 Found 235 hits with Last Name = 'balasubramanian' and Initial = 's'
Found 235 hits with Last Name = 'balasubramanian' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM24621
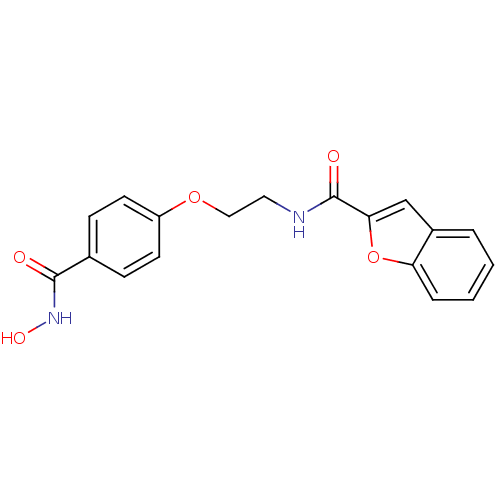
(CG-003 | N-{2-[4-(hydroxycarbamoyl)phenoxy]ethyl}-...)Show InChI InChI=1S/C18H16N2O5/c21-17(20-23)12-5-7-14(8-6-12)24-10-9-19-18(22)16-11-13-3-1-2-4-15(13)25-16/h1-8,11,23H,9-10H2,(H,19,22)(H,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 4 | -47.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics
| Assay Description
HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... |
Mol Cancer Ther 5: 1309-17 (2006)
Article DOI: 10.1158/1535-7163.MCT-05-0442
BindingDB Entry DOI: 10.7270/Q2DF6PJJ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM24620
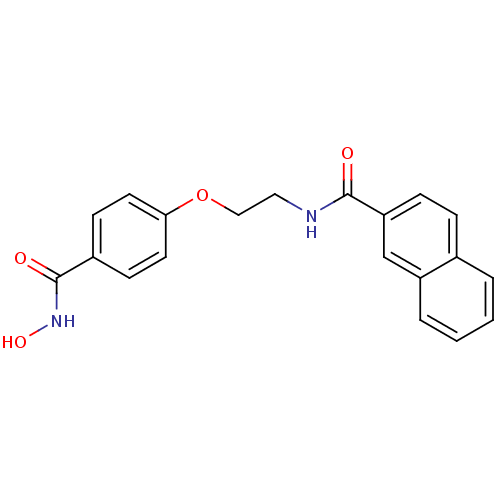
(CG-002 | N-{2-[4-(hydroxycarbamoyl)phenoxy]ethyl}n...)Show InChI InChI=1S/C20H18N2O4/c23-19(17-6-5-14-3-1-2-4-16(14)13-17)21-11-12-26-18-9-7-15(8-10-18)20(24)22-25/h1-10,13,25H,11-12H2,(H,21,23)(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | -46.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics
| Assay Description
HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... |
Mol Cancer Ther 5: 1309-17 (2006)
Article DOI: 10.1158/1535-7163.MCT-05-0442
BindingDB Entry DOI: 10.7270/Q2DF6PJJ |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM424912
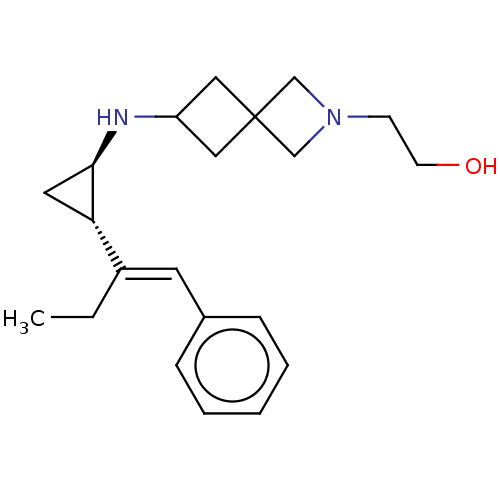
(2-(6-(((1R,2S)-2-((E)-1-phenylbut-1-en-2-yl)cyclop...)Show SMILES CC\C(=C/c1ccccc1)[C@@H]1C[C@H]1NC1CC2(C1)CN(CCO)C2 |r| Show InChI InChI=1S/C21H30N2O/c1-2-17(10-16-6-4-3-5-7-16)19-11-20(19)22-18-12-21(13-18)14-23(15-21)8-9-24/h3-7,10,18-20,22,24H,2,8-9,11-15H2,1H3/b17-10+/t19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Time dependant inhibition of recombinant N-terminal His-tagged LSD1 (unknown origin) expressed in Escherichia coli expression system assessed as inhi... |
ACS Med Chem Lett 11: 1213-1220 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00060
BindingDB Entry DOI: 10.7270/Q22N55VZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM24622

(3-[(dimethylamino)methyl]-N-{2-[4-(hydroxycarbamoy...)Show SMILES CN(C)Cc1c(oc2ccccc12)C(=O)NCCOc1ccc(cc1)C(=O)NO Show InChI InChI=1S/C21H23N3O5/c1-24(2)13-17-16-5-3-4-6-18(16)29-19(17)21(26)22-11-12-28-15-9-7-14(8-10-15)20(25)23-27/h3-10,27H,11-13H2,1-2H3,(H,22,26)(H,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7 | -46.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics
| Assay Description
HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... |
Mol Cancer Ther 5: 1309-17 (2006)
Article DOI: 10.1158/1535-7163.MCT-05-0442
BindingDB Entry DOI: 10.7270/Q2DF6PJJ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM24622

(3-[(dimethylamino)methyl]-N-{2-[4-(hydroxycarbamoy...)Show SMILES CN(C)Cc1c(oc2ccccc12)C(=O)NCCOc1ccc(cc1)C(=O)NO Show InChI InChI=1S/C21H23N3O5/c1-24(2)13-17-16-5-3-4-6-18(16)29-19(17)21(26)22-11-12-28-15-9-7-14(8-10-15)20(25)23-27/h3-10,27H,11-13H2,1-2H3,(H,22,26)(H,23,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.20 | -45.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics
| Assay Description
HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... |
Mol Cancer Ther 5: 1309-17 (2006)
Article DOI: 10.1158/1535-7163.MCT-05-0442
BindingDB Entry DOI: 10.7270/Q2DF6PJJ |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50158869
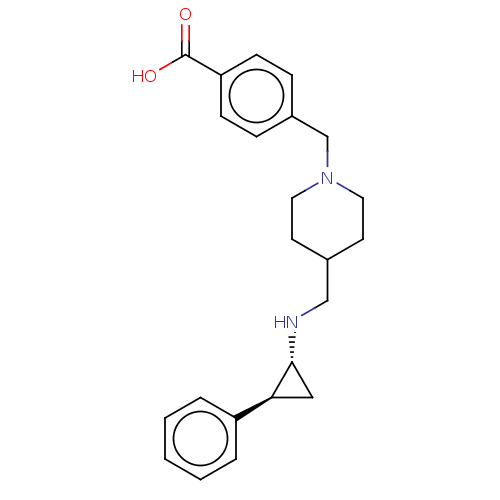
(CHEMBL3786182 | US10836743, Compound GSK-2879552 |...)Show SMILES OC(=O)c1ccc(CN2CCC(CN[C@@H]3C[C@H]3c3ccccc3)CC2)cc1 |r| Show InChI InChI=1S/C23H28N2O2/c26-23(27)20-8-6-18(7-9-20)16-25-12-10-17(11-13-25)15-24-22-14-21(22)19-4-2-1-3-5-19/h1-9,17,21-22,24H,10-16H2,(H,26,27)/t21-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Time dependant inhibition of recombinant N-terminal His-tagged LSD1 (unknown origin) expressed in Escherichia coli expression system assessed as inhi... |
ACS Med Chem Lett 11: 1213-1220 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00060
BindingDB Entry DOI: 10.7270/Q22N55VZ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM24622

