 Found 1293 hits with Last Name = 'baker' and Initial = 'c'
Found 1293 hits with Last Name = 'baker' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Alpha-2C adrenergic receptor
(OK) | BDBM81811
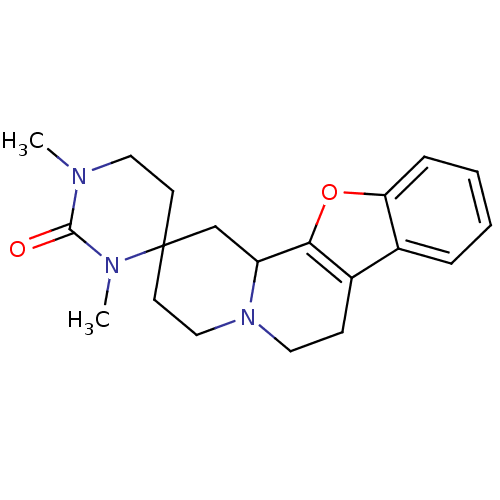
(CAS_123679 | L-657,743 | MK-912 | NSC_123679)Show InChI InChI=1S/C20H25N3O2/c1-21-11-8-20(22(2)19(21)24)9-12-23-10-7-15-14-5-3-4-6-17(14)25-18(15)16(23)13-20/h3-6,16H,7-13H2,1-2H3 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(OK) | BDBM50013515

((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...)Show SMILES COC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 |r| Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15-,17-,18-,19+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(OK) | BDBM81806
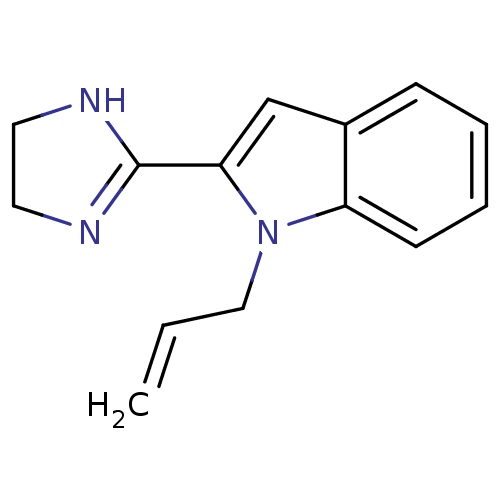
(2-(4,5-dihydro-1h-imidazol-2-yl)-2,3-dihydro-1-(2-...)Show InChI InChI=1S/C14H15N3/c1-2-9-17-12-6-4-3-5-11(12)10-13(17)14-15-7-8-16-14/h2-6,10H,1,7-9H2,(H,15,16) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM81806

(2-(4,5-dihydro-1h-imidazol-2-yl)-2,3-dihydro-1-(2-...)Show InChI InChI=1S/C14H15N3/c1-2-9-17-12-6-4-3-5-11(12)10-13(17)14-15-7-8-16-14/h2-6,10H,1,7-9H2,(H,15,16) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM81804
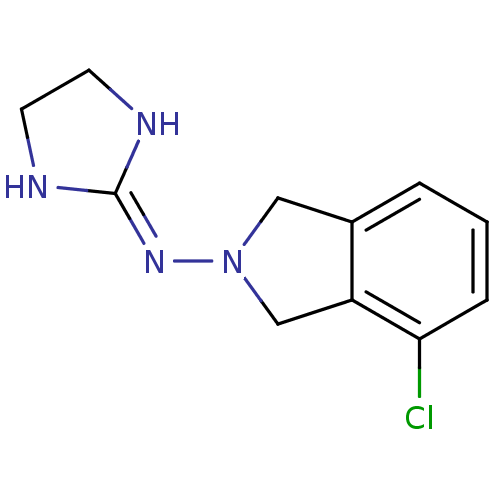
(4-chloro-2-(2-imidazolin-2-ylamino)isoindoline | B...)Show SMILES Clc1cccc2-[#6]-[#7](-[#6]-c12)\[#7]=[#6]-1\[#7]-[#6]-[#6]-[#7]-1 Show InChI InChI=1S/C11H13ClN4/c12-10-3-1-2-8-6-16(7-9(8)10)15-11-13-4-5-14-11/h1-3H,4-7H2,(H2,13,14,15) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(OK) | BDBM81804

(4-chloro-2-(2-imidazolin-2-ylamino)isoindoline | B...)Show SMILES Clc1cccc2-[#6]-[#7](-[#6]-c12)\[#7]=[#6]-1\[#7]-[#6]-[#6]-[#7]-1 Show InChI InChI=1S/C11H13ClN4/c12-10-3-1-2-8-6-16(7-9(8)10)15-11-13-4-5-14-11/h1-3H,4-7H2,(H2,13,14,15) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(OPOSSUM) | BDBM50013515

((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...)Show SMILES COC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 |r| Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15-,17-,18-,19+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50581191

(CHEMBL5070876)Show SMILES COc1ccc(cc1OC)[C@H](Cc1c(Cl)c[n+]([O-])cc1Cl)OC(=O)c1ccc(CN(C(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)s1 |r,wU:10.22,wD:33.34,(21.99,-25.56,;23.33,-26.33,;24.66,-25.56,;25.99,-26.33,;27.33,-25.56,;27.32,-24.01,;25.99,-23.25,;24.66,-24.02,;23.33,-23.25,;23.33,-21.71,;28.66,-23.24,;29.99,-24,;31.32,-23.23,;32.66,-24,;32.66,-25.54,;33.99,-23.22,;33.98,-21.68,;35.31,-20.91,;32.64,-20.92,;31.31,-21.7,;29.97,-20.94,;28.65,-21.7,;27.31,-20.93,;25.98,-21.71,;27.3,-19.39,;26.05,-18.5,;26.52,-17.03,;28.06,-17.02,;28.95,-15.77,;28.32,-14.37,;29.22,-13.12,;28.59,-11.71,;30.69,-13.23,;31.65,-12.02,;33.18,-12.26,;34.14,-11.06,;32.92,-10.1,;32.4,-11.6,;31.09,-10.59,;32.06,-9.39,;33.58,-9.63,;26.79,-14.22,;26.16,-12.81,;24.63,-12.66,;23.73,-13.91,;24.37,-15.32,;25.9,-15.47,;28.54,-18.49,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00204
BindingDB Entry DOI: 10.7270/Q2DN48WC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50581204

(CHEMBL5076637)Show SMILES COc1ccc(cc1OC)[C@H](Cc1c(Cl)c[n+]([O-])cc1Cl)OC(=O)c1ccc(CNC(C(=O)O[C@H]2CN3CCC2CC3)c2ccccc2F)s1 |r,wU:10.22,wD:34.35,(20.54,-23.22,;21.88,-23.99,;23.21,-23.22,;24.54,-23.99,;25.88,-23.22,;25.87,-21.67,;24.54,-20.9,;23.21,-21.68,;21.88,-20.91,;21.88,-19.37,;27.21,-20.89,;28.54,-21.66,;29.87,-20.88,;31.21,-21.65,;31.21,-23.19,;32.54,-20.88,;32.53,-19.34,;33.86,-18.56,;31.19,-18.57,;29.86,-19.35,;28.52,-18.59,;27.2,-19.35,;25.86,-18.59,;24.53,-19.36,;25.85,-17.05,;24.61,-16.14,;25.08,-14.68,;26.65,-14.7,;27.52,-13.42,;26.84,-12.04,;27.7,-10.76,;29.24,-10.88,;29.91,-12.26,;30.1,-9.59,;31.63,-9.71,;32.31,-11.1,;33.83,-11.22,;32.55,-10.34,;33.76,-9.31,;32.5,-8.44,;34.04,-8.56,;34.7,-9.95,;27.02,-9.38,;25.5,-9.27,;24.82,-7.9,;25.69,-6.61,;27.22,-6.72,;27.9,-8.11,;29.43,-8.22,;27.1,-16.14,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00204
BindingDB Entry DOI: 10.7270/Q2DN48WC |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM129590
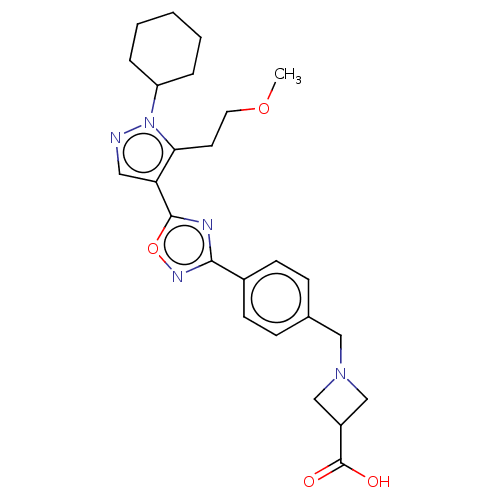
(US8802663, 120)Show SMILES COCCc1c(cnn1C1CCCCC1)-c1nc(no1)-c1ccc(CN2CC(C2)C(O)=O)cc1 Show InChI InChI=1S/C25H31N5O4/c1-33-12-11-22-21(13-26-30(22)20-5-3-2-4-6-20)24-27-23(28-34-24)18-9-7-17(8-10-18)14-29-15-19(16-29)25(31)32/h7-10,13,19-20H,2-6,11-12,14-16H2,1H3,(H,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.100 | -53.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
Merck Serono SA
US Patent
| Assay Description
Receptor binding assay: Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 355-GTPgammaS binding studies. Cells wer... |
US Patent US8802663 (2014)
BindingDB Entry DOI: 10.7270/Q2ZC81K5 |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM577

