

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50314676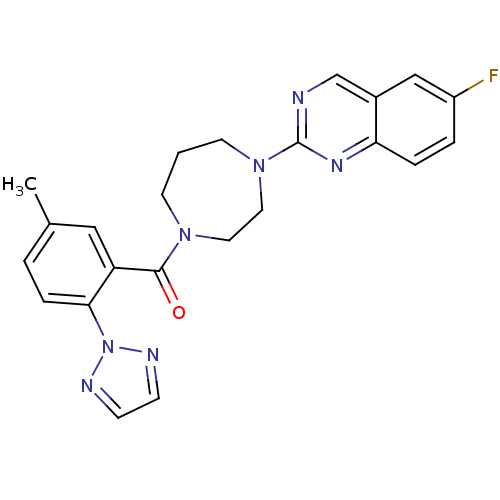 ((4-(6-fluoroquinazolin-2-yl)-1,4-diazepan-1-yl)(5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to OX2 receptor by radioligand displacement assay | Bioorg Med Chem Lett 20: 2311-5 (2010) Article DOI: 10.1016/j.bmcl.2010.01.138 BindingDB Entry DOI: 10.7270/Q2MK6D26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50004167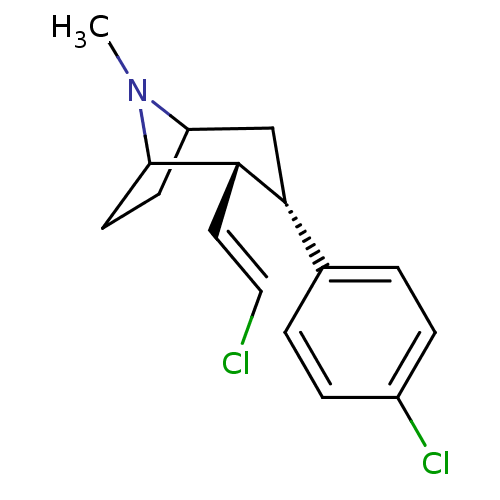 ((2R,3S)-3-(4-Chloro-phenyl)-2-((E)-2-chloro-vinyl)...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding constant for the ability to displace [3H]mazindol to dopamine receptor was calculated from the Cheng-Prusoff relationship | Bioorg Med Chem Lett 3: 1327-1332 (1993) Article DOI: 10.1016/S0960-894X(00)80341-8 BindingDB Entry DOI: 10.7270/Q2RJ4JZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50314681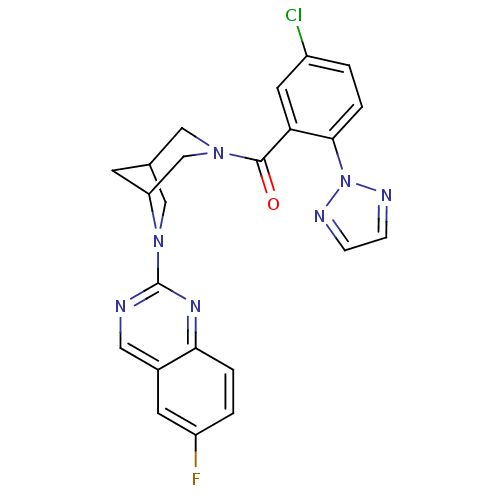 ((5-chloro-2-(2H-1,2,3-triazol-2-yl)phenyl)(6-(6-fl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to OX1 receptor by radioligand displacement assay | Bioorg Med Chem Lett 20: 2311-5 (2010) Article DOI: 10.1016/j.bmcl.2010.01.138 BindingDB Entry DOI: 10.7270/Q2MK6D26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50258741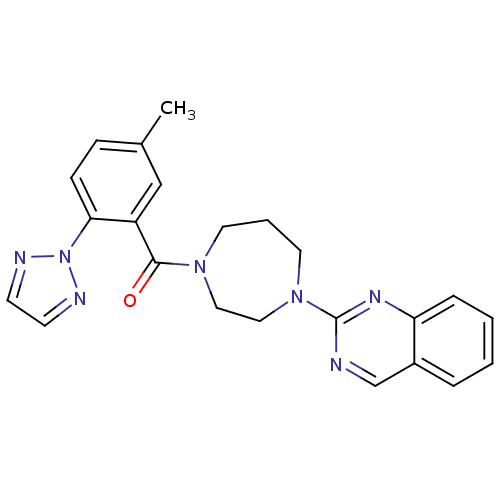 ((5-methyl-2-(2H-1,2,3-triazol-2-yl)phenyl)(4-(quin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to OX2 receptor by radioligand displacement assay | Bioorg Med Chem Lett 20: 2311-5 (2010) Article DOI: 10.1016/j.bmcl.2010.01.138 BindingDB Entry DOI: 10.7270/Q2MK6D26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50314681 ((5-chloro-2-(2H-1,2,3-triazol-2-yl)phenyl)(6-(6-fl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to OX2 receptor by radioligand displacement assay | Bioorg Med Chem Lett 20: 2311-5 (2010) Article DOI: 10.1016/j.bmcl.2010.01.138 BindingDB Entry DOI: 10.7270/Q2MK6D26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50258741 ((5-methyl-2-(2H-1,2,3-triazol-2-yl)phenyl)(4-(quin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to OX2 receptor (unknown origin) | Bioorg Med Chem Lett 19: 2997-3001 (2009) Article DOI: 10.1016/j.bmcl.2009.04.026 BindingDB Entry DOI: 10.7270/Q22J6BR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50369890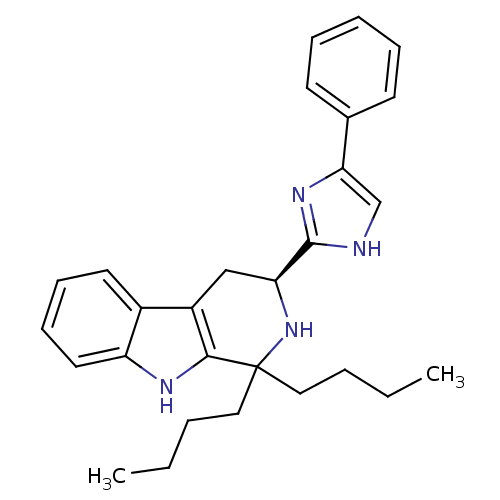 (CHEMBL1237140 | CHEMBL1788167) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]SS14 from human SST3 expressed in