 Found 2239 hits with Last Name = 'bakshi' and Initial = 'r'
Found 2239 hits with Last Name = 'bakshi' and Initial = 'r' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50400528
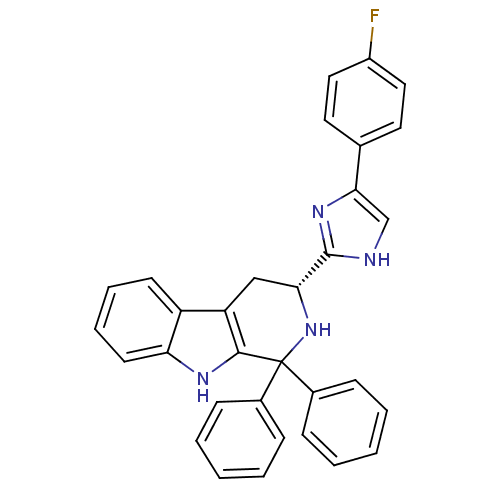
(CHEMBL2204934)Show SMILES Fc1ccc(cc1)-c1c[nH]c(n1)[C@H]1Cc2c([nH]c3ccccc23)C(N1)(c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C32H25FN4/c33-24-17-15-21(16-18-24)29-20-34-31(36-29)28-19-26-25-13-7-8-14-27(25)35-30(26)32(37-28,22-9-3-1-4-10-22)23-11-5-2-6-12-23/h1-18,20,28,35,37H,19H2,(H,34,36)/t28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled MK-499 from human ERG channel |
ACS Med Chem Lett 3: 484-489 (2012)
Article DOI: 10.1021/ml300063m
BindingDB Entry DOI: 10.7270/Q2V9897C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50400518

(CHEMBL2204942)Show SMILES Cn1cc(cn1)[C@H]1N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C24H21FN6/c1-31-13-15(11-27-31)22-23-18(17-4-2-3-5-19(17)28-23)10-20(29-22)24-26-12-21(30-24)14-6-8-16(25)9-7-14/h2-9,11-13,20,22,28-29H,10H2,1H3,(H,26,30)/t20-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled MK-499 from human ERG channel |
ACS Med Chem Lett 3: 484-489 (2012)
Article DOI: 10.1021/ml300063m
BindingDB Entry DOI: 10.7270/Q2V9897C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50389590
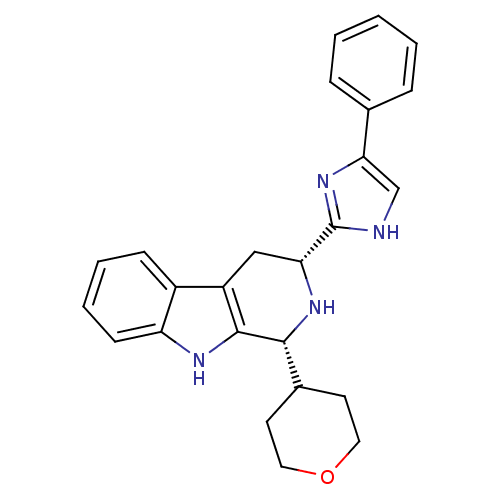
(CHEMBL2069502)Show SMILES C1CC(CCO1)[C@H]1N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccccc1 |r| Show InChI InChI=1S/C25H26N4O/c1-2-6-16(7-3-1)22-15-26-25(29-22)21-14-19-18-8-4-5-9-20(18)27-24(19)23(28-21)17-10-12-30-13-11-17/h1-9,15,17,21,23,27-28H,10-14H2,(H,26,29)/t21-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 369 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled MK-499 from human ERG channel |
ACS Med Chem Lett 3: 484-489 (2012)
Article DOI: 10.1021/ml300063m
BindingDB Entry DOI: 10.7270/Q2V9897C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50400519

(CHEMBL2204941)Show SMILES Fc1ccc(cc1)-c1c[nH]c(n1)[C@H]1Cc2c([nH]c3ccccc23)[C@H](N1)C1CCOCC1 |r| Show InChI InChI=1S/C25H25FN4O/c26-17-7-5-15(6-8-17)22-14-27-25(30-22)21-13-19-18-3-1-2-4-20(18)28-24(19)23(29-21)16-9-11-31-12-10-16/h1-8,14,16,21,23,28-29H,9-13H2,(H,27,30)/t21-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 377 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled MK-499 from human ERG channel |
ACS Med Chem Lett 3: 484-489 (2012)
Article DOI: 10.1021/ml300063m
BindingDB Entry DOI: 10.7270/Q2V9897C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50400520

(CHEMBL2204932)Show SMILES Cc1nc(no1)[C@H]1N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C23H19FN6O/c1-12-26-23(30-31-12)21-20-16(15-4-2-3-5-17(15)27-20)10-18(28-21)22-25-11-19(29-22)13-6-8-14(24)9-7-13/h2-9,11,18,21,27-28H,10H2,1H3,(H,25,29)/t18-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 385 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled MK-499 from human ERG channel |
ACS Med Chem Lett 3: 484-489 (2012)
Article DOI: 10.1021/ml300063m
BindingDB Entry DOI: 10.7270/Q2V9897C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50400526

