 Found 850 hits with Last Name = 'balestra' and Initial = 'm'
Found 850 hits with Last Name = 'balestra' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50130561
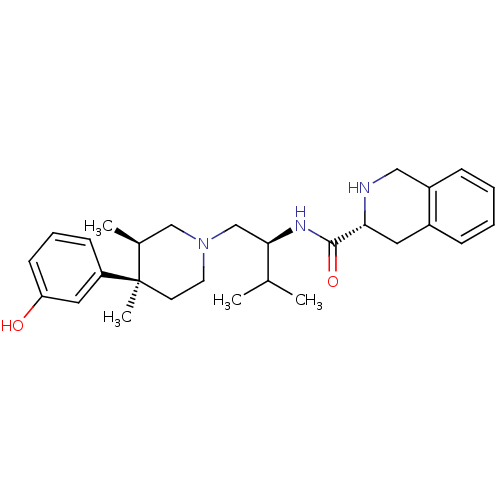
((R)-N-((S)-1-((3R,4R)-4-(3-hydroxyphenyl)-3,4-dime...)Show SMILES CC(C)[C@@H](CN1CC[C@](C)([C@@H](C)C1)c1cccc(O)c1)NC(=O)[C@H]1Cc2ccccc2CN1 Show InChI InChI=1S/C28H39N3O2/c1-19(2)26(30-27(33)25-14-21-8-5-6-9-22(21)16-29-25)18-31-13-12-28(4,20(3)17-31)23-10-7-11-24(32)15-23/h5-11,15,19-20,25-26,29,32H,12-14,16-18H2,1-4H3,(H,30,33)/t20-,25+,26+,28+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50327257
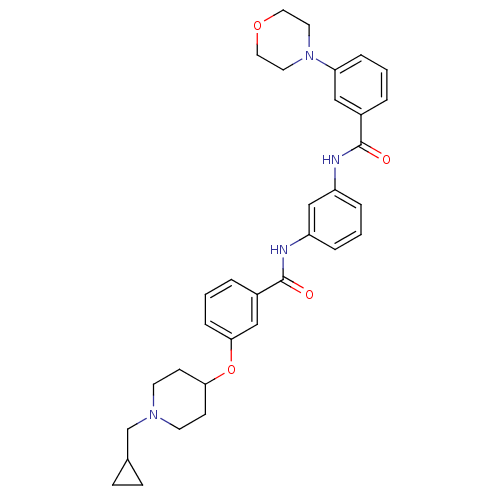
(3-(1-(cyclopropylmethyl)piperidin-4-yloxy)-N-(3-(3...)Show SMILES O=C(Nc1cccc(NC(=O)c2cccc(c2)N2CCOCC2)c1)c1cccc(OC2CCN(CC3CC3)CC2)c1 Show InChI InChI=1S/C33H38N4O4/c38-32(25-4-1-8-29(20-25)37-16-18-40-19-17-37)34-27-6-3-7-28(22-27)35-33(39)26-5-2-9-31(21-26)41-30-12-14-36(15-13-30)23-24-10-11-24/h1-9,20-22,24,30H,10-19,23H2,(H,34,38)(H,35,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM82551

(C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)c1[nH]c2[C@@H]4Oc6c7c(C[C@H]8N(CC9CC9)CC[C@@]47[C@@]8(O)Cc2c1C[C@@]35O)ccc6O |r| Show InChI InChI=1S/C40H43N3O6/c44-25-7-5-21-13-27-39(46)15-23-24-16-40(47)28-14-22-6-8-26(45)34-30(22)38(40,10-12-43(28)18-20-3-4-20)36(49-34)32(24)41-31(23)35-37(39,29(21)33(25)48-35)9-11-42(27)17-19-1-2-19/h5-8,19-20,27-28,35-36,41,44-47H,1-4,9-18H2/t27-,28-,35+,36+,37+,38+,39-,40-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM82070

(CAS_29790-52-1 | NICOTINE-L (BASE) | Nicotine-D sa...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3/t10-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Binding affinity in rat forebrain against Nicotinic acetylcholine receptor alpha4-beta2 |
J Med Chem 43: 4045-50 (2000)
BindingDB Entry DOI: 10.7270/Q24Q7T88 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50325855
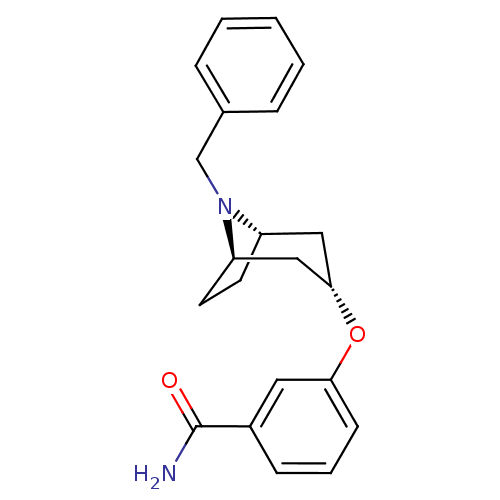
(CHEMBL1223951 | exo-3-((1R,3s,5S)-8-benzyl-8-azabi...)Show SMILES NC(=O)c1cccc(O[C@H]2C[C@@H]3CC[C@H](C2)N3Cc2ccccc2)c1 |r,TLB:17:16:15.9.10:13.12| Show InChI InChI=1S/C21H24N2O2/c22-21(24)16-7-4-8-19(11-16)25-20-12-17-9-10-18(13-20)23(17)14-15-5-2-1-3-6-15/h1-8,11,17-18,20H,9-10,12-14H2,(H2,22,24)/t17-,18+,20- | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50327259
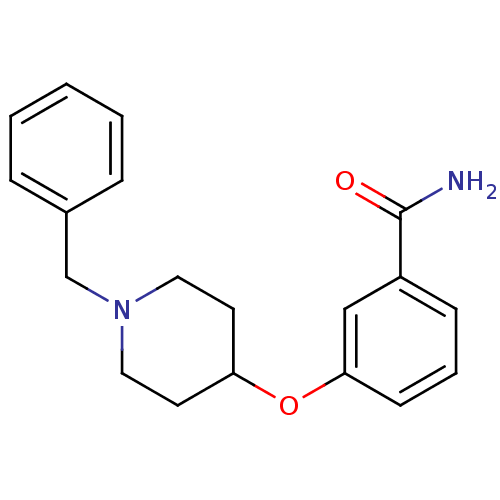
(3-(1-benzylpiperidin-4-yloxy)benzamide | CHEMBL125...)Show InChI InChI=1S/C19H22N2O2/c20-19(22)16-7-4-8-18(13-16)23-17-9-11-21(12-10-17)14-15-5-2-1-3-6-15/h1-8,13,17H,9-12,14H2,(H2,20,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50093255

((+)-Spiro[1-azabicyclo[2.2.2]octane-3,5'-oxazolidi...)Show SMILES O=C1NCC2(CN3CCC2CC3)O1 |(13.73,-1.27,;14.13,.22,;13.16,1.42,;14.01,2.72,;15.5,2.31,;15.5,3.85,;16.83,4.63,;18.16,3.85,;18.16,2.31,;16.83,1.55,;16.25,2.54,;17.59,3.3,;15.58,.77,)| Show InChI InChI=1S/C9H14N2O2/c12-8-10-5-9(13-8)6-11-3-1-7(9)2-4-11/h7H,1-6H2,(H,10,12) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
binding affinity in rat hippocampi against alpha7 nicotinic receptor |
J Med Chem 43: 4045-50 (2000)
BindingDB Entry DOI: 10.7270/Q24Q7T88 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50093255