CHO membrane after 60 to 90 mins by scintillation counting | ACS Med Chem Lett 5: 690-5 (2014) Article DOI: 10.1021/ml500079u BindingDB Entry DOI: 10.7270/Q22N53V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50021074 (CHEMBL3287628) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.815 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]SS14 from human SST3 expressed in CHO membrane after 60 to 90 mins by scintillation counting | ACS Med Chem Lett 5: 690-5 (2014) Article DOI: 10.1021/ml500079u BindingDB Entry DOI: 10.7270/Q22N53V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent dopamine transporter (Homo sapiens (Human)) | BDBM50004167 ((2R,3S)-3-(4-Chloro-phenyl)-2-((E)-2-chloro-vinyl)...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Binding constant for [3H]dopamine uptake was calculated from the Cheng-Prusoff relationship | Bioorg Med Chem Lett 3: 1327-1332 (1993) Article DOI: 10.1016/S0960-894X(00)80341-8 BindingDB Entry DOI: 10.7270/Q2RJ4JZN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50205990 (CHEMBL395429 | OXYTOCIN) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Curated by ChEMBL | Assay Description Binding affinity against oxytocin receptor in rat uterus | J Med Chem 33: 2321-3 (1990) BindingDB Entry DOI: 10.7270/Q2F76D60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GABA-A receptor; alpha-5/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50065229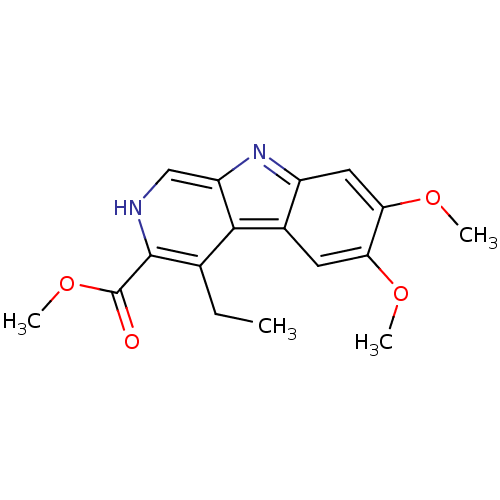 (4-Ethyl-6,7-dimethoxy-9H-beta-carboline-3-carboxyl...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]-Ro-15-1788 binding to human GABA A receptor (alpha5-beta3-gamma2) stably expressed in L(tk-) cells. | J Med Chem 45: 1887-900 (2002) BindingDB Entry DOI: 10.7270/Q2KP81HZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50314676 ((4-(6-fluoroquinazolin-2-yl)-1,4-diazepan-1-yl)(5-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to OX1 receptor by radioligand displacement assay | Bioorg Med Chem Lett 20: 2311-5 (2010) Article DOI: 10.1016/j.bmcl.2010.01.138 BindingDB Entry DOI: 10.7270/Q2MK6D26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50368134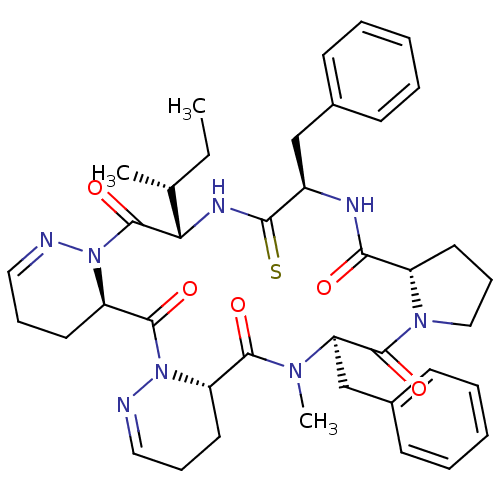 (CHEMBL1790544) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Curated by ChEMBL | Assay Description Binding affinity against oxytocin receptor in rat uterus | J Med Chem 33: 2321-3 (1990) BindingDB Entry DOI: 10.7270/Q2F76D60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50258741 ((5-methyl-2-(2H-1,2,3-triazol-2-yl)phenyl)(4-(quin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to OX1 receptor (unknown origin) | Bioorg Med Chem Lett 19: 2997-3001 (2009) Article DOI: 10.1016/j.bmcl.2009.04.026 BindingDB Entry DOI: 10.7270/Q22J6BR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50258741 ((5-methyl-2-(2H-1,2,3-triazol-2-yl)phenyl)(4-(quin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to OX1 receptor by radioligand displacement assay | Bioorg Med Chem Lett 20: 2311-5 (2010) Article DOI: 10.1016/j.bmcl.2010.01.138 BindingDB Entry DOI: 10.7270/Q2MK6D26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Homo sapiens (Human)) | BDBM50002827 (1-{3-(3-Hydroxy-propoxy)-2-propoxy-5-[(2S,5S)-5-(3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of the binding of [3H]C18-Platelet activating factor to human platelet membrane preparation | J Med Chem 35: 3474-82 (1992) BindingDB Entry DOI: 10.7270/Q2445N3B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50258742 ((5-methyl-2-(2H-1,2,3-triazol-2-yl)phenyl)(4-(naph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to OX2 receptor (unknown origin) | Bioorg Med Chem Lett 19: 2997-3001 (2009) Article DOI: 10.1016/j.bmcl.2009.04.026 BindingDB Entry DOI: 10.7270/Q22J6BR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50258742 ((5-methyl-2-(2H-1,2,3-triazol-2-yl)phenyl)(4-(naph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to OX1 receptor (unknown origin) | Bioorg Med Chem Lett 19: 2997-3001 (2009) Article DOI: 10.1016/j.bmcl.2009.04.026 BindingDB Entry DOI: 10.7270/Q22J6BR1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50314677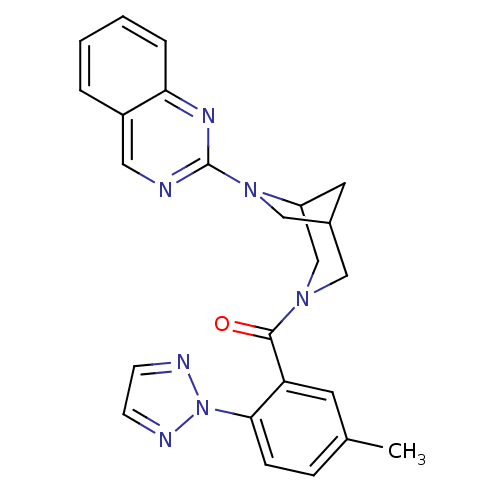 ((5-methyl-2-(2H-1,2,3-triazol-2-yl)phenyl)(6-(quin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to OX1 receptor by radioligand displacement assay | Bioorg Med Chem Lett 20: 2311-5 (2010) Article DOI: 10.1016/j.bmcl.2010.01.138 BindingDB Entry DOI: 10.7270/Q2MK6D26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50314677 ((5-methyl-2-(2H-1,2,3-triazol-2-yl)phenyl)(6-(quin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to OX1 receptor by radioligand displacement assay | Bioorg Med Chem Lett 20: 2311-5 (2010) Article DOI: 10.1016/j.bmcl.2010.01.138 BindingDB Entry DOI: 10.7270/Q2MK6D26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Homo sapiens (Human)) | BDBM50002828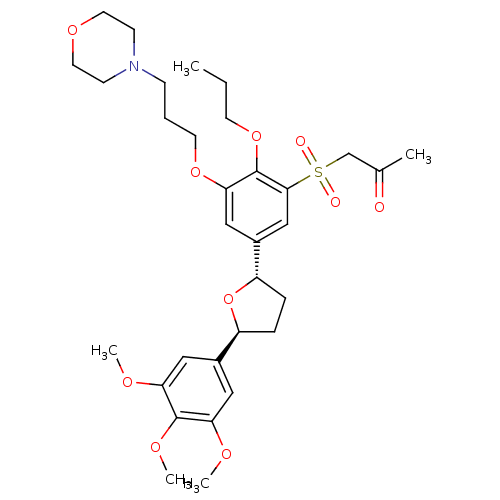 (1-{3-(3-Morpholin-4-yl-propoxy)-2-propoxy-5-[(2S,5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of the binding of [3H]C18-Platelet activating factor to human platelet membrane preparation | J Med Chem 35: 3474-82 (1992) BindingDB Entry DOI: 10.7270/Q2445N3B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Homo sapiens (Human)) | BDBM50002827 (1-{3-(3-Hydroxy-propoxy)-2-propoxy-5-[(2S,5S)-5-(3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro effect on inhibition of the binding of [3H]C18-Platelet activating factor to human PMN membranes preparation | J Med Chem 35: 3474-82 (1992) BindingDB Entry DOI: 10.7270/Q2445N3B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Homo sapiens (Human)) | BDBM50002824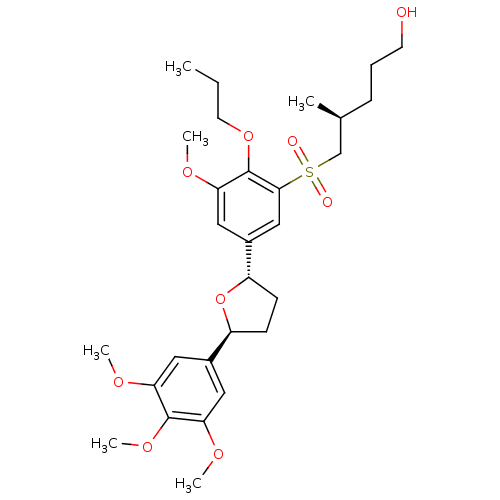 ((S)-5-{3-Methoxy-2-propoxy-5-[(2S,5S)-5-(3,4,5-tri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of the binding of [3H]C18-Platelet activating factor to human platelet membrane preparation | J Med Chem 35: 3474-82 (1992) BindingDB Entry DOI: 10.7270/Q2445N3B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50021075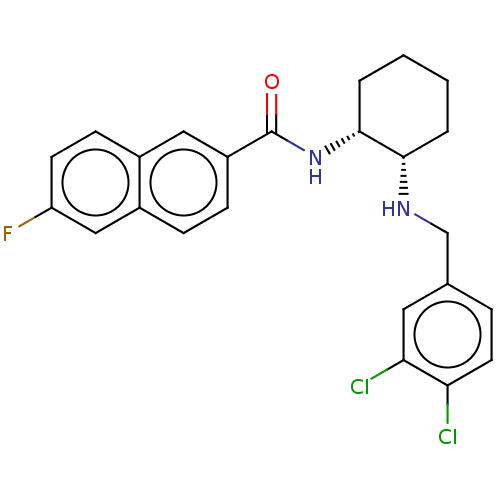 (CHEMBL3287629) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]SS14 from human SST3 expressed in CHO membrane after 60 to 90 mins by scintillation counting | ACS Med Chem Lett 5: 690-5 (2014) Article DOI: 10.1021/ml500079u BindingDB Entry DOI: 10.7270/Q22N53V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Homo sapiens (Human)) | BDBM50002826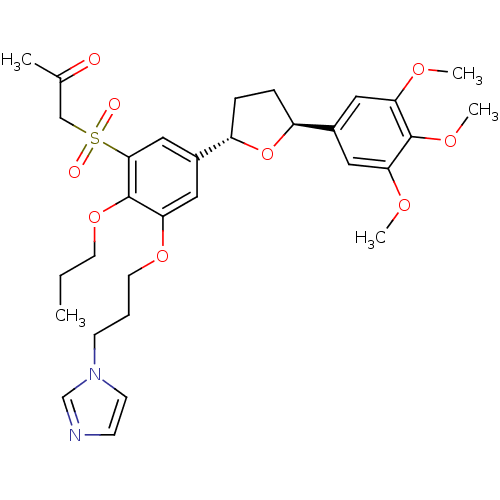 (1-{3-(3-Imidazol-1-yl-propoxy)-2-propoxy-5-[(2S,5S...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of the binding of [3H]C18-Platelet activating factor to human platelet membrane preparation | J Med Chem 35: 3474-82 (1992) BindingDB Entry DOI: 10.7270/Q2445N3B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Homo sapiens (Human)) | BDBM50002823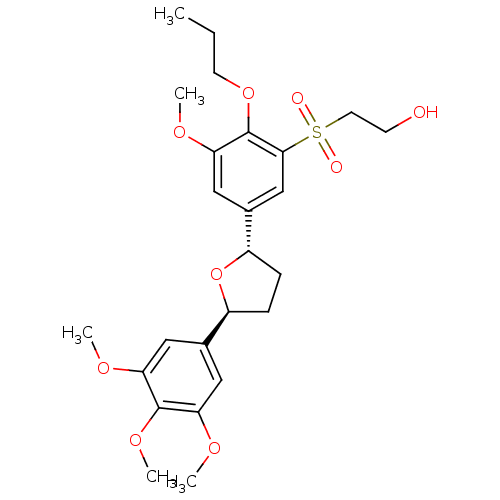 (2-{3-Methoxy-2-propoxy-5-[(2S,5S)-5-(3,4,5-trimeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro effect on inhibition of the binding of [3H]C18-Platelet activating factor to human PMN membranes preparation | J Med Chem 35: 3474-82 (1992) BindingDB Entry DOI: 10.7270/Q2445N3B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50368130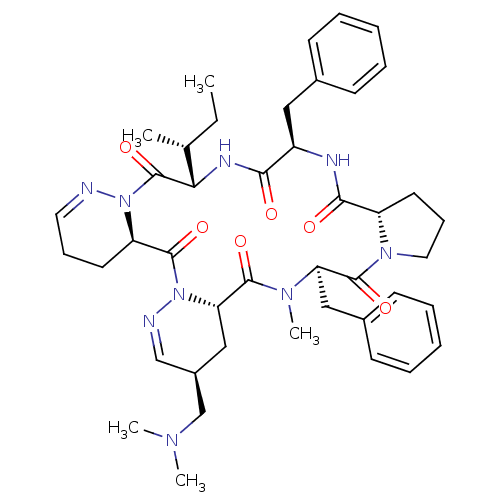 (CHEMBL1790551) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Curated by ChEMBL | Assay Description Binding affinity against oxytocin receptor in rat uterus | J Med Chem 33: 2321-3 (1990) BindingDB Entry DOI: 10.7270/Q2F76D60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-3/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50112233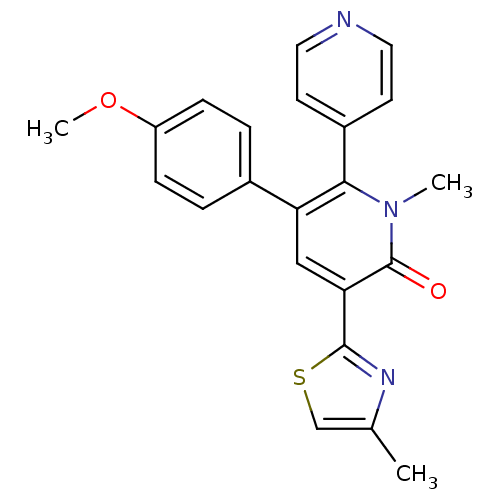 (3-(4-Methoxy-phenyl)-1-methyl-5-(4-methyl-thiazol-...