BindingDB Entry DOI: 10.7270/Q2C53N7W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM514526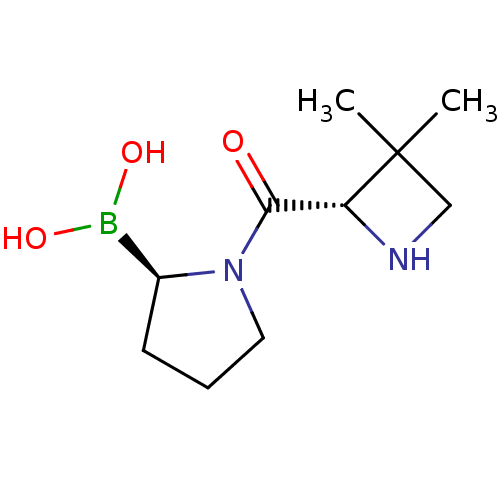 (US11096924, DASH-inhibitors 5362 | US11583516, Cmp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Materials: Enzymes Recombinant human DPPIV (R&D Systems, Cat. No. 1180-SE). Recombinant human DPP8 (Enzo Life Sciences, Cat. No. BML-SE527). Recombin... | Citation and Details BindingDB Entry DOI: 10.7270/Q27P93BT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM50050525 ((R)-1-((S)-2-aminopropanoyl)pyrrolidin-2-ylboronic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description This assay is described in Bachovchin et al. Nature Chemical Biology 10, 656-663 (2014). Briefly, purified enzymes are coupled to Luminex microsphere... | Citation and Details BindingDB Entry DOI: 10.7270/Q28W3J7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 4 (Homo sapiens (Human)) | BDBM514526 (US11096924, DASH-inhibitors 5362 | US11583516, Cmp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description This assay is described in Bachovchin et al. Nature Chemical Biology 10, 656-663 (2014). Briefly, purified enzymes are coupled to Luminex microsphere... | Citation and Details BindingDB Entry DOI: 10.7270/Q28W3J7Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1289 total ) | Next | Last >> |