(CHEMBL2204937)Show SMILES Cc1nc(no1)[C@]1(N[C@@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cc1)c1cnn(C)c1 |r| Show InChI InChI=1S/C27H23FN8O/c1-15-31-26(35-37-15)27(17-12-30-36(2)14-17)24-20(19-5-3-4-6-21(19)32-24)11-22(34-27)25-29-13-23(33-25)16-7-9-18(28)10-8-16/h3-10,12-14,22,32,34H,11H2,1-2H3,(H,29,33)/t22-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 462 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled MK-499 from human ERG channel |
ACS Med Chem Lett 3: 484-489 (2012)
Article DOI: 10.1021/ml300063m
BindingDB Entry DOI: 10.7270/Q2V9897C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50400525
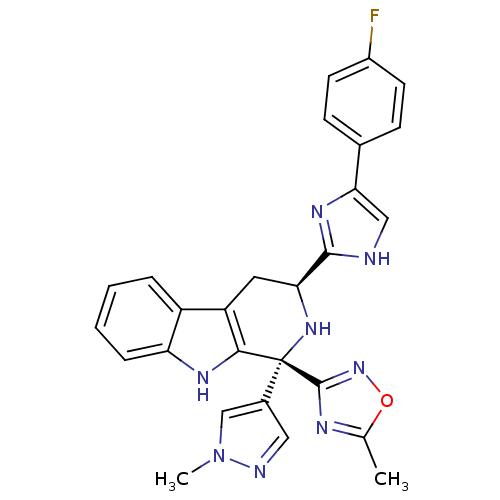
(CHEMBL2204938)Show SMILES Cc1nc(no1)[C@@]1(N[C@@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cc1)c1cnn(C)c1 |r| Show InChI InChI=1S/C27H23FN8O/c1-15-31-26(35-37-15)27(17-12-30-36(2)14-17)24-20(19-5-3-4-6-21(19)32-24)11-22(34-27)25-29-13-23(33-25)16-7-9-18(28)10-8-16/h3-10,12-14,22,32,34H,11H2,1-2H3,(H,29,33)/t22-,27+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 543 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled MK-499 from human ERG channel |
ACS Med Chem Lett 3: 484-489 (2012)
Article DOI: 10.1021/ml300063m
BindingDB Entry DOI: 10.7270/Q2V9897C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50021345
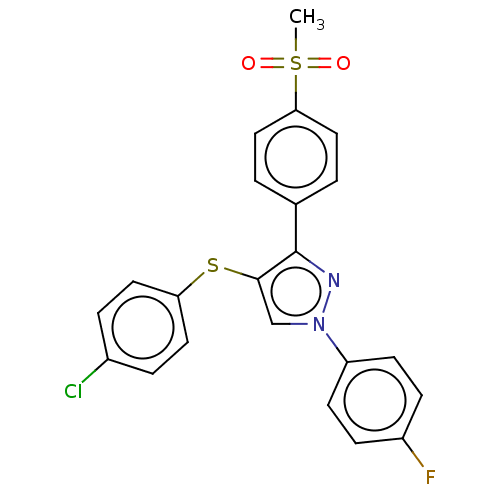
(CHEMBL3287928)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1nn(cc1Sc1ccc(Cl)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C22H16ClFN2O2S2/c1-30(27,28)20-12-2-15(3-13-20)22-21(29-19-10-4-16(23)5-11-19)14-26(25-22)18-8-6-17(24)7-9-18/h2-14H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 637 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK-499 from human ERG |
ACS Med Chem Lett 5: 717-21 (2014)
Article DOI: 10.1021/ml5001239
BindingDB Entry DOI: 10.7270/Q2JQ12KN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50021331
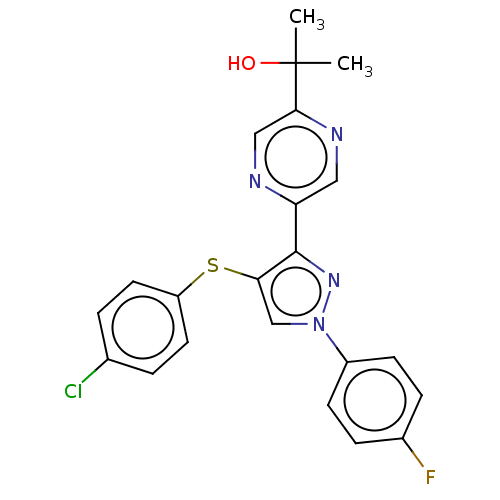
(CHEMBL3287930)Show SMILES CC(C)(O)c1cnc(cn1)-c1nn(cc1Sc1ccc(Cl)cc1)-c1ccc(F)cc1 Show InChI InChI=1S/C22H18ClFN4OS/c1-22(2,29)20-12-25-18(11-26-20)21-19(30-17-9-3-14(23)4-10-17)13-28(27-21)16-7-5-15(24)6-8-16/h3-13,29H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK-499 from human ERG |
ACS Med Chem Lett 5: 717-21 (2014)
Article DOI: 10.1021/ml5001239
BindingDB Entry DOI: 10.7270/Q2JQ12KN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50350538
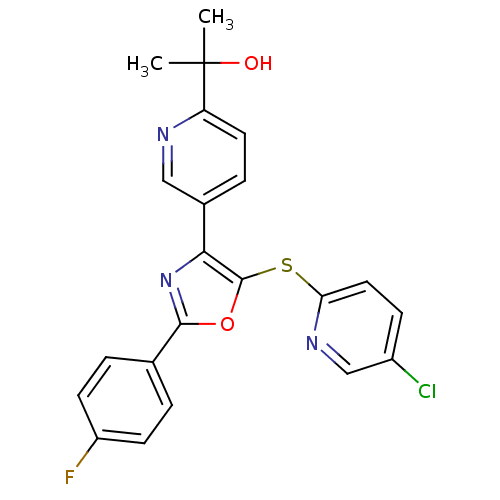
(CHEMBL1812717)Show SMILES CC(C)(O)c1ccc(cn1)-c1nc(oc1Sc1ccc(Cl)cn1)-c1ccc(F)cc1 Show InChI InChI=1S/C22H17ClFN3O2S/c1-22(2,28)17-9-5-14(11-25-17)19-21(30-18-10-6-15(23)12-26-18)29-20(27-19)13-3-7-16(24)8-4-13/h3-12,28H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK-499 from human ERG |
ACS Med Chem Lett 5: 717-21 (2014)
Article DOI: 10.1021/ml5001239
BindingDB Entry DOI: 10.7270/Q2JQ12KN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50400523
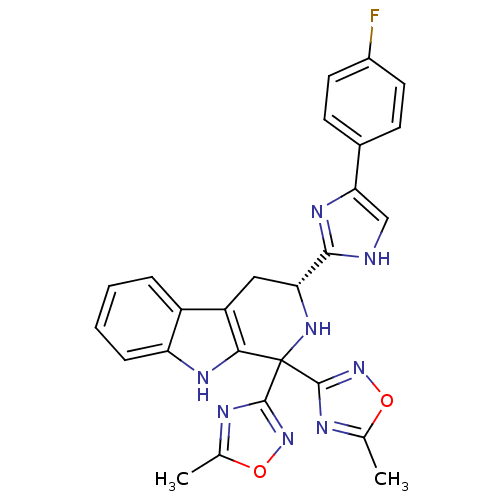
(CHEMBL2204940)Show SMILES Cc1nc(no1)C1(N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cc1)c1noc(C)n1 |r| Show InChI InChI=1S/C26H21FN8O2/c1-13-29-24(34-36-13)26(25-30-14(2)37-35-25)22-18(17-5-3-4-6-19(17)31-22)11-20(33-26)23-28-12-21(32-23)15-7-9-16(27)10-8-15/h3-10,12,20,31,33H,11H2,1-2H3,(H,28,32)/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled MK-499 from human ERG channel |
ACS Med Chem Lett 3: 484-489 (2012)
Article DOI: 10.1021/ml300063m
BindingDB Entry DOI: 10.7270/Q2V9897C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50021346
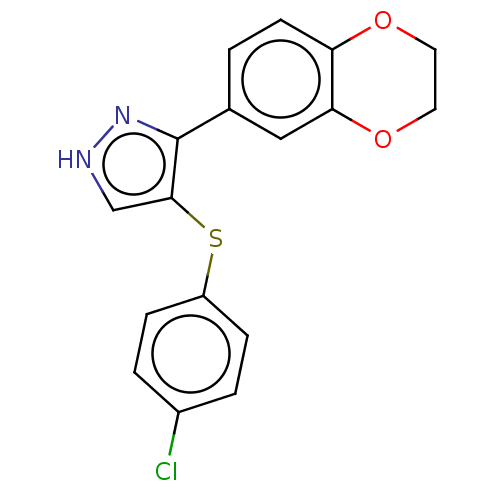
(CHEMBL3287926)Show InChI InChI=1S/C17H13ClN2O2S/c18-12-2-4-13(5-3-12)23-16-10-19-20-17(16)11-1-6-14-15(9-11)22-8-7-21-14/h1-6,9-10H,7-8H2,(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK-499 from human ERG |
ACS Med Chem Lett 5: 717-21 (2014)
Article DOI: 10.1021/ml5001239
BindingDB Entry DOI: 10.7270/Q2JQ12KN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50400527
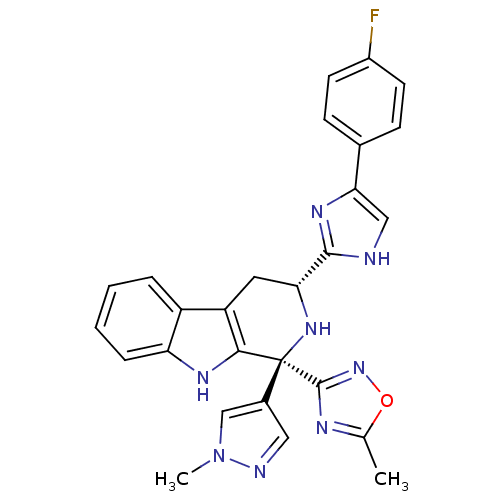
(CHEMBL2204936)Show SMILES Cc1nc(no1)[C@]1(N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cc1)c1cnn(C)c1 |r| Show InChI InChI=1S/C27H23FN8O/c1-15-31-26(35-37-15)27(17-12-30-36(2)14-17)24-20(19-5-3-4-6-21(19)32-24)11-22(34-27)25-29-13-23(33-25)16-7-9-18(28)10-8-16/h3-10,12-14,22,32,34H,11H2,1-2H3,(H,29,33)/t22-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled MK-499 from human ERG channel |
ACS Med Chem Lett 3: 484-489 (2012)
Article DOI: 10.1021/ml300063m
BindingDB Entry DOI: 10.7270/Q2V9897C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50400517
