

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tripeptidyl-peptidase 2 (Rattus norvegicus) | BDBM50176918 ((2S)-aminobutyryl-(2S)-indoline carboxylic acid pr...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition of TPPII in rat cererbral membrane | J Med Chem 48: 7333-42 (2005) Article DOI: 10.1021/jm0500830 BindingDB Entry DOI: 10.7270/Q2KK9CMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tripeptidyl-peptidase 2 (Rattus norvegicus) | BDBM50121282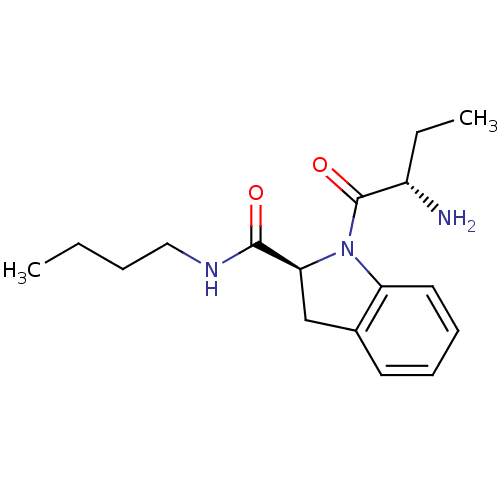 ((S)-1-((S)-2-aminobutanoyl)-N-butylindoline-2-carb...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition of TPPII in rat cererbral membrane | J Med Chem 48: 7333-42 (2005) Article DOI: 10.1021/jm0500830 BindingDB Entry DOI: 10.7270/Q2KK9CMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tripeptidyl-peptidase 2 (Rattus norvegicus) | BDBM50176940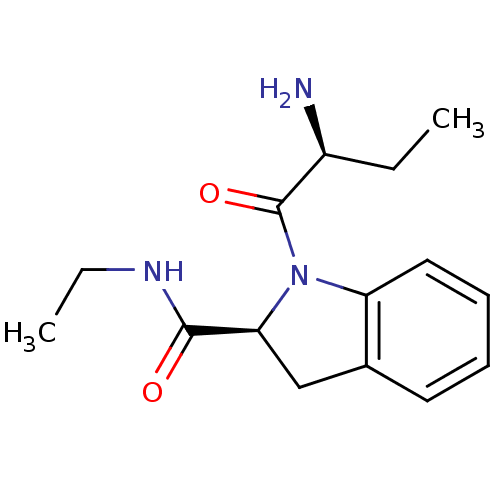 ((2S)-aminobutyryl-(2S)-indoline carboxylic acid et...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition of TPPII in rat cererbral membrane | J Med Chem 48: 7333-42 (2005) Article DOI: 10.1021/jm0500830 BindingDB Entry DOI: 10.7270/Q2KK9CMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tripeptidyl-peptidase 2 (Rattus norvegicus) | BDBM50176934 ((2S)-aminobutyryl-L-proline n-butylamide | CHEMBL2...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition of TPPII in rat cererbral membrane | J Med Chem 48: 7333-42 (2005) Article DOI: 10.1021/jm0500830 BindingDB Entry DOI: 10.7270/Q2KK9CMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tripeptidyl-peptidase 2 (Rattus norvegicus) | BDBM50176913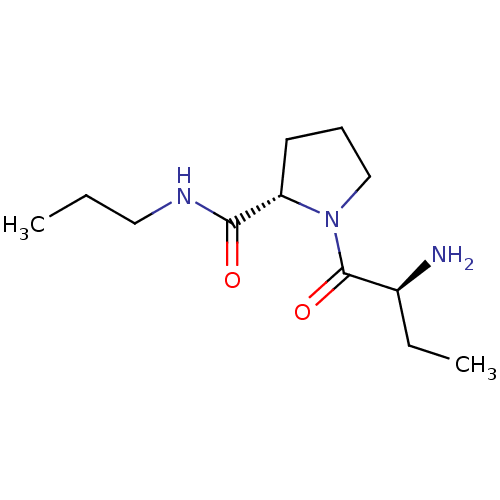 ((2S)-aminobutyryl-L-proline n-propylamide | CHEMBL...