(3-[(dimethylamino)methyl]-N-{2-[4-(hydroxycarbamoy...)Show SMILES CN(C)Cc1c(oc2ccccc12)C(=O)NCCOc1ccc(cc1)C(=O)NO Show InChI InChI=1S/C21H23N3O5/c1-24(2)13-17-16-5-3-4-6-18(16)29-19(17)21(26)22-11-12-28-15-9-7-14(8-10-15)20(25)23-27/h3-10,27H,11-13H2,1-2H3,(H,22,26)(H,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 17 | -43.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics
| Assay Description
HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... |
Mol Cancer Ther 5: 1309-17 (2006)
Article DOI: 10.1158/1535-7163.MCT-05-0442
BindingDB Entry DOI: 10.7270/Q2DF6PJJ |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM24622

(3-[(dimethylamino)methyl]-N-{2-[4-(hydroxycarbamoy...)Show SMILES CN(C)Cc1c(oc2ccccc12)C(=O)NCCOc1ccc(cc1)C(=O)NO Show InChI InChI=1S/C21H23N3O5/c1-24(2)13-17-16-5-3-4-6-18(16)29-19(17)21(26)22-11-12-28-15-9-7-14(8-10-15)20(25)23-27/h3-10,27H,11-13H2,1-2H3,(H,22,26)(H,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 19 | -43.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics
| Assay Description
HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... |
Mol Cancer Ther 5: 1309-17 (2006)
Article DOI: 10.1158/1535-7163.MCT-05-0442
BindingDB Entry DOI: 10.7270/Q2DF6PJJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM468103
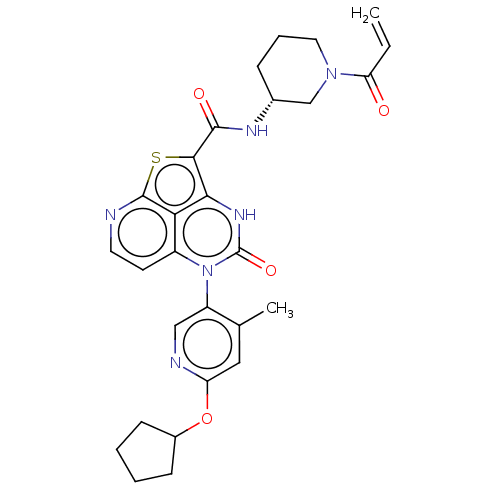
((R)-N-(1-Acryloylpiperidin-3-yl)-5-(*S)-(6-(cyclop...)Show SMILES Cc1cc(OC2CCCC2)ncc1-n1c2ccnc3sc(C(=O)N[C@@H]4CCCN(C4)C(=O)C=C)c([nH]c1=O)c23 |r,wD:24.25,(-1.92,5.75,;-3.25,4.98,;-4.59,5.75,;-5.92,4.98,;-7.25,5.75,;-8.59,4.98,;-9.99,5.6,;-11.02,4.46,;-10.25,3.13,;-8.75,3.45,;-5.92,3.44,;-4.59,2.67,;-3.25,3.44,;-1.92,2.67,;-1.92,1.13,;-3.25,.36,;-3.25,-1.18,;-1.92,-1.95,;-.59,-1.18,;.88,-1.66,;1.78,-.41,;3.32,-.41,;4.09,.92,;4.09,-1.75,;5.63,-1.75,;6.4,-.41,;7.94,-.41,;8.71,-1.75,;7.94,-3.08,;6.4,-3.08,;8.71,-4.41,;7.94,-5.75,;10.25,-4.41,;11.02,-5.75,;.75,1.13,;.75,2.67,;-.59,3.44,;-.59,4.98,;-.59,.36,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01026
BindingDB Entry DOI: 10.7270/Q2MK6HZ3 |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM24622