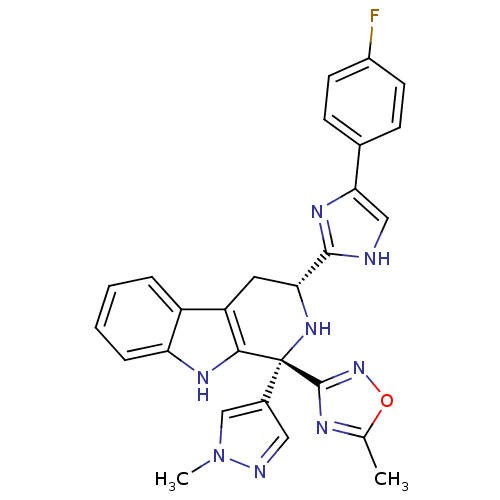
(CHEMBL2204935)Show SMILES Cc1nc(no1)[C@@]1(N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cc1)c1cnn(C)c1 |r| Show InChI InChI=1S/C27H23FN8O/c1-15-31-26(35-37-15)27(17-12-30-36(2)14-17)24-20(19-5-3-4-6-21(19)32-24)11-22(34-27)25-29-13-23(33-25)16-7-9-18(28)10-8-16/h3-10,12-14,22,32,34H,11H2,1-2H3,(H,29,33)/t22-,27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled MK-499 from human ERG channel |
ACS Med Chem Lett 3: 484-489 (2012)
Article DOI: 10.1021/ml300063m
BindingDB Entry DOI: 10.7270/Q2V9897C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50400522
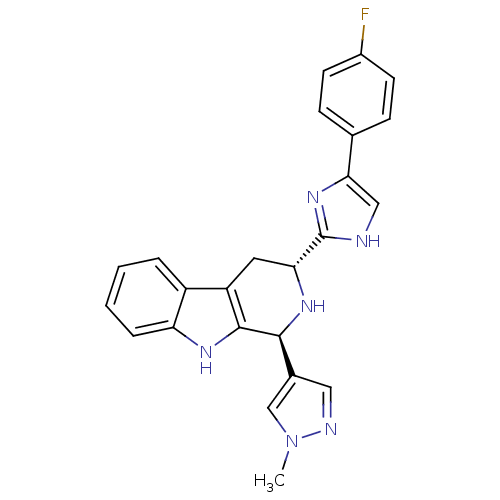
(CHEMBL2204931)Show SMILES Cn1cc(cn1)[C@@H]1N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C24H21FN6/c1-31-13-15(11-27-31)22-23-18(17-4-2-3-5-19(17)28-23)10-20(29-22)24-26-12-21(30-24)14-6-8-16(25)9-7-14/h2-9,11-13,20,22,28-29H,10H2,1H3,(H,26,30)/t20-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled MK-499 from human ERG channel |
ACS Med Chem Lett 3: 484-489 (2012)
Article DOI: 10.1021/ml300063m
BindingDB Entry DOI: 10.7270/Q2V9897C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50400521
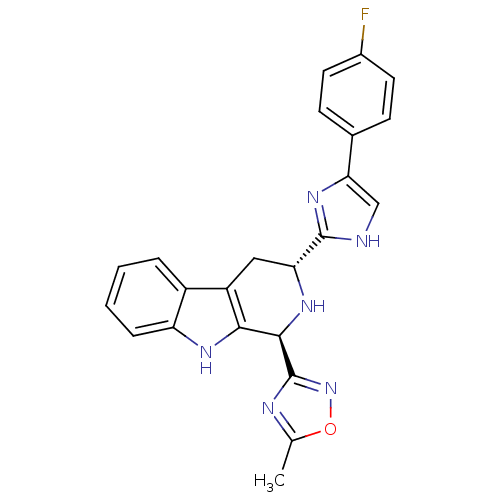
(CHEMBL2204933)Show SMILES Cc1nc(no1)[C@@H]1N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cc1 |r| Show InChI InChI=1S/C23H19FN6O/c1-12-26-23(30-31-12)21-20-16(15-4-2-3-5-17(15)27-20)10-18(28-21)22-25-11-19(29-22)13-6-8-14(24)9-7-13/h2-9,11,18,21,27-28H,10H2,1H3,(H,25,29)/t18-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled MK-499 from human ERG channel |
ACS Med Chem Lett 3: 484-489 (2012)
Article DOI: 10.1021/ml300063m
BindingDB Entry DOI: 10.7270/Q2V9897C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50021329
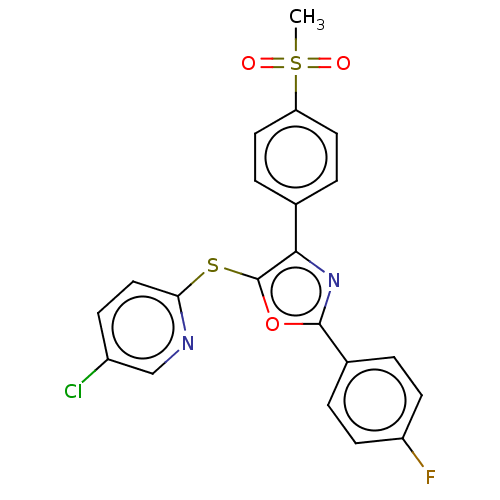
(CHEMBL3287932)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1nc(oc1Sc1ccc(Cl)cn1)-c1ccc(F)cc1 Show InChI InChI=1S/C21H14ClFN2O3S2/c1-30(26,27)17-9-4-13(5-10-17)19-21(29-18-11-6-15(22)12-24-18)28-20(25-19)14-2-7-16(23)8-3-14/h2-12H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK-499 from human ERG |
ACS Med Chem Lett 5: 717-21 (2014)
Article DOI: 10.1021/ml5001239
BindingDB Entry DOI: 10.7270/Q2JQ12KN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50400524
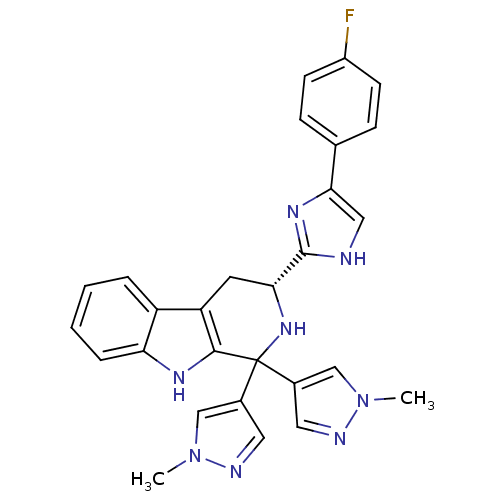
(CHEMBL2204939)Show SMILES Cn1cc(cn1)C1(N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cc1)c1cnn(C)c1 |r| Show InChI InChI=1S/C28H25FN8/c1-36-15-18(12-31-36)28(19-13-32-37(2)16-19)26-22(21-5-3-4-6-23(21)33-26)11-24(35-28)27-30-14-25(34-27)17-7-9-20(29)10-8-17/h3-10,12-16,24,33,35H,11H2,1-2H3,(H,30,34)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of radiolabeled MK-499 from human ERG channel |
ACS Med Chem Lett 3: 484-489 (2012)
Article DOI: 10.1021/ml300063m
BindingDB Entry DOI: 10.7270/Q2V9897C |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50021344
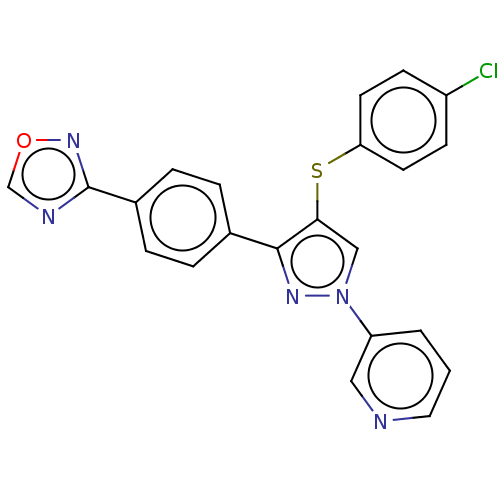
(CHEMBL3287929)Show SMILES Clc1ccc(Sc2cn(nc2-c2ccc(cc2)-c2ncon2)-c2cccnc2)cc1 Show InChI InChI=1S/C22H14ClN5OS/c23-17-7-9-19(10-8-17)30-20-13-28(18-2-1-11-24-12-18)26-21(20)15-3-5-16(6-4-15)22-25-14-29-27-22/h1-14H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.21E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK-499 from human ERG |
ACS Med Chem Lett 5: 717-21 (2014)
Article DOI: 10.1021/ml5001239
BindingDB Entry DOI: 10.7270/Q2JQ12KN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50021334
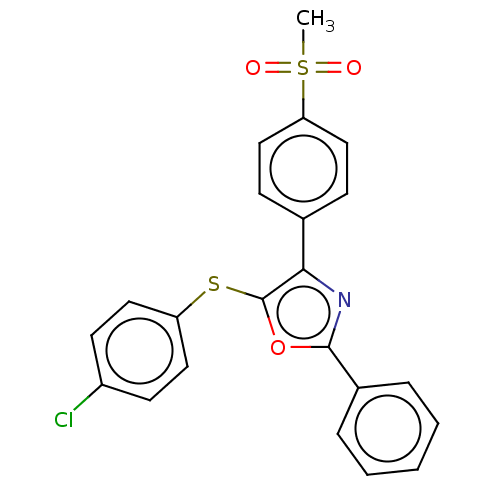
(CHEMBL3287931)Show SMILES CS(=O)(=O)c1ccc(cc1)-c1nc(oc1Sc1ccc(Cl)cc1)-c1ccccc1 Show InChI InChI=1S/C22H16ClNO3S2/c1-29(25,26)19-13-7-15(8-14-19)20-22(28-18-11-9-17(23)10-12-18)27-21(24-20)16-5-3-2-4-6-16/h2-14H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.85E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK-499 from human ERG |
ACS Med Chem Lett 5: 717-21 (2014)
Article DOI: 10.1021/ml5001239
BindingDB Entry DOI: 10.7270/Q2JQ12KN |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50021330
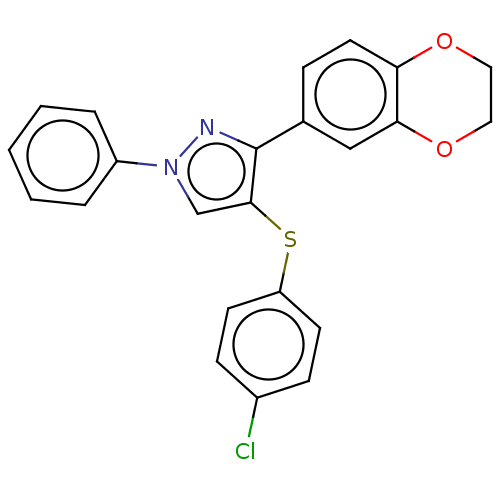
(CHEMBL3287927)Show SMILES Clc1ccc(Sc2cn(nc2-c2ccc3OCCOc3c2)-c2ccccc2)cc1 Show InChI InChI=1S/C23H17ClN2O2S/c24-17-7-9-19(10-8-17)29-22-15-26(18-4-2-1-3-5-18)25-23(22)16-6-11-20-21(14-16)28-13-12-27-20/h1-11,14-15H,12-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [35S]MK-499 from human ERG |
ACS Med Chem Lett 5: 717-21 (2014)
Article DOI: 10.1021/ml5001239
BindingDB Entry DOI: 10.7270/Q2JQ12KN |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50032782

