

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aurora kinase A (Mus musculus (mouse)) | BDBM50277545 (4-(9-chloro-7-(2-fluoro-6-methoxyphenyl)-5H-benzo[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States. Curated by ChEMBL | Assay Description Competitive inhibition of recombinant mouse aurora kinase A expressed in insect Sf9 cells in presence of ATP | ACS Med Chem Lett 6: 630-4 (2015) Article DOI: 10.1021/ml500409n BindingDB Entry DOI: 10.7270/Q2WS8W1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Mus musculus (mouse)) | BDBM31093 (4-[[7-[2,6-bis(fluoranyl)phenyl]-9-chloranyl-5H-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States. Curated by ChEMBL | Assay Description Competitive inhibition of recombinant mouse aurora kinase A expressed in insect Sf9 cells in presence of ATP | ACS Med Chem Lett 6: 630-4 (2015) Article DOI: 10.1021/ml500409n BindingDB Entry DOI: 10.7270/Q2WS8W1V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase A (Mus musculus (mouse)) | BDBM50277545 (4-(9-chloro-7-(2-fluoro-6-methoxyphenyl)-5H-benzo[...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant mouse aurora kinase A expressed in insect Sf9 cells using biotin-GLRRASLG as substrate in presence of [gamma-33P]ATP | ACS Med Chem Lett 6: 630-4 (2015) Article DOI: 10.1021/ml500409n BindingDB Entry DOI: 10.7270/Q2WS8W1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2278 (3-(benzenesulfonyl)-5-chloro-1H-indole-2-carboxami...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 8.2 | n/a |
Merck Research Laboratories | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 1291-4 (1993) Article DOI: 10.1021/jm00061a022 BindingDB Entry DOI: 10.7270/Q2RV0KWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Mus musculus (mouse)) | BDBM31093 (4-[[7-[2,6-bis(fluoranyl)phenyl]-9-chloranyl-5H-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of mouse recombinant Aurora A kinase expressed in insect Sf9 cells by radioactive flashplate assay | Proc Natl Acad Sci USA 104: 4106-11 (2007) Article DOI: 10.1073/pnas.0608798104 BindingDB Entry DOI: 10.7270/Q25B03CS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50277545 (4-(9-chloro-7-(2-fluoro-6-methoxyphenyl)-5H-benzo[...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States. Curated by ChEMBL | Assay Description Inhibition of aurora kinase A autophosphorylation at T288 in human HCT116 cells by immunofluorescence analysis | ACS Med Chem Lett 6: 630-4 (2015) Article DOI: 10.1021/ml500409n BindingDB Entry DOI: 10.7270/Q2WS8W1V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Aurora kinase A (Mus musculus (mouse)) | BDBM50097413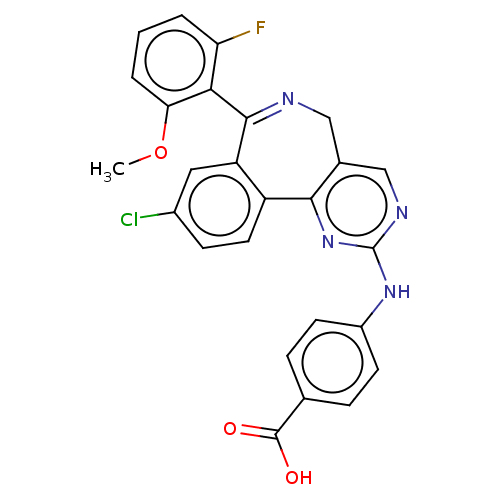 (CHEMBL3586473) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant mouse aurora kinase A expressed in insect Sf9 cells using biotin-GLRRASLG as substrate in presence of [gamma-33P]ATP | ACS Med Chem Lett 6: 630-4 (2015) Article DOI: 10.1021/ml500409n BindingDB Entry DOI: 10.7270/Q2WS8W1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2284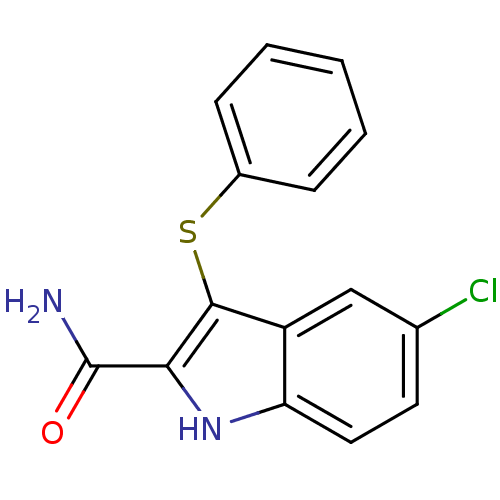 (5-chloro-3-(phenylsulfanyl)-1H-indole-2-carboxamid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | 8.2 | n/a |
Merck Research Laboratories | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 1291-4 (1993) Article DOI: 10.1021/jm00061a022 BindingDB Entry DOI: 10.7270/Q2RV0KWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50097413 (CHEMBL3586473) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States. Curated by ChEMBL | Assay Description Inhibition of aurora kinase A autophosphorylation at T288 in human HCT116 cells by immunofluorescence analysis | ACS Med Chem Lett 6: 630-4 (2015) Article DOI: 10.1021/ml500409n BindingDB Entry DOI: 10.7270/Q2WS8W1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2287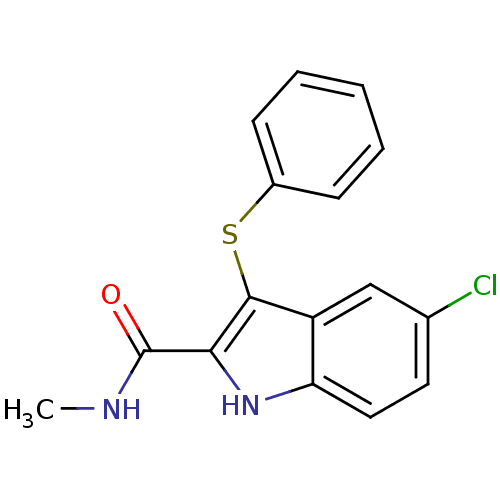 (5-chloro-N-methyl-3-(phenylsulfanyl)-1H-indole-2-c...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | 8.2 | n/a |
Merck Research Laboratories | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 1291-4 (1993) Article DOI: 10.1021/jm00061a022 BindingDB Entry DOI: 10.7270/Q2RV0KWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2288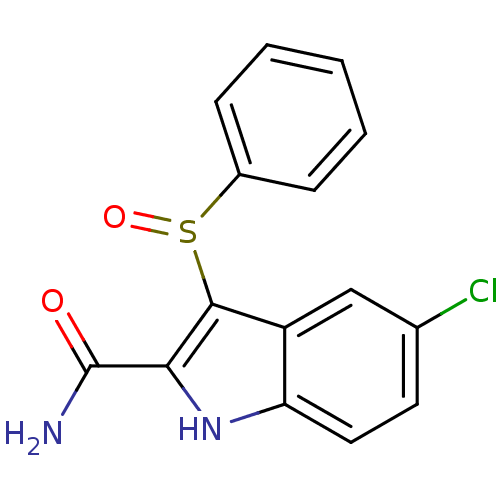 (3-(benzenesulfinyl)-5-chloro-1H-indole-2-carboxami...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | 8.2 | n/a |
Merck Research Laboratories | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 1291-4 (1993) Article DOI: 10.1021/jm00061a022 BindingDB Entry DOI: 10.7270/Q2RV0KWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Mus musculus (mouse)) | BDBM31093 (4-[[7-[2,6-bis(fluoranyl)phenyl]-9-chloranyl-5H-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant mouse aurora kinase A expressed in insect Sf9 cells using biotin-GLRRASLG as substrate in presence of [gamma-33P]ATP | ACS Med Chem Lett 6: 630-4 (2015) Article DOI: 10.1021/ml500409n BindingDB Entry DOI: 10.7270/Q2WS8W1V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase A (Mus musculus (mouse)) | BDBM50097414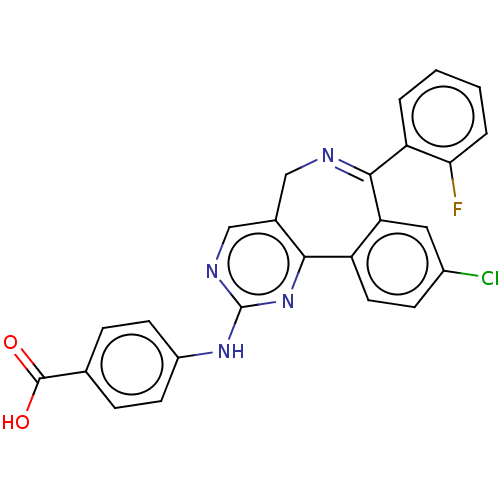 (CHEMBL3586468) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States. Curated by ChEMBL | Assay Description Inhibition of recombinant mouse aurora kinase A expressed in insect Sf9 cells using biotin-GLRRASLG as substrate in presence of [gamma-33P]ATP | ACS Med Chem Lett 6: 630-4 (2015) Article DOI: 10.1021/ml500409n BindingDB Entry DOI: 10.7270/Q2WS8W1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM31093 (4-[[7-[2,6-bis(fluoranyl)phenyl]-9-chloranyl-5H-py...