((3S)-oxolan-3-yl N-[(2S,3R)-4-[(4-aminobenzene)(2-...)Show SMILES CC(C)CN(C[C@@H](O)[C@H](Cc1ccccc1)NC(=O)O[C@H]1CCOC1)S(=O)(=O)c1ccc(N)cc1 |r| Show InChI InChI=1S/C25H35N3O6S/c1-18(2)15-28(35(31,32)22-10-8-20(26)9-11-22)16-24(29)23(14-19-6-4-3-5-7-19)27-25(30)34-21-12-13-33-17-21/h3-11,18,21,23-24,29H,12-17,26H2,1-2H3,(H,27,30)/t21-,23-,24+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
PubMed
| <0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals Inc
Curated by ChEMBL
| Assay Description
Binding affinity to HIV-1 protease was determined |
Bioorg Med Chem Lett 8: 3631-6 (1999)
BindingDB Entry DOI: 10.7270/Q2MC8Z5V |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50581204

(CHEMBL5076637)Show SMILES COc1ccc(cc1OC)[C@H](Cc1c(Cl)c[n+]([O-])cc1Cl)OC(=O)c1ccc(CNC(C(=O)O[C@H]2CN3CCC2CC3)c2ccccc2F)s1 |r,wU:10.22,wD:34.35,(20.54,-23.22,;21.88,-23.99,;23.21,-23.22,;24.54,-23.99,;25.88,-23.22,;25.87,-21.67,;24.54,-20.9,;23.21,-21.68,;21.88,-20.91,;21.88,-19.37,;27.21,-20.89,;28.54,-21.66,;29.87,-20.88,;31.21,-21.65,;31.21,-23.19,;32.54,-20.88,;32.53,-19.34,;33.86,-18.56,;31.19,-18.57,;29.86,-19.35,;28.52,-18.59,;27.2,-19.35,;25.86,-18.59,;24.53,-19.36,;25.85,-17.05,;24.61,-16.14,;25.08,-14.68,;26.65,-14.7,;27.52,-13.42,;26.84,-12.04,;27.7,-10.76,;29.24,-10.88,;29.91,-12.26,;30.1,-9.59,;31.63,-9.71,;32.31,-11.1,;33.83,-11.22,;32.55,-10.34,;33.76,-9.31,;32.5,-8.44,;34.04,-8.56,;34.7,-9.95,;27.02,-9.38,;25.5,-9.27,;24.82,-7.9,;25.69,-6.61,;27.22,-6.72,;27.9,-8.11,;29.43,-8.22,;27.1,-16.14,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00204
BindingDB Entry DOI: 10.7270/Q2DN48WC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50581203
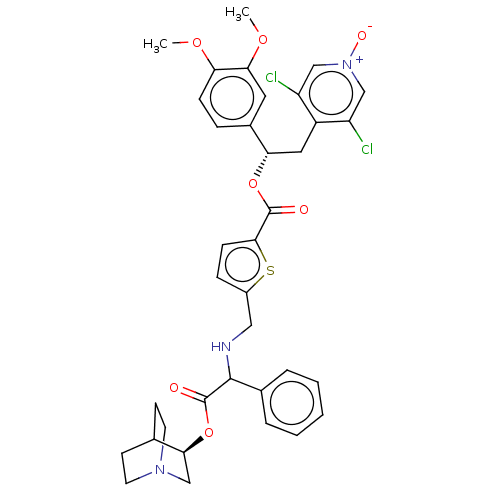
(CHEMBL5074599)Show SMILES COc1ccc(cc1OC)[C@H](Cc1c(Cl)c[n+]([O-])cc1Cl)OC(=O)c1ccc(CNC(C(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)s1 |r,wU:10.22,wD:34.35,(20.54,-23.22,;21.88,-23.99,;23.21,-23.22,;24.54,-23.99,;25.88,-23.22,;25.87,-21.67,;24.54,-20.9,;23.21,-21.68,;21.88,-20.91,;21.88,-19.37,;27.21,-20.89,;28.54,-21.66,;29.87,-20.88,;31.21,-21.65,;31.21,-23.19,;32.54,-20.88,;32.53,-19.34,;33.86,-18.56,;31.19,-18.57,;29.86,-19.35,;28.52,-18.59,;27.2,-19.35,;25.86,-18.59,;24.53,-19.36,;25.85,-17.05,;24.61,-16.14,;25.08,-14.68,;26.65,-14.7,;27.52,-13.42,;26.84,-12.04,;27.7,-10.76,;29.24,-10.88,;29.91,-12.26,;30.1,-9.59,;31.63,-9.71,;32.31,-11.1,;33.83,-11.22,;32.55,-10.34,;33.76,-9.31,;32.5,-8.44,;34.04,-8.56,;34.7,-9.95,;27.02,-9.38,;27.9,-8.11,;27.22,-6.72,;25.69,-6.61,;24.82,-7.9,;25.5,-9.27,;27.1,-16.14,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00204
BindingDB Entry DOI: 10.7270/Q2DN48WC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50581209
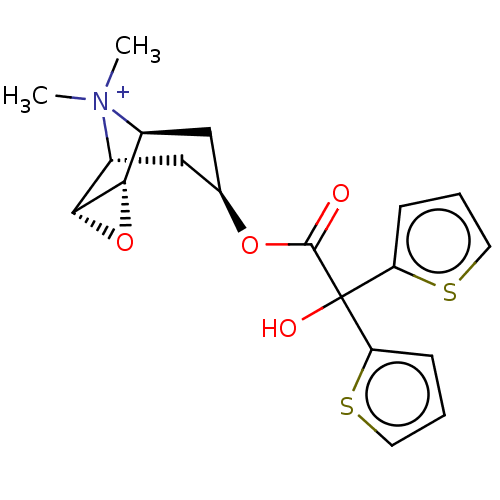
(CHEMBL4650755)Show SMILES [H][C@]12O[C@@]1([H])[C@]1([H])C[C@H](C[C@@]2([H])[N+]1(C)C)OC(=O)C(O)(c1cccs1)c1cccs1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00204
BindingDB Entry DOI: 10.7270/Q2DN48WC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50581192

(CHEMBL5091461)Show SMILES COc1ccc(cc1OC)[C@H](Cc1c(Cl)c[n+]([O-])cc1Cl)OC(=O)c1ccc(CN(C(=O)O[C@H]2CN3CCC2CC3)c2ccccc2F)s1 |r,wU:10.22,wD:33.34,(21.47,-24.81,;22.8,-25.58,;24.14,-24.81,;25.47,-25.58,;26.8,-24.81,;26.8,-23.26,;25.47,-22.49,;24.14,-23.26,;22.81,-22.49,;22.81,-20.95,;28.14,-22.48,;29.47,-23.25,;30.8,-22.47,;32.14,-23.24,;32.14,-24.78,;33.47,-22.47,;33.46,-20.93,;34.79,-20.15,;32.12,-20.16,;30.79,-20.94,;29.45,-20.18,;28.13,-20.94,;26.79,-20.18,;25.46,-20.95,;26.78,-18.64,;25.53,-17.74,;25.99,-16.27,;27.53,-16.27,;28.43,-15.02,;27.8,-13.61,;28.7,-12.36,;28.06,-10.96,;30.17,-12.47,;31.13,-11.27,;32.65,-11.5,;33.62,-10.31,;32.4,-9.34,;31.88,-10.85,;30.57,-9.84,;31.53,-8.64,;33.06,-8.87,;26.27,-13.46,;25.38,-14.71,;23.85,-14.57,;23.21,-13.16,;24.11,-11.9,;25.64,-12.06,;26.54,-10.81,;28.02,-17.73,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00204
BindingDB Entry DOI: 10.7270/Q2DN48WC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50581203