((4aR,6aS)-4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,...)Show SMILES C[C@]12CCC3C(CCC4NC(=O)C=C[C@]34C)C1CCC2C(=O)Nc1cccc(c1)C(F)(F)F |c:12| Show InChI InChI=1S/C26H31F3N2O2/c1-24-12-10-19-17(6-9-21-25(19,2)13-11-22(32)31-21)18(24)7-8-20(24)23(33)30-16-5-3-4-15(14-16)26(27,28)29/h3-5,11,13-14,17-21H,6-10,12H2,1-2H3,(H,30,33)(H,31,32)/t17?,18?,19?,20?,21?,24-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human Steroid 5-alpha-reductase type 2 |
J Med Chem 38: 3189-92 (1995)
BindingDB Entry DOI: 10.7270/Q2G161G8 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50032773

((4aR,6aS)-4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,...)Show SMILES Cc1ccc(NC(=O)C2CCC3C4CCC5NC(=O)C=C[C@]5(C)C4CC[C@]23C)cc1 |c:19| Show InChI InChI=1S/C26H34N2O2/c1-16-4-6-17(7-5-16)27-24(30)21-10-9-19-18-8-11-22-26(3,15-13-23(29)28-22)20(18)12-14-25(19,21)2/h4-7,13,15,18-22H,8-12,14H2,1-3H3,(H,27,30)(H,28,29)/t18?,19?,20?,21?,22?,25-,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human Steroid 5-alpha-reductase type 2 |
J Med Chem 38: 3189-92 (1995)
BindingDB Entry DOI: 10.7270/Q2G161G8 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50032789