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of Aurora A Thr288 autophosphorylation in human HeLa cells after 1 hr | Proc Natl Acad Sci USA 104: 4106-11 (2007) Article DOI: 10.1073/pnas.0608798104 BindingDB Entry DOI: 10.7270/Q25B03CS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM31093 (4-[[7-[2,6-bis(fluoranyl)phenyl]-9-chloranyl-5H-py...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States. Curated by ChEMBL | Assay Description Inhibition of aurora kinase A autophosphorylation at T288 in human HCT116 cells by immunofluorescence analysis | ACS Med Chem Lett 6: 630-4 (2015) Article DOI: 10.1021/ml500409n BindingDB Entry DOI: 10.7270/Q2WS8W1V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM1317 (3-[[(4,7-Dichlorobenzoxazol-2-yl)-methyl]amino]-5-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | 8.2 | n/a |
Merck Research Laboratories | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 1291-4 (1993) Article DOI: 10.1021/jm00061a022 BindingDB Entry DOI: 10.7270/Q2RV0KWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2279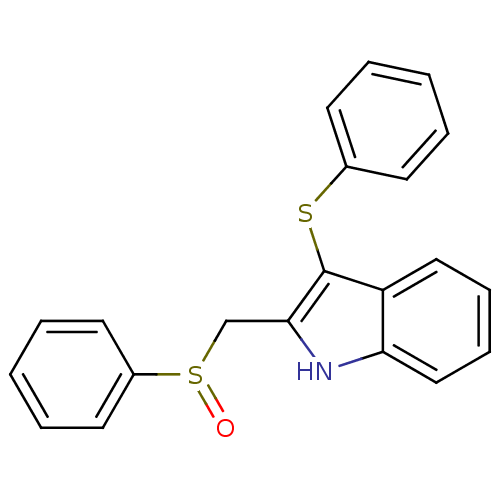 (2-[(benzenesulfinyl)methyl]-3-(phenylsulfanyl)-1H-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 63 | n/a | n/a | n/a | n/a | 8.2 | n/a |
Merck Research Laboratories | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 1291-4 (1993) Article DOI: 10.1021/jm00061a022 BindingDB Entry DOI: 10.7270/Q2RV0KWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1127,Y768C]/[588-1147,Y768C] (Human immunodeficiency virus type 1) | BDBM2278 (3-(benzenesulfonyl)-5-chloro-1H-indole-2-carboxami...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | 8.2 | n/a |
Merck Research Laboratories | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 1291-4 (1993) Article DOI: 10.1021/jm00061a022 BindingDB Entry DOI: 10.7270/Q2RV0KWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Long-chain fatty acid transport protein 4 (Homo sapiens (Human)) | BDBM50186007 ((E)-hept-2-enyl 6-methyl-4-(4-nitrophenyl)-2-oxo-1...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human FATP4-mediated 12-BODIPY-lauric acid uptake in HEK293 cells | Bioorg Med Chem Lett 16: 3504-9 (2006) Article DOI: 10.1016/j.bmcl.2006.03.102 BindingDB Entry DOI: 10.7270/Q2K9373C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM1434 (11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 101 | n/a | n/a | n/a | n/a | 8.2 | n/a |