(CHEMBL5074599)Show SMILES COc1ccc(cc1OC)[C@H](Cc1c(Cl)c[n+]([O-])cc1Cl)OC(=O)c1ccc(CNC(C(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)s1 |r,wU:10.22,wD:34.35,(20.54,-23.22,;21.88,-23.99,;23.21,-23.22,;24.54,-23.99,;25.88,-23.22,;25.87,-21.67,;24.54,-20.9,;23.21,-21.68,;21.88,-20.91,;21.88,-19.37,;27.21,-20.89,;28.54,-21.66,;29.87,-20.88,;31.21,-21.65,;31.21,-23.19,;32.54,-20.88,;32.53,-19.34,;33.86,-18.56,;31.19,-18.57,;29.86,-19.35,;28.52,-18.59,;27.2,-19.35,;25.86,-18.59,;24.53,-19.36,;25.85,-17.05,;24.61,-16.14,;25.08,-14.68,;26.65,-14.7,;27.52,-13.42,;26.84,-12.04,;27.7,-10.76,;29.24,-10.88,;29.91,-12.26,;30.1,-9.59,;31.63,-9.71,;32.31,-11.1,;33.83,-11.22,;32.55,-10.34,;33.76,-9.31,;32.5,-8.44,;34.04,-8.56,;34.7,-9.95,;27.02,-9.38,;27.9,-8.11,;27.22,-6.72,;25.69,-6.61,;24.82,-7.9,;25.5,-9.27,;27.1,-16.14,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00204
BindingDB Entry DOI: 10.7270/Q2DN48WC |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(OK) | BDBM81815
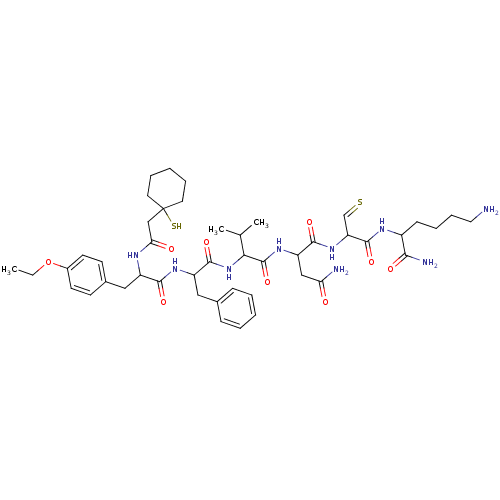
(CAS_196343 | L-654,284 | NSC_196343)Show SMILES CCOc1ccc(CC(NC(=O)CC2(S)CCCCC2)C(=O)NC(Cc2ccccc2)C(=O)NC(C(C)C)C(=O)NC(CC(N)=O)C(=O)NC(C=S)C(=O)NC(CCCCN)C(N)=O)cc1 Show InChI InChI=1S/C46H67N9O9S2/c1-4-64-31-18-16-30(17-19-31)24-33(50-38(57)26-46(66)20-10-6-11-21-46)41(59)52-34(23-29-13-7-5-8-14-29)43(61)55-39(28(2)3)45(63)53-35(25-37(48)56)42(60)54-36(27-65)44(62)51-32(40(49)58)15-9-12-22-47/h5,7-8,13-14,16-19,27-28,32-36,39,66H,4,6,9-12,15,20-26,47H2,1-3H3,(H2,48,56)(H2,49,58)(H,50,57)(H,51,62)(H,52,59)(H,53,63)(H,54,60)(H,55,61) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Alpha-2B adrenergic receptor
(NEONATAL RAT) | BDBM81804

(4-chloro-2-(2-imidazolin-2-ylamino)isoindoline | B...)Show SMILES Clc1cccc2-[#6]-[#7](-[#6]-c12)\[#7]=[#6]-1\[#7]-[#6]-[#6]-[#7]-1 Show InChI InChI=1S/C11H13ClN4/c12-10-3-1-2-8-6-16(7-9(8)10)15-11-13-4-5-14-11/h1-3H,4-7H2,(H2,13,14,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50581185

(CHEMBL5076558)Show SMILES COc1ccc(cc1OC)[C@H](Cc1c(Cl)c[n+]([O-])cc1Cl)OC(=O)c1cccc(CNC(C(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)c1 |r,wU:10.22,wD:35.36,(49.9,-16.29,;51.24,-17.06,;52.57,-16.29,;53.91,-17.06,;55.24,-16.29,;55.24,-14.74,;53.9,-13.98,;52.57,-14.75,;51.24,-13.98,;51.24,-12.44,;56.57,-13.96,;57.91,-14.73,;59.24,-13.95,;59.22,-12.42,;57.89,-11.66,;60.55,-11.65,;61.89,-12.41,;63.22,-11.63,;61.9,-13.95,;60.57,-14.73,;60.57,-16.27,;56.56,-12.42,;55.23,-11.66,;53.9,-12.43,;55.22,-10.12,;53.89,-9.36,;53.88,-7.83,;55.21,-7.04,;56.55,-7.81,;57.88,-7.04,;57.88,-5.5,;59.21,-4.72,;60.54,-5.49,;60.55,-7.03,;61.87,-4.71,;63.21,-5.48,;63.21,-7.03,;64.54,-7.79,;65.87,-7.02,;65.87,-5.48,;64.54,-4.71,;65.29,-6.04,;63.75,-6.45,;59.2,-3.18,;60.54,-2.41,;60.53,-.87,;59.19,-.1,;57.86,-.89,;57.87,-2.42,;56.55,-9.35,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00204
BindingDB Entry DOI: 10.7270/Q2DN48WC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50581209

(CHEMBL4650755)Show SMILES [H][C@]12O[C@@]1([H])[C@]1([H])C[C@H](C[C@@]2([H])[N+]1(C)C)OC(=O)C(O)(c1cccs1)c1cccs1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-N-methyl Scopolamine Chloride from human M2 receptor membranes incubated for 2 hrs by scintillation counting analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00204
BindingDB Entry DOI: 10.7270/Q2DN48WC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50581189
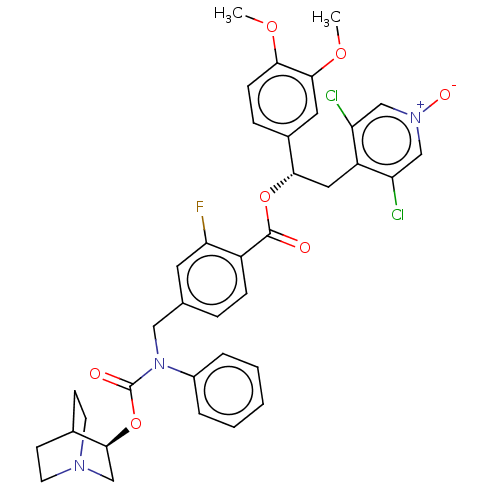
(CHEMBL5075132)Show SMILES COc1ccc(cc1OC)[C@H](Cc1c(Cl)c[n+]([O-])cc1Cl)OC(=O)c1ccc(CN(C(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)cc1F |r,wU:10.22,wD:33.34,(45.59,-53.85,;46.93,-54.62,;48.26,-53.85,;49.6,-54.62,;50.93,-53.85,;50.93,-52.3,;49.59,-51.54,;48.26,-52.31,;46.93,-51.54,;46.93,-50,;52.26,-51.53,;53.59,-52.29,;54.93,-51.52,;54.91,-49.99,;53.57,-49.23,;56.24,-49.21,;57.58,-49.97,;58.91,-49.2,;57.59,-51.52,;56.26,-52.29,;56.26,-53.83,;52.25,-49.99,;50.92,-49.22,;49.59,-50,;50.91,-47.68,;52.24,-46.91,;52.24,-45.37,;50.9,-44.61,;50.92,-43.07,;52.27,-42.32,;53.59,-43.1,;54.93,-42.35,;53.57,-44.64,;54.89,-45.43,;54.86,-46.97,;56.18,-47.76,;57.53,-47.01,;57.55,-45.47,;56.23,-44.68,;55.41,-46.11,;56.97,-46.42,;52.29,-40.78,;53.63,-40.03,;53.66,-38.49,;52.33,-37.7,;50.98,-38.46,;50.97,-40,;49.57,-45.39,;49.58,-46.93,;48.25,-47.71,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00204
BindingDB Entry DOI: 10.7270/Q2DN48WC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50581187