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Binding affinity by displacement of [3H]-Ro-15-1788 from recombinant human gamma-aminobutyric-acid A receptor alpha3,beta3,gamma2 stably expressed in... | J Med Chem 45: 1887-900 (2002) BindingDB Entry DOI: 10.7270/Q2KP81HZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-3/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50112224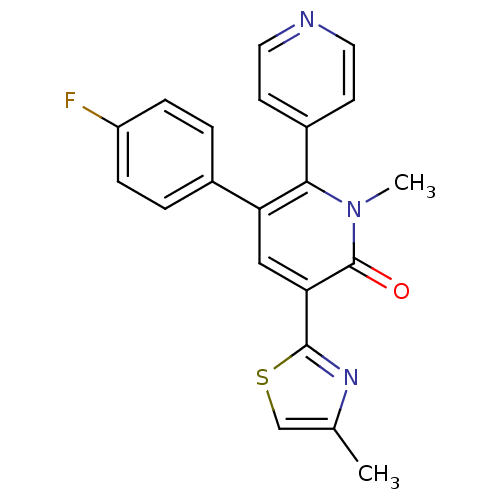 (3-(4-Fluoro-phenyl)-1-methyl-5-(4-methyl-thiazol-2...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Binding affinity by displacement of [3H]-Ro-15-1788 from recombinant human gamma-aminobutyric-acid A receptor alpha3,beta3,gamma2 stably expressed in... | J Med Chem 45: 1887-900 (2002) BindingDB Entry DOI: 10.7270/Q2KP81HZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50314677 ((5-methyl-2-(2H-1,2,3-triazol-2-yl)phenyl)(6-(quin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to OX2 receptor by radioligand displacement assay | Bioorg Med Chem Lett 20: 2311-5 (2010) Article DOI: 10.1016/j.bmcl.2010.01.138 BindingDB Entry DOI: 10.7270/Q2MK6D26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50314677 ((5-methyl-2-(2H-1,2,3-triazol-2-yl)phenyl)(6-(quin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to OX2 receptor by radioligand displacement assay | Bioorg Med Chem Lett 20: 2311-5 (2010) Article DOI: 10.1016/j.bmcl.2010.01.138 BindingDB Entry DOI: 10.7270/Q2MK6D26 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50021090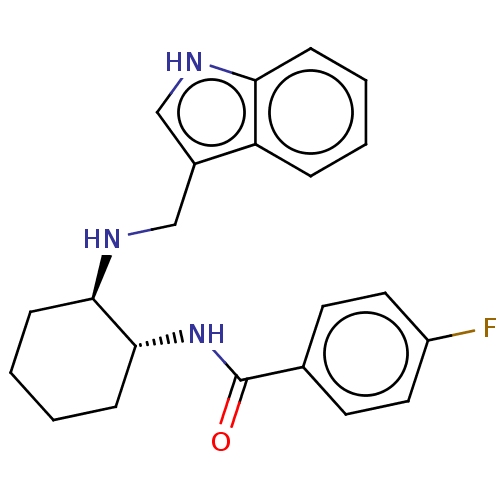 (CHEMBL3287632) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]SS14 from human SST3 expressed in CHO membrane after 60 to 90 mins by scintillation counting | ACS Med Chem Lett 5: 690-5 (2014) Article DOI: 10.1021/ml500079u BindingDB Entry DOI: 10.7270/Q22N53V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Homo sapiens (Human)) | BDBM50002825 ((R)-5-{3-Methoxy-2-propoxy-5-[(2S,5S)-5-(3,4,5-tri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of the binding of [3H]C18-Platelet activating factor to human platelet membrane preparation | J Med Chem 35: 3474-82 (1992) BindingDB Entry DOI: 