(3-[(dimethylamino)methyl]-N-{2-[4-(hydroxycarbamoy...)Show SMILES CN(C)Cc1c(oc2ccccc12)C(=O)NCCOc1ccc(cc1)C(=O)NO Show InChI InChI=1S/C21H23N3O5/c1-24(2)13-17-16-5-3-4-6-18(16)29-19(17)21(26)22-11-12-28-15-9-7-14(8-10-15)20(25)23-27/h3-10,27H,11-13H2,1-2H3,(H,22,26)(H,23,25) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 24 | -43.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics
| Assay Description
HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... |
Mol Cancer Ther 5: 1309-17 (2006)
Article DOI: 10.1158/1535-7163.MCT-05-0442
BindingDB Entry DOI: 10.7270/Q2DF6PJJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM468000
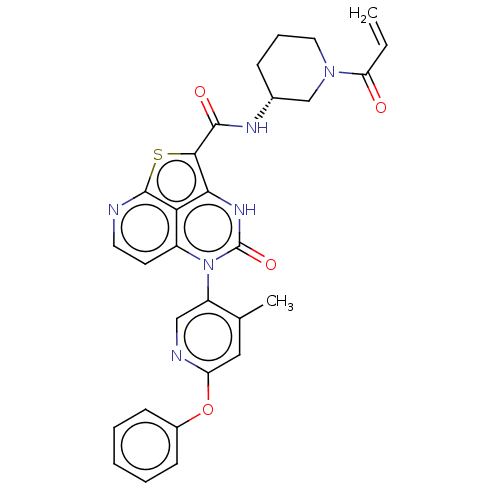
((R)-N-(1-Acryloylpiperidin-3-yl)-5-(*S)-(4-methyl-...)Show SMILES Cc1cc(Oc2ccccc2)ncc1-n1c2ccnc3sc(C(=O)N[C@@H]4CCCN(C4)C(=O)C=C)c([nH]c1=O)c23 |r,wU:25.26,(-4.48,-.77,;-4.48,.77,;-5.82,1.54,;-5.82,3.08,;-7.15,3.85,;-8.48,3.08,;-9.82,3.85,;-11.15,3.08,;-11.15,1.54,;-9.82,.77,;-8.48,1.54,;-4.48,3.85,;-3.15,3.08,;-3.15,1.54,;-1.82,.77,;-1.82,-.77,;-3.15,-1.54,;-3.15,-3.08,;-1.82,-3.85,;-.48,-3.08,;1.01,-3.48,;1.94,-1.86,;3.48,-1.86,;4.25,-3.19,;4.25,-.53,;5.79,-.53,;6.56,-1.86,;8.1,-1.86,;8.87,-.53,;8.1,.81,;6.56,.81,;8.87,2.14,;8.1,3.48,;10.41,2.14,;11.15,3.43,;.85,-.77,;.85,.77,;-.48,1.54,;-.48,3.08,;-.48,-1.54,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01026
BindingDB Entry DOI: 10.7270/Q2MK6HZ3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM467718
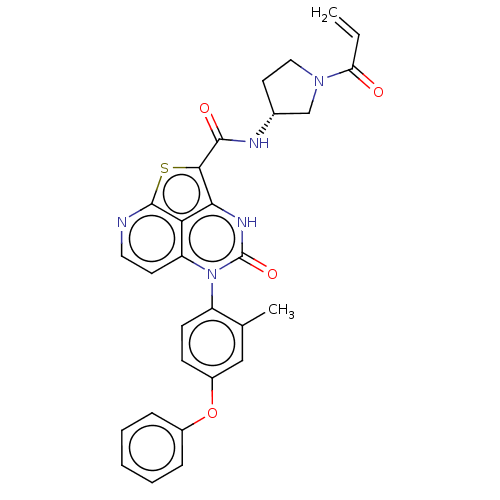
((R)-N-(1-Acryloylpyrrolidin-3-yl)-5-(2-methyl-4-(2...)Show SMILES Cc1cc(Oc2ccccc2)ccc1-n1c2ccnc3sc(C(=O)N[C@@H]4CCN(C4)C(=O)C=C)c([nH]c1=O)c23 |r,wD:25.26,(-1,4.5,;-2.28,3.77,;-3.62,4.54,;-4.95,3.77,;-6.28,4.54,;-7.62,3.77,;-8.95,4.54,;-10.28,3.77,;-10.28,2.23,;-8.95,1.46,;-7.62,2.23,;-4.95,2.23,;-3.62,1.46,;-2.28,2.23,;-.95,1.46,;-.95,-.08,;-2.28,-.85,;-2.28,-2.39,;-.95,-3.16,;.39,-2.39,;1.85,-2.87,;2.76,-1.62,;4.29,-1.65,;5.09,-.33,;5.04,-3,;6.58,-3,;7.49,-1.75,;8.95,-2.23,;8.95,-3.77,;7.49,-4.24,;10.28,-4.54,;10.28,-6.08,;11.62,-3.77,;11.62,-2.23,;1.72,-.08,;1.72,1.46,;.39,2.23,;.39,3.77,;.39,-.85,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01026
BindingDB Entry DOI: 10.7270/Q2MK6HZ3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM467367
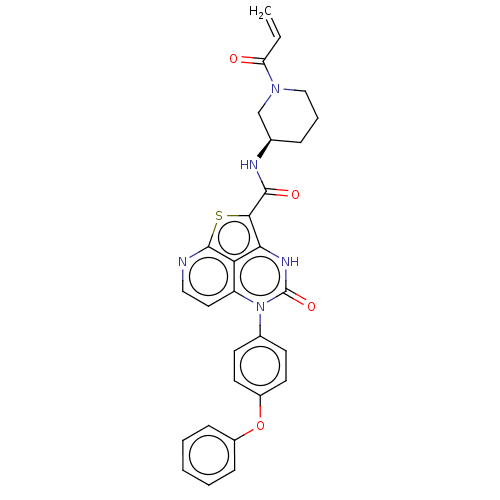
((R)-N-(1-Acryloylpiperidin-3-yl)-4-oxo-5-(4-phenox...)Show SMILES C=CC(=O)N1CCC[C@H](C1)NC(=O)c1sc2nccc3n(-c4ccc(Oc5ccccc5)cc4)c(=O)[nH]c1c23 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01026
BindingDB Entry DOI: 10.7270/Q2MK6HZ3 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM24618
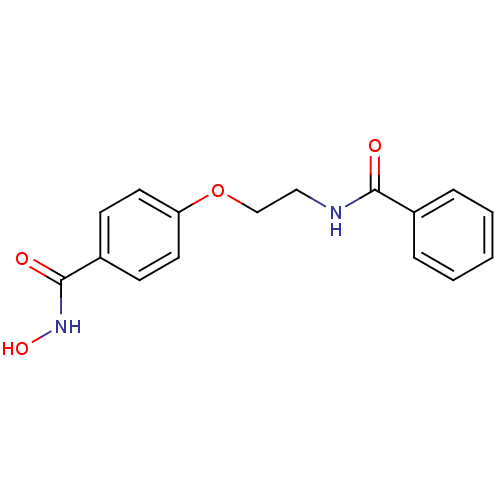
(CG-001 | N-hydroxy-4-[2-(phenylformamido)ethoxy]be...)Show InChI InChI=1S/C16H16N2O4/c19-15(12-4-2-1-3-5-12)17-10-11-22-14-8-6-13(7-9-14)16(20)18-21/h1-9,21H,10-11H2,(H,17,19)(H,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 43 | -41.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics
| Assay Description
HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... |
Mol Cancer Ther 5: 1309-17 (2006)
Article DOI: 10.1158/1535-7163.MCT-05-0442
BindingDB Entry DOI: 10.7270/Q2DF6PJJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50601991
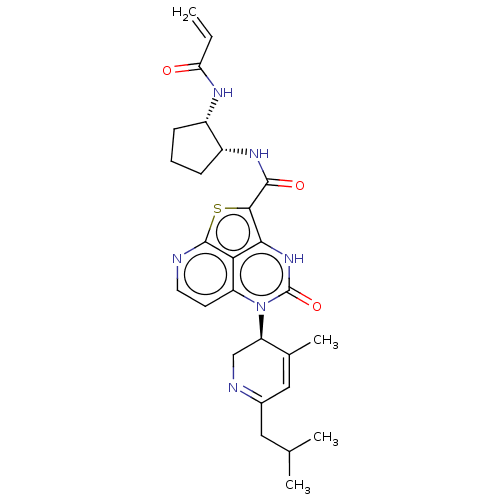
(CHEMBL5206366)Show SMILES CC(C)CC1=NC[C@H](C(C)=C1)n1c2ccnc3sc(C(=O)N[C@@H]4CCC[C@@H]4NC(=O)C=C)c([nH]c1=O)c23 |r,c:9,t:4| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01026
BindingDB Entry DOI: 10.7270/Q2MK6HZ3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM468010