(CHEMBL5077161)Show SMILES COc1ccc(cc1OC)[C@H](Cc1c(Cl)c[n+]([O-])cc1Cl)OC(=O)c1ccc(CN(C(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)cc1 |r,wU:10.22,wD:33.34,(3.32,-53.1,;4.65,-53.87,;5.98,-53.1,;7.32,-53.87,;8.66,-53.1,;8.65,-51.55,;7.31,-50.79,;5.99,-51.56,;4.65,-50.79,;4.65,-49.25,;9.98,-50.78,;11.32,-51.54,;12.65,-50.77,;12.64,-49.24,;11.3,-48.48,;13.96,-48.46,;15.31,-49.22,;16.64,-48.45,;15.31,-50.76,;13.98,-51.54,;13.98,-53.08,;9.98,-49.24,;8.64,-48.47,;7.31,-49.25,;8.63,-46.93,;9.97,-46.16,;9.96,-44.62,;8.62,-43.85,;8.64,-42.32,;9.99,-41.56,;11.31,-42.35,;12.66,-41.6,;11.29,-43.89,;12.61,-44.68,;12.59,-46.22,;13.9,-47.01,;15.25,-46.26,;15.28,-44.72,;13.95,-43.92,;13.13,-45.36,;14.7,-45.67,;10.01,-40.02,;11.36,-39.28,;11.38,-37.74,;10.05,-36.95,;8.71,-37.71,;8.69,-39.25,;7.29,-44.64,;7.3,-46.17,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00204
BindingDB Entry DOI: 10.7270/Q2DN48WC |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(OK) | BDBM50013515

((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...)Show SMILES COC(=O)[C@H]1[C@@H](O)CC[C@H]2CN3CCc4c([nH]c5ccccc45)[C@@H]3C[C@H]12 |r| Show InChI InChI=1S/C21H26N2O3/c1-26-21(25)19-15-10-17-20-14(13-4-2-3-5-16(13)22-20)8-9-23(17)11-12(15)6-7-18(19)24/h2-5,12,15,17-19,22,24H,6-11H2,1H3/t12-,15-,17-,18-,19+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50581203

(CHEMBL5074599)Show SMILES COc1ccc(cc1OC)[C@H](Cc1c(Cl)c[n+]([O-])cc1Cl)OC(=O)c1ccc(CNC(C(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)s1 |r,wU:10.22,wD:34.35,(20.54,-23.22,;21.88,-23.99,;23.21,-23.22,;24.54,-23.99,;25.88,-23.22,;25.87,-21.67,;24.54,-20.9,;23.21,-21.68,;21.88,-20.91,;21.88,-19.37,;27.21,-20.89,;28.54,-21.66,;29.87,-20.88,;31.21,-21.65,;31.21,-23.19,;32.54,-20.88,;32.53,-19.34,;33.86,-18.56,;31.19,-18.57,;29.86,-19.35,;28.52,-18.59,;27.2,-19.35,;25.86,-18.59,;24.53,-19.36,;25.85,-17.05,;24.61,-16.14,;25.08,-14.68,;26.65,-14.7,;27.52,-13.42,;26.84,-12.04,;27.7,-10.76,;29.24,-10.88,;29.91,-12.26,;30.1,-9.59,;31.63,-9.71,;32.31,-11.1,;33.83,-11.22,;32.55,-10.34,;33.76,-9.31,;32.5,-8.44,;34.04,-8.56,;34.7,-9.95,;27.02,-9.38,;27.9,-8.11,;27.22,-6.72,;25.69,-6.61,;24.82,-7.9,;25.5,-9.27,;27.1,-16.14,)| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-N-methyl Scopolamine Chloride from human M2 receptor membranes incubated for 2 hrs by scintillation counting analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00204
BindingDB Entry DOI: 10.7270/Q2DN48WC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50581193
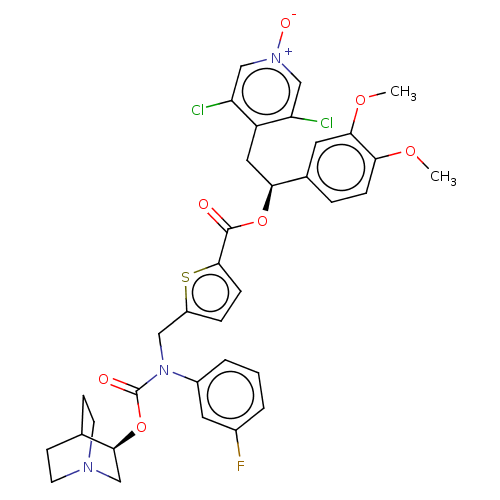
(CHEMBL5084383)Show SMILES COc1ccc(cc1OC)[C@H](Cc1c(Cl)c[n+]([O-])cc1Cl)OC(=O)c1ccc(CN(C(=O)O[C@H]2CN3CCC2CC3)c2cccc(F)c2)s1 |r,wU:10.22,wD:33.34,(21.47,-24.81,;22.8,-25.58,;24.14,-24.81,;25.47,-25.58,;26.8,-24.81,;26.8,-23.26,;25.47,-22.49,;24.14,-23.26,;22.81,-22.49,;22.81,-20.95,;28.14,-22.48,;29.47,-23.25,;30.8,-22.47,;32.14,-23.24,;32.14,-24.78,;33.47,-22.47,;33.46,-20.93,;34.79,-20.15,;32.12,-20.16,;30.79,-20.94,;29.45,-20.18,;28.13,-20.94,;26.79,-20.18,;25.46,-20.95,;26.78,-18.64,;25.53,-17.74,;25.99,-16.27,;27.53,-16.27,;28.43,-15.02,;27.8,-13.61,;28.7,-12.36,;28.06,-10.96,;30.17,-12.47,;31.13,-11.27,;32.65,-11.5,;33.62,-10.31,;32.4,-9.34,;31.88,-10.85,;30.57,-9.84,;31.53,-8.64,;33.06,-8.87,;26.27,-13.46,;25.38,-14.71,;23.85,-14.57,;23.21,-13.16,;24.11,-11.9,;23.48,-10.5,;25.64,-12.06,;28.02,-17.73,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00204
BindingDB Entry DOI: 10.7270/Q2DN48WC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50581202