Merck Research Laboratories | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 1291-4 (1993) Article DOI: 10.1021/jm00061a022 BindingDB Entry DOI: 10.7270/Q2RV0KWR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2280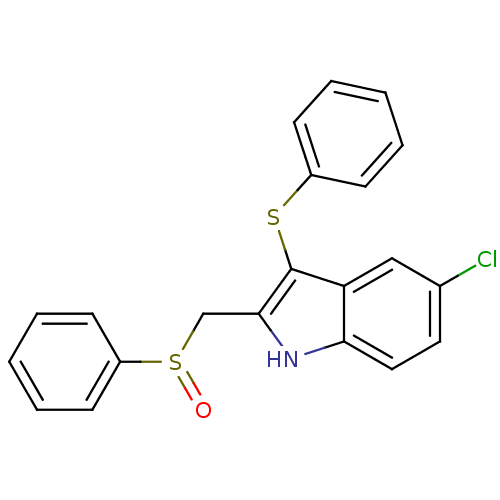 (2-[(benzenesulfinyl)methyl]-5-chloro-3-(phenylsulf...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 112 | n/a | n/a | n/a | n/a | 8.2 | n/a |
Merck Research Laboratories | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 1291-4 (1993) Article DOI: 10.1021/jm00061a022 BindingDB Entry DOI: 10.7270/Q2RV0KWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027,K690N]/[588-1147,K690N] (Human immunodeficiency virus type 1) | BDBM2278 (3-(benzenesulfonyl)-5-chloro-1H-indole-2-carboxami...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 116 | n/a | n/a | n/a | n/a | 8.2 | n/a |
Merck Research Laboratories | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 1291-4 (1993) Article DOI: 10.1021/jm00061a022 BindingDB Entry DOI: 10.7270/Q2RV0KWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Long-chain fatty acid transport protein 4 (Homo sapiens (Human)) | BDBM50186041 ((trans)-4-ethylcyclohexyl 6-methyl-4-(4-nitropheny...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human FATP4-mediated 12-BODIPY-lauric acid uptake in HEK293 cells | Bioorg Med Chem Lett 16: 3504-9 (2006) Article DOI: 10.1016/j.bmcl.2006.03.102 BindingDB Entry DOI: 10.7270/Q2K9373C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-1 (Homo sapiens (Human)) | BDBM50097413 (CHEMBL3586473) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States. Curated by ChEMBL | Assay Description Binding affinity to GABAA alpha-1 benzodiazepine binding site (unknown origin) | ACS Med Chem Lett 6: 630-4 (2015) Article DOI: 10.1021/ml500409n BindingDB Entry DOI: 10.7270/Q2WS8W1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2283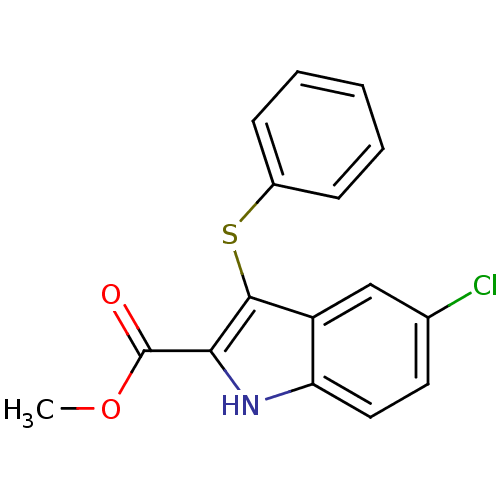 (5Cl3PhS-2IndolCONH2 inhibitor 9b | methyl 5-chloro...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 156 | n/a | n/a | n/a | n/a | 8.2 | n/a |
Merck Research Laboratories | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 1291-4 (1993) Article DOI: 10.1021/jm00061a022 BindingDB Entry DOI: 10.7270/Q2RV0KWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase A (Homo sapiens (Human)) | BDBM50097414 (CHEMBL3586468) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States. Curated by ChEMBL | Assay Description Inhibition of aurora kinase A autophosphorylation at T288 in human HCT116 cells by immunofluorescence analysis | ACS Med Chem Lett 6: 630-4 (2015) Article DOI: 10.1021/ml500409n BindingDB Entry DOI: 10.7270/Q2WS8W1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aurora kinase B (Mus musculus) | BDBM31093 (4-[[7-[2,6-bis(fluoranyl)phenyl]-9-chloranyl-5H-py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 