((+)-Spiro[1-azabicyclo[2.2.2]octane-3,5'-oxazolidi...)Show SMILES O=C1NCC2(CN3CCC2CC3)O1 |(13.73,-1.27,;14.13,.22,;13.16,1.42,;14.01,2.72,;15.5,2.31,;15.5,3.85,;16.83,4.63,;18.16,3.85,;18.16,2.31,;16.83,1.55,;16.25,2.54,;17.59,3.3,;15.58,.77,)| Show InChI InChI=1S/C9H14N2O2/c12-8-10-5-9(13-8)6-11-3-1-7(9)2-4-11/h7H,1-6H2,(H,10,12) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
binding affinity in rat hippocampi against alpha7 nicotinic receptor |
J Med Chem 43: 4045-50 (2000)
BindingDB Entry DOI: 10.7270/Q24Q7T88 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50327258
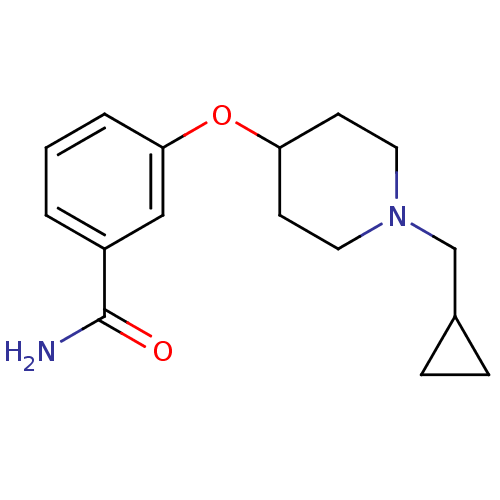
(3-(1-(cyclopropylmethyl)piperidin-4-yloxy)benzamid...)Show InChI InChI=1S/C16H22N2O2/c17-16(19)13-2-1-3-15(10-13)20-14-6-8-18(9-7-14)11-12-4-5-12/h1-3,10,12,14H,4-9,11H2,(H2,17,19) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 146 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity to kappa opioid receptor |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM50093259
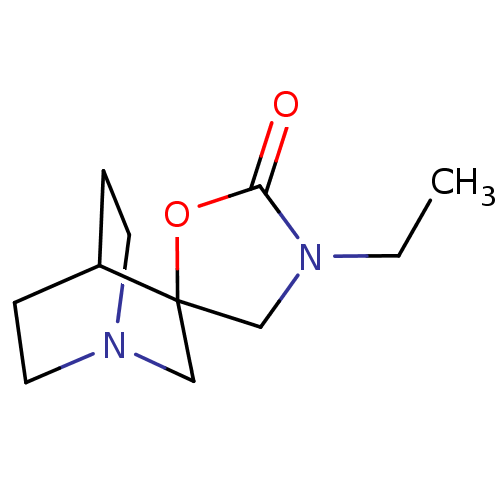
(3'-Ethylspiro[1-azabicyclo[2.2.2]octane-3,5'-oxazo...)Show SMILES CCN1CC2(CN3CCC2CC3)OC1=O |TLB:3:4:10.11:8.7,THB:12:4:10.11:8.7,(7.97,-7.56,;8.38,-6.07,;7.29,-4.98,;5.75,-4.98,;5.26,-3.5,;4.15,-4.09,;3.57,-2.87,;1.98,-3.39,;2.56,-2.35,;4.27,-1.93,;4.63,-.97,;3.57,-1.7,;6.52,-2.59,;7.77,-3.5,;9.25,-3.03,)| Show InChI InChI=1S/C11H18N2O2/c1-2-13-8-11(15-10(13)14)7-12-5-3-9(11)4-6-12/h9H,2-8H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Binding affinity in rat forebrain against Nicotinic acetylcholine receptor alpha4-beta2 |
J Med Chem 43: 4045-50 (2000)
BindingDB Entry DOI: 10.7270/Q24Q7T88 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50093253
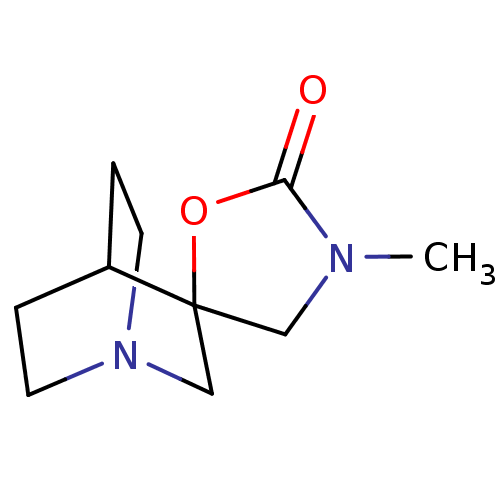
((+)-3'-Methylspiro[1-azabicyclo[2.2.2]octane-3,5'-...)Show SMILES CN1CC2(CN3CCC2CC3)OC1=O |TLB:2:3:9.10:7.6,THB:11:3:9.10:7.6,(8.37,-6.07,;7.28,-4.97,;5.74,-4.97,;5.25,-3.5,;4.14,-4.08,;3.57,-2.86,;1.97,-3.38,;2.56,-2.34,;4.26,-1.93,;4.62,-.97,;3.57,-1.69,;6.51,-2.58,;7.76,-3.5,;9.23,-3.02,)| Show InChI InChI=1S/C10H16N2O2/c1-11-6-10(14-9(11)13)7-12-4-2-8(10)3-5-12/h8H,2-7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
binding affinity in rat hippocampi against alpha7 nicotinic receptor |
J Med Chem 43: 4045-50 (2000)
BindingDB Entry DOI: 10.7270/Q24Q7T88 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50156071
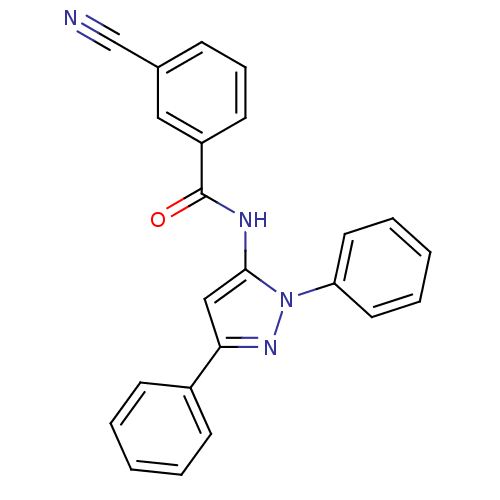
(3-Cyano-N-(2,5-diphenyl-2H-pyrazol-3-yl)-benzamide...)Show SMILES O=C(Nc1cc(nn1-c1ccccc1)-c1ccccc1)c1cccc(c1)C#N Show InChI InChI=1S/C23H16N4O/c24-16-17-8-7-11-19(14-17)23(28)25-22-15-21(18-9-3-1-4-10-18)26-27(22)20-12-5-2-6-13-20/h1-15H,(H,25,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-MPEP from human mGluR5 |
Bioorg Med Chem Lett 20: 7381-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.036
BindingDB Entry DOI: 10.7270/Q27S7P13 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50093253

((+)-3'-Methylspiro[1-azabicyclo[2.2.2]octane-3,5'-...)Show SMILES CN1CC2(CN3CCC2CC3)OC1=O |TLB:2:3:9.10:7.6,THB:11:3:9.10:7.6,(8.37,-6.07,;7.28,-4.97,;5.74,-4.97,;5.25,-3.5,;4.14,-4.08,;3.57,-2.86,;1.97,-3.38,;2.56,-2.34,;4.26,-1.93,;4.62,-.97,;3.57,-1.69,;6.51,-2.58,;7.76,-3.5,;9.23,-3.02,)| Show InChI InChI=1S/C10H16N2O2/c1-11-6-10(14-9(11)13)7-12-4-2-8(10)3-5-12/h8H,2-7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
binding affinity in rat hippocampi against alpha7 nicotinic receptor |
J Med Chem 43: 4045-50 (2000)
BindingDB Entry DOI: 10.7270/Q24Q7T88 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM50093253