10.7270/Q2445N3B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Homo sapiens (Human)) | BDBM50002824 ((S)-5-{3-Methoxy-2-propoxy-5-[(2S,5S)-5-(3,4,5-tri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description In vitro effect on inhibition of the binding of [3H]C18-Platelet activating factor to human PMN membranes preparation | J Med Chem 35: 3474-82 (1992) BindingDB Entry DOI: 10.7270/Q2445N3B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Homo sapiens (Human)) | BDBM50002825 ((R)-5-{3-Methoxy-2-propoxy-5-[(2S,5S)-5-(3,4,5-tri...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.72 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of the binding of [3H]C18-Platelet activating factor to human platelet membrane preparation | J Med Chem 35: 3474-82 (1992) BindingDB Entry DOI: 10.7270/Q2445N3B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50021063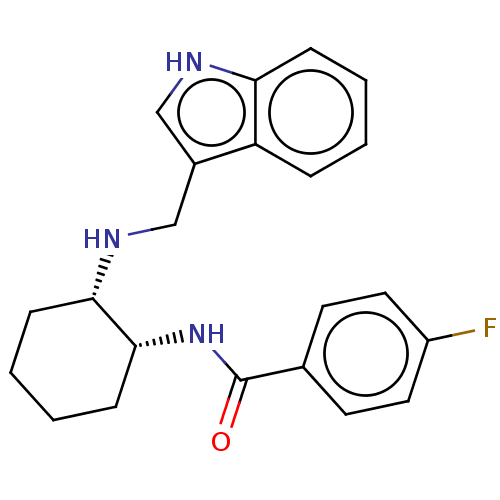 (CHEMBL3287613) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]SS14 from human SST3 expressed in CHO membrane after 60 to 90 mins by scintillation counting | ACS Med Chem Lett 5: 690-5 (2014) Article DOI: 10.1021/ml500079u BindingDB Entry DOI: 10.7270/Q22N53V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-3/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50112218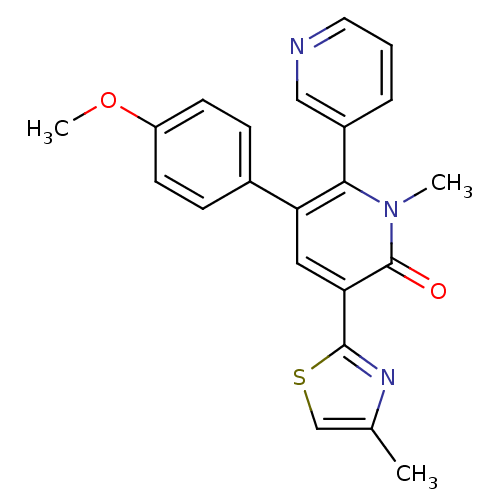 (3-(4-Methoxy-phenyl)-1-methyl-5-(4-methyl-thiazol-...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Binding affinity by displacement of [3H]-Ro-15-1788 from recombinant human gamma-aminobutyric-acid A receptor alpha3,beta3,gamma2 stably expressed in... | J Med Chem 45: 1887-900 (2002) BindingDB Entry DOI: 10.7270/Q2KP81HZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-3/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50065229 (4-Ethyl-6,7-dimethoxy-9H-beta-carboline-3-carboxyl...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Binding affinity by displacement of [3H]-Ro-15-1788 from recombinant human gamma-aminobutyric-acid A receptor alpha3,beta3,gamma2 stably expressed in... | J Med Chem 45: 1887-900 (2002) BindingDB Entry DOI: 10.7270/Q2KP81HZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-3/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50112232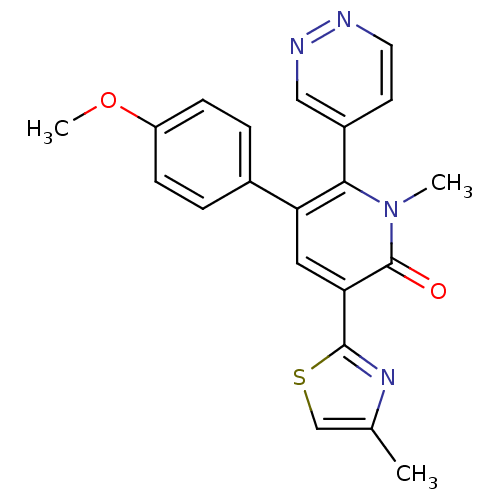 (5-(4-Methoxy-phenyl)-1-methyl-3-(4-methyl-thiazol-...