((4aR,6aS)-4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,...)Show SMILES C[C@]12CCC3C(CCC4NC(=O)C=C[C@]34C)C1CCC2C(=O)Nc1cccc(Cl)c1 |c:12| Show InChI InChI=1S/C25H31ClN2O2/c1-24-12-10-19-17(6-9-21-25(19,2)13-11-22(29)28-21)18(24)7-8-20(24)23(30)27-16-5-3-4-15(26)14-16/h3-5,11,13-14,17-21H,6-10,12H2,1-2H3,(H,27,30)(H,28,29)/t17?,18?,19?,20?,21?,24-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human Steroid 5-alpha-reductase type 2 |
J Med Chem 38: 3189-92 (1995)
BindingDB Entry DOI: 10.7270/Q2G161G8 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(MOUSE) | BDBM50400525

(CHEMBL2204938)Show SMILES Cc1nc(no1)[C@@]1(N[C@@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cc1)c1cnn(C)c1 |r| Show InChI InChI=1S/C27H23FN8O/c1-15-31-26(35-37-15)27(17-12-30-36(2)14-17)24-20(19-5-3-4-6-21(19)32-24)11-22(34-27)25-29-13-23(33-25)16-7-9-18(28)10-8-16/h3-10,12-14,22,32,34H,11H2,1-2H3,(H,29,33)/t22-,27+/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of mouse SSTR3 transfected in CHO cells assessed as inhibition of SRIF-induced reduction of cAMP accumulation after 45 mins |
ACS Med Chem Lett 3: 484-489 (2012)
Article DOI: 10.1021/ml300063m
BindingDB Entry DOI: 10.7270/Q2V9897C |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(Homo sapiens (Human)) | BDBM50054462

(CHEMBL3323076)Show SMILES CCOC(=O)c1cncc(c1)[C@@]1(N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cc1)c1cnn(C)c1 |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at human SSTR3 expressed in CHO cells assessed as cAMP level by fluorescence analysis |
ACS Med Chem Lett 5: 748-53 (2014)
Article DOI: 10.1021/ml500028c
BindingDB Entry DOI: 10.7270/Q2NV9KXP |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 3
(MOUSE) | BDBM50053739
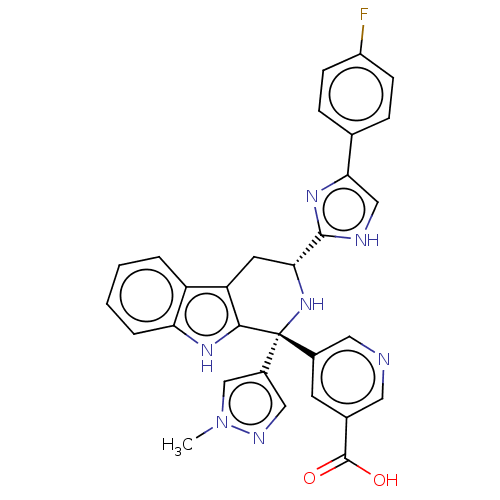
(CHEMBL3323084)Show SMILES Cn1cc(cn1)[C@]1(N[C@H](Cc2c1[nH]c1ccccc21)c1nc(c[nH]1)-c1ccc(F)cc1)c1cncc(c1)C(O)=O |r| | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Antagonist activity at mouse SSTR3 expressed in CHO cells assessed as cAMP level by fluorescence analysis |
ACS Med Chem Lett 5: 748-53 (2014)
Article DOI: 10.1021/ml500028c
BindingDB Entry DOI: 10.7270/Q2NV9KXP |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM275221
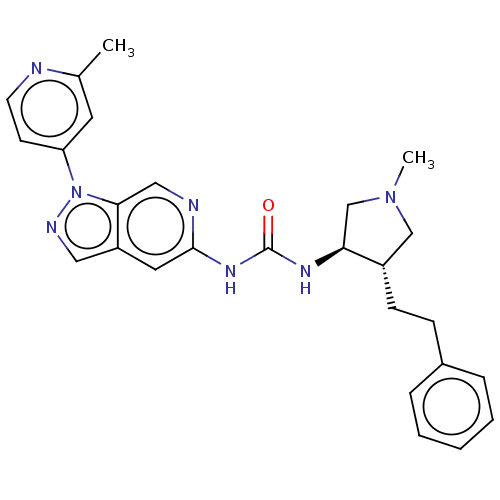
(1-[(3R,4S)-1-methyl-4-(2- phenylethyl)pyrrolidin-3...)Show SMILES CN1C[C@H](CCc2ccccc2)[C@H](C1)NC(=O)Nc1cc2cnn(-c3ccnc(C)c3)c2cn1 |r| Show InChI InChI=1S/C26H29N7O/c1-18-12-22(10-11-27-18)33-24-15-28-25(13-21(24)14-29-33)31-26(34)30-23-17-32(2)16-20(23)9-8-19-6-4-3-5-7-19/h3-7,10-15,20,23H,8-9,16-17H2,1-2H3,(H2,28,30,31,34)/t20-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.
US Patent
| Assay Description
Activated ERK2 activity was determined in an IMAP-FP assay (Molecular Devices). Using this assay format, the potency (IC50) of each compound was dete... |
US Patent US9884048 (2018)
BindingDB Entry DOI: 10.7270/Q29G5PT5 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM201601

(US9233979, 42)Show SMILES CN1C[C@@H](NC(=O)Nc2cc3[nH]nc(N4CCOCC4)c3cn2)[C@H](C1)c1ccccc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Activated ERK2 activity was determined in an IMAP-FP assay (Molecular
Devices). Using this assay format, the potency (IC50) of each compound
was de... |
US Patent US9233979 (2016)
BindingDB Entry DOI: 10.7270/Q2B27T3B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM201669

(US9233979, 97)Show SMILES C[C@@H](O)[C@@H](NC(=O)Nc1cc2[nH]nc(N3CCOCC3)c2cn1)c1ccc(F)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Activated ERK2 activity was determined in an IMAP-FP assay (Molecular
Devices). Using this assay format, the potency (IC50) of each compound
was de... |
US Patent US9233979 (2016)
BindingDB Entry DOI: 10.7270/Q2B27T3B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM201670