((+)-3'-Methylspiro[1-azabicyclo[2.2.2]octane-3,5'-...)Show SMILES CN1CC2(CN3CCC2CC3)OC1=O |TLB:2:3:9.10:7.6,THB:11:3:9.10:7.6,(8.37,-6.07,;7.28,-4.97,;5.74,-4.97,;5.25,-3.5,;4.14,-4.08,;3.57,-2.86,;1.97,-3.38,;2.56,-2.34,;4.26,-1.93,;4.62,-.97,;3.57,-1.69,;6.51,-2.58,;7.76,-3.5,;9.23,-3.02,)| Show InChI InChI=1S/C10H16N2O2/c1-11-6-10(14-9(11)13)7-12-4-2-8(10)3-5-12/h8H,2-7H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
binding affinity in rat forebrain against alpha4-beta2 nicotinic receptor |
J Med Chem 43: 4045-50 (2000)
BindingDB Entry DOI: 10.7270/Q24Q7T88 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM50093253

((+)-3'-Methylspiro[1-azabicyclo[2.2.2]octane-3,5'-...)Show SMILES CN1CC2(CN3CCC2CC3)OC1=O |TLB:2:3:9.10:7.6,THB:11:3:9.10:7.6,(8.37,-6.07,;7.28,-4.97,;5.74,-4.97,;5.25,-3.5,;4.14,-4.08,;3.57,-2.86,;1.97,-3.38,;2.56,-2.34,;4.26,-1.93,;4.62,-.97,;3.57,-1.69,;6.51,-2.58,;7.76,-3.5,;9.23,-3.02,)| Show InChI InChI=1S/C10H16N2O2/c1-11-6-10(14-9(11)13)7-12-4-2-8(10)3-5-12/h8H,2-7H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
binding affinity in rat forebrain against alpha4-beta2 nicotinic receptor |
J Med Chem 43: 4045-50 (2000)
BindingDB Entry DOI: 10.7270/Q24Q7T88 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM82070

(CAS_29790-52-1 | NICOTINE-L (BASE) | Nicotine-D sa...)Show InChI InChI=1S/C10H14N2/c1-12-7-3-5-10(12)9-4-2-6-11-8-9/h2,4,6,8,10H,3,5,7H2,1H3/t10-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Binding affinity in rat hippocampi against Nicotinic acetylcholine receptor alpha7 |
J Med Chem 43: 4045-50 (2000)
BindingDB Entry DOI: 10.7270/Q24Q7T88 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50093251
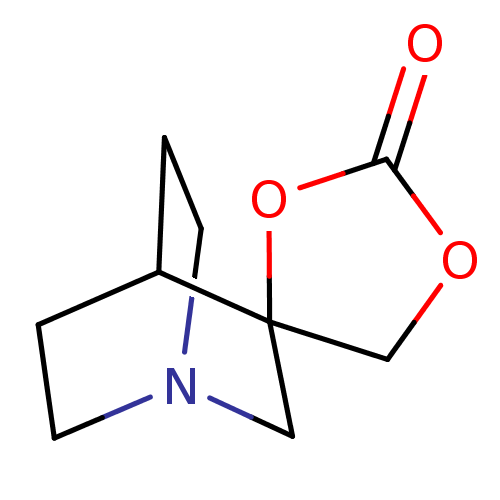
(CHEMBL338698 | Spiro[1-azabicyclo[2.2.2]octane-3,5...)Show SMILES O=C1OCC2(CN3CCC2CC3)O1 |TLB:3:4:10.11:8.7,THB:12:4:10.11:8.7,(9.83,-3.22,;8.26,-3.72,;7.75,-5.29,;6.11,-5.29,;5.59,-3.72,;4.41,-4.35,;3.8,-3.05,;2.1,-3.6,;2.73,-2.49,;4.54,-2.05,;4.92,-1.03,;3.8,-1.8,;6.93,-2.75,)| Show InChI InChI=1S/C9H13NO3/c11-8-12-6-9(13-8)5-10-3-1-7(9)2-4-10/h7H,1-6H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Binding affinity in rat hippocampi against Nicotinic acetylcholine receptor alpha7 |
J Med Chem 43: 4045-50 (2000)
BindingDB Entry DOI: 10.7270/Q24Q7T88 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50093259

(3'-Ethylspiro[1-azabicyclo[2.2.2]octane-3,5'-oxazo...)Show SMILES CCN1CC2(CN3CCC2CC3)OC1=O |TLB:3:4:10.11:8.7,THB:12:4:10.11:8.7,(7.97,-7.56,;8.38,-6.07,;7.29,-4.98,;5.75,-4.98,;5.26,-3.5,;4.15,-4.09,;3.57,-2.87,;1.98,-3.39,;2.56,-2.35,;4.27,-1.93,;4.63,-.97,;3.57,-1.7,;6.52,-2.59,;7.77,-3.5,;9.25,-3.03,)| Show InChI InChI=1S/C11H18N2O2/c1-2-13-8-11(15-10(13)14)7-12-5-3-9(11)4-6-12/h9H,2-8H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Binding affinity in rat hippocampi against Nicotinic acetylcholine receptor alpha7 |
J Med Chem 43: 4045-50 (2000)
BindingDB Entry DOI: 10.7270/Q24Q7T88 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM50093251