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Binding affinity by displacement of [3H]-Ro-15-1788 from recombinant human gamma-aminobutyric-acid A receptor alpha3,beta3,gamma2 stably expressed in... | J Med Chem 45: 1887-900 (2002) BindingDB Entry DOI: 10.7270/Q2KP81HZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50021109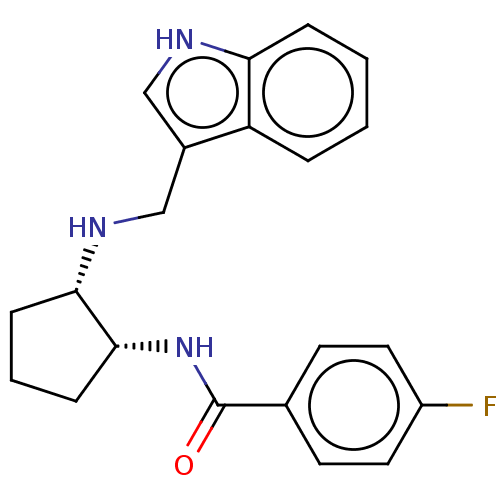 (CHEMBL3287633) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]SS14 from human SST3 expressed in CHO membrane after 60 to 90 mins by scintillation counting | ACS Med Chem Lett 5: 690-5 (2014) Article DOI: 10.1021/ml500079u BindingDB Entry DOI: 10.7270/Q22N53V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50021073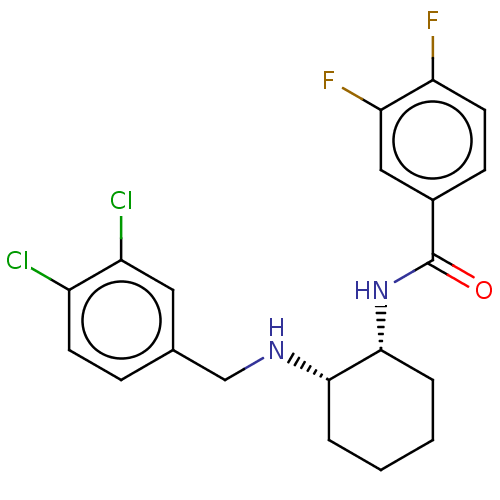 (CHEMBL3287627) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]SS14 from human SST3 expressed in CHO membrane after 60 to 90 mins by scintillation counting | ACS Med Chem Lett 5: 690-5 (2014) Article DOI: 10.1021/ml500079u BindingDB Entry DOI: 10.7270/Q22N53V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GABA-A receptor; alpha-2/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50112218 (3-(4-Methoxy-phenyl)-1-methyl-5-(4-methyl-thiazol-...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-Ro-15-1788 from human Gamma-aminobutyric-acid A receptor alpha2-beta3-gamma2 stably expressed in L(tk-) cells | J Med Chem 45: 1887-900 (2002) BindingDB Entry DOI: 10.7270/Q2KP81HZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-1/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50065229 (4-Ethyl-6,7-dimethoxy-9H-beta-carboline-3-carboxyl...) | GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Inhibition of [3H]-Ro-15-1788 binding to human GABA A receptor (alpha1-beta3-gamma2) stably expressed in L(tk-) cells. | J Med Chem 45: 1887-900 (2002) BindingDB Entry DOI: 10.7270/Q2KP81HZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-3/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50112220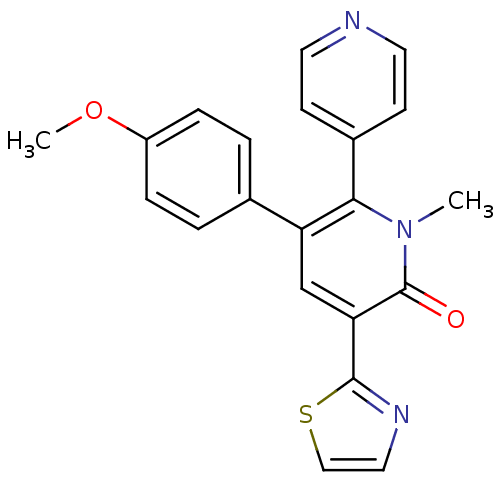 (3-(4-Methoxy-phenyl)-1-methyl-5-thiazol-2-yl-1H-[2...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Binding affinity by displacement of [3H]-Ro-15-1788 from recombinant human gamma-aminobutyric-acid A receptor alpha3,beta3,gamma2 stably expressed in... | J Med Chem 45: 1887-900 (2002) BindingDB Entry DOI: 10.7270/Q2KP81HZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet-activating factor receptor (Homo sapiens (Human)) | BDBM50002823 (2-{3-Methoxy-2-propoxy-5-[(2S,5S)-5-(3,4,5-trimeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of the binding of [3H]C18-Platelet activating factor to human platelet membrane preparation | J Med Chem 35: 3474-82 (1992) BindingDB Entry DOI: 10.7270/Q2445N3B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GABA-A receptor; alpha-2/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50112232 (5-(4-Methoxy-phenyl)-1-methyl-3-(4-methyl-thiazol-...