((R)-N-(1-Acryloylpiperidin-3-yl)-5-(6-cyclobutoxy-...)Show SMILES Cc1cc(OC2CCC2)ncc1-n1c2ccnc3sc(C(=O)N[C@@H]4CCCN(C4)C(=O)C=C)c([nH]c1=O)c23 |r,wD:23.24,(-1.8,6.31,;-3.13,5.54,;-4.47,6.31,;-5.8,5.54,;-7.14,6.31,;-8.47,5.54,;-9.96,5.93,;-10.36,4.45,;-8.87,4.05,;-5.8,4,;-4.47,3.23,;-3.13,4,;-1.8,3.23,;-1.8,1.69,;-3.13,.92,;-3.13,-.62,;-1.8,-1.39,;-.47,-.62,;1.14,-.89,;1.88,.36,;3.42,.36,;4.19,1.7,;4.19,-.97,;5.73,-.97,;6.5,.36,;8.04,.36,;8.82,-.97,;8.04,-2.3,;6.5,-2.3,;8.82,-3.64,;10.36,-3.64,;8.04,-4.97,;8.82,-6.31,;.87,1.69,;.87,3.23,;-.47,4,;-.47,5.54,;-.47,.92,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01026
BindingDB Entry DOI: 10.7270/Q2MK6HZ3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM467836
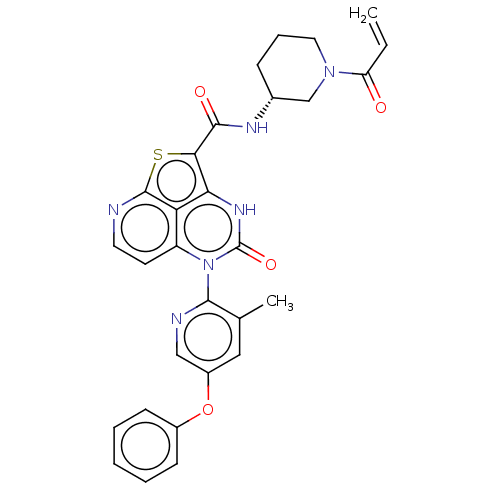
((R)-N-(1-Acryloylpiperidin-3-yl)-5-(3-methyl-5-phe...)Show SMILES Cc1cc(Oc2ccccc2)cnc1-n1c2ccnc3sc(C(=O)N[C@@H]4CCCN(C4)C(=O)C=C)c([nH]c1=O)c23 |r,wD:25.26,(-4.18,1.77,;-4.18,3.31,;-5.51,4.08,;-5.51,5.62,;-6.85,6.39,;-8.18,5.62,;-9.51,6.39,;-10.85,5.62,;-10.85,4.08,;-9.51,3.31,;-8.18,4.08,;-4.18,6.39,;-2.84,5.62,;-2.84,4.08,;-1.51,3.31,;-1.51,1.77,;-2.84,1,;-2.84,-.54,;-1.51,-1.31,;-.18,-.54,;1.16,-1.31,;2.37,.26,;3.91,.27,;4.66,1.61,;4.69,-1.06,;6.23,-1.06,;7,.28,;8.54,.28,;9.31,-1.06,;8.54,-2.39,;7,-2.39,;9.31,-3.72,;10.85,-3.72,;8.54,-5.06,;9.31,-6.39,;1.16,1.77,;1.16,3.31,;-.18,4.08,;-.18,5.62,;-.18,1,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01026
BindingDB Entry DOI: 10.7270/Q2MK6HZ3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM468007
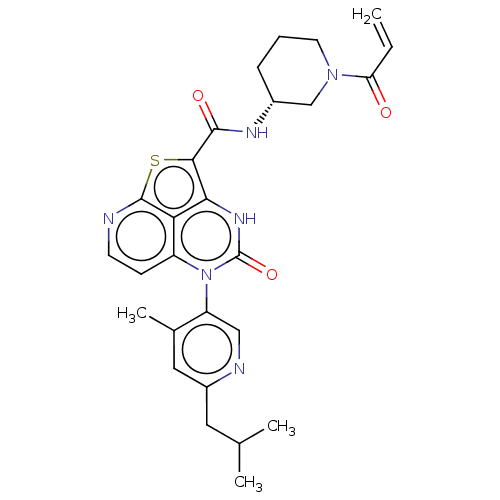
((R)-N-(1-Acryloylpiperidin-3-yl)-5-(*S)-(6-isobuty...)Show SMILES CC(C)Cc1cc(C)c(cn1)-n1c2ccnc3sc(C(=O)N[C@@H]4CCCN(C4)C(=O)C=C)c([nH]c1=O)c23 |r,wD:22.22,(-9.16,1.88,;-9.16,3.42,;-10.5,4.19,;-7.83,4.19,;-6.5,3.42,;-6.5,1.88,;-5.16,1.11,;-5.16,-.49,;-3.83,1.88,;-3.83,3.42,;-5.16,4.19,;-2.5,1.11,;-2.5,-.43,;-3.83,-1.2,;-3.83,-2.74,;-2.5,-3.51,;-1.16,-2.74,;.32,-3.14,;1.26,-1.52,;2.8,-1.52,;3.57,-2.85,;3.57,-.19,;5.11,-.19,;5.88,1.15,;7.42,1.15,;8.19,-.19,;7.42,-1.52,;5.88,-1.52,;8.19,-2.85,;7.42,-4.19,;9.73,-2.85,;10.5,-1.52,;.17,-.43,;.17,1.11,;-1.16,1.88,;-1.16,3.42,;-1.16,-1.2,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 145 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01026
BindingDB Entry DOI: 10.7270/Q2MK6HZ3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM468124
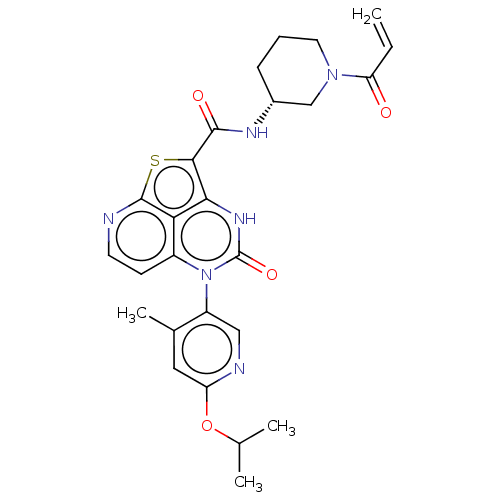
((R)-N-(1-Acryloylpiperidin-3-yl)-5-(*S)-(6-isoprop...)Show SMILES CC(C)Oc1cc(C)c(cn1)-n1c2ccnc3sc(C(=O)N[C@@H]4CCCN(C4)C(=O)C=C)c([nH]c1=O)c23 |r,wD:22.22,(-10.22,5.69,;-8.88,4.92,;-8.88,3.38,;-7.55,5.69,;-6.22,4.92,;-4.88,5.69,;-3.55,4.92,;-2.22,5.69,;-3.55,3.38,;-4.88,2.61,;-6.22,3.38,;-2.22,2.61,;-2.22,1.07,;-3.55,.3,;-3.55,-1.24,;-2.22,-2.01,;-.88,-1.24,;.49,-1.84,;1.22,-.35,;2.76,-.35,;3.53,.98,;3.53,-1.69,;4.83,-1.69,;5.6,-.35,;7.14,-.35,;7.91,-1.69,;7.14,-3.02,;5.6,-3.02,;7.91,-4.35,;7.14,-5.69,;9.45,-4.35,;10.22,-5.69,;.45,1.07,;.45,2.61,;-.88,3.38,;-.88,4.92,;-.88,.3,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 208 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01026
BindingDB Entry DOI: 10.7270/Q2MK6HZ3 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM24622