(CHEMBL338698 | Spiro[1-azabicyclo[2.2.2]octane-3,5...)Show SMILES O=C1OCC2(CN3CCC2CC3)O1 |TLB:3:4:10.11:8.7,THB:12:4:10.11:8.7,(9.83,-3.22,;8.26,-3.72,;7.75,-5.29,;6.11,-5.29,;5.59,-3.72,;4.41,-4.35,;3.8,-3.05,;2.1,-3.6,;2.73,-2.49,;4.54,-2.05,;4.92,-1.03,;3.8,-1.8,;6.93,-2.75,)| Show InChI InChI=1S/C9H13NO3/c11-8-12-6-9(13-8)5-10-3-1-7(9)2-4-10/h7H,1-6H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Binding affinity in rat forebrain against Nicotinic acetylcholine receptor alpha4-beta2 |
J Med Chem 43: 4045-50 (2000)
BindingDB Entry DOI: 10.7270/Q24Q7T88 |
More data for this
Ligand-Target Pair | |
Metabotropic glutamate receptor 5
(Homo sapiens (Human)) | BDBM50331975
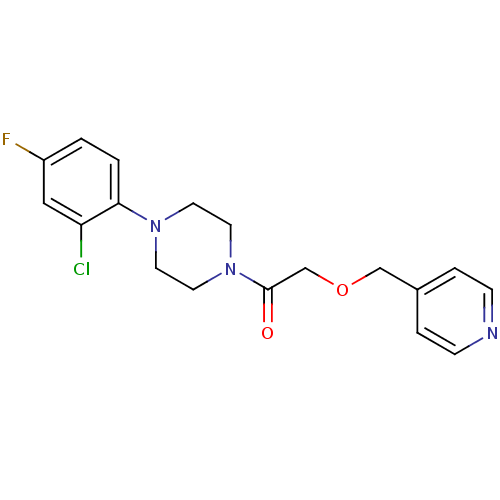
(1-(4-(2-chloro-4-fluorophenyl)piperazin-1-yl)-2-(p...)Show InChI InChI=1S/C18H19ClFN3O2/c19-16-11-15(20)1-2-17(16)22-7-9-23(10-8-22)18(24)13-25-12-14-3-5-21-6-4-14/h1-6,11H,7-10,12-13H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of [3H]-MPEP from human mGluR5 |
Bioorg Med Chem Lett 20: 7381-4 (2010)
Article DOI: 10.1016/j.bmcl.2010.10.036
BindingDB Entry DOI: 10.7270/Q27S7P13 |
More data for this
Ligand-Target Pair | |
Carnitine O-palmitoyltransferase 1, liver isoform
(Rattus norvegicus) | BDBM50033117
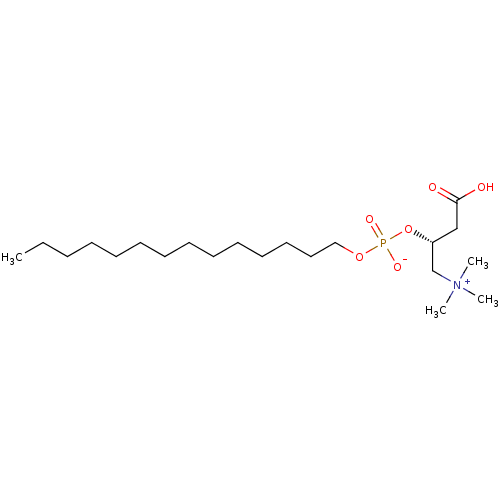
(CHEMBL114113 | [3-Carboxy-2-(hydroxy-tetradecyloxy...)Show SMILES CCCCCCCCCCCCCCOP([O-])(=O)O[C@H](CC(O)=O)C[N+](C)(C)C Show InChI InChI=1S/C21H44NO6P/c1-5-6-7-8-9-10-11-12-13-14-15-16-17-27-29(25,26)28-20(18-21(23)24)19-22(2,3)4/h20H,5-19H2,1-4H3,(H-,23,24,25,26)/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sandoz Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibition of carnitine palmitoyltransferase I withrespect to palmitoyl CoA in rat |
J Med Chem 38: 3448-50 (1995)
BindingDB Entry DOI: 10.7270/Q2VQ31R1 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50093260
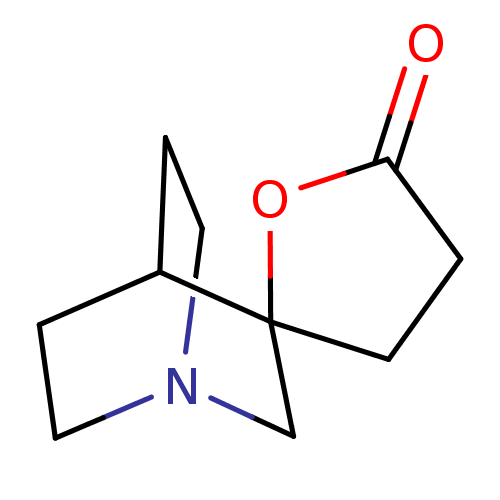
(CHEMBL133295 | Spiro[1-azabicyclo[2.2.2]octane-3,5...)Show SMILES O=C1CCC2(CN3CCC2CC3)O1 |TLB:3:4:10.11:8.7,THB:12:4:10.11:8.7,(9.2,-3.01,;7.73,-3.48,;7.26,-4.95,;5.72,-4.95,;5.23,-3.48,;4.13,-4.07,;3.55,-2.85,;1.97,-3.37,;2.55,-2.33,;4.25,-1.92,;4.6,-.96,;3.55,-1.69,;6.49,-2.57,)| Show InChI InChI=1S/C10H15NO2/c12-9-1-4-10(13-9)7-11-5-2-8(10)3-6-11/h8H,1-7H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Binding affinity in rat hippocampi against Nicotinic acetylcholine receptor alpha7 |
J Med Chem 43: 4045-50 (2000)
BindingDB Entry DOI: 10.7270/Q24Q7T88 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50093257

(CHEMBL131574 | Spiro[1-azabicyclo[2.2.2]octane-3,4...)Show SMILES O=C1CC2(CN1)CN1CCC2CC1 |TLB:4:3:11.12:9.8,THB:2:3:11.12:9.8,(9.19,-3.01,;7.72,-3.48,;6.48,-2.57,;5.23,-3.48,;5.71,-4.95,;7.25,-4.95,;4.13,-4.06,;3.55,-2.85,;1.97,-3.36,;2.55,-2.33,;4.24,-1.92,;4.6,-.96,;3.55,-1.69,)| Show InChI InChI=1S/C10H16N2O/c13-9-5-10(6-11-9)7-12-3-1-8(10)2-4-12/h8H,1-7H2,(H,11,13) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Binding affinity in rat hippocampi against Nicotinic acetylcholine receptor alpha7 |
J Med Chem 43: 4045-50 (2000)
BindingDB Entry DOI: 10.7270/Q24Q7T88 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50093256
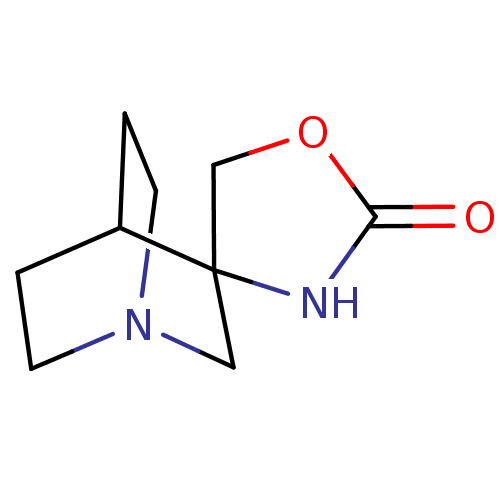
(CHEMBL130205 | Spiro[1-azabicyclo[2.2.2]octane-3,4...)Show SMILES O=C1NC2(CO1)CN1CCC2CC1 |TLB:4:3:11.12:9.8,THB:2:3:11.12:9.8,(24.72,-6.48,;23.15,-7,;21.81,-6.02,;20.48,-7,;20.98,-8.57,;22.63,-8.57,;19.28,-7.62,;18.67,-6.32,;16.97,-6.87,;17.6,-5.76,;19.41,-5.32,;19.8,-4.3,;18.67,-5.07,)| Show InChI InChI=1S/C9H14N2O2/c12-8-10-9(6-13-8)5-11-3-1-7(9)2-4-11/h7H,1-6H2,(H,10,12) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| >4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Binding affinity in rat hippocampi against Nicotinic acetylcholine receptor alpha7 |
J Med Chem 43: 4045-50 (2000)
BindingDB Entry DOI: 10.7270/Q24Q7T88 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50093255

