

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 1/2 (Rattus norvegicus (Rat)-Rattus norvegicus (rat)) | BDBM50229939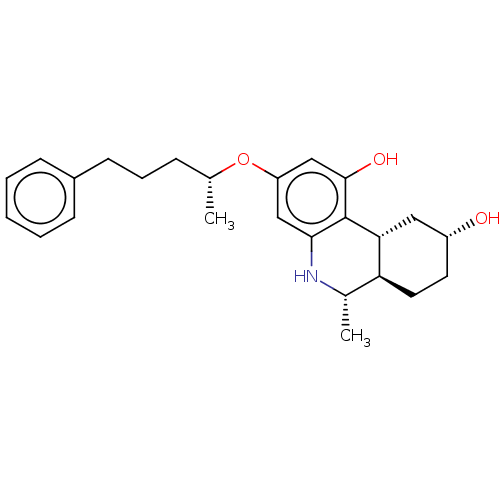 (CHEMBL423481) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Research Group Curated by ChEMBL | Assay Description Concentration required to displace 50% of 0.5 nM [3H](aminoalkyl)indole binding to cannabinoid receptor in rat cerebellum membranes | J Med Chem 35: 124-35 (1992) BindingDB Entry DOI: 10.7270/Q2SF2ZDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1/2 (Rattus norvegicus (Rat)-Rattus norvegicus (rat)) | BDBM50229940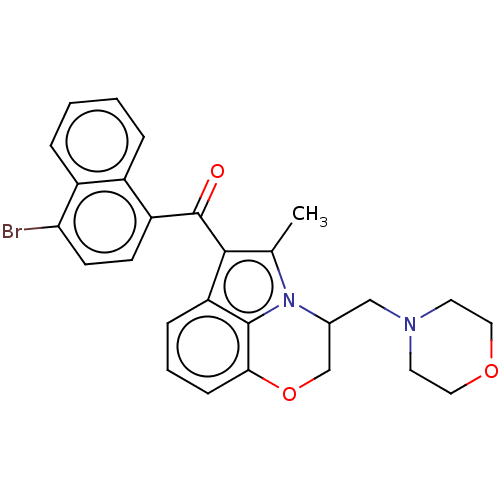 (CHEMBL286248) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Research Group Curated by ChEMBL | Assay Description Concentration required to displace 50% of 0.5 nM [3H](aminoalkyl)indole binding to cannabinoid receptor in rat cerebellum membranes | J Med Chem 35: 124-35 (1992) BindingDB Entry DOI: 10.7270/Q2SF2ZDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1/2 (Rattus norvegicus (Rat)-Rattus norvegicus (rat)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 5.56 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Research Group Curated by ChEMBL | Assay Description Concentration required to displace 50% of 0.5 nM [3H](aminoalkyl)indole binding to cannabinoid receptor in rat cerebellum membranes | J Med Chem 35: 124-35 (1992) BindingDB Entry DOI: 10.7270/Q2SF2ZDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1/2 (Rattus norvegicus (Rat)-Rattus norvegicus (rat)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Research Group Curated by ChEMBL | Assay Description Concentration required to displace 50% of 0.5 nM [3H](aminoalkyl)indole binding to cannabinoid receptor in rat cerebellum membranes | J Med Chem 35: 124-35 (1992) BindingDB Entry DOI: 10.7270/Q2SF2ZDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1/2 (Rattus norvegicus (Rat)-Rattus norvegicus (rat)) | BDBM50229943 (CHEMBL27388) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Research Group Curated by ChEMBL | Assay Description Concentration required to displace 50% of 0.5 nM [3H](aminoalkyl)indole binding to cannabinoid receptor in rat cerebellum membranes | J Med Chem 35: 124-35 (1992) BindingDB Entry DOI: 10.7270/Q2SF2ZDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1/2 (Rattus norvegicus (Rat)-Rattus norvegicus (rat)) | BDBM50229941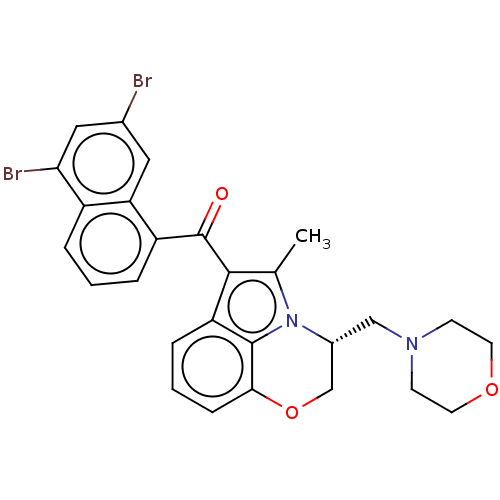 (CHEMBL1744071) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Research Group Curated by ChEMBL | Assay Description Concentration required to displace 