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition of TPPII in rat cererbral membrane | J Med Chem 48: 7333-42 (2005) Article DOI: 10.1021/jm0500830 BindingDB Entry DOI: 10.7270/Q2KK9CMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tripeptidyl-peptidase 2 (Rattus norvegicus) | BDBM50176939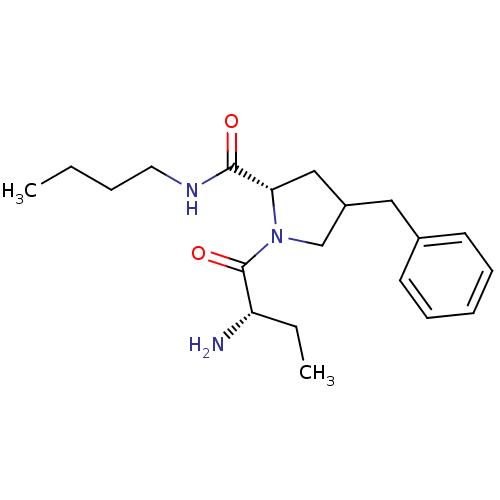 ((2S)-1-((S)-2-aminobutanoyl)-4-benzyl-N-butylpyrro...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition of TPPII in rat cererbral membrane | J Med Chem 48: 7333-42 (2005) Article DOI: 10.1021/jm0500830 BindingDB Entry DOI: 10.7270/Q2KK9CMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tripeptidyl-peptidase 2 (Rattus norvegicus) | BDBM50176936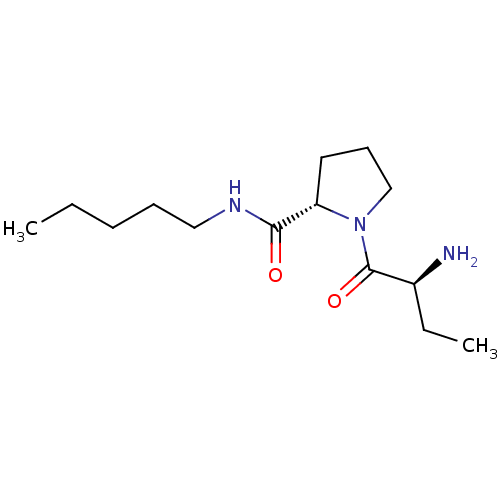 ((2S)-aminobutyryl-L-proline n-pentylamide | CHEMBL...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition of TPPII in rat cererbral membrane | J Med Chem 48: 7333-42 (2005) Article DOI: 10.1021/jm0500830 BindingDB Entry DOI: 10.7270/Q2KK9CMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tripeptidyl-peptidase 2 (Rattus norvegicus) | BDBM50176927 ((2S)-aminobutyryl-L-proline 3-methylthiopropylamid...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition of TPPII in rat cererbral membrane | J Med Chem 48: 7333-42 (2005) Article DOI: 10.1021/jm0500830 BindingDB Entry DOI: 10.7270/Q2KK9CMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tripeptidyl-peptidase 