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-Ro-15-1788 from human Gamma-aminobutyric-acid A receptor alpha2-beta3-gamma2 stably expressed in L(tk-) cells | J Med Chem 45: 1887-900 (2002) BindingDB Entry DOI: 10.7270/Q2KP81HZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gamma-aminobutyric acid receptor subunit alpha-3/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50112237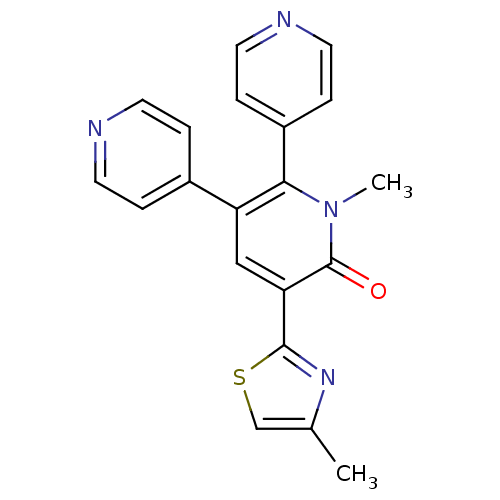 (1'-Methyl-5'-(4-methyl-thiazol-2-yl)-1'H-[4,2';3',...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Binding affinity by displacement of [3H]-Ro-15-1788 from recombinant human gamma-aminobutyric-acid A receptor alpha3,beta3,gamma2 stably expressed in... | J Med Chem 45: 1887-900 (2002) BindingDB Entry DOI: 10.7270/Q2KP81HZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Somatostatin receptor type 3 (Homo sapiens (Human)) | BDBM50021064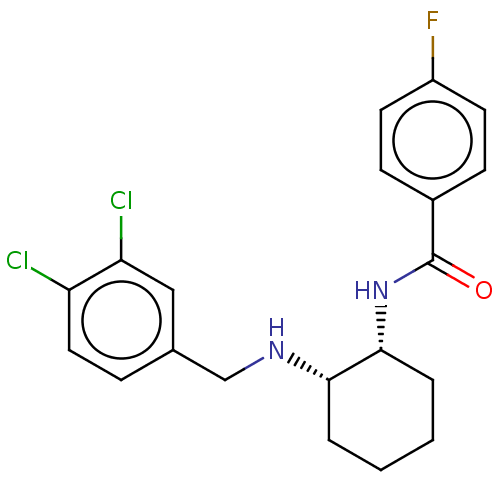 (CHEMBL3287614) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [125I]SS14 from human SST3 expressed in CHO membrane after 60 to 90 mins by scintillation counting | ACS Med Chem Lett 5: 690-5 (2014) Article DOI: 10.1021/ml500079u BindingDB Entry DOI: 10.7270/Q22N53V4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxytocin receptor (RAT) | BDBM50368132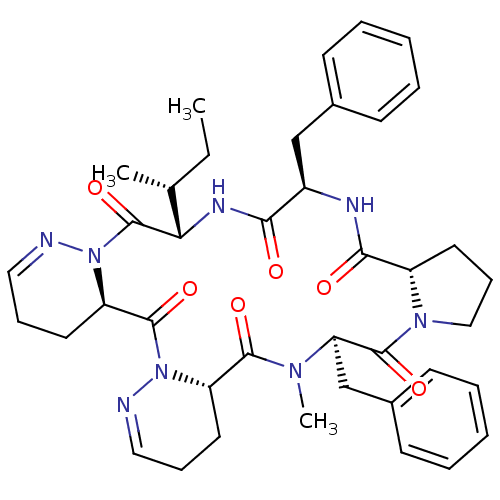 (CHEMBL1790546) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Curated by ChEMBL | Assay Description Binding affinity against oxytocin receptor in rat uterus | J Med Chem 33: 2321-3 (1990) BindingDB Entry DOI: 10.7270/Q2F76D60 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| GABA-A receptor; alpha-2/beta-3/gamma-2 (Homo sapiens (Human)) | BDBM50112224 (3-(4-Fluoro-phenyl)-1-methyl-5-(4-methyl-thiazol-2...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Sharp & Dohme Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]-Ro-15-1788 from human Gamma-aminobutyric-acid A receptor alpha2-beta3-gamma2 stably expressed in L(tk-) cells | J Med Chem 45: 1887-900 (2002) BindingDB Entry DOI: 10.7270/Q2KP81HZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1403 total ) | Next | Last >> |