172 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals Inc. Curated by ChEMBL | Assay Description Inhibition of mouse recombinant Aurora B kinase expressed in insect Sf9 cells by radioactive flashplate assay | Proc Natl Acad Sci USA 104: 4106-11 (2007) Article DOI: 10.1073/pnas.0608798104 BindingDB Entry DOI: 10.7270/Q25B03CS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Long-chain fatty acid transport protein 4 (Homo sapiens (Human)) | BDBM50185996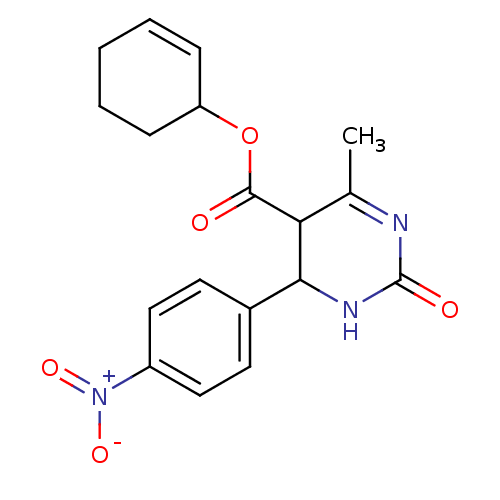 (CHEMBL426323 | cyclohex-2-enyl 6-methyl-4-(4-nitro...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human FATP4-mediated 12-BODIPY-lauric acid uptake in HEK293 cells | Bioorg Med Chem Lett 16: 3504-9 (2006) Article DOI: 10.1016/j.bmcl.2006.03.102 BindingDB Entry DOI: 10.7270/Q2K9373C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Long-chain fatty acid transport protein 4 (Homo sapiens (Human)) | BDBM50186030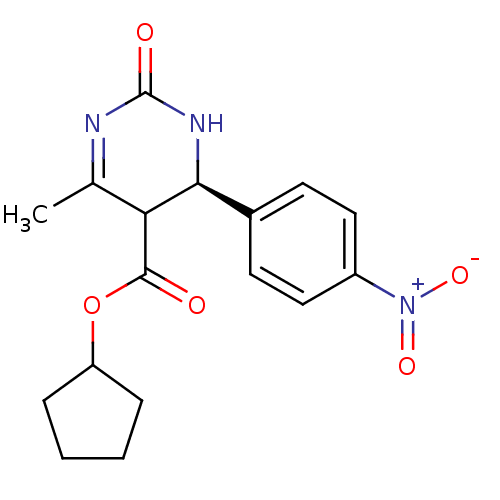 ((S)-cyclopentyl 6-methyl-4-(4-nitrophenyl)-2-oxo-1...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human FATP4-mediated 12-BODIPY-lauric acid uptake in HEK293 cells | Bioorg Med Chem Lett 16: 3504-9 (2006) Article DOI: 10.1016/j.bmcl.2006.03.102 BindingDB Entry DOI: 10.7270/Q2K9373C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Long-chain fatty acid transport protein 4 (Homo sapiens (Human)) | BDBM50186031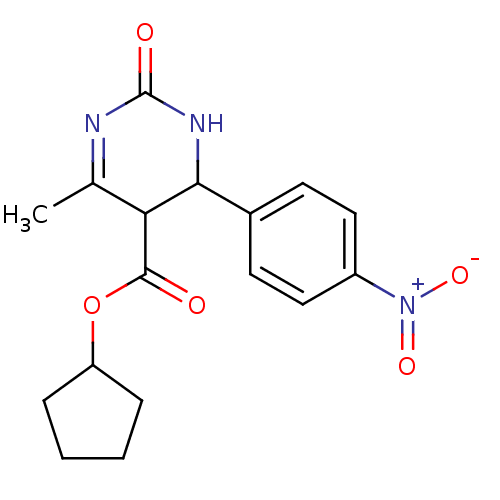 (CHEMBL211864 | cyclopentyl 6-methyl-4-(4-nitrophen...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human FATP4-mediated 12-BODIPY-lauric acid uptake in HEK293 cells | Bioorg Med Chem Lett 16: 3504-9 (2006) Article DOI: 10.1016/j.bmcl.2006.03.102 BindingDB Entry DOI: 10.7270/Q2K9373C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Long-chain fatty acid transport protein 4 (Homo sapiens (Human)) | BDBM50186028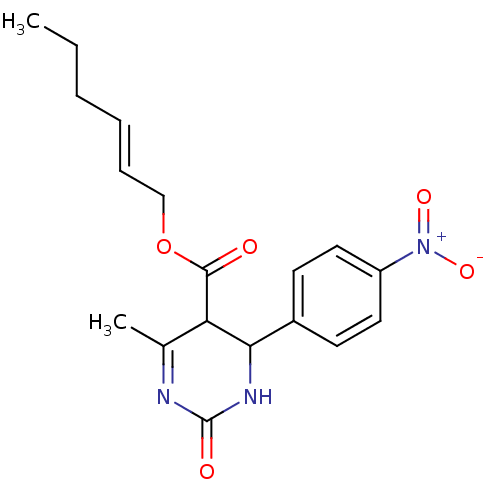 ((E)-hex-2-enyl 6-methyl-4-(4-nitrophenyl)-2-oxo-1,...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human FATP4-mediated 12-BODIPY-lauric acid uptake in HEK293 cells | Bioorg Med Chem Lett 16: 3504-9 (2006) Article DOI: 10.1016/j.bmcl.2006.03.102 BindingDB Entry DOI: 10.7270/Q2K9373C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM1868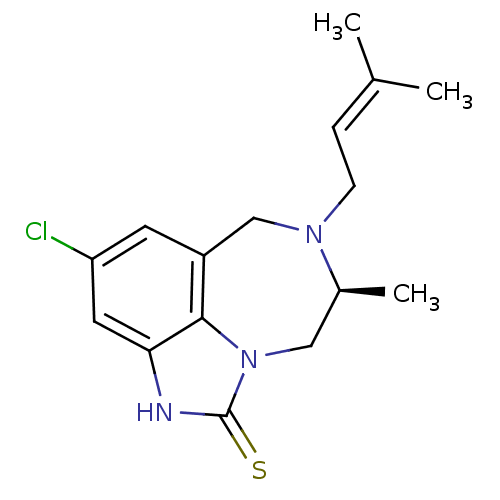 ((+)-(5S)-4,5,6,7-tetrahydro-9-chloro 5-methyl-6-(3...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | MMDB PDB Article PubMed | n/a | n/a | 311 | n/a | n/a | n/a | n/a | 8.2 | n/a |