2 (Rattus norvegicus) | BDBM50176935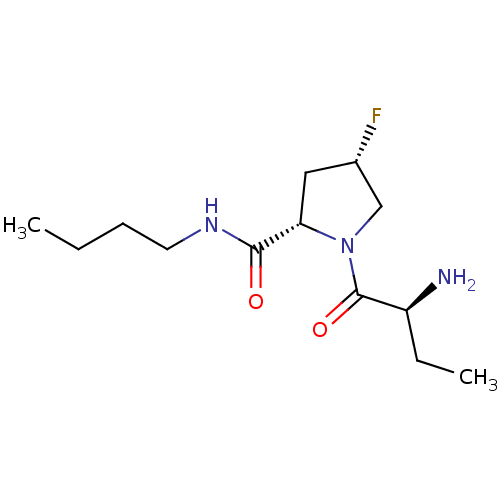 ((2S,4S)-1-((S)-2-aminobutanoyl)-N-butyl-4-fluoropy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition of TPPII in rat cererbral membrane | J Med Chem 48: 7333-42 (2005) Article DOI: 10.1021/jm0500830 BindingDB Entry DOI: 10.7270/Q2KK9CMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tripeptidyl-peptidase 2 (Rattus norvegicus) | BDBM50176945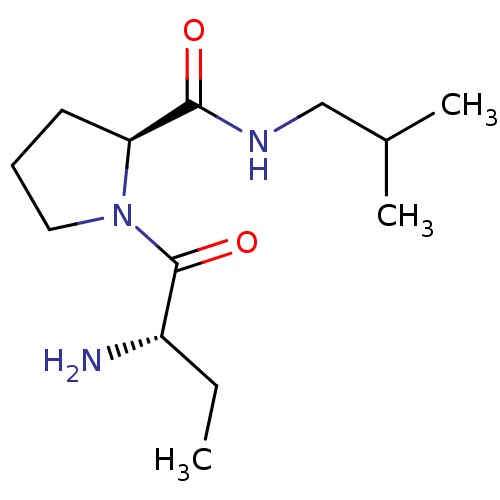 ((2S)-aminobutyryl-L-proline isobutylamide | CHEMBL...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition of TPPII in rat cererbral membrane | J Med Chem 48: 7333-42 (2005) Article DOI: 10.1021/jm0500830 BindingDB Entry DOI: 10.7270/Q2KK9CMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tripeptidyl-peptidase 2 (Rattus norvegicus) | BDBM50176922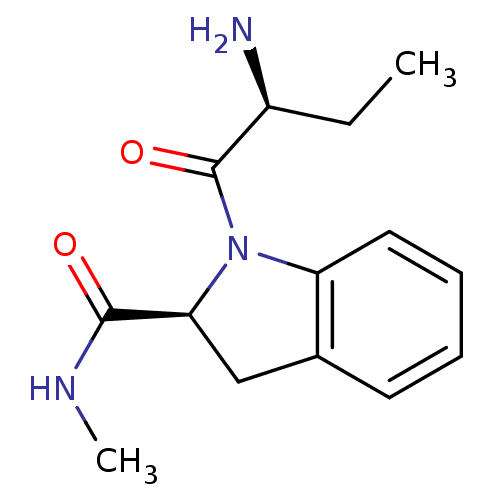 ((2S)-aminobutyryl-(2S)-indoline carboxylic acid me...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition of TPPII in rat cererbral membrane | J Med Chem 48: 7333-42 (2005) Article DOI: 10.1021/jm0500830 BindingDB Entry DOI: 10.7270/Q2KK9CMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tripeptidyl-peptidase 2 (Rattus norvegicus) | BDBM50176914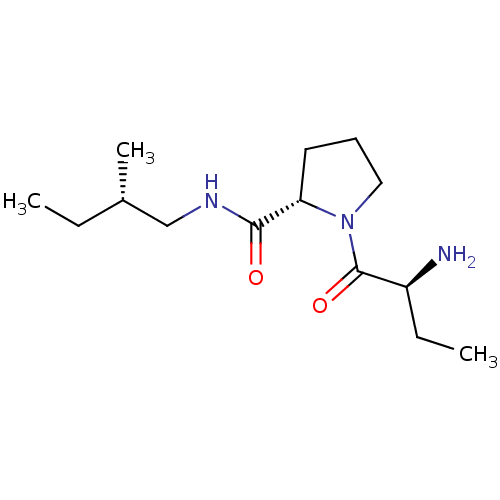 ((2S)-aminobutyryl-L-proline-(2S)-methylbutylamide ...