((+)-Spiro[1-azabicyclo[2.2.2]octane-3,5'-oxazolidi...)Show SMILES O=C1NCC2(CN3CCC2CC3)O1 |(13.73,-1.27,;14.13,.22,;13.16,1.42,;14.01,2.72,;15.5,2.31,;15.5,3.85,;16.83,4.63,;18.16,3.85,;18.16,2.31,;16.83,1.55,;16.25,2.54,;17.59,3.3,;15.58,.77,)| Show InChI InChI=1S/C9H14N2O2/c12-8-10-5-9(13-8)6-11-3-1-7(9)2-4-11/h7H,1-6H2,(H,10,12) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
binding affinity in rat hippocampi against alpha7 nicotinic receptor |
J Med Chem 43: 4045-50 (2000)
BindingDB Entry DOI: 10.7270/Q24Q7T88 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM50093252
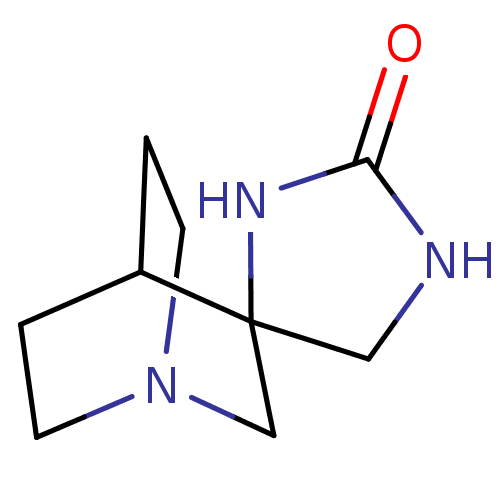
(CHEMBL422259 | Spiro[1-azabicyclo[2.2.2]octane-3,4...)Show SMILES O=C1NCC2(CN3CCC2CC3)N1 |TLB:3:4:10.11:8.7,THB:12:4:10.11:8.7,(24.72,-6.48,;23.15,-7,;22.63,-8.57,;20.98,-8.57,;20.48,-7,;19.28,-7.62,;18.67,-6.32,;16.97,-6.87,;17.6,-5.76,;19.41,-5.32,;19.8,-4.3,;18.67,-5.07,;21.81,-6.02,)| Show InChI InChI=1S/C9H15N3O/c13-8-10-5-9(11-8)6-12-3-1-7(9)2-4-12/h7H,1-6H2,(H2,10,11,13) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Binding affinity in rat forebrain against Nicotinic acetylcholine receptor alpha4-beta2 |
J Med Chem 43: 4045-50 (2000)
BindingDB Entry DOI: 10.7270/Q24Q7T88 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50093252

(CHEMBL422259 | Spiro[1-azabicyclo[2.2.2]octane-3,4...)Show SMILES O=C1NCC2(CN3CCC2CC3)N1 |TLB:3:4:10.11:8.7,THB:12:4:10.11:8.7,(24.72,-6.48,;23.15,-7,;22.63,-8.57,;20.98,-8.57,;20.48,-7,;19.28,-7.62,;18.67,-6.32,;16.97,-6.87,;17.6,-5.76,;19.41,-5.32,;19.8,-4.3,;18.67,-5.07,;21.81,-6.02,)| Show InChI InChI=1S/C9H15N3O/c13-8-10-5-9(11-8)6-12-3-1-7(9)2-4-12/h7H,1-6H2,(H2,10,11,13) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Binding affinity in rat hippocampi against Nicotinic acetylcholine receptor alpha7 |
J Med Chem 43: 4045-50 (2000)
BindingDB Entry DOI: 10.7270/Q24Q7T88 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50093258

(3'-Methylspiro[1-azabicyclo[2.2.2]octane-3,4'-2H-t...)Show SMILES CN1C(=O)NCC11CN2CCC1CC2 |TLB:5:6:12.13:10.9,THB:1:6:12.13:10.9,(20.42,-4.1,;20.42,-5.64,;21.67,-6.55,;23.15,-6.07,;21.19,-8.03,;19.64,-8.03,;19.18,-6.55,;18.05,-7.14,;17.48,-5.92,;15.89,-6.44,;16.48,-5.39,;18.17,-4.98,;18.54,-4.03,;17.48,-4.75,)| Show InChI InChI=1S/C10H17N3O/c1-12-9(14)11-6-10(12)7-13-4-2-8(10)3-5-13/h8H,2-7H2,1H3,(H,11,14) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Binding affinity in rat hippocampi against Nicotinic acetylcholine receptor alpha7 |
J Med Chem 43: 4045-50 (2000)
BindingDB Entry DOI: 10.7270/Q24Q7T88 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM50093255

((+)-Spiro[1-azabicyclo[2.2.2]octane-3,5'-oxazolidi...)Show SMILES O=C1NCC2(CN3CCC2CC3)O1 |(13.73,-1.27,;14.13,.22,;13.16,1.42,;14.01,2.72,;15.5,2.31,;15.5,3.85,;16.83,4.63,;18.16,3.85,;18.16,2.31,;16.83,1.55,;16.25,2.54,;17.59,3.3,;15.58,.77,)| Show InChI InChI=1S/C9H14N2O2/c12-8-10-5-9(13-8)6-11-3-1-7(9)2-4-11/h7H,1-6H2,(H,10,12) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
binding affinity in rat forebrain against alpha4-beta2 nicotinic receptor |
J Med Chem 43: 4045-50 (2000)
BindingDB Entry DOI: 10.7270/Q24Q7T88 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM50093255

((+)-Spiro[1-azabicyclo[2.2.2]octane-3,5'-oxazolidi...)Show SMILES O=C1NCC2(CN3CCC2CC3)O1 |(13.73,-1.27,;14.13,.22,;13.16,1.42,;14.01,2.72,;15.5,2.31,;15.5,3.85,;16.83,4.63,;18.16,3.85,;18.16,2.31,;16.83,1.55,;16.25,2.54,;17.59,3.3,;15.58,.77,)| Show InChI InChI=1S/C9H14N2O2/c12-8-10-5-9(13-8)6-11-3-1-7(9)2-4-11/h7H,1-6H2,(H,10,12) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
binding affinity in rat forebrain against alpha4-beta2 nicotinic receptor |
J Med Chem 43: 4045-50 (2000)
BindingDB Entry DOI: 10.7270/Q24Q7T88 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50093254
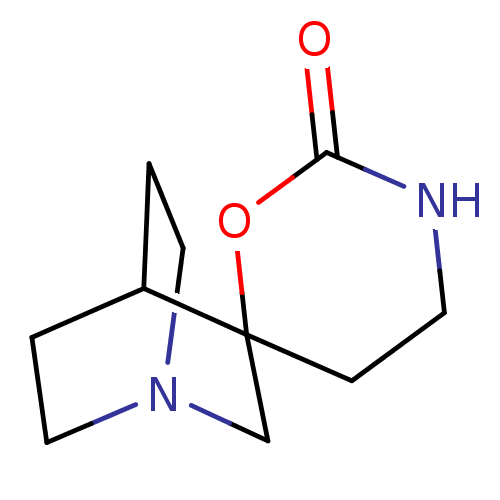
(CHEMBL336197 | Spiro[1-azabicyclo[2.2.2]octane-3,6...)Show SMILES O=C1NCCC2(CN3CCC2CC3)O1 |TLB:4:5:11.12:9.8,THB:13:5:11.12:9.8,(9.22,-3.02,;7.75,-3.49,;7.74,-5.03,;6.58,-5.81,;5.25,-5.03,;5.25,-3.49,;4.14,-4.08,;3.56,-2.86,;1.97,-3.37,;2.56,-2.34,;4.26,-1.93,;4.61,-.97,;3.56,-1.69,;6.5,-2.58,)| Show InChI InChI=1S/C10H16N2O2/c13-9-11-4-3-10(14-9)7-12-5-1-8(10)2-6-12/h8H,1-7H2,(H,11,13) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 2.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Binding affinity in rat hippocampi against Nicotinic acetylcholine receptor alpha7; value range is 1000. |
J Med Chem 43: 4045-50 (2000)
BindingDB Entry DOI: 10.7270/Q24Q7T88 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM50093253