50% of 0.5 nM [3H](aminoalkyl)indole binding to cannabinoid receptor in rat cerebellum membranes | J Med Chem 35: 124-35 (1992) BindingDB Entry DOI: 10.7270/Q2SF2ZDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1/2 (Rattus norvegicus (Rat)-Rattus norvegicus (rat)) | BDBM50229942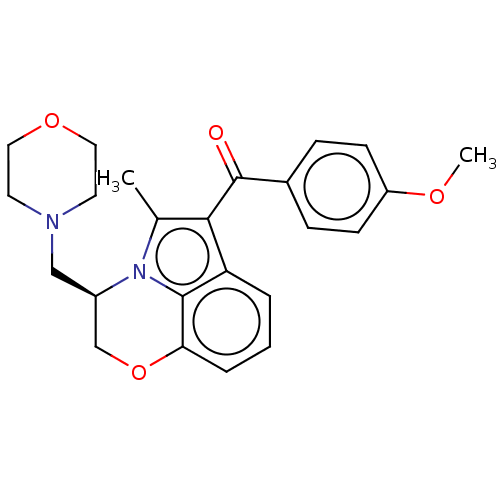 (CHEMBL343532) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 106 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Research Group Curated by ChEMBL | Assay Description Concentration required to displace 50% of 0.5 nM [3H](aminoalkyl)indole binding to cannabinoid receptor in rat cerebellum membranes | J Med Chem 35: 124-35 (1992) BindingDB Entry DOI: 10.7270/Q2SF2ZDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1/2 (Rattus norvegicus (Rat)-Rattus norvegicus (rat)) | BDBM50229935 (CHEMBL284678) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 133 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Research Group Curated by ChEMBL | Assay Description Concentration required to displace 50% of 0.5 nM [3H](aminoalkyl)indole binding to cannabinoid receptor in rat cerebellum membranes | J Med Chem 35: 124-35 (1992) BindingDB Entry DOI: 10.7270/Q2SF2ZDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1/2 (Rattus norvegicus (Rat)-Rattus norvegicus (rat)) | BDBM50229934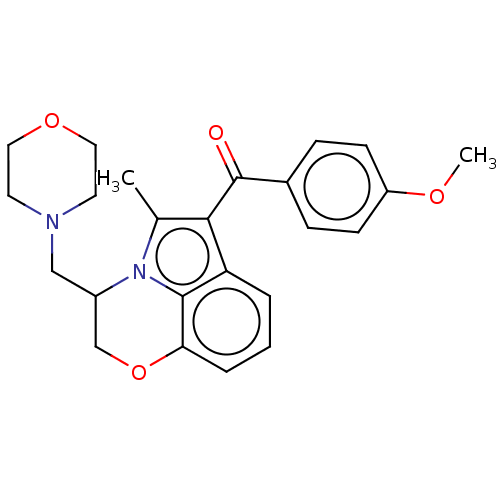 (CHEMBL440651) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 152 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Research Group Curated by ChEMBL | Assay Description Concentration required to displace 50% of 0.5 nM [3H](aminoalkyl)indole binding to cannabinoid receptor in rat cerebellum membranes | J Med Chem 35: 124-35 (1992) BindingDB Entry DOI: 10.7270/Q2SF2ZDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1/2 (Rattus norvegicus (Rat)-Rattus norvegicus (rat)) | BDBM50229944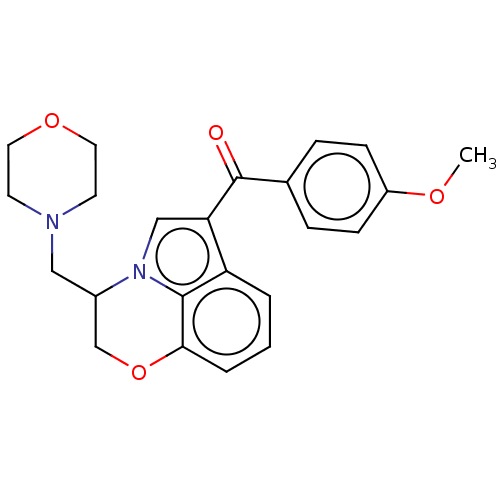 (CHEMBL25847) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 249 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Research Group Curated by ChEMBL | Assay Description Concentration required to displace 50% of 0.5 nM [3H](aminoalkyl)indole binding to cannabinoid receptor in rat cerebellum membranes | J Med Chem 35: 124-35 (1992) BindingDB Entry DOI: 10.7270/Q2SF2ZDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1/2 (Rattus norvegicus (Rat)-Rattus norvegicus (rat)) | BDBM50229937 (CHEMBL280991) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Research Group Curated by ChEMBL | Assay Description Concentration required to displace 50% of 0.5 nM [3H](aminoalkyl)indole binding to cannabinoid receptor in rat cerebellum membranes | J Med Chem 35: 124-35 (1992) BindingDB Entry DOI: 10.7270/Q2SF2ZDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesterol side-chain cleavage enzyme, mitochondrial (Rattus norvegicus) | BDBM50218278 (CHEMBL279571) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Advanced Medicine, Inc. Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of peptidoglycan synthesis in Enterococcus faecalis using [14C]lysine radioligand | Bioorg Med Chem Lett 10: 2251-4 (2000) BindingDB Entry DOI: 10.7270/Q2D79DM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesterol side-chain cleavage enzyme, mitochondrial (Rattus norvegicus) | BDBM50335519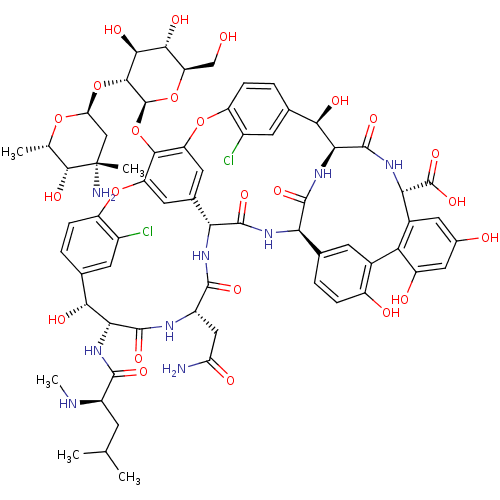 ((S)-3,6-Diamino-hexanoic acid {(3S,9S,12S,15S)-3-(...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Advanced Medicine, Inc. Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of peptidoglycan synthesis in Enterococcus faecalis using [14C]lysine radioligand | Bioorg Med Chem Lett 10: 2251-4 (2000) BindingDB Entry DOI: 10.7270/Q2D79DM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1/2 (Rattus norvegicus (Rat)-Rattus norvegicus (rat)) | BDBM50008022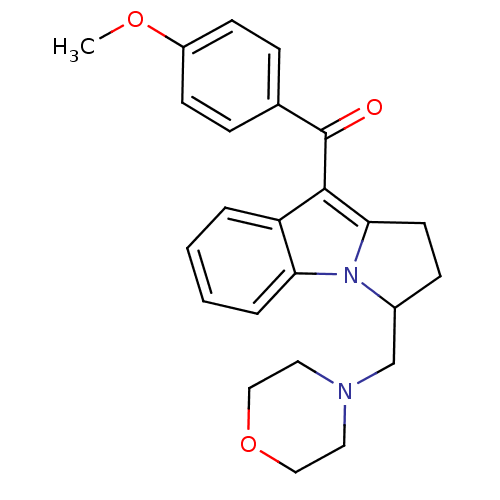 ((4-Methoxy-phenyl)-(3-morpholin-4-ylmethyl-2,3-dih...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Research Group Curated by ChEMBL | Assay Description Concentration required to displace 50% of 0.5 nM [3H](aminoalkyl)indole binding to cannabinoid receptor in rat cerebellum membranes | J Med Chem 35: 124-35 (1992) BindingDB Entry DOI: 10.7270/Q2SF2ZDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1/2 (Rattus norvegicus (Rat)-Rattus norvegicus (rat)) | BDBM50008029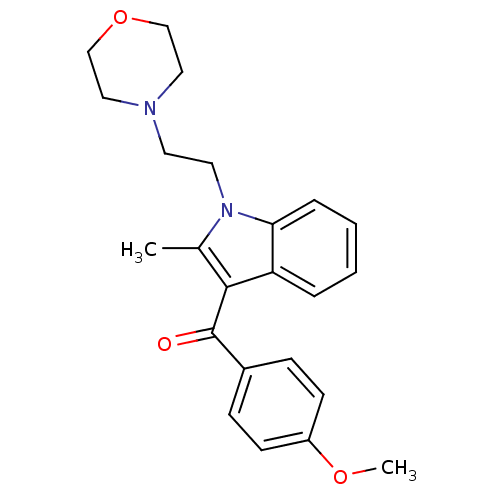 ((4-Methoxy-phenyl)-[2-methyl-1-(2-morpholin-4-yl-e...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Research Group Curated by ChEMBL | Assay Description Concentration required to displace 50% of 0.5 nM [3H](aminoalkyl)indole binding to cannabinoid receptor in rat cerebellum membranes | J Med Chem 35: 124-35 (1992) BindingDB Entry DOI: 10.7270/Q2SF2ZDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1/2 (Rattus norvegicus (Rat)-Rattus norvegicus (rat)) | BDBM50229938 (CHEMBL1592888) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Research Group Curated by ChEMBL | Assay Description Concentration required to displace 50% of 0.5 nM [3H](aminoalkyl)indole binding to cannabinoid receptor in rat cerebellum membranes | J Med Chem 35: 124-35 (1992) BindingDB Entry DOI: 10.7270/Q2SF2ZDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1/2 (Rattus norvegicus (Rat)-Rattus norvegicus (rat)) | BDBM50229936 (CHEMBL1788279) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.75E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Research Group Curated by ChEMBL | Assay Description Concentration required to displace 50% of 0.5 nM [3H](aminoalkyl)indole binding to cannabinoid receptor in rat cerebellum membranes | J Med Chem 35: 124-35 (1992) BindingDB Entry DOI: 10.7270/Q2SF2ZDZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesterol side-chain cleavage enzyme, mitochondrial (Rattus norvegicus) | BDBM50218323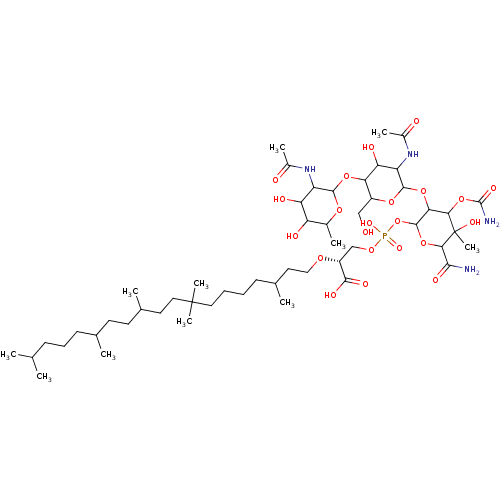 (CHEMBL406969) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Advanced Medicine, Inc. Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of peptidoglycan synthesis in Enterococcus faecalis using [14C]lysine radioligand | Bioorg Med Chem Lett 10: 2251-4 (2000) BindingDB Entry DOI: 10.7270/Q2D79DM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesterol side-chain cleavage enzyme, mitochondrial (Rattus norvegicus) | BDBM50218322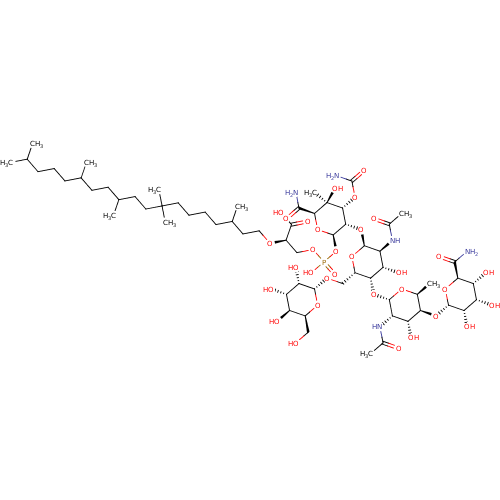 (CHEMBL2029021) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >7.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Advanced Medicine, Inc. Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of peptidoglycan synthesis in Enterococcus faecalis using [14C]lysine radioligand | Bioorg Med Chem Lett 10: 2251-4 (2000) BindingDB Entry DOI: 10.7270/Q2D79DM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesterol side-chain cleavage enzyme, mitochondrial (Rattus norvegicus) | BDBM50218324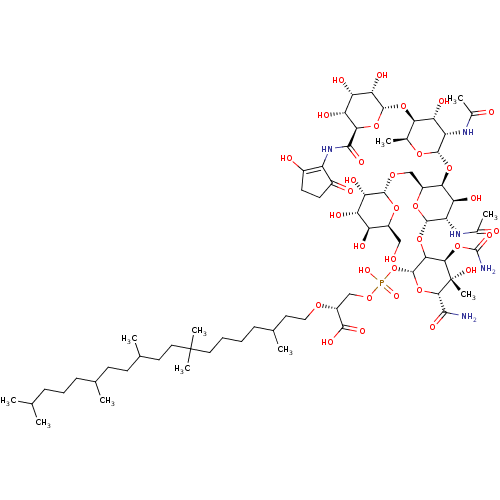 (CHEMBL2029019) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >7.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Advanced Medicine, Inc. Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of peptidoglycan synthesis in Enterococcus faecalis using [14C]lysine radioligand | Bioorg Med Chem Lett 10: 2251-4 (2000) BindingDB Entry DOI: 10.7270/Q2D79DM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholesterol side-chain cleavage enzyme, mitochondrial (Rattus norvegicus) | BDBM50218279 (MOENOMYCIN) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >7.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Advanced Medicine, Inc. Curated by ChEMBL | Assay Description Compound was evaluated for inhibition of peptidoglycan synthesis in Enterococcus faecalis using [14C]lysine radioligand | Bioorg Med Chem Lett 10: 2251-4 (2000) BindingDB Entry DOI: 10.7270/Q2D79DM6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||