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition of TPPII in rat cererbral membrane | J Med Chem 48: 7333-42 (2005) Article DOI: 10.1021/jm0500830 BindingDB Entry DOI: 10.7270/Q2KK9CMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tripeptidyl-peptidase 2 (Rattus norvegicus) | BDBM50176932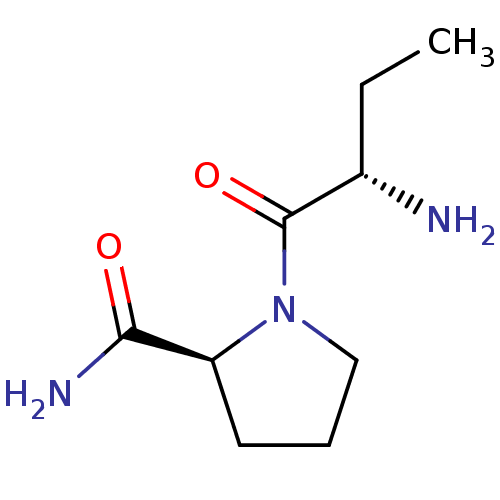 ((2S)-aminobutyryl-L-prolinamide | CHEMBL224063) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition of TPPII in rat cererbral membrane | J Med Chem 48: 7333-42 (2005) Article DOI: 10.1021/jm0500830 BindingDB Entry DOI: 10.7270/Q2KK9CMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tripeptidyl-peptidase 2 (Rattus norvegicus) | BDBM50176920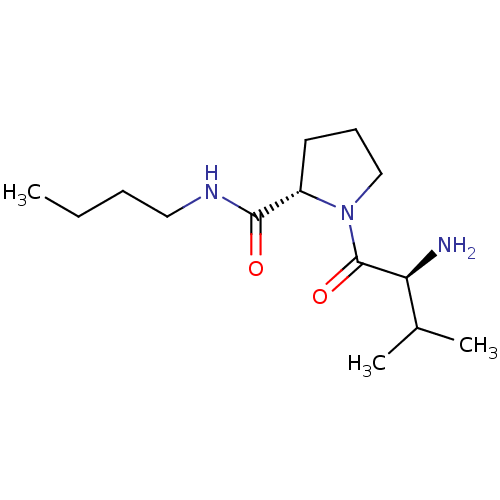 ((S)-1-((S)-2-amino-3-methylbutanoyl)-N-butylpyrrol...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition of TPPII in rat cererbral membrane | J Med Chem 48: 7333-42 (2005) Article DOI: 10.1021/jm0500830 BindingDB Entry DOI: 10.7270/Q2KK9CMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tripeptidyl-peptidase 2 (Rattus norvegicus) | BDBM50176928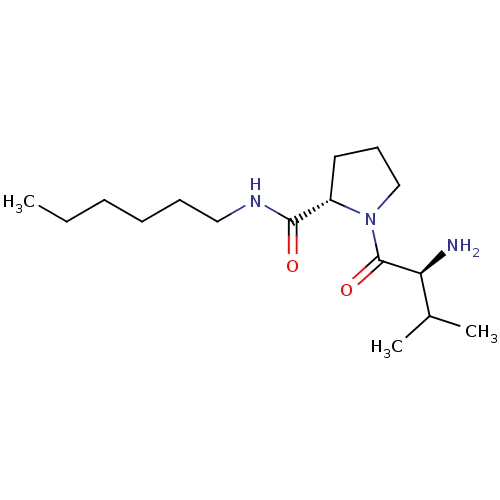 (CHEMBL375490 | L-valyl-L-proline hexylamide) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition of TPPII in rat cererbral membrane | J Med Chem 48: 7333-42 (2005) Article DOI: 10.1021/jm0500830 BindingDB Entry DOI: 10.7270/Q2KK9CMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tripeptidyl-peptidase 2 (Rattus norvegicus) | BDBM50085083 (2-{[1-(2-Amino-3-methyl-pentanoyl)-pyrrolidine-2-c...