((+)-3'-Methylspiro[1-azabicyclo[2.2.2]octane-3,5'-...)Show SMILES CN1CC2(CN3CCC2CC3)OC1=O |TLB:2:3:9.10:7.6,THB:11:3:9.10:7.6,(8.37,-6.07,;7.28,-4.97,;5.74,-4.97,;5.25,-3.5,;4.14,-4.08,;3.57,-2.86,;1.97,-3.38,;2.56,-2.34,;4.26,-1.93,;4.62,-.97,;3.57,-1.69,;6.51,-2.58,;7.76,-3.5,;9.23,-3.02,)| Show InChI InChI=1S/C10H16N2O2/c1-11-6-10(14-9(11)13)7-12-4-2-8(10)3-5-12/h8H,2-7H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
binding affinity in rat forebrain against alpha4-beta2 nicotinic receptor; value range is 1000. |
J Med Chem 43: 4045-50 (2000)
BindingDB Entry DOI: 10.7270/Q24Q7T88 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-7
(Rattus norvegicus (Rat)) | BDBM50093253

((+)-3'-Methylspiro[1-azabicyclo[2.2.2]octane-3,5'-...)Show SMILES CN1CC2(CN3CCC2CC3)OC1=O |TLB:2:3:9.10:7.6,THB:11:3:9.10:7.6,(8.37,-6.07,;7.28,-4.97,;5.74,-4.97,;5.25,-3.5,;4.14,-4.08,;3.57,-2.86,;1.97,-3.38,;2.56,-2.34,;4.26,-1.93,;4.62,-.97,;3.57,-1.69,;6.51,-2.58,;7.76,-3.5,;9.23,-3.02,)| Show InChI InChI=1S/C10H16N2O2/c1-11-6-10(14-9(11)13)7-12-4-2-8(10)3-5-12/h8H,2-7H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
binding affinity in rat hippocampi against alpha7 nicotinic receptor |
J Med Chem 43: 4045-50 (2000)
BindingDB Entry DOI: 10.7270/Q24Q7T88 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Rattus norvegicus (Rat)) | BDBM50093258

(3'-Methylspiro[1-azabicyclo[2.2.2]octane-3,4'-2H-t...)Show SMILES CN1C(=O)NCC11CN2CCC1CC2 |TLB:5:6:12.13:10.9,THB:1:6:12.13:10.9,(20.42,-4.1,;20.42,-5.64,;21.67,-6.55,;23.15,-6.07,;21.19,-8.03,;19.64,-8.03,;19.18,-6.55,;18.05,-7.14,;17.48,-5.92,;15.89,-6.44,;16.48,-5.39,;18.17,-4.98,;18.54,-4.03,;17.48,-4.75,)| Show InChI InChI=1S/C10H17N3O/c1-12-9(14)11-6-10(12)7-13-4-2-8(10)3-5-13/h8H,2-7H2,1H3,(H,11,14) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Boston
Curated by ChEMBL
| Assay Description
Binding affinity in rat forebrain against Nicotinic acetylcholine receptor alpha4-beta2 |
J Med Chem 43: 4045-50 (2000)
BindingDB Entry DOI: 10.7270/Q24Q7T88 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50327248
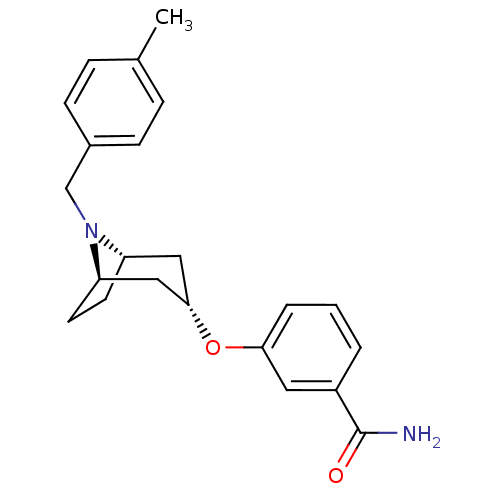
(CHEMBL1257821 | endo-3-(8-(4-methylbenzyl)-8-azabi...)Show SMILES Cc1ccc(CN2[C@H]3CC[C@@H]2C[C@H](C3)Oc2cccc(c2)C(N)=O)cc1 |r,TLB:5:6:12.11.13:9.8| Show InChI InChI=1S/C22H26N2O2/c1-15-5-7-16(8-6-15)14-24-18-9-10-19(24)13-21(12-18)26-20-4-2-3-17(11-20)22(23)25/h2-8,11,18-19,21H,9-10,12-14H2,1H3,(H2,23,25)/t18-,19+,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50327247
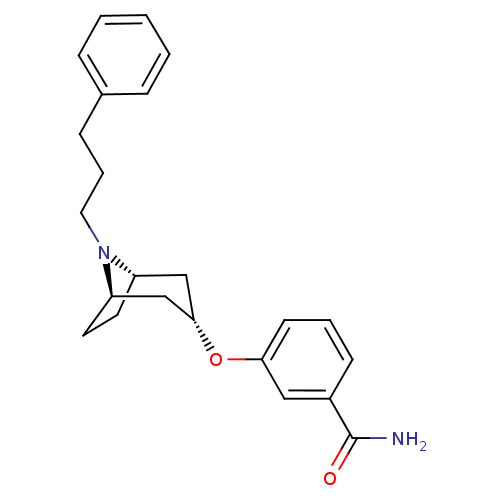
(CHEMBL1257820 | endo-3-(8-(3-phenylpropyl)-8-azabi...)Show SMILES NC(=O)c1cccc(O[C@H]2C[C@@H]3CC[C@H](C2)N3CCCc2ccccc2)c1 |r,TLB:17:16:9.15.10:13.12| Show InChI InChI=1S/C23H28N2O2/c24-23(26)18-9-4-10-21(14-18)27-22-15-19-11-12-20(16-22)25(19)13-5-8-17-6-2-1-3-7-17/h1-4,6-7,9-10,14,19-20,22H,5,8,11-13,15-16H2,(H2,24,26)/t19-,20+,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50327244

(3-Endo-(8-((5-methylthiophen-2-yl)methyl)-8-azabic...)Show SMILES Cc1ccc(CN2[C@H]3CC[C@@H]2C[C@H](C3)Oc2cccc(c2)C(N)=O)s1 |r,TLB:5:6:12.11.13:9.8| Show InChI InChI=1S/C20H24N2O2S/c1-13-5-8-19(25-13)12-22-15-6-7-16(22)11-18(10-15)24-17-4-2-3-14(9-17)20(21)23/h2-5,8-9,15-16,18H,6-7,10-12H2,1H3,(H2,21,23)/t15-,16+,18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50327246
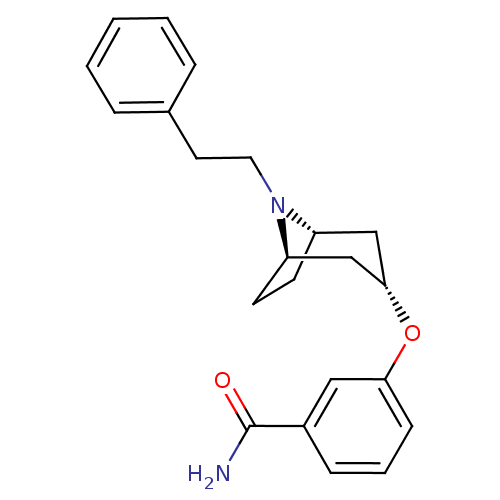
(CHEMBL1257698 | endo-3-(8-phenethyl-8-azabicyclo[3...)Show SMILES NC(=O)c1cccc(O[C@H]2C[C@@H]3CC[C@H](C2)N3CCc2ccccc2)c1 |r,TLB:17:16:9.15.10:13.12| Show InChI InChI=1S/C22H26N2O2/c23-22(25)17-7-4-8-20(13-17)26-21-14-18-9-10-19(15-21)24(18)12-11-16-5-2-1-3-6-16/h1-8,13,18-19,21H,9-12,14-15H2,(H2,23,25)/t18-,19+,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50327243
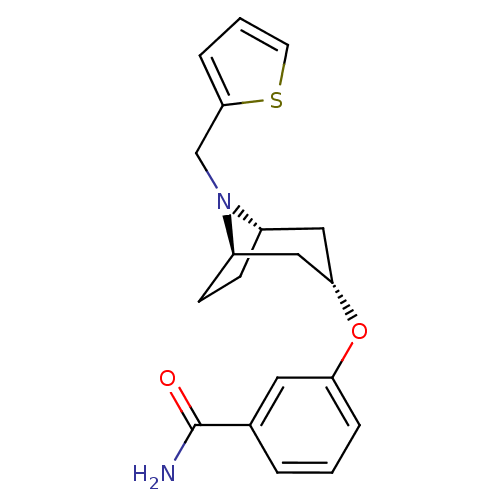
(CHEMBL1257577 | endo-3-(8-(thiophen-2-ylmethyl)-8-...)Show SMILES NC(=O)c1cccc(O[C@H]2C[C@@H]3CC[C@H](C2)N3Cc2cccs2)c1 |r,TLB:17:16:9.15.10:13.12| Show InChI InChI=1S/C19H22N2O2S/c20-19(22)13-3-1-4-16(9-13)23-17-10-14-6-7-15(11-17)21(14)12-18-5-2-8-24-18/h1-5,8-9,14-15,17H,6-7,10-12H2,(H2,20,22)/t14-,15+,17- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50130561