(3-[(dimethylamino)methyl]-N-{2-[4-(hydroxycarbamoy...)Show SMILES CN(C)Cc1c(oc2ccccc12)C(=O)NCCOc1ccc(cc1)C(=O)NO Show InChI InChI=1S/C21H23N3O5/c1-24(2)13-17-16-5-3-4-6-18(16)29-19(17)21(26)22-11-12-28-15-9-7-14(8-10-15)20(25)23-27/h3-10,27H,11-13H2,1-2H3,(H,22,26)(H,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 280 | -37.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics
| Assay Description
HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... |
Mol Cancer Ther 5: 1309-17 (2006)
Article DOI: 10.1158/1535-7163.MCT-05-0442
BindingDB Entry DOI: 10.7270/Q2DF6PJJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM467845

((R)-N-(1-Acryloylpiperidin-3-yl)-4-oxo-5-(6-phenox...)Show SMILES C=CC(=O)N1CCC[C@H](C1)NC(=O)c1sc2nccc3n(-c4ccc(Oc5ccccc5)nc4)c(=O)[nH]c1c23 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 354 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01026
BindingDB Entry DOI: 10.7270/Q2MK6HZ3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM467852

((R)-N-(1-Acryloylpiperidin-3-yl)-4-oxo-5-(5-phenox...)Show SMILES C=CC(=O)N1CCC[C@H](C1)NC(=O)c1sc2nccc3n(-c4ccc(Oc5ccccc5)cn4)c(=O)[nH]c1c23 |r| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 456 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01026
BindingDB Entry DOI: 10.7270/Q2MK6HZ3 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM468084

((R)-N-(1-Acryloylpyrrolidin-3-yl)-5-(*S)-(6-isobut...)Show SMILES CC(C)Cc1cc(C)c(cn1)-n1c2ccnc3sc(C(=O)N[C@@H]4CCN(C4)C(=O)C=C)c([nH]c1=O)c23 |r,wD:22.22,(-10.96,3.85,;-9.63,3.08,;-9.63,1.54,;-8.3,3.85,;-6.96,3.08,;-6.96,1.54,;-5.63,.77,;-5.63,-.81,;-4.29,1.54,;-4.29,3.08,;-5.63,3.85,;-2.96,.77,;-2.96,-.77,;-4.29,-1.54,;-4.29,-3.08,;-2.96,-3.85,;-1.63,-3.08,;-.16,-3.56,;.74,-1.95,;2.28,-1.95,;3.05,-3.28,;3.05,-.61,;4.59,-.61,;5.5,.63,;6.96,.16,;6.96,-1.38,;5.5,-1.86,;8.3,-2.15,;8.3,-3.69,;9.63,-1.38,;10.96,-2.15,;-.29,-.77,;-.29,.77,;-1.63,1.54,;-1.63,3.08,;-1.63,-1.54,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01026
BindingDB Entry DOI: 10.7270/Q2MK6HZ3 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM24623

(4-[2-({3-[(dimethylamino)methyl]-1-benzofuran-2-yl...)Show SMILES CN(C)Cc1c(oc2ccccc12)C(=O)NCCOc1ccc(cc1)C(O)=O Show InChI InChI=1S/C21H22N2O5/c1-23(2)13-17-16-5-3-4-6-18(16)28-19(17)20(24)22-11-12-27-15-9-7-14(8-10-15)21(25)26/h3-10H,11-13H2,1-2H3,(H,22,24)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >5.00E+4 | >-24.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Celera Genomics
| Assay Description
HDAC activity was measured using a continuous trypsin-coupled assay. For inhibitor characterization, measurements were done using 96-well assay plat... |
Mol Cancer Ther 5: 1309-17 (2006)
Article DOI: 10.1158/1535-7163.MCT-05-0442
BindingDB Entry DOI: 10.7270/Q2DF6PJJ |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50601990

(CHEMBL5179679)Show SMILES CC(C)Cc1cc(C)c(cn1)-n1c2ccnc3sc(C(=O)NCCNC(=O)C=C)c([nH]c1=O)c23 |(-10.11,3.45,;-8.77,2.68,;-8.77,1.14,;-7.44,3.45,;-6.11,2.68,;-6.11,1.14,;-4.78,.37,;-4.78,-1.17,;-3.44,1.14,;-3.44,2.69,;-4.78,3.45,;-2.11,.37,;-2.11,-1.17,;-3.44,-1.94,;-3.44,-3.48,;-2.11,-4.25,;-.77,-3.48,;.88,-3.59,;1.65,-2.25,;3.17,-2.05,;4.11,-3.27,;3.76,-.63,;5.29,-.43,;5.88,1,;7.4,1.2,;7.99,2.62,;6.9,3.71,;9.52,2.82,;10.11,4.25,;.56,-1.17,;.56,.37,;-.78,1.14,;-.78,2.68,;-.77,-1.94,)| | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2.43E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01026
BindingDB Entry DOI: 10.7270/Q2MK6HZ3 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50563820

(CHEMBL4779123)Show SMILES COc1cc(C)[nH]c(=O)c1CNC(=O)c1c(C)n([C@H](C)C2CCN(CC2)C(=O)OC(C)C)c2ccccc12 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of wild-type EZH2 in PRC2 complex (unknown origin) using RKQLATKAARK(Me3)SAPATGGVKKP-NH2 peptide substrate preincubated for 30 mins follow... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QZ2FQB |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50110359
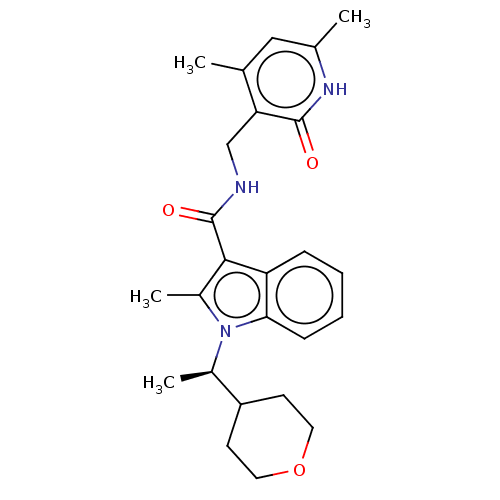
(CHEMBL3605453)Show SMILES C[C@H](C1CCOCC1)n1c(C)c(C(=O)NCc2c(C)cc(C)[nH]c2=O)c2ccccc12 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 (unknown origin) using biotinylated nucleosome, H3K27me3 activator and [3H]-SAM incubated for 60 mins by top-count based method |
Bioorg Med Chem Lett 25: 3644-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.056
BindingDB Entry DOI: 10.7270/Q2Q24205 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50563822