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition potency against Cholecystokinin-8-Inactivating Peptidase (Serine Peptidase). | J Med Chem 43: 664-74 (2000) BindingDB Entry DOI: 10.7270/Q2RX9CTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tripeptidyl-peptidase 2 (Rattus norvegicus) | BDBM50176926 (CHEMBL224628 | L-norvalyl-L-prolinamide) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition of TPPII in rat cererbral membrane | J Med Chem 48: 7333-42 (2005) Article DOI: 10.1021/jm0500830 BindingDB Entry DOI: 10.7270/Q2KK9CMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tripeptidyl-peptidase 2 (Rattus norvegicus) | BDBM50176919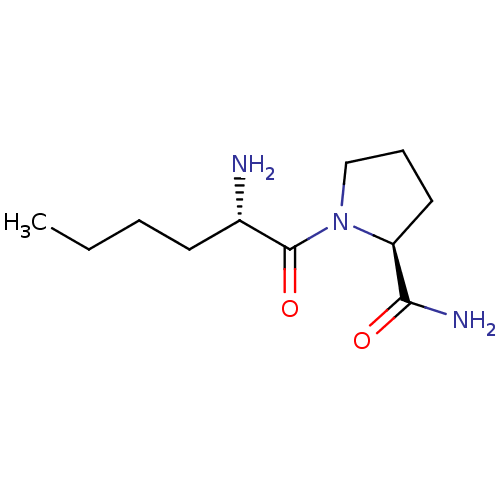 (CHEMBL388843 | L-norleucyl-L-prolinamide) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition of TPPII in rat cererbral membrane | J Med Chem 48: 7333-42 (2005) Article DOI: 10.1021/jm0500830 BindingDB Entry DOI: 10.7270/Q2KK9CMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tripeptidyl-peptidase 2 (Rattus norvegicus) | BDBM50176925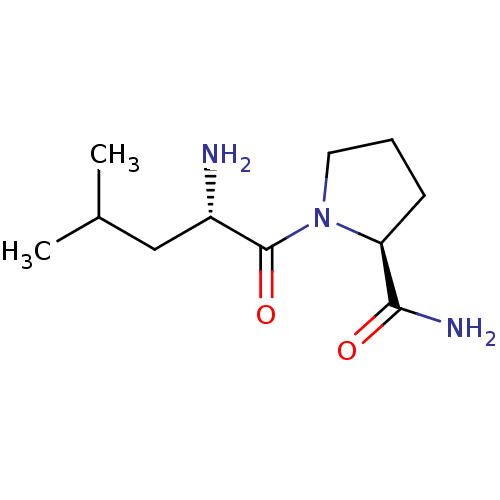 (CHEMBL224340 | L-leucyl-L-prolinamide) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition of TPPII in rat cererbral membrane | J Med Chem 48: 7333-42 (2005) Article DOI: 10.1021/jm0500830 BindingDB Entry DOI: 10.7270/Q2KK9CMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tripeptidyl-peptidase 2 (Rattus norvegicus) | BDBM50176943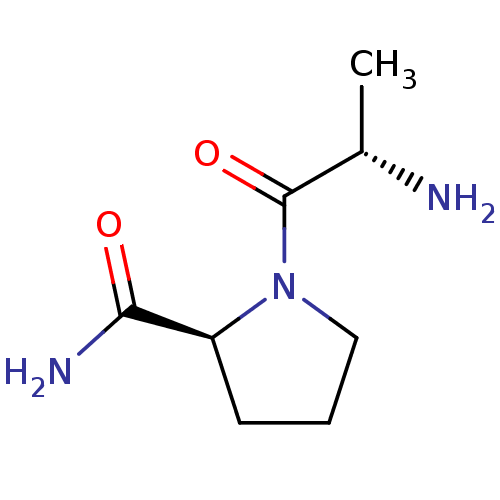 ((S)-1-((S)-2-aminopropanoyl)pyrrolidine-2-carboxam...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition of TPPII in rat cererbral membrane | J Med Chem 48: 7333-42 (2005) Article DOI: 10.1021/jm0500830 BindingDB Entry DOI: 10.7270/Q2KK9CMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tripeptidyl-peptidase 2 (Rattus norvegicus) | BDBM50085116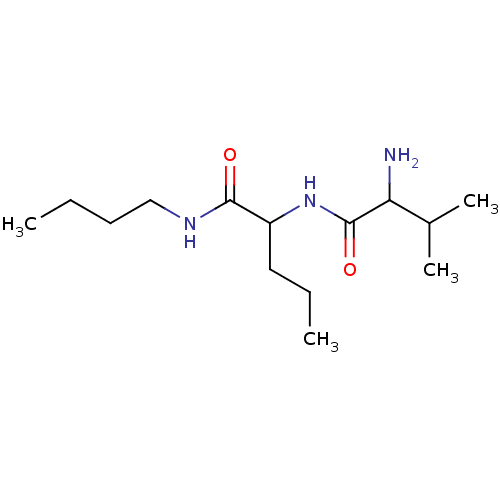 (2-(2-Amino-3-methyl-butyrylamino)-pentanoic acid b...