((R)-N-((S)-1-((3R,4R)-4-(3-hydroxyphenyl)-3,4-dime...)Show SMILES CC(C)[C@@H](CN1CC[C@](C)([C@@H](C)C1)c1cccc(O)c1)NC(=O)[C@H]1Cc2ccccc2CN1 Show InChI InChI=1S/C28H39N3O2/c1-19(2)26(30-27(33)25-14-21-8-5-6-9-22(21)16-29-25)18-31-13-12-28(4,20(3)17-31)23-10-7-11-24(32)15-23/h5-11,15,19-20,25-26,29,32H,12-14,16-18H2,1-4H3,(H,30,33)/t20-,25+,26+,28+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Antagonist activity at human kappa opioid receptor assessed as inhibition of dynorphin A-induced [35S]GTPgammaS binding |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50327249
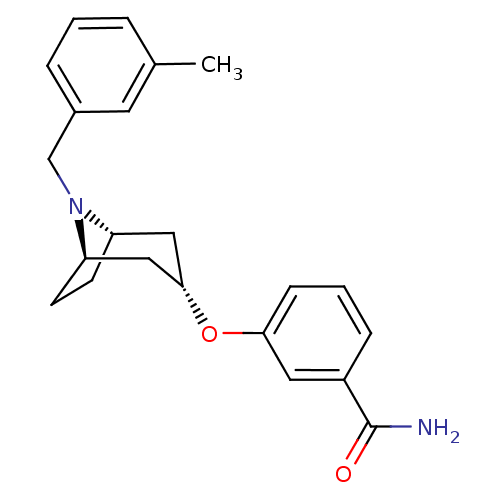
(CHEMBL1257937 | endo-3-(8-(3-methylbenzyl)-8-azabi...)Show SMILES Cc1cccc(CN2[C@H]3CC[C@@H]2C[C@H](C3)Oc2cccc(c2)C(N)=O)c1 |r,TLB:6:7:13.12.14:10.9| Show InChI InChI=1S/C22H26N2O2/c1-15-4-2-5-16(10-15)14-24-18-8-9-19(24)13-21(12-18)26-20-7-3-6-17(11-20)22(23)25/h2-7,10-11,18-19,21H,8-9,12-14H2,1H3,(H2,23,25)/t18-,19+,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50325855

(CHEMBL1223951 | exo-3-((1R,3s,5S)-8-benzyl-8-azabi...)Show SMILES NC(=O)c1cccc(O[C@H]2C[C@@H]3CC[C@H](C2)N3Cc2ccccc2)c1 |r,TLB:17:16:15.9.10:13.12| Show InChI InChI=1S/C21H24N2O2/c22-21(24)16-7-4-8-19(11-16)25-20-12-17-9-10-18(13-20)23(17)14-15-5-2-1-3-6-15/h1-8,11,17-18,20H,9-10,12-14H2,(H2,22,24)/t17-,18+,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50327250
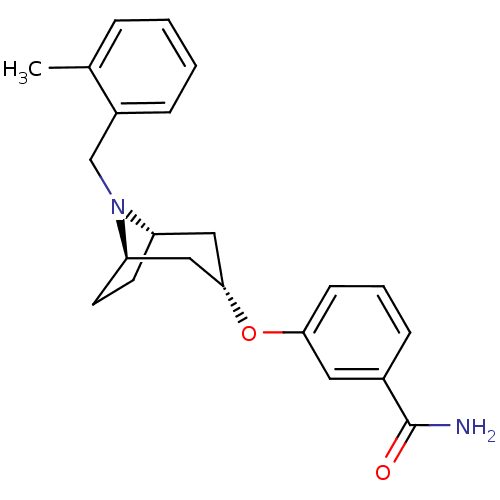
(CHEMBL1257938 | endo-3-(8-(2-methylbenzyl)-8-azabi...)Show SMILES Cc1ccccc1CN1[C@H]2CC[C@@H]1C[C@H](C2)Oc1cccc(c1)C(N)=O |r,TLB:7:8:14.13.15:11.10| Show InChI InChI=1S/C22H26N2O2/c1-15-5-2-3-6-17(15)14-24-18-9-10-19(24)13-21(12-18)26-20-8-4-7-16(11-20)22(23)25/h2-8,11,18-19,21H,9-10,12-14H2,1H3,(H2,23,25)/t18-,19+,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50327256
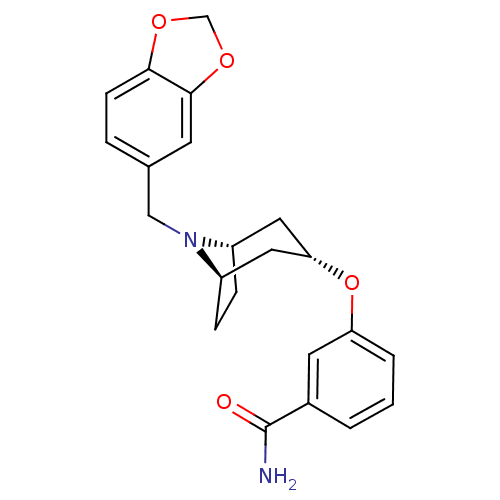
(CHEMBL1258280 | endo-3-(8-(benzo[d][1,3]dioxol-5-y...)Show SMILES NC(=O)c1cccc(O[C@H]2C[C@@H]3CC[C@H](C2)N3Cc2ccc3OCOc3c2)c1 |r,TLB:17:16:9.15.10:13.12| Show InChI InChI=1S/C22H24N2O4/c23-22(25)15-2-1-3-18(9-15)28-19-10-16-5-6-17(11-19)24(16)12-14-4-7-20-21(8-14)27-13-26-20/h1-4,7-9,16-17,19H,5-6,10-13H2,(H2,23,25)/t16-,17+,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50327264
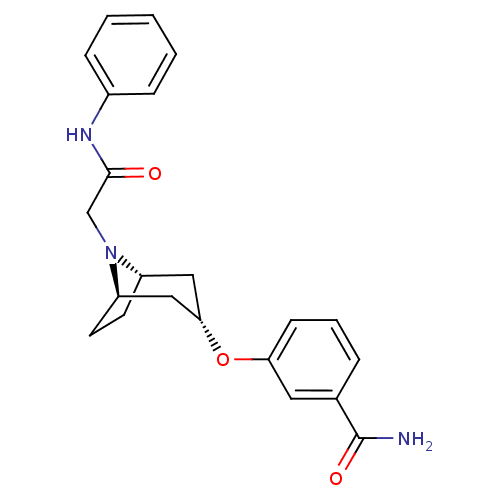
(CHEMBL1258503 | endo-3-(8-(2-oxo-2-(phenylamino)et...)Show SMILES NC(=O)c1cccc(O[C@H]2C[C@@H]3CC[C@H](C2)N3CC(=O)Nc2ccccc2)c1 |r,TLB:17:16:9.15.10:13.12| Show InChI InChI=1S/C22H25N3O3/c23-22(27)15-5-4-8-19(11-15)28-20-12-17-9-10-18(13-20)25(17)14-21(26)24-16-6-2-1-3-7-16/h1-8,11,17-18,20H,9-10,12-14H2,(H2,23,27)(H,24,26)/t17-,18+,20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50327268
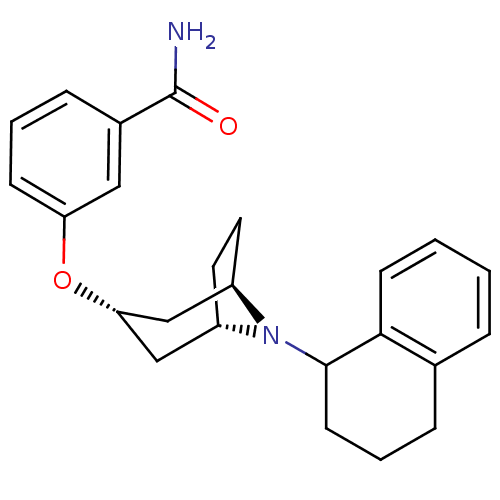
(CHEMBL1258725 | endo-3-(8-(1,2,3,4-tetrahydronapht...)Show SMILES NC(=O)c1cccc(O[C@H]2C[C@@H]3CC[C@H](C2)N3C2CCCc3ccccc23)c1 |r,TLB:17:16:9.15.10:13.12| Show InChI InChI=1S/C24H28N2O2/c25-24(27)17-7-3-8-20(13-17)28-21-14-18-11-12-19(15-21)26(18)23-10-4-6-16-5-1-2-9-22(16)23/h1-3,5,7-9,13,18-19,21,23H,4,6,10-12,14-15H2,(H2,25,27)/t18-,19+,21-,23? | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 11B2, mitochondrial
(Homo sapiens (Human)) | BDBM473831