(CHEMBL4779426)Show SMILES COc1cc(C)[nH]c(=O)c1CNC(=O)c1c(C)n([C@H](C)C2CCN(CC2)C2COC2)c2ccccc12 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of wild-type EZH2 in PRC2 complex (unknown origin) using RKQLATKAARK(Me3)SAPATGGVKKP-NH2 peptide substrate preincubated for 30 mins follow... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QZ2FQB |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50563823

(CHEMBL4799137)Show SMILES CCOC(=O)CN1CCC(CC1)[C@@H](C)n1c(C)c(C(=O)NCc2c(OC)cc(C)[nH]c2=O)c2ccccc12 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of wild-type EZH2 in PRC2 complex (unknown origin) using RKQLATKAARK(Me3)SAPATGGVKKP-NH2 peptide substrate preincubated for 30 mins follow... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QZ2FQB |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50110357
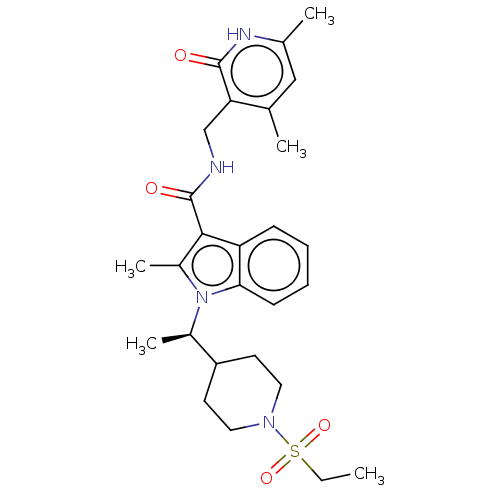
(CHEMBL3605455)Show SMILES CCS(=O)(=O)N1CCC(CC1)[C@@H](C)n1c(C)c(C(=O)NCc2c(C)cc(C)[nH]c2=O)c2ccccc12 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 (unknown origin) using biotinylated nucleosome, H3K27me3 activator and [3H]-SAM incubated for 60 mins by top-count based method |
Bioorg Med Chem Lett 25: 3644-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.056
BindingDB Entry DOI: 10.7270/Q2Q24205 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM172038
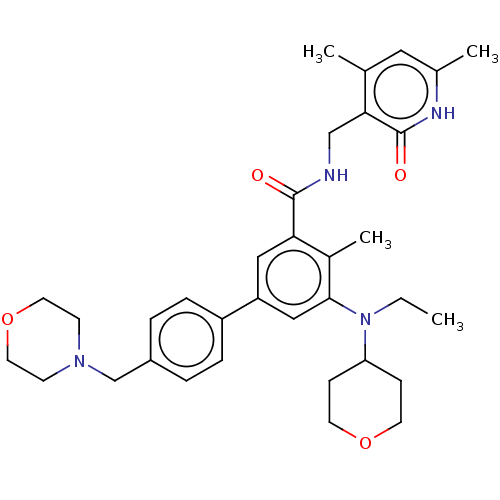
(US10155002, Compound 44 | US10647700, Compound EPZ...)Show SMILES CCN(C1CCOCC1)c1cc(cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C)-c1ccc(CN2CCOCC2)cc1 Show InChI InChI=1S/C34H44N4O4/c1-5-38(29-10-14-41-15-11-29)32-20-28(27-8-6-26(7-9-27)22-37-12-16-42-17-13-37)19-30(25(32)4)33(39)35-21-31-23(2)18-24(3)36-34(31)40/h6-9,18-20,29H,5,10-17,21-22H2,1-4H3,(H,35,39)(H,36,40) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 (unknown origin) using biotinylated nucleosome, H3K27me3 activator and [3H]-SAM incubated for 60 mins by top-count based method |
Bioorg Med Chem Lett 25: 3644-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.056
BindingDB Entry DOI: 10.7270/Q2Q24205 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50563825
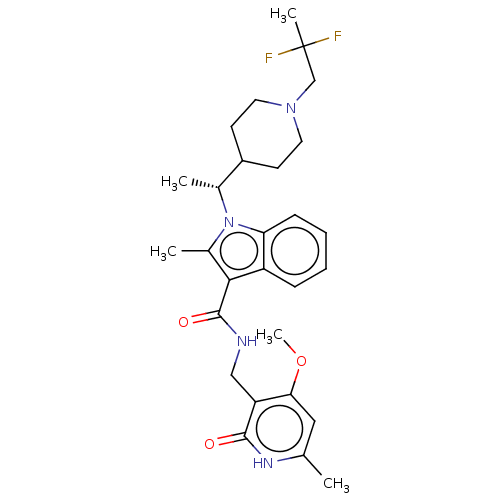
(CHEMBL4794859)Show SMILES COc1cc(C)[nH]c(=O)c1CNC(=O)c1c(C)n([C@H](C)C2CCN(CC(C)(F)F)CC2)c2ccccc12 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of wild-type EZH2 in PRC2 complex (unknown origin) using RKQLATKAARK(Me3)SAPATGGVKKP-NH2 peptide substrate preincubated for 30 mins follow... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QZ2FQB |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM172038

(US10155002, Compound 44 | US10647700, Compound EPZ...)Show SMILES CCN(C1CCOCC1)c1cc(cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C)-c1ccc(CN2CCOCC2)cc1 Show InChI InChI=1S/C34H44N4O4/c1-5-38(29-10-14-41-15-11-29)32-20-28(27-8-6-26(7-9-27)22-37-12-16-42-17-13-37)19-30(25(32)4)33(39)35-21-31-23(2)18-24(3)36-34(31)40/h6-9,18-20,29H,5,10-17,21-22H2,1-4H3,(H,35,39)(H,36,40) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 Y641N mutant (unknown origin) using biotinylated nucleosome, H3K27me3 activator and [3H]-SAM incubated for 60 mins by top-count ba... |
Bioorg Med Chem Lett 25: 3644-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.056
BindingDB Entry DOI: 10.7270/Q2Q24205 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50563818