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition potency against Cholecystokinin-8-Inactivating Peptidase (Serine Peptidase). | J Med Chem 43: 664-74 (2000) BindingDB Entry DOI: 10.7270/Q2RX9CTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tripeptidyl-peptidase 2 (Rattus norvegicus) | BDBM50085127 (2-{[1-(2-Amino-propionyl)-pyrrolidine-2-carbonyl]-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition potency against Cholecystokinin-8-Inactivating Peptidase (Serine Peptidase). | J Med Chem 43: 664-74 (2000) BindingDB Entry DOI: 10.7270/Q2RX9CTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tripeptidyl-peptidase 2 (Rattus norvegicus) | BDBM50176931 (CHEMBL376375 | L-valyl-L-proline benzylamide) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition of TPPII in rat cererbral membrane | J Med Chem 48: 7333-42 (2005) Article DOI: 10.1021/jm0500830 BindingDB Entry DOI: 10.7270/Q2KK9CMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tripeptidyl-peptidase 2 (Rattus norvegicus) | BDBM50176924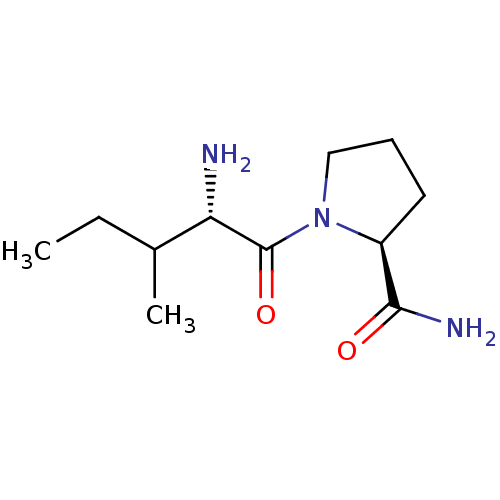 (CHEMBL224341 | L-isoleucyl-L-prolinamide) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition of TPPII in rat cererbral membrane | J Med Chem 48: 7333-42 (2005) Article DOI: 10.1021/jm0500830 BindingDB Entry DOI: 10.7270/Q2KK9CMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tripeptidyl-peptidase 2 (Rattus norvegicus) | BDBM50176930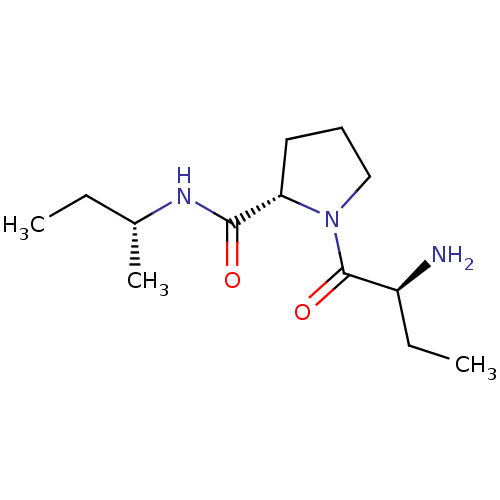 ((2S)-aminobutyryl-L-proline (R)-sec-butylamide | C...