(CHEMBL5090464)Show SMILES [O-][n+]1cc(Cl)c(C[C@H](OC(=O)c2ccc(CN(C(=O)O[C@H]3CN4CCC3CC4)c3ccccc3F)s2)c2ccc(OC(F)F)c(OCC3CC3)c2)c(Cl)c1 |r,wU:7.7,wD:20.19,(41.59,-14.5,;40.26,-15.27,;40.26,-16.81,;38.94,-17.59,;38.94,-19.13,;37.6,-16.82,;36.27,-17.59,;34.94,-16.83,;34.93,-15.29,;33.59,-14.52,;32.26,-15.3,;33.58,-12.98,;32.33,-12.09,;32.8,-10.62,;34.34,-10.62,;35.24,-9.36,;34.6,-7.96,;35.5,-6.71,;34.87,-5.31,;36.97,-6.82,;37.93,-5.62,;39.45,-5.85,;40.42,-4.65,;39.2,-3.69,;38.68,-5.2,;37.37,-4.19,;38.33,-2.99,;39.86,-3.22,;33.06,-7.82,;32.18,-9.07,;30.65,-8.94,;30,-7.53,;30.89,-6.27,;32.43,-6.41,;33.32,-5.16,;34.82,-12.08,;33.6,-17.6,;33.61,-19.15,;32.27,-19.92,;30.94,-19.15,;29.61,-19.92,;28.27,-19.15,;28.27,-17.61,;26.94,-19.92,;30.94,-17.61,;29.61,-16.84,;29.61,-15.3,;28.28,-14.53,;27.5,-13.2,;26.73,-14.54,;32.27,-16.84,;37.59,-15.29,;36.25,-14.53,;38.92,-14.51,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00204
BindingDB Entry DOI: 10.7270/Q2DN48WC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50581190
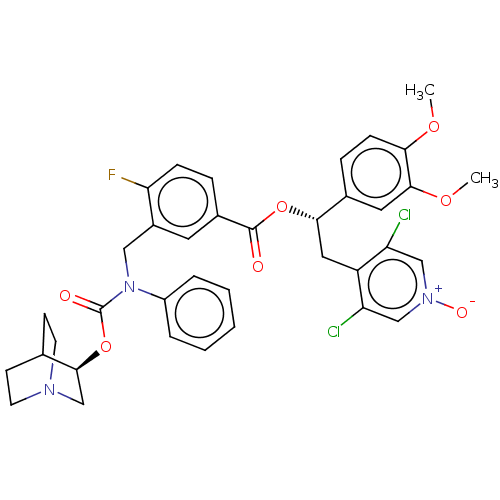
(CHEMBL5076266)Show SMILES COc1ccc(cc1OC)[C@H](Cc1c(Cl)c[n+]([O-])cc1Cl)OC(=O)c1ccc(F)c(CN(C(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)c1 |r,wU:10.22,wD:35.36,(65.48,-54.2,;66.82,-54.98,;68.15,-54.21,;69.48,-54.98,;70.82,-54.21,;70.82,-52.65,;69.48,-51.89,;68.15,-52.66,;66.82,-51.89,;66.82,-50.35,;72.15,-51.88,;73.48,-52.64,;74.81,-51.87,;74.8,-50.34,;73.46,-49.58,;76.13,-49.56,;77.47,-50.33,;78.8,-49.55,;77.48,-51.87,;76.15,-52.64,;76.15,-54.18,;72.14,-50.34,;70.8,-49.58,;69.47,-50.35,;70.8,-48.04,;69.47,-47.28,;69.45,-45.74,;70.79,-44.96,;69.92,-43.68,;72.13,-45.73,;73.46,-44.95,;73.45,-43.41,;74.78,-42.64,;74.78,-41.1,;76.12,-43.4,;77.45,-42.63,;78.79,-43.4,;80.11,-42.63,;80.12,-41.09,;78.78,-40.32,;77.44,-41.09,;78.3,-42.5,;79.33,-41.29,;72.12,-42.65,;72.12,-41.1,;70.79,-40.34,;69.45,-41.11,;69.46,-42.66,;70.8,-43.42,;72.13,-47.26,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00204
BindingDB Entry DOI: 10.7270/Q2DN48WC |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM81803
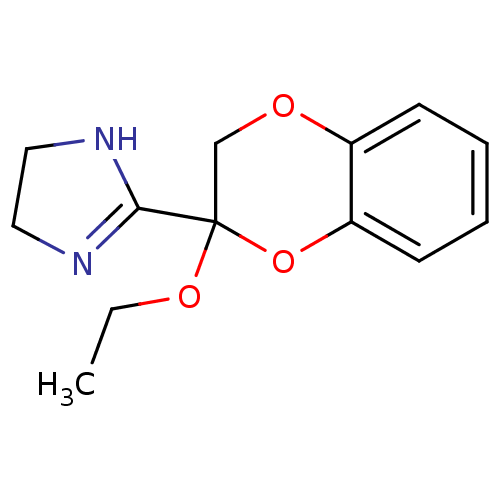
(CAS_125992 | NSC_125992 | RX 811033)Show InChI InChI=1S/C13H16N2O3/c1-2-17-13(12-14-7-8-15-12)9-16-10-5-3-4-6-11(10)18-13/h3-6H,2,7-9H2,1H3,(H,14,15) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM325610
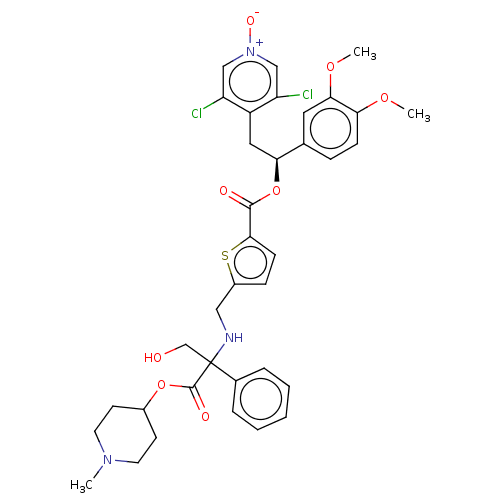
(US9636336, Example 105c)Show SMILES COc1ccc(cc1OC)[C@H](Cc1c(Cl)c[n+]([O-])cc1Cl)OC(=O)c1ccc(CNC(CO)(C(=O)OC2CCN(C)CC2)c2ccccc2)s1 |r| Show InChI InChI=1S/C36H39Cl2N3O8S/c1-40-15-13-25(14-16-40)48-35(44)36(22-42,24-7-5-4-6-8-24)39-19-26-10-12-33(50-26)34(43)49-31(18-27-28(37)20-41(45)21-29(27)38)23-9-11-30(46-2)32(17-23)47-3/h4-12,17,20-21,25,31,39,42H,13-16,18-19,22H2,1-3H3/t31-,36?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CHIESI FARMACEUTICI S.p.A.
US Patent
| Assay Description
Human M3 receptor membranes (15 μg/well) from Perkin Elmer are incubated with 0.52 nM Scopolamine Methyl Chloride, [N-methyl-3H] with or without... |
US Patent US9636336 (2017)
BindingDB Entry DOI: 10.7270/Q2JS9SH5 |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(OK) | BDBM81803

(CAS_125992 | NSC_125992 | RX 811033)Show InChI InChI=1S/C13H16N2O3/c1-2-17-13(12-14-7-8-15-12)9-16-10-5-3-4-6-11(10)18-13/h3-6H,2,7-9H2,1H3,(H,14,15) | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50581199

(CHEMBL5090179)Show SMILES COc1cc(ccc1OC(F)F)[C@H](Cc1c(Cl)c[n+]([O-])cc1Cl)OC(=O)c1ccc(CN(C(=O)O[C@H]2CN3CCC2CC3)c2ccccc2F)s1 |r,wU:12.24,wD:35.36,(22.8,-20.95,;22.8,-22.49,;24.13,-23.26,;25.46,-22.49,;26.79,-23.25,;26.79,-24.8,;25.46,-25.57,;24.13,-24.8,;22.8,-25.57,;21.46,-24.8,;21.46,-23.26,;20.13,-25.57,;28.12,-22.47,;29.46,-23.24,;30.79,-22.46,;32.13,-23.23,;32.13,-24.77,;33.45,-22.46,;33.45,-20.92,;34.77,-20.14,;32.1,-20.16,;30.77,-20.93,;29.44,-20.18,;28.12,-20.93,;26.78,-20.17,;25.45,-20.94,;26.77,-18.63,;25.52,-17.73,;25.99,-16.27,;27.52,-16.26,;28.42,-15.01,;27.79,-13.61,;28.69,-12.36,;28.05,-10.95,;30.16,-12.47,;31.12,-11.26,;32.64,-11.5,;33.6,-10.3,;32.39,-9.34,;31.86,-10.85,;30.56,-9.84,;31.52,-8.63,;33.04,-8.87,;26.25,-13.46,;25.37,-14.72,;23.84,-14.58,;23.19,-13.17,;24.08,-11.92,;25.62,-12.06,;26.51,-10.81,;28.01,-17.72,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00204
BindingDB Entry DOI: 10.7270/Q2DN48WC |
More data for this
Ligand-Target Pair | |
Alpha-2B adrenergic receptor
(NEONATAL RAT) | BDBM81811