(CHEMBL4781657)Show SMILES COc1cc(C)[nH]c(=O)c1CNC(=O)c1c(C)n([C@H](C)C2CCN(C)CC2)c2ccccc12 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of wild-type EZH2 in PRC2 complex (unknown origin) using RKQLATKAARK(Me3)SAPATGGVKKP-NH2 peptide substrate preincubated for 30 mins follow... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QZ2FQB |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50110355
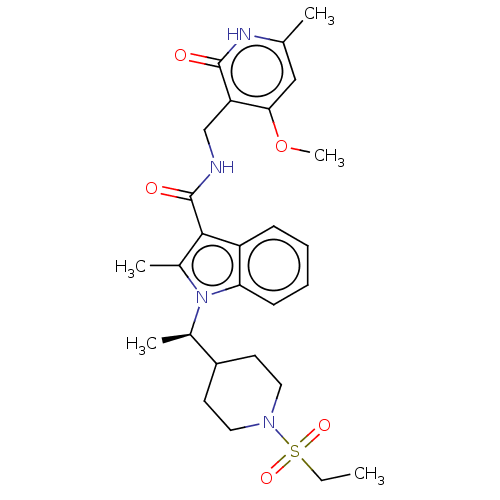
(CHEMBL3605457)Show SMILES CCS(=O)(=O)N1CCC(CC1)[C@@H](C)n1c(C)c(C(=O)NCc2c(OC)cc(C)[nH]c2=O)c2ccccc12 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of wild-type EZH2 in PRC2 complex (unknown origin) using RKQLATKAARK(Me3)SAPATGGVKKP-NH2 peptide substrate preincubated for 30 mins follow... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QZ2FQB |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50563825

(CHEMBL4794859)Show SMILES COc1cc(C)[nH]c(=O)c1CNC(=O)c1c(C)n([C@H](C)C2CCN(CC(C)(F)F)CC2)c2ccccc12 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EZH2 Y641F mutant in PRC2 complex (unknown origin) using RKQLATKAARK(Me3)SAPATGGVKKP-NH2 peptide substrate preincubated for 30 mins fol... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QZ2FQB |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50017293

(CHEMBL3287735 | US10647700, Compound GSK126)Show SMILES CC[C@H](C)n1cc(C)c2c(cc(cc12)-c1ccc(nc1)N1CCNCC1)C(=O)NCc1c(C)cc(C)[nH]c1=O |r| Show InChI InChI=1S/C31H38N6O2/c1-6-22(5)37-18-20(3)29-25(30(38)34-17-26-19(2)13-21(4)35-31(26)39)14-24(15-27(29)37)23-7-8-28(33-16-23)36-11-9-32-10-12-36/h7-8,13-16,18,22,32H,6,9-12,17H2,1-5H3,(H,34,38)(H,35,39)/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 (unknown origin) using biotinylated nucleosome, H3K27me3 activator and [3H]-SAM incubated for 60 mins by top-count based method |
Bioorg Med Chem Lett 25: 3644-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.056
BindingDB Entry DOI: 10.7270/Q2Q24205 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50563826

(CHEMBL4782143)Show SMILES COc1cc(C)[nH]c(=O)c1CNC(=O)c1c(C)n([C@H](C)C2CCN(CC(F)(F)C(F)F)CC2)c2ccccc12 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of wild-type EZH2 in PRC2 complex (unknown origin) using RKQLATKAARK(Me3)SAPATGGVKKP-NH2 peptide substrate preincubated for 30 mins follow... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QZ2FQB |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50563822

(CHEMBL4779426)Show SMILES COc1cc(C)[nH]c(=O)c1CNC(=O)c1c(C)n([C@H](C)C2CCN(CC2)C2COC2)c2ccccc12 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EZH2 Y641F mutant in PRC2 complex (unknown origin) using RKQLATKAARK(Me3)SAPATGGVKKP-NH2 peptide substrate preincubated for 30 mins fol... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QZ2FQB |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50563818

(CHEMBL4781657)Show SMILES COc1cc(C)[nH]c(=O)c1CNC(=O)c1c(C)n([C@H](C)C2CCN(C)CC2)c2ccccc12 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of EZH2 Y641F mutant in PRC2 complex (unknown origin) using RKQLATKAARK(Me3)SAPATGGVKKP-NH2 peptide substrate preincubated for 30 mins fol... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QZ2FQB |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50563827

(CHEMBL4797333)Show SMILES COc1cc(C)[nH]c(=O)c1CNC(=O)c1c(C)n([C@H](C)C2CCN(CC(F)F)CC2)c2ccccc12 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of wild-type EZH2 in PRC2 complex (unknown origin) using RKQLATKAARK(Me3)SAPATGGVKKP-NH2 peptide substrate preincubated for 30 mins follow... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QZ2FQB |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50545727

(CHEMBL4637906)Show SMILES [H][C@@]1(CC[C@@]([H])(CC1)N[C@@H]1C[C@H]1\C(CC)=C\c1ccccc1)NCCO |r,wU:4.4,9.9,wD:1.0,11.13,(70.57,-28.79,;70.57,-30.33,;70.57,-31.87,;69.24,-32.64,;67.91,-31.87,;67.91,-33.41,;67.91,-30.33,;69.24,-29.56,;66.58,-32.64,;65.24,-31.87,;64.47,-30.54,;63.7,-31.87,;62.37,-32.64,;62.37,-34.18,;63.7,-34.95,;61.03,-31.87,;59.7,-32.64,;58.36,-31.87,;57.04,-32.65,;57.04,-34.18,;58.37,-34.95,;59.7,-34.18,;71.91,-29.56,;73.24,-30.33,;74.57,-29.56,;75.91,-30.33,)| Show InChI InChI=1S/C21H32N2O/c1-2-17(14-16-6-4-3-5-7-16)20-15-21(20)23-19-10-8-18(9-11-19)22-12-13-24/h3-7,14,18-24H,2,8-13,15H2,1H3/b17-14+/t18-,19-,20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of recombinant N-terminal His-tagged LSD1 (unknown origin) expressed in Escherichia coli expression system using ART-K(Me1)-QTARKSTGGKAPRK... |
ACS Med Chem Lett 11: 1213-1220 (2020)
Article DOI: 10.1021/acsmedchemlett.0c00060
BindingDB Entry DOI: 10.7270/Q22N55VZ |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50110358
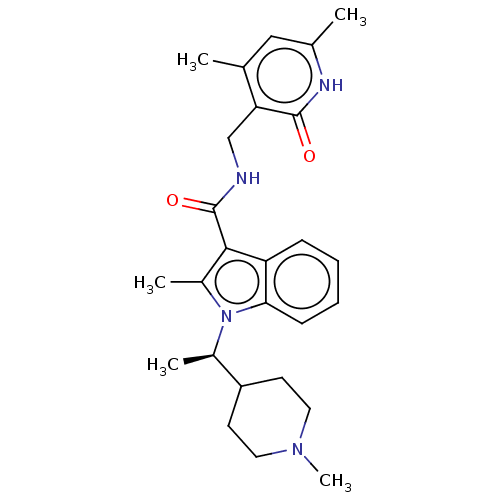
(CHEMBL3605454)Show SMILES C[C@H](C1CCN(C)CC1)n1c(C)c(C(=O)NCc2c(C)cc(C)[nH]c2=O)c2ccccc12 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 (unknown origin) using biotinylated nucleosome, H3K27me3 activator and [3H]-SAM incubated for 60 mins by top-count based method |
Bioorg Med Chem Lett 25: 3644-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.056
BindingDB Entry DOI: 10.7270/Q2Q24205 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50110355