(US9233979, 98)Show SMILES C[C@H](O)[C@@H](NC(=O)Nc1cc2[nH]nc(N3CCOCC3)c2cn1)c1ccc(F)cc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Activated ERK2 activity was determined in an IMAP-FP assay (Molecular
Devices). Using this assay format, the potency (IC50) of each compound
was de... |
US Patent US9233979 (2016)
BindingDB Entry DOI: 10.7270/Q2B27T3B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM201695
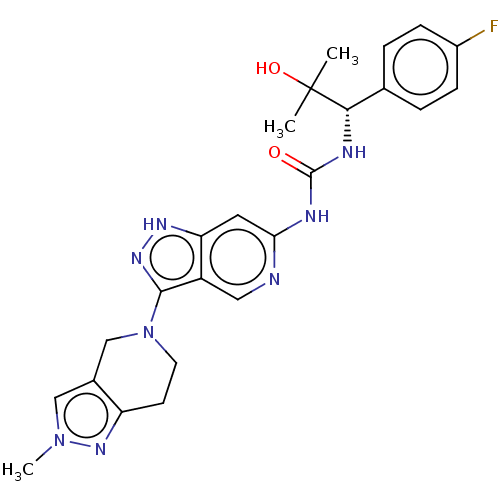
(US9233979, 126)Show SMILES Cn1cc2CN(CCc2n1)c1n[nH]c2cc(NC(=O)N[C@@H](c3ccc(F)cc3)C(C)(C)O)ncc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Activated ERK2 activity was determined in an IMAP-FP assay (Molecular
Devices). Using this assay format, the potency (IC50) of each compound
was de... |
US Patent US9233979 (2016)
BindingDB Entry DOI: 10.7270/Q2B27T3B |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50032763
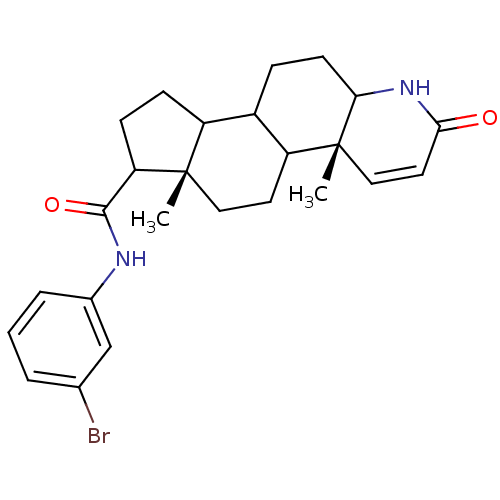
((4aR,6aS)-4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,...)Show SMILES C[C@]12CCC3C(CCC4NC(=O)C=C[C@]34C)C1CCC2C(=O)Nc1cccc(Br)c1 |c:12| Show InChI InChI=1S/C25H31BrN2O2/c1-24-12-10-19-17(6-9-21-25(19,2)13-11-22(29)28-21)18(24)7-8-20(24)23(30)27-16-5-3-4-15(26)14-16/h3-5,11,13-14,17-21H,6-10,12H2,1-2H3,(H,27,30)(H,28,29)/t17?,18?,19?,20?,21?,24-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human Steroid 5-alpha-reductase type 2 |
J Med Chem 38: 3189-92 (1995)
BindingDB Entry DOI: 10.7270/Q2G161G8 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50032781

((4aR,6aS)-4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,...)Show SMILES C[C@]12CCC3C(CCC4NC(=O)C=C[C@]34C)C1CCC2C(=O)Nc1ccc(F)cc1 |c:12| Show InChI InChI=1S/C25H31FN2O2/c1-24-13-11-19-17(7-10-21-25(19,2)14-12-22(29)28-21)18(24)8-9-20(24)23(30)27-16-5-3-15(26)4-6-16/h3-6,12,14,17-21H,7-11,13H2,1-2H3,(H,27,30)(H,28,29)/t17?,18?,19?,20?,21?,24-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human Steroid 5-alpha-reductase type 2 |
J Med Chem 38: 3189-92 (1995)
BindingDB Entry DOI: 10.7270/Q2G161G8 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50032785