Merck Research Laboratories | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 1291-4 (1993) Article DOI: 10.1021/jm00061a022 BindingDB Entry DOI: 10.7270/Q2RV0KWR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-1 (Homo sapiens (Human)) | BDBM31093 (4-[[7-[2,6-bis(fluoranyl)phenyl]-9-chloranyl-5H-py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States. Curated by ChEMBL | Assay Description Binding affinity to GABAA alpha-1 benzodiazepine binding site (unknown origin) | ACS Med Chem Lett 6: 630-4 (2015) Article DOI: 10.1021/ml500409n BindingDB Entry DOI: 10.7270/Q2WS8W1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-1 (Homo sapiens (Human)) | BDBM50277545 (4-(9-chloro-7-(2-fluoro-6-methoxyphenyl)-5H-benzo[...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda Pharmaceuticals International Co. , 40 Landsdowne Street, Cambridge, Massachusetts 02139, United States. Curated by ChEMBL | Assay Description Binding affinity to GABAA alpha-1 benzodiazepine binding site (unknown origin) | ACS Med Chem Lett 6: 630-4 (2015) Article DOI: 10.1021/ml500409n BindingDB Entry DOI: 10.7270/Q2WS8W1V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Long-chain fatty acid transport protein 4 (Homo sapiens (Human)) | BDBM50186035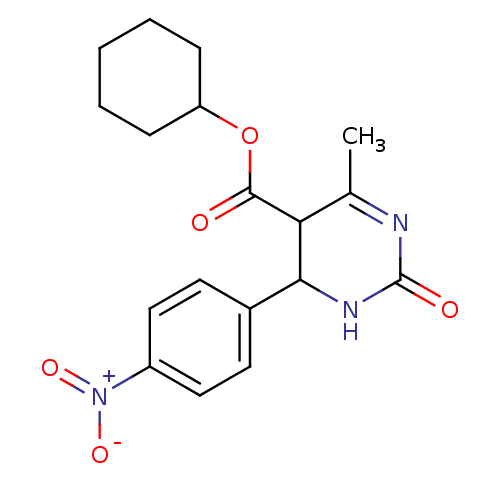 (CHEMBL437303 | cyclohexyl 6-methyl-4-(4-nitropheny...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human FATP4-mediated 12-BODIPY-lauric acid uptake in HEK293 cells | Bioorg Med Chem Lett 16: 3504-9 (2006) Article DOI: 10.1016/j.bmcl.2006.03.102 BindingDB Entry DOI: 10.7270/Q2K9373C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027,K690N]/[588-1147,K690N] (Human immunodeficiency virus type 1) | BDBM1317 (3-[[(4,7-Dichlorobenzoxazol-2-yl)-methyl]amino]-5-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 545 | n/a | n/a | n/a | n/a | 8.2 | n/a |
Merck Research Laboratories | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 1291-4 (1993) Article DOI: 10.1021/jm00061a022 BindingDB Entry DOI: 10.7270/Q2RV0KWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Long-chain fatty acid transport protein 4 (Homo sapiens (Human)) | BDBM50186009 ((S)-cyclopentyl 6-methyl-2-oxo-4-(4-(trifluorometh...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human FATP4-mediated 12-BODIPY-lauric acid uptake in HEK293 cells | Bioorg Med Chem Lett 16: 3504-9 (2006) Article DOI: 10.1016/j.bmcl.2006.03.102 BindingDB Entry DOI: 10.7270/Q2K9373C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Long-chain fatty acid transport protein 4 (Homo sapiens (Human)) | BDBM50186034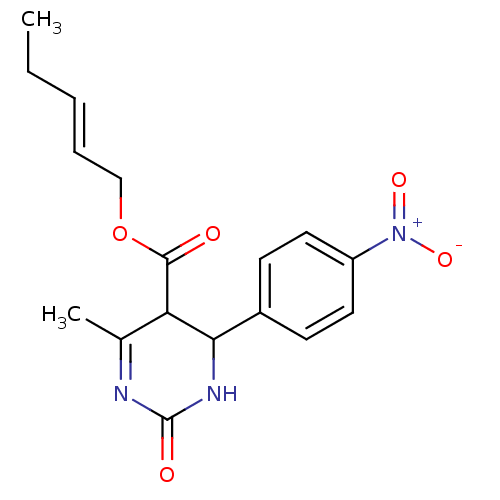 ((E)-pent-2-enyl 6-methyl-4-(4-nitrophenyl)-2-oxo-1...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 640 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human FATP4-mediated 12-BODIPY-lauric acid uptake in HEK293 cells | Bioorg Med Chem Lett 16: 3504-9 (2006) Article DOI: 10.1016/j.bmcl.2006.03.102 BindingDB Entry DOI: 10.7270/Q2K9373C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Long-chain fatty acid transport protein 4 (Homo sapiens (Human)) | BDBM50186032 ((E)-but-2-enyl 6-methyl-4-(4-nitrophenyl)-2-oxo-1,...