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition of TPPII in rat cererbral membrane | J Med Chem 48: 7333-42 (2005) Article DOI: 10.1021/jm0500830 BindingDB Entry DOI: 10.7270/Q2KK9CMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tripeptidyl-peptidase 2 (Rattus norvegicus) | BDBM50176938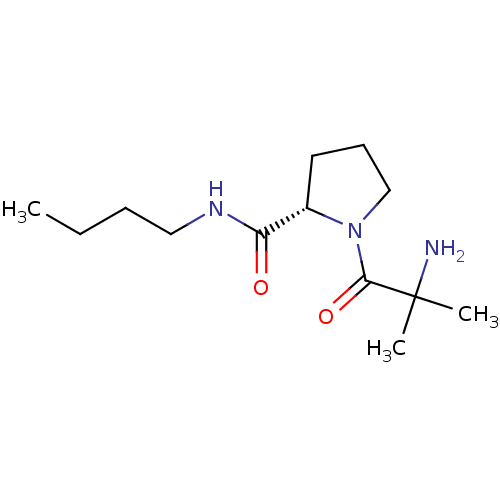 (CHEMBL388062 | alpha-methylalanyl-L-proline butyla...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition of TPPII in rat cererbral membrane | J Med Chem 48: 7333-42 (2005) Article DOI: 10.1021/jm0500830 BindingDB Entry DOI: 10.7270/Q2KK9CMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tripeptidyl-peptidase 2 (Rattus norvegicus) | BDBM50176944 (1-((S)-2-aminobutanoyl)-N-butylazepane-2-carboxami...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition of TPPII in rat cererbral membrane | J Med Chem 48: 7333-42 (2005) Article DOI: 10.1021/jm0500830 BindingDB Entry DOI: 10.7270/Q2KK9CMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tripeptidyl-peptidase 2 (Rattus norvegicus) | BDBM50176929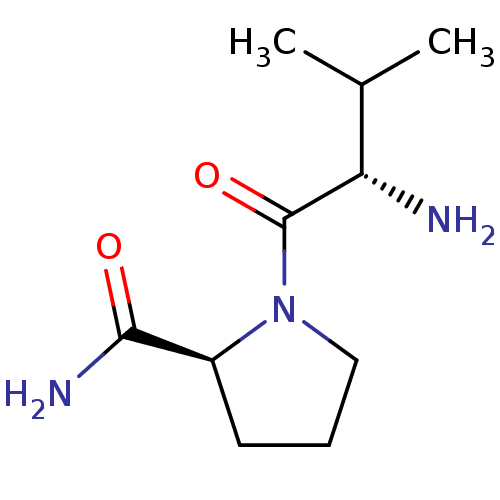 (CHEMBL375933 | L-valyl-L-prolinamide) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition of TPPII in rat cererbral membrane | J Med Chem 48: 7333-42 (2005) Article DOI: 10.1021/jm0500830 BindingDB Entry DOI: 10.7270/Q2KK9CMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tripeptidyl-peptidase 2 (Rattus norvegicus) | BDBM50085111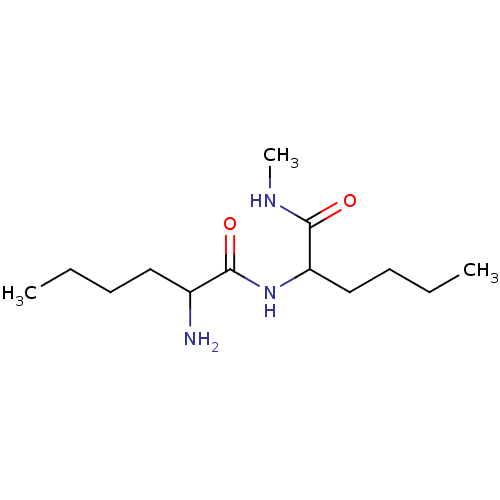 (2-Amino-hexanoic acid (1-methylcarbamoyl-pentyl)-a...