((4aR,6aS)-4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,...)Show SMILES C[C@]12CCC3C(CCC4NC(=O)C=C[C@]34C)C1CCC2C(=O)Nc1cccc2ccccc12 |c:12| Show InChI InChI=1S/C29H34N2O2/c1-28-16-14-22-20(10-13-25-29(22,2)17-15-26(32)31-25)21(28)11-12-23(28)27(33)30-24-9-5-7-18-6-3-4-8-19(18)24/h3-9,15,17,20-23,25H,10-14,16H2,1-2H3,(H,30,33)(H,31,32)/t20?,21?,22?,23?,25?,28-,29+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human Steroid 5-alpha-reductase type 2 |
J Med Chem 38: 3189-92 (1995)
BindingDB Entry DOI: 10.7270/Q2G161G8 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50032786
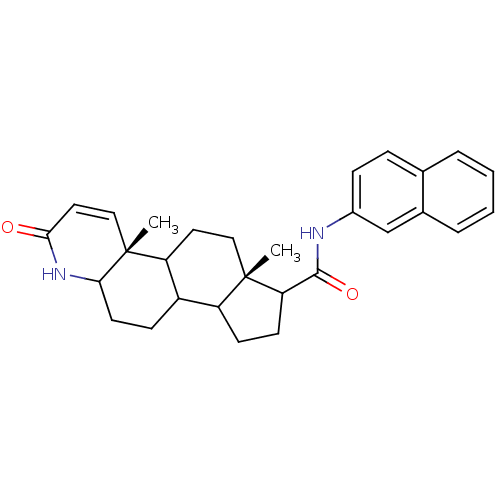
((4aR,6aS)-4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,...)Show SMILES C[C@]12CCC3C(CCC4NC(=O)C=C[C@]34C)C1CCC2C(=O)Nc1ccc2ccccc2c1 |c:12| Show InChI InChI=1S/C29H34N2O2/c1-28-15-13-23-21(9-12-25-29(23,2)16-14-26(32)31-25)22(28)10-11-24(28)27(33)30-20-8-7-18-5-3-4-6-19(18)17-20/h3-8,14,16-17,21-25H,9-13,15H2,1-2H3,(H,30,33)(H,31,32)/t21?,22?,23?,24?,25?,28-,29+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human Steroid 5-alpha-reductase type 2 |
J Med Chem 38: 3189-92 (1995)
BindingDB Entry DOI: 10.7270/Q2G161G8 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50032778
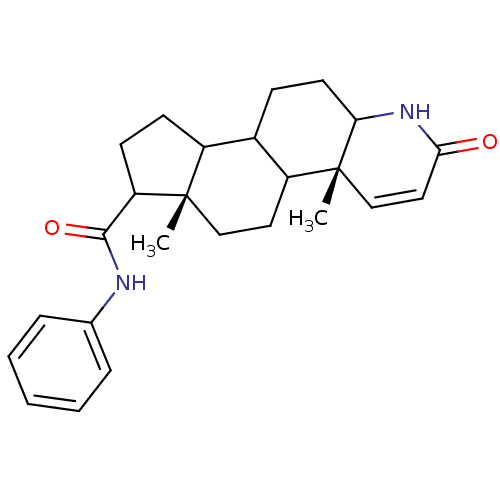
((4aR,6aS)-4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,...)Show SMILES C[C@]12CCC3C(CCC4NC(=O)C=C[C@]34C)C1CCC2C(=O)Nc1ccccc1 |c:12| Show InChI InChI=1S/C25H32N2O2/c1-24-14-12-19-17(8-11-21-25(19,2)15-13-22(28)27-21)18(24)9-10-20(24)23(29)26-16-6-4-3-5-7-16/h3-7,13,15,17-21H,8-12,14H2,1-2H3,(H,26,29)(H,27,28)/t17?,18?,19?,20?,21?,24-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human Steroid 5-alpha-reductase type 2 |
J Med Chem 38: 3189-92 (1995)
BindingDB Entry DOI: 10.7270/Q2G161G8 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50032787
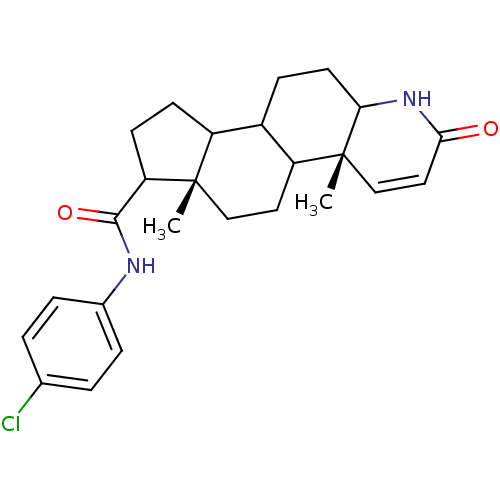
((4aR,6aS)-4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,...)Show SMILES C[C@]12CCC3C(CCC4NC(=O)C=C[C@]34C)C1CCC2C(=O)Nc1ccc(Cl)cc1 |c:12| Show InChI InChI=1S/C25H31ClN2O2/c1-24-13-11-19-17(7-10-21-25(19,2)14-12-22(29)28-21)18(24)8-9-20(24)23(30)27-16-5-3-15(26)4-6-16/h3-6,12,14,17-21H,7-11,13H2,1-2H3,(H,27,30)(H,28,29)/t17?,18?,19?,20?,21?,24-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human Steroid 5-alpha-reductase type 2 |
J Med Chem 38: 3189-92 (1995)
BindingDB Entry DOI: 10.7270/Q2G161G8 |
More data for this
Ligand-Target Pair | |
3-oxo-5-alpha-steroid 4-dehydrogenase 2
(Homo sapiens (Human)) | BDBM50032774
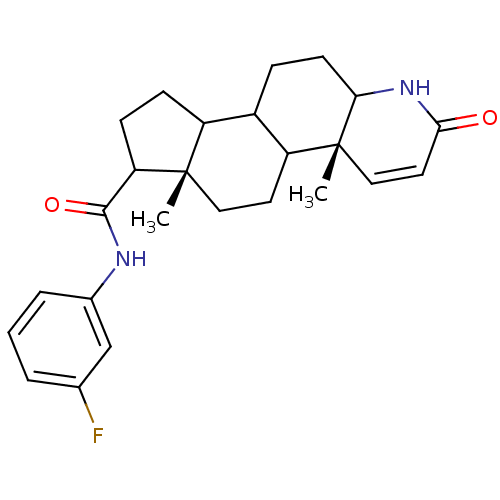
((4aR,6aS)-4a,6a-Dimethyl-2-oxo-2,4a,4b,5,6,6a,7,8,...)Show SMILES C[C@]12CCC3C(CCC4NC(=O)C=C[C@]34C)C1CCC2C(=O)Nc1cccc(F)c1 |c:12| Show InChI InChI=1S/C25H31FN2O2/c1-24-12-10-19-17(6-9-21-25(19,2)13-11-22(29)28-21)18(24)7-8-20(24)23(30)27-16-5-3-4-15(26)14-16/h3-5,11,13-14,17-21H,6-10,12H2,1-2H3,(H,27,30)(H,28,29)/t17?,18?,19?,20?,21?,24-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human Steroid 5-alpha-reductase type 2 |
J Med Chem 38: 3189-92 (1995)
BindingDB Entry DOI: 10.7270/Q2G161G8 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM275295
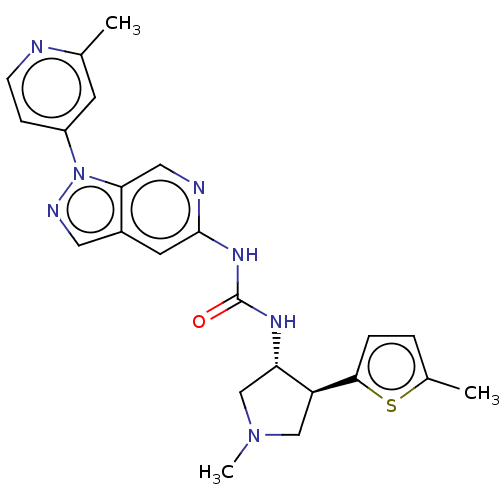
(1-[(3R,4S)-1-methyl-4-(5- methylthiophen-2-yl)pyrr...)Show SMILES CN1C[C@H](NC(=O)Nc2cc3cnn(-c4ccnc(C)c4)c3cn2)[C@H](C1)c1ccc(C)s1 |r| Show InChI InChI=1S/C23H25N7OS/c1-14-8-17(6-7-24-14)30-20-11-25-22(9-16(20)10-26-30)28-23(31)27-19-13-29(3)12-18(19)21-5-4-15(2)32-21/h4-11,18-19H,12-13H2,1-3H3,(H2,25,27,28,31)/t18-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.
US Patent
| Assay Description
Activated ERK2 activity was determined in an IMAP-FP assay (Molecular Devices). Using this assay format, the potency (IC50) of each compound was dete... |
US Patent US9884048 (2018)
BindingDB Entry DOI: 10.7270/Q29G5PT5 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM275140
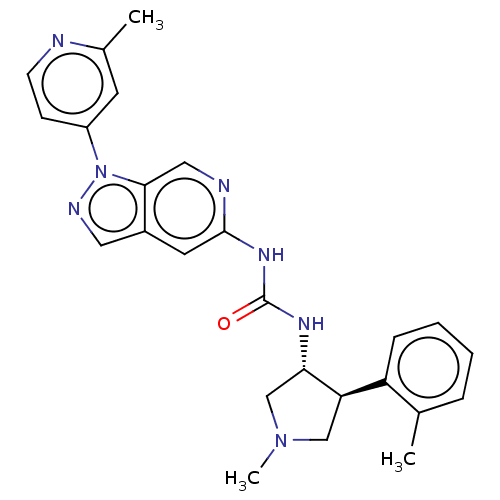
(1-[(3R,4S) or (3S, 4R)-1- methyl-4-(2- methylpheny...)Show SMILES CN1C[C@H](NC(=O)Nc2cc3cnn(-c4ccnc(C)c4)c3cn2)[C@H](C1)c1ccccc1C |r| Show InChI InChI=1S/C25H27N7O/c1-16-6-4-5-7-20(16)21-14-31(3)15-22(21)29-25(33)30-24-11-18-12-28-32(23(18)13-27-24)19-8-9-26-17(2)10-19/h4-13,21-22H,14-15H2,1-3H3,(H2,27,29,30,33)/t21-,22+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.
US Patent
| Assay Description
Activated ERK2 activity was determined in an IMAP-FP assay (Molecular Devices). Using this assay format, the potency (IC50) of each compound was dete... |
US Patent US9884048 (2018)
BindingDB Entry DOI: 10.7270/Q29G5PT5 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM202013

(US9233979, 445 | US9233979, 455 | US9233979, 456)Show SMILES CC(O)[C@@H](NC(=O)Nc1cc2[nH]nc(N3CCOCC3)c2cn1)c1ccccc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Activated ERK2 activity was determined in an IMAP-FP assay (Molecular
Devices). Using this assay format, the potency (IC50) of each compound
was de... |
US Patent US9233979 (2016)
BindingDB Entry DOI: 10.7270/Q2B27T3B |
More data for this
Ligand-Target Pair | |
Fatty-acid amide hydrolase 1
(Homo sapiens (Human)) | BDBM50434318