(CHEMBL3605457)Show SMILES CCS(=O)(=O)N1CCC(CC1)[C@@H](C)n1c(C)c(C(=O)NCc2c(OC)cc(C)[nH]c2=O)c2ccccc12 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 (unknown origin) using biotinylated nucleosome, H3K27me3 activator and [3H]-SAM incubated for 60 mins by top-count based method |
Bioorg Med Chem Lett 25: 3644-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.056
BindingDB Entry DOI: 10.7270/Q2Q24205 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50110356
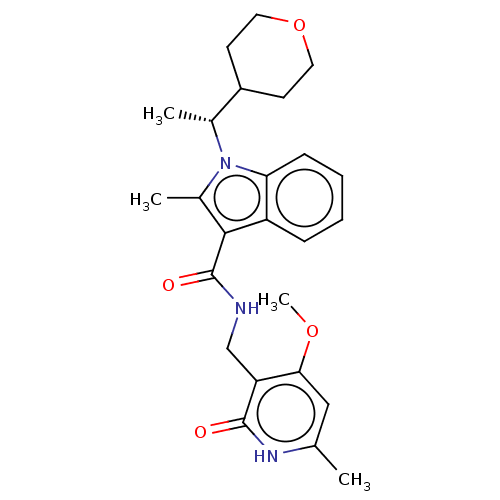
(CHEMBL3605456)Show SMILES COc1cc(C)[nH]c(=O)c1CNC(=O)c1c(C)n([C@H](C)C2CCOCC2)c2ccccc12 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 (unknown origin) using biotinylated nucleosome, H3K27me3 activator and [3H]-SAM incubated for 60 mins by top-count based method |
Bioorg Med Chem Lett 25: 3644-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.056
BindingDB Entry DOI: 10.7270/Q2Q24205 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50017293

(CHEMBL3287735 | US10647700, Compound GSK126)Show SMILES CC[C@H](C)n1cc(C)c2c(cc(cc12)-c1ccc(nc1)N1CCNCC1)C(=O)NCc1c(C)cc(C)[nH]c1=O |r| Show InChI InChI=1S/C31H38N6O2/c1-6-22(5)37-18-20(3)29-25(30(38)34-17-26-19(2)13-21(4)35-31(26)39)14-24(15-27(29)37)23-7-8-28(33-16-23)36-11-9-32-10-12-36/h7-8,13-16,18,22,32H,6,9-12,17H2,1-5H3,(H,34,38)(H,35,39)/t22-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Constellation Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of EZH2 Y641N mutant (unknown origin) using biotinylated nucleosome, H3K27me3 activator and [3H]-SAM incubated for 60 mins by top-count ba... |
Bioorg Med Chem Lett 25: 3644-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.06.056
BindingDB Entry DOI: 10.7270/Q2Q24205 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50563817
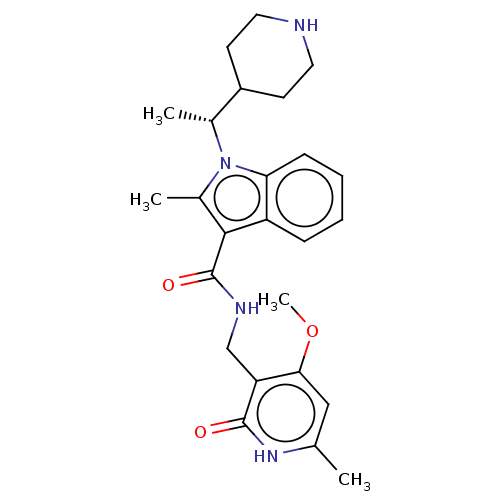
(CHEMBL4787149)Show SMILES COc1cc(C)[nH]c(=O)c1CNC(=O)c1c(C)n([C@H](C)C2CCNCC2)c2ccccc12 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of wild-type EZH2 in PRC2 complex (unknown origin) using RKQLATKAARK(Me3)SAPATGGVKKP-NH2 peptide substrate preincubated for 30 mins follow... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QZ2FQB |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50541920

(Cpi-1205 | Lirametostat)Show SMILES COc1cc(C)[nH]c(=O)c1CNC(=O)c1c(C)n([C@H](C)C2CCN(CC(F)(F)F)CC2)c2ccccc12 Show InChI InChI=1S/C27H33F3N4O3/c1-16-13-23(37-4)21(25(35)32-16)14-31-26(36)24-18(3)34(22-8-6-5-7-20(22)24)17(2)19-9-11-33(12-10-19)15-27(28,29)30/h5-8,13,17,19H,9-12,14-15H2,1-4H3,(H,31,36)(H,32,35)/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of wild-type EZH2 in PRC2 complex (unknown origin) using RKQLATKAARK(Me3)SAPATGGVKKP-NH2 peptide substrate preincubated for 30 mins follow... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QZ2FQB |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50563824
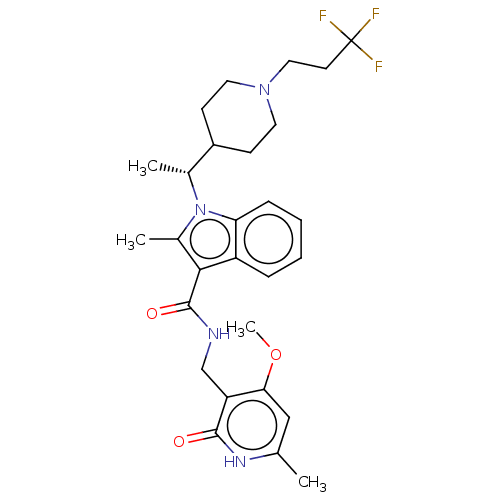
(CHEMBL4777385)Show SMILES COc1cc(C)[nH]c(=O)c1CNC(=O)c1c(C)n([C@H](C)C2CCN(CCC(F)(F)F)CC2)c2ccccc12 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of wild-type EZH2 in PRC2 complex (unknown origin) using RKQLATKAARK(Me3)SAPATGGVKKP-NH2 peptide substrate preincubated for 30 mins follow... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QZ2FQB |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50563819

(CHEMBL4800227)Show SMILES COc1cc(C)[nH]c(=O)c1CNC(=O)c1c(C)n([C@H](C)C2CCN(CC2)C(=O)C(C)C)c2ccccc12 |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of wild-type EZH2 in PRC2 complex (unknown origin) using RKQLATKAARK(Me3)SAPATGGVKKP-NH2 peptide substrate preincubated for 30 mins follow... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2QZ2FQB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data