(CAS_123679 | L-657,743 | MK-912 | NSC_123679)Show InChI InChI=1S/C20H25N3O2/c1-21-11-8-20(22(2)19(21)24)9-12-23-10-7-15-14-5-3-4-6-17(14)25-18(15)16(23)13-20/h3-6,16H,7-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(OK) | BDBM81444
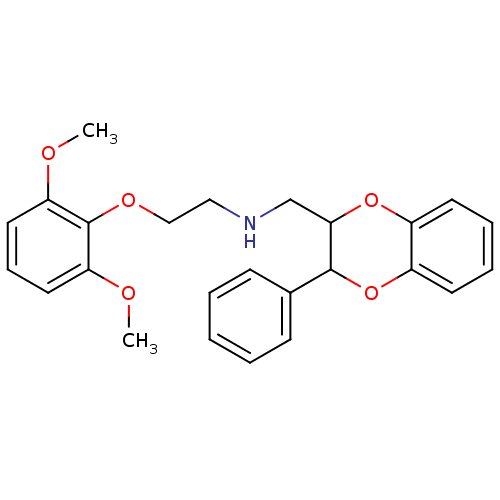
(CAS_185453 | NSC_185453 | WB 4101 | WB-4101)Show InChI InChI=1S/C25H27NO5/c1-27-21-13-8-14-22(28-2)25(21)29-16-15-26-17-23-24(18-9-4-3-5-10-18)31-20-12-7-6-11-19(20)30-23/h3-14,23-24,26H,15-17H2,1-2H3 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Alpha-2B adrenergic receptor
(NEONATAL RAT) | BDBM81806

(2-(4,5-dihydro-1h-imidazol-2-yl)-2,3-dihydro-1-(2-...)Show InChI InChI=1S/C14H15N3/c1-2-9-17-12-6-4-3-5-11(12)10-13(17)14-15-7-8-16-14/h2-6,10H,1,7-9H2,(H,15,16) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM325606

(US9636336, Example 87c)Show SMILES COc1ccc(cc1OC)[C@H](Cc1c(Cl)c[n+]([O-])cc1Cl)OC(=O)c1ccc(CNC(C(=O)OC2CCN(C)CC2)c2ccccc2)s1 |r| Show InChI InChI=1S/C35H37Cl2N3O7S/c1-39-15-13-24(14-16-39)46-35(42)33(22-7-5-4-6-8-22)38-19-25-10-12-32(48-25)34(41)47-30(18-26-27(36)20-40(43)21-28(26)37)23-9-11-29(44-2)31(17-23)45-3/h4-12,17,20-21,24,30,33,38H,13-16,18-19H2,1-3H3/t30-,33?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CHIESI FARMACEUTICI S.p.A.
US Patent
| Assay Description
Human M3 receptor membranes (15 μg/well) from Perkin Elmer are incubated with 0.52 nM Scopolamine Methyl Chloride, [N-methyl-3H] with or without... |
US Patent US9636336 (2017)
BindingDB Entry DOI: 10.7270/Q2JS9SH5 |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(OK) | BDBM30993
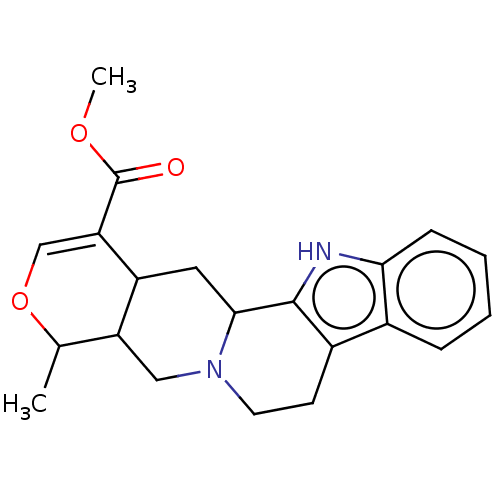
(Ajmalicine | MLS000111555 | Raubasine | SMR0001074...)Show SMILES COC(=O)C1=COC(C)C2CN3CCc4c([nH]c5ccccc45)C3CC12 |t:4| Show InChI InChI=1S/C21H24N2O3/c1-12-16-10-23-8-7-14-13-5-3-4-6-18(13)22-20(14)19(23)9-15(16)17(11-26-12)21(24)25-2/h3-6,11-12,15-16,19,22H,7-10H2,1-2H3 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM182716
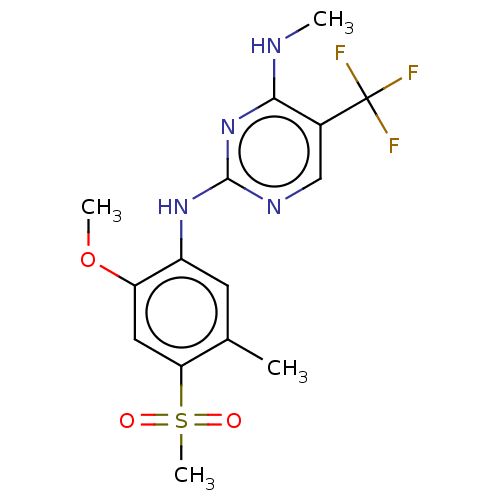
(US9145402, 23 | US9145402, 36)Show SMILES CNc1nc(Nc2cc(C)c(cc2OC)S(C)(=O)=O)ncc1C(F)(F)F Show InChI InChI=1S/C15H17F3N4O3S/c1-8-5-10(11(25-3)6-12(8)26(4,23)24)21-14-20-7-9(15(16,17)18)13(19-2)22-14/h5-7H,1-4H3,(H2,19,20,21,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
his assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 38... |
US Patent US9145402 (2015)
BindingDB Entry DOI: 10.7270/Q2J67FQ3 |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM196757

(US9212173, 35)Show SMILES CCNc1nc(Nc2cn(nc2C)C(C)(C)c2ccnn2C)ncc1C(F)(F)F Show InChI InChI=1S/C18H23F3N8/c1-6-22-15-12(18(19,20)21)9-23-16(26-15)25-13-10-29(27-11(13)2)17(3,4)14-7-8-24-28(14)5/h7-10H,6H2,1-5H3,(H2,22,23,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 3... |
US Patent US9212173 (2015)
BindingDB Entry DOI: 10.7270/Q2222SKT |
More data for this
Ligand-Target Pair | |
Leucine-rich repeat serine/threonine-protein kinase 2
(Homo sapiens (Human)) | BDBM196826

(US9212186, 23)Show InChI InChI=1S/C15H19F3N6O/c1-4-19-12-9(15(16,17)18)5-20-13(23-12)22-10-6-21-24-8-14(2,3)25-7-11(10)24/h5-6H,4,7-8H2,1-3H3,(H2,19,20,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech, Inc.
US Patent
| Assay Description
This assay was used to determine a compound's potency in inhibiting activity of LRRK2 by determining, Kiapp, IC50, or percent inhibition values. In 3... |
US Patent US9212186 (2015)
BindingDB Entry DOI: 10.7270/Q2NS0SPX |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50581186
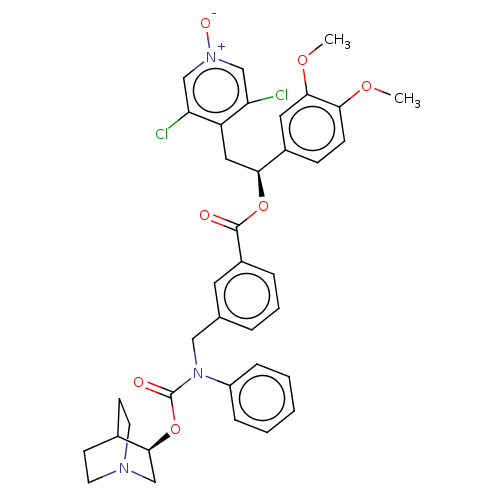
(CHEMBL5088742)Show SMILES COc1ccc(cc1OC)[C@H](Cc1c(Cl)c[n+]([O-])cc1Cl)OC(=O)c1cccc(CN(C(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)c1 |r,wU:10.22,wD:34.35,(67,-31.8,;68.33,-32.57,;69.66,-31.81,;71,-32.58,;72.34,-31.8,;72.33,-30.25,;70.99,-29.49,;69.67,-30.26,;68.33,-29.49,;68.33,-27.95,;73.66,-29.48,;75,-30.24,;76.33,-29.47,;76.32,-27.94,;74.98,-27.18,;77.64,-27.16,;78.99,-27.93,;80.32,-27.15,;78.99,-29.47,;77.66,-30.24,;77.66,-31.78,;73.66,-27.94,;72.32,-27.17,;70.99,-27.95,;72.31,-25.63,;70.98,-24.88,;70.97,-23.34,;72.3,-22.56,;73.64,-23.33,;74.97,-22.55,;74.97,-21.01,;76.3,-20.24,;76.29,-18.7,;77.63,-21,;78.97,-20.23,;80.3,-21,;81.63,-20.23,;81.63,-18.69,;80.3,-17.92,;78.96,-18.69,;79.81,-20.1,;80.84,-18.89,;73.63,-20.25,;73.64,-18.7,;72.3,-17.94,;70.97,-18.71,;70.98,-20.26,;72.31,-21.02,;73.65,-24.86,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00204
BindingDB Entry DOI: 10.7270/Q2DN48WC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50581188