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human FATP4-mediated 12-BODIPY-lauric acid uptake in HEK293 cells | Bioorg Med Chem Lett 16: 3504-9 (2006) Article DOI: 10.1016/j.bmcl.2006.03.102 BindingDB Entry DOI: 10.7270/Q2K9373C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Long-chain fatty acid transport protein 4 (Homo sapiens (Human)) | BDBM50186019 ((Z)-pent-2-enyl 6-methyl-4-(4-nitrophenyl)-2-oxo-1...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human FATP4-mediated 12-BODIPY-lauric acid uptake in HEK293 cells | Bioorg Med Chem Lett 16: 3504-9 (2006) Article DOI: 10.1016/j.bmcl.2006.03.102 BindingDB Entry DOI: 10.7270/Q2K9373C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2286 (5Cl3PhS-2IndolCONH2 inhibitor 12 | N-methyl-3-(phe...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 746 | n/a | n/a | n/a | n/a | 8.2 | n/a |
Merck Research Laboratories | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 1291-4 (1993) Article DOI: 10.1021/jm00061a022 BindingDB Entry DOI: 10.7270/Q2RV0KWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Long-chain fatty acid transport protein 4 (Homo sapiens (Human)) | BDBM50186010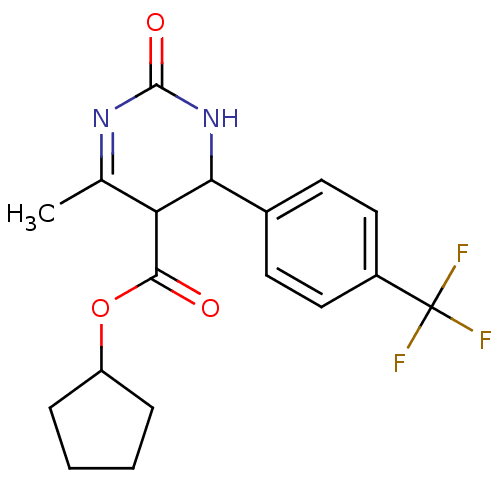 (CHEMBL211656 | cyclopentyl 6-methyl-2-oxo-4-(4-(tr...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human FATP4-mediated 12-BODIPY-lauric acid uptake in HEK293 cells | Bioorg Med Chem Lett 16: 3504-9 (2006) Article DOI: 10.1016/j.bmcl.2006.03.102 BindingDB Entry DOI: 10.7270/Q2K9373C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Long-chain fatty acid transport protein 4 (Homo sapiens (Human)) | BDBM50186014 (CHEMBL208649 | cyclopentyl 4-(4-chlorophenyl)-6-me...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human FATP4-mediated 12-BODIPY-lauric acid uptake in HEK293 cells | Bioorg Med Chem Lett 16: 3504-9 (2006) Article DOI: 10.1016/j.bmcl.2006.03.102 BindingDB Entry DOI: 10.7270/Q2K9373C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Long-chain fatty acid transport protein 4 (Homo sapiens (Human)) | BDBM50186002 (CHEMBL211182 | cyclopentyl 4-(4-bromophenyl)-6-met...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human FATP4-mediated 12-BODIPY-lauric acid uptake in HEK293 cells | Bioorg Med Chem Lett 16: 3504-9 (2006) Article DOI: 10.1016/j.bmcl.2006.03.102 BindingDB Entry DOI: 10.7270/Q2K9373C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1127,Y768C]/[588-1147,Y768C] (Human immunodeficiency virus type 1) | BDBM2284 (5-chloro-3-(phenylsulfanyl)-1H-indole-2-carboxamid...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.04E+3 | n/a | n/a | n/a | n/a | 8.2 | n/a |