(CHEMBL2386566)Show SMILES CN(C)C(=O)[C@H]1C[C@@H]1c1ccc(cc1)-c1ncn(C)c1Sc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C22H22ClN3OS/c1-25(2)21(27)19-12-18(19)14-4-6-15(7-5-14)20-22(26(3)13-24-20)28-17-10-8-16(23)9-11-17/h4-11,13,18-19H,12H2,1-3H3/t18-,19+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human FAAH expressed in CHO cell lysates assessed as arachidonyl-7-amino-4-methylcoumarin amide hydrolysis to 7-amino 4-methyl coumarin... |
ACS Med Chem Lett 4: 509-13 (2013)
Article DOI: 10.1021/ml4000996
BindingDB Entry DOI: 10.7270/Q21R6RWJ |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM201962

(US9233979, 394)Show SMILES COC(=O)Nc1n[nH]c2cc(NC(=O)N[C@@H]3[C@H](O)CCOc4ccccc34)ncc12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Activated ERK2 activity was determined in an IMAP-FP assay (Molecular
Devices). Using this assay format, the potency (IC50) of each compound
was de... |
US Patent US9233979 (2016)
BindingDB Entry DOI: 10.7270/Q2B27T3B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM201668

(BDBM202039 | US9233979, 96)Show SMILES C[C@@H](O)[C@@H](NC(=O)Nc1cc2[nH]nc(N3CCOCC3)c2cn1)c1ccccc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Activated ERK2 activity was determined in an IMAP-FP assay (Molecular
Devices). Using this assay format, the potency (IC50) of each compound
was de... |
US Patent US9233979 (2016)
BindingDB Entry DOI: 10.7270/Q2B27T3B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM201679

(US9233979, 107)Show SMILES Cc1nc(NC(=O)N[C@@H](c2ccccc2)C(C)(C)O)cc2[nH]nc(N3CCOCC3)c12 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Activated ERK2 activity was determined in an IMAP-FP assay (Molecular
Devices). Using this assay format, the potency (IC50) of each compound
was de... |
US Patent US9233979 (2016)
BindingDB Entry DOI: 10.7270/Q2B27T3B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM201693

(US9233979, 124)Show SMILES CC(C)(O)[C@@H](NC(=O)Nc1cc2[nH]nc(N3CCc4ncsc4C3)c2cn1)c1ccccc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Activated ERK2 activity was determined in an IMAP-FP assay (Molecular
Devices). Using this assay format, the potency (IC50) of each compound
was de... |
US Patent US9233979 (2016)
BindingDB Entry DOI: 10.7270/Q2B27T3B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM201696
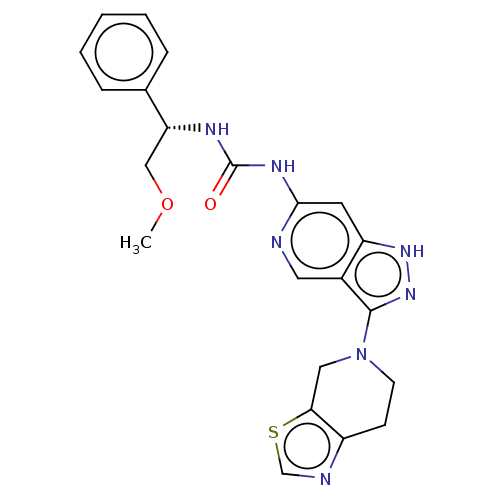
(US9233979, 127)Show SMILES COC[C@@H](NC(=O)Nc1cc2[nH]nc(N3CCc4ncsc4C3)c2cn1)c1ccccc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Activated ERK2 activity was determined in an IMAP-FP assay (Molecular
Devices). Using this assay format, the potency (IC50) of each compound
was de... |
US Patent US9233979 (2016)
BindingDB Entry DOI: 10.7270/Q2B27T3B |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM201681
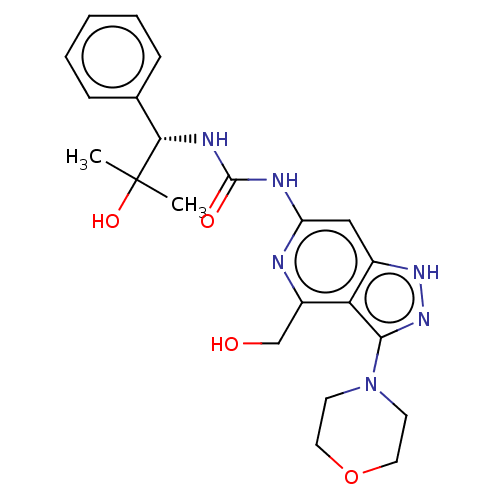
(US9233979, 109)Show SMILES CC(C)(O)[C@@H](NC(=O)Nc1cc2[nH]nc(N3CCOCC3)c2c(CO)n1)c1ccccc1 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Corp.
US Patent
| Assay Description
Activated ERK2 activity was determined in an IMAP-FP assay (Molecular
Devices). Using this assay format, the potency (IC50) of each compound
was de... |
US Patent US9233979 (2016)
BindingDB Entry DOI: 10.7270/Q2B27T3B |
More data for this
Ligand-Target Pair | |
Melanocortin receptor 4
(Homo sapiens (Human)) | BDBM50119367
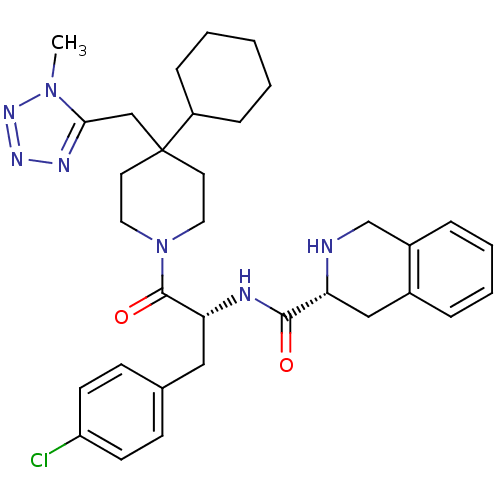
((3R)-N-[(2R)-3-(4-chlorophenyl)-1-{4-cyclohexyl-4-...)Show SMILES Cn1nnnc1CC1(CCN(CC1)C(=O)[C@@H](Cc1ccc(Cl)cc1)NC(=O)[C@H]1Cc2ccccc2CN1)C1CCCCC1 Show InChI InChI=1S/C33H42ClN7O2/c1-40-30(37-38-39-40)21-33(26-9-3-2-4-10-26)15-17-41(18-16-33)32(43)29(19-23-11-13-27(34)14-12-23)36-31(42)28-20-24-7-5-6-8-25(24)22-35-28/h5-8,11-14,26,28-29,35H,2-4,9-10,15-22H2,1H3,(H,36,42)/t28-,29-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co.
Curated by ChEMBL
| Assay Description
Evaluated for binding affinity against Melanocortin-4 receptor by displacing [125I]-NDP-alpha-MSH radioligand expressed in CHO cells |
J Med Chem 45: 4589-93 (2002)
BindingDB Entry DOI: 10.7270/Q2GT5MH9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data