(2-Chloro-4-(5,5-dimethyl-7- oxo-4,5,6,7-tetrahydro...)Show InChI InChI=1S/C16H14ClN3O/c1-16(2)6-13-15(14(21)7-16)20(9-19-13)11-4-3-10(8-18)12(17)5-11/h3-5,9H,6-7H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim International GmbH
US Patent
| Assay Description
Assays are performed in 96-well format in a final volume of 60 microL/well, containing 100 mM potassium phosphate, pH 7.4, 1% (v/v) DMSO, and additio... |
US Patent US10858342 (2020)
BindingDB Entry DOI: 10.7270/Q2MG7SKT |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50327251
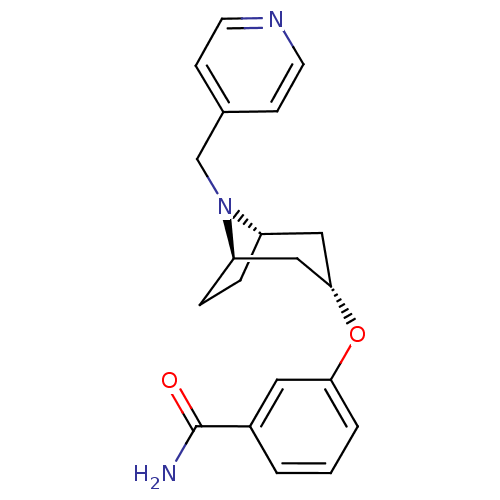
(CHEMBL1258047 | endo-3-(8-(pyridin-4-ylmethyl)-8-a...)Show SMILES NC(=O)c1cccc(O[C@H]2C[C@@H]3CC[C@H](C2)N3Cc2ccncc2)c1 |r,TLB:17:16:9.15.10:13.12| Show InChI InChI=1S/C20H23N3O2/c21-20(24)15-2-1-3-18(10-15)25-19-11-16-4-5-17(12-19)23(16)13-14-6-8-22-9-7-14/h1-3,6-10,16-17,19H,4-5,11-13H2,(H2,21,24)/t16-,17+,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50327266
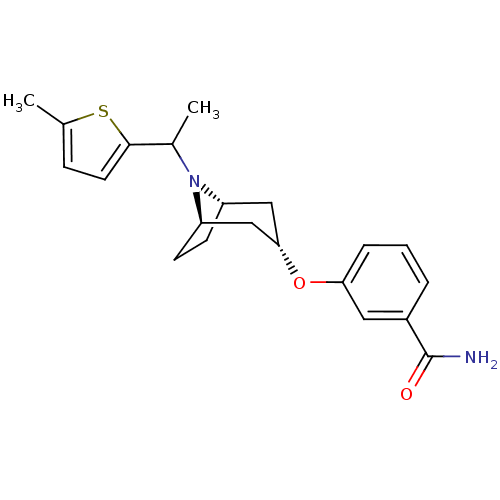
(CHEMBL1258616 | endo-3-(8-(1-(5-methylthiophen-2-y...)Show SMILES CC(N1[C@H]2CC[C@@H]1C[C@H](C2)Oc1cccc(c1)C(N)=O)c1ccc(C)s1 |r,TLB:1:2:8.7.9:5.4| Show InChI InChI=1S/C21H26N2O2S/c1-13-6-9-20(26-13)14(2)23-16-7-8-17(23)12-19(11-16)25-18-5-3-4-15(10-18)21(22)24/h3-6,9-10,14,16-17,19H,7-8,11-12H2,1-2H3,(H2,22,24)/t14?,16-,17+,19- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50327274
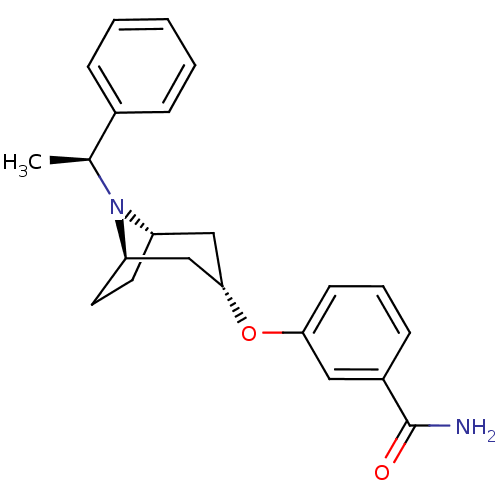
(CHEMBL1257227 | endo-3-(8-((S)-1-phenylethyl)-8-az...)Show SMILES C[C@H](N1[C@H]2CC[C@@H]1C[C@H](C2)Oc1cccc(c1)C(N)=O)c1ccccc1 |r,TLB:1:2:8.7.9:5.4| Show InChI InChI=1S/C22H26N2O2/c1-15(16-6-3-2-4-7-16)24-18-10-11-19(24)14-21(13-18)26-20-9-5-8-17(12-20)22(23)25/h2-9,12,15,18-19,21H,10-11,13-14H2,1H3,(H2,23,25)/t15-,18-,19+,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of human ERG |
Bioorg Med Chem Lett 20: 5847-52 (2010)
Article DOI: 10.1016/j.bmcl.2010.07.113
BindingDB Entry DOI: 10.7270/Q23F4PV1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data