Merck Research Laboratories | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 1291-4 (1993) Article DOI: 10.1021/jm00061a022 BindingDB Entry DOI: 10.7270/Q2RV0KWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Long-chain fatty acid transport protein 4 (Homo sapiens (Human)) | BDBM50186029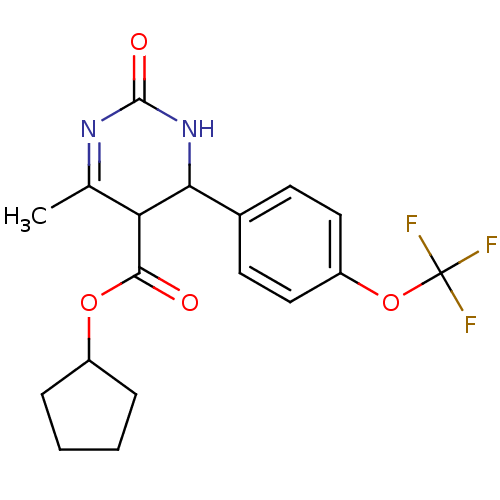 (CHEMBL212361 | cyclopentyl 6-methyl-2-oxo-4-(4-(tr...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human FATP4-mediated 12-BODIPY-lauric acid uptake in HEK293 cells | Bioorg Med Chem Lett 16: 3504-9 (2006) Article DOI: 10.1016/j.bmcl.2006.03.102 BindingDB Entry DOI: 10.7270/Q2K9373C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Long-chain fatty acid transport protein 4 (Homo sapiens (Human)) | BDBM50186036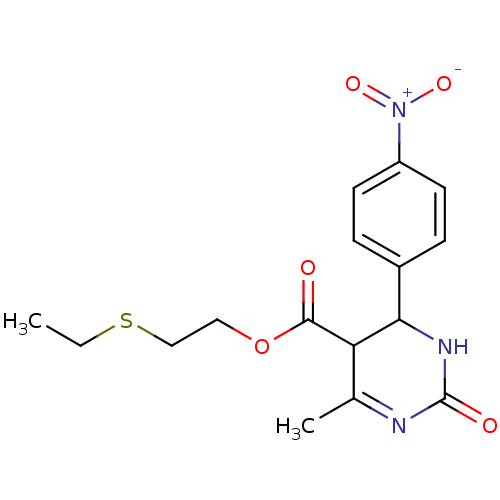 (2-(ethylthio)ethyl 6-methyl-4-(4-nitrophenyl)-2-ox...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human FATP4-mediated 12-BODIPY-lauric acid uptake in HEK293 cells | Bioorg Med Chem Lett 16: 3504-9 (2006) Article DOI: 10.1016/j.bmcl.2006.03.102 BindingDB Entry DOI: 10.7270/Q2K9373C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Long-chain fatty acid transport protein 4 (Homo sapiens (Human)) | BDBM50186001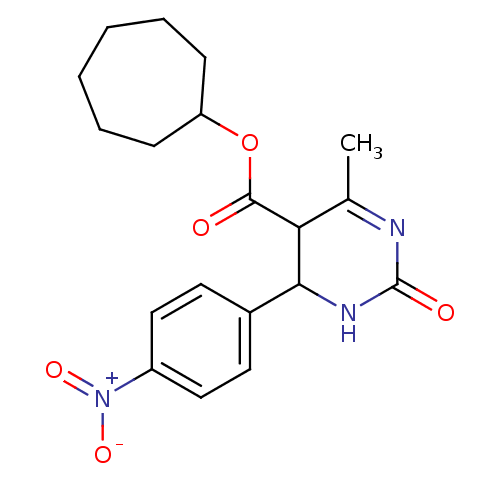 (CHEMBL436931 | cycloheptyl 6-methyl-4-(4-nitrophen...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human FATP4-mediated 12-BODIPY-lauric acid uptake in HEK293 cells | Bioorg Med Chem Lett 16: 3504-9 (2006) Article DOI: 10.1016/j.bmcl.2006.03.102 BindingDB Entry DOI: 10.7270/Q2K9373C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Long-chain fatty acid transport protein 4 (Homo sapiens (Human)) | BDBM50186017 (CHEMBL211547 | cyclobutyl 6-methyl-4-(4-nitropheny...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Millennium Pharmaceuticals, Inc. Curated by ChEMBL | Assay Description Inhibition of human FATP4-mediated 12-BODIPY-lauric acid uptake in HEK293 cells | Bioorg Med Chem Lett 16: 3504-9 (2006) Article DOI: 10.1016/j.bmcl.2006.03.102 BindingDB Entry DOI: 10.7270/Q2K9373C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM1437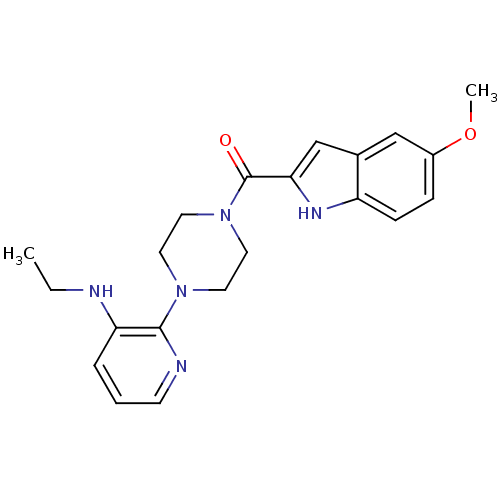 (5-methoxyindole-2-carboxylic acid [N -[3-(aminoeth...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.32E+3 | n/a | n/a | n/a | n/a | 8.2 | n/a |
Merck Research Laboratories | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 36: 1291-4 (1993) Article DOI: 10.1021/jm00061a022 BindingDB Entry DOI: 10.7270/Q2RV0KWR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 108 total ) | Next | Last >> |