(CHEMBL5076680)Show SMILES COc1ccc(cc1OC)[C@H](Cc1c(Cl)c[n+]([O-])cc1Cl)OC(=O)Cc1ccc(CN(C(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)cc1 |r,wU:10.22,wD:34.35,(26.37,-52.58,;27.7,-53.35,;29.04,-52.58,;30.37,-53.35,;31.71,-52.58,;31.7,-51.03,;30.37,-50.26,;29.04,-51.03,;27.7,-50.26,;27.7,-48.72,;33.03,-50.25,;34.37,-51.02,;35.7,-50.24,;35.69,-48.71,;34.35,-47.95,;37.01,-47.93,;38.36,-48.7,;39.69,-47.92,;38.36,-50.24,;37.03,-51.01,;37.04,-52.55,;33.03,-48.71,;31.69,-47.95,;30.36,-48.72,;31.68,-46.41,;30.34,-45.64,;30.28,-44.1,;28.92,-43.39,;27.62,-44.21,;26.27,-43.48,;26.23,-41.94,;27.54,-41.14,;27.51,-39.6,;28.89,-41.87,;30.21,-41.07,;31.56,-41.82,;32.87,-41.02,;32.84,-39.48,;31.49,-38.74,;30.17,-39.54,;31.05,-40.93,;32.06,-39.69,;24.88,-41.2,;24.85,-39.66,;23.5,-38.92,;22.18,-39.72,;22.22,-41.27,;23.58,-42,;27.69,-45.76,;29.05,-46.47,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00204
BindingDB Entry DOI: 10.7270/Q2DN48WC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50581201

(CHEMBL5085717)Show SMILES CC(C)Oc1cc(ccc1OC(F)F)[C@H](Cc1c(Cl)c[n+]([O-])cc1Cl)OC(=O)c1ccc(CN(C(=O)O[C@H]2CN3CCC2CC3)c2ccccc2F)s1 |r,wU:14.26,wD:37.38,(8.35,-14.65,;9.68,-15.42,;11.02,-14.65,;9.68,-16.95,;11.02,-17.72,;12.34,-16.95,;13.67,-17.72,;13.68,-19.27,;12.34,-20.04,;11.01,-19.27,;9.68,-20.04,;8.34,-19.27,;8.34,-17.73,;7.01,-20.04,;15.01,-16.94,;16.34,-17.71,;17.67,-16.93,;19.01,-17.7,;19.01,-19.24,;20.34,-16.93,;20.33,-15.39,;21.66,-14.61,;18.99,-14.63,;17.66,-15.4,;16.32,-14.64,;15,-15.4,;13.66,-14.64,;12.33,-15.41,;13.66,-13.1,;12.4,-12.2,;12.87,-10.74,;14.41,-10.73,;15.31,-9.48,;14.67,-8.08,;15.57,-6.83,;14.94,-5.42,;17.05,-6.94,;18,-5.73,;19.53,-5.97,;20.49,-4.77,;19.27,-3.81,;18.75,-5.31,;17.44,-4.31,;18.41,-3.1,;19.93,-3.34,;13.14,-7.93,;12.25,-9.19,;10.72,-9.05,;10.07,-7.64,;10.97,-6.38,;12.5,-6.53,;13.4,-5.28,;14.9,-12.19,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00204
BindingDB Entry DOI: 10.7270/Q2DN48WC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50581200
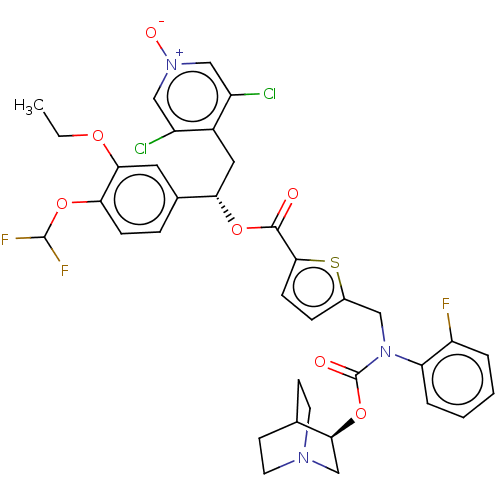
(CHEMBL5084829)Show SMILES CCOc1cc(ccc1OC(F)F)[C@H](Cc1c(Cl)c[n+]([O-])cc1Cl)OC(=O)c1ccc(CN(C(=O)O[C@H]2CN3CCC2CC3)c2ccccc2F)s1 |r,wU:13.25,wD:36.37,(8.35,-14.65,;9.68,-15.42,;9.68,-16.95,;11.02,-17.72,;12.34,-16.95,;13.67,-17.72,;13.68,-19.27,;12.34,-20.04,;11.01,-19.27,;9.68,-20.04,;8.34,-19.27,;8.34,-17.73,;7.01,-20.04,;15.01,-16.94,;16.34,-17.71,;17.67,-16.93,;19.01,-17.7,;19.01,-19.24,;20.34,-16.93,;20.33,-15.39,;21.66,-14.61,;18.99,-14.63,;17.66,-15.4,;16.32,-14.64,;15,-15.4,;13.66,-14.64,;12.33,-15.41,;13.66,-13.1,;12.4,-12.2,;12.87,-10.74,;14.41,-10.73,;15.31,-9.48,;14.67,-8.08,;15.57,-6.83,;14.94,-5.42,;17.05,-6.94,;18,-5.73,;19.53,-5.97,;20.49,-4.77,;19.27,-3.81,;18.75,-5.31,;17.44,-4.31,;18.41,-3.1,;19.93,-3.34,;13.14,-7.93,;12.25,-9.19,;10.72,-9.05,;10.07,-7.64,;10.97,-6.38,;12.5,-6.53,;13.4,-5.28,;14.9,-12.19,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00204
BindingDB Entry DOI: 10.7270/Q2DN48WC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50581198
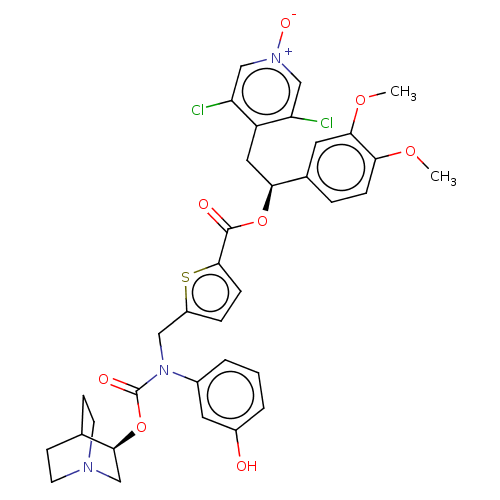
(CHEMBL5086769)Show SMILES COc1ccc(cc1OC)[C@H](Cc1c(Cl)c[n+]([O-])cc1Cl)OC(=O)c1ccc(CN(C(=O)O[C@H]2CN3CCC2CC3)c2cccc(O)c2)s1 |r,wU:10.22,wD:33.34,(21.46,-24.8,;22.8,-25.57,;24.13,-24.8,;25.46,-25.57,;26.79,-24.8,;26.79,-23.25,;25.46,-22.49,;24.13,-23.26,;22.8,-22.49,;22.8,-20.95,;28.12,-22.47,;29.46,-23.24,;30.79,-22.46,;32.13,-23.23,;32.13,-24.77,;33.45,-22.46,;33.45,-20.92,;34.77,-20.14,;32.1,-20.16,;30.77,-20.93,;29.44,-20.18,;28.12,-20.93,;26.78,-20.17,;25.45,-20.94,;26.77,-18.63,;25.52,-17.73,;25.99,-16.27,;27.52,-16.26,;28.42,-15.01,;27.79,-13.61,;28.69,-12.36,;28.05,-10.95,;30.16,-12.47,;31.12,-11.26,;32.64,-11.5,;33.6,-10.3,;32.39,-9.34,;31.86,-10.85,;30.56,-9.84,;31.52,-8.63,;33.04,-8.87,;26.25,-13.46,;25.37,-14.72,;23.84,-14.58,;23.19,-13.17,;24.08,-11.92,;23.44,-10.51,;25.62,-12.06,;28.01,-17.72,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00204
BindingDB Entry DOI: 10.7270/Q2DN48WC |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM81807
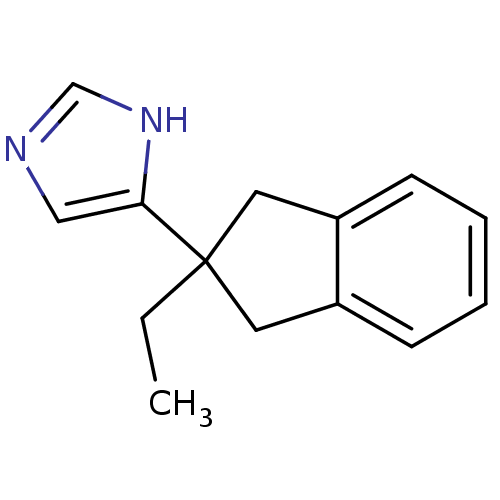
(ATIPAMEZOLE | CAS_104054-27-5 | NSC_71310)Show InChI InChI=1S/C14H16N2/c1-2-14(13-9-15-10-16-13)7-11-5-3-4-6-12(11)8-14/h3-6,9-10H,2,7-8H2,1H3,(H,15,16) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Alpha-2B adrenergic receptor
(NEONATAL RAT) | BDBM50020192
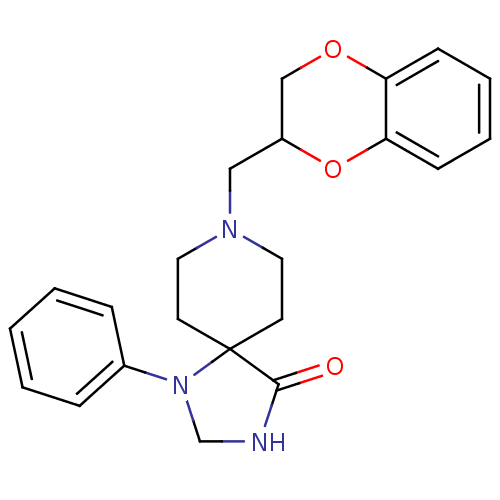
(8-(2,3-Dihydro-benzo[1,4]dioxin-2-ylmethyl)-1-phen...)Show SMILES O=C1NCN(c2ccccc2)C11CCN(CC2COc3ccccc3O2)CC1 Show InChI InChI=1S/C22H25N3O3/c26-21-22(25(16-23-21)17-6-2-1-3-7-17)10-12-24(13-11-22)14-18-15-27-19-8-4-5-9-20(19)28-18/h1-9,18H,10-16H2,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM81815

(CAS_196343 | L-654,284 | NSC_196343)Show SMILES CCOc1ccc(CC(NC(=O)CC2(S)CCCCC2)C(=O)NC(Cc2ccccc2)C(=O)NC(C(C)C)C(=O)NC(CC(N)=O)C(=O)NC(C=S)C(=O)NC(CCCCN)C(N)=O)cc1 Show InChI InChI=1S/C46H67N9O9S2/c1-4-64-31-18-16-30(17-19-31)24-33(50-38(57)26-46(66)20-10-6-11-21-46)41(59)52-34(23-29-13-7-5-8-14-29)43(61)55-39(28(2)3)45(63)53-35(25-37(48)56)42(60)54-36(27-65)44(62)51-32(40(49)58)15-9-12-22-47/h5,7-8,13-14,16-19,27-28,32-36,39,66H,4,6,9-12,15,20-26,47H2,1-3H3,(H2,48,56)(H2,49,58)(H,50,57)(H,51,62)(H,52,59)(H,53,63)(H,54,60)(H,55,61) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Alpha-2C adrenergic receptor
(OK) | BDBM81773
(BAM 1303 | BAM-1303 | CAS_115219-10-8)Show SMILES CN1C[C@H](Cn2ccnc2-c2ccccc2)C[C@H]2[C@H]1Cc1c(Br)[nH]c3cccc2c13 |r| Show InChI InChI=1S/C25H25BrN4/c1-29-14-16(15-30-11-10-27-25(30)17-6-3-2-4-7-17)12-19-18-8-5-9-21-23(18)20(13-22(19)29)24(26)28-21/h2-11,16,19,22,28H,12-15H2,1H3/t16-,19-,22-/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50581185

(CHEMBL5076558)Show SMILES COc1ccc(cc1OC)[C@H](Cc1c(Cl)c[n+]([O-])cc1Cl)OC(=O)c1cccc(CNC(C(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)c1 |r,wU:10.22,wD:35.36,(49.9,-16.29,;51.24,-17.06,;52.57,-16.29,;53.91,-17.06,;55.24,-16.29,;55.24,-14.74,;53.9,-13.98,;52.57,-14.75,;51.24,-13.98,;51.24,-12.44,;56.57,-13.96,;57.91,-14.73,;59.24,-13.95,;59.22,-12.42,;57.89,-11.66,;60.55,-11.65,;61.89,-12.41,;63.22,-11.63,;61.9,-13.95,;60.57,-14.73,;60.57,-16.27,;56.56,-12.42,;55.23,-11.66,;53.9,-12.43,;55.22,-10.12,;53.89,-9.36,;53.88,-7.83,;55.21,-7.04,;56.55,-7.81,;57.88,-7.04,;57.88,-5.5,;59.21,-4.72,;60.54,-5.49,;60.55,-7.03,;61.87,-4.71,;63.21,-5.48,;63.21,-7.03,;64.54,-7.79,;65.87,-7.02,;65.87,-5.48,;64.54,-4.71,;65.29,-6.04,;63.75,-6.45,;59.2,-3.18,;60.54,-2.41,;60.53,-.87,;59.19,-.1,;57.86,-.89,;57.87,-2.42,;56.55,-9.35,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00204
BindingDB Entry DOI: 10.7270/Q2DN48WC |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(Homo sapiens (Human)) | BDBM50581183

(CHEMBL5087564)Show SMILES COc1ccc(cc1OC)[C@H](Cc1c(Cl)c[n+]([O-])cc1Cl)OC(=O)c1cccc(NC(C(=O)O[C@H]2CN3CCC2CC3)c2ccccc2)c1 |r,wU:10.22,wD:34.35,(2.97,-32.16,;4.31,-32.93,;5.64,-32.16,;6.98,-32.94,;8.31,-32.16,;8.31,-30.61,;6.97,-29.85,;5.64,-30.62,;4.31,-29.85,;4.31,-28.31,;9.64,-29.84,;10.98,-30.6,;12.31,-29.83,;12.29,-28.3,;10.95,-27.54,;13.62,-27.52,;14.96,-28.28,;16.29,-27.51,;14.97,-29.83,;13.64,-30.6,;13.64,-32.14,;9.63,-28.3,;8.3,-27.53,;6.97,-28.31,;8.29,-25.99,;6.96,-25.24,;6.95,-23.7,;8.28,-22.92,;9.62,-23.68,;10.95,-22.91,;10.95,-21.37,;12.28,-20.59,;12.27,-19.06,;13.61,-21.36,;14.94,-20.59,;16.28,-21.36,;17.61,-20.59,;17.61,-19.05,;16.27,-18.28,;14.93,-19.05,;15.79,-20.46,;16.82,-19.25,;9.61,-20.6,;9.61,-19.06,;8.28,-18.3,;6.95,-19.07,;6.96,-20.62,;8.29,-21.38,;9.62,-25.22,)| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-N-methyl Scopolamine Chloride from human M3 receptor membranes incubated for 2 hrs by scintillation counting analysis |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c00204
BindingDB Entry DOI: 10.7270/Q2DN48WC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data