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition potency against Cholecystokinin-8-Inactivating Peptidase (Serine Peptidase). | J Med Chem 43: 664-74 (2000) BindingDB Entry DOI: 10.7270/Q2RX9CTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tripeptidyl-peptidase 2 (Rattus norvegicus) | BDBM50085117 (2-Amino-N-[2-(4-hydroxy-phenyl)-1-methylcarbamoyl-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition potency against Cholecystokinin-8-Inactivating Peptidase (Serine Peptidase). | J Med Chem 43: 664-74 (2000) BindingDB Entry DOI: 10.7270/Q2RX9CTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tripeptidyl-peptidase 2 (Rattus norvegicus) | BDBM50176923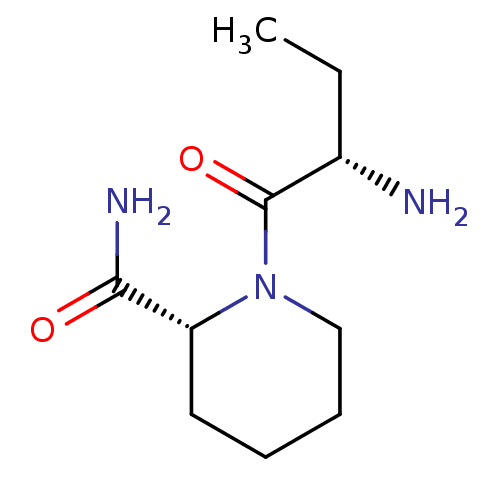 ((2S)-aminobutyryl-(R)-pipecolinic acid amide | CHE...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition of TPPII in rat cererbral membrane | J Med Chem 48: 7333-42 (2005) Article DOI: 10.1021/jm0500830 BindingDB Entry DOI: 10.7270/Q2KK9CMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tripeptidyl-peptidase 2 (Rattus norvegicus) | BDBM50085075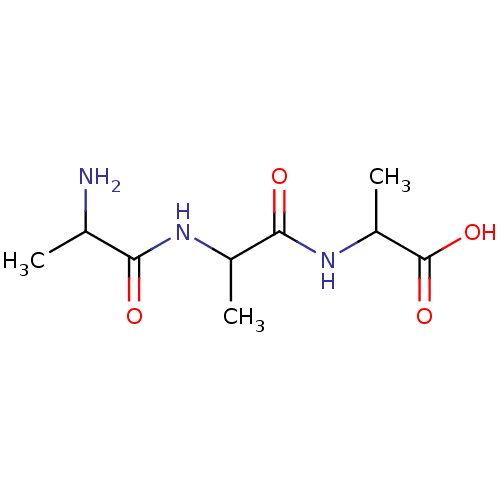 (2-[2-(2-Amino-propionylamino)-propionylamino]-prop...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition potency against Cholecystokinin-8-Inactivating Peptidase (Serine Peptidase). | J Med Chem 43: 664-74 (2000) BindingDB Entry DOI: 10.7270/Q2RX9CTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tripeptidyl-peptidase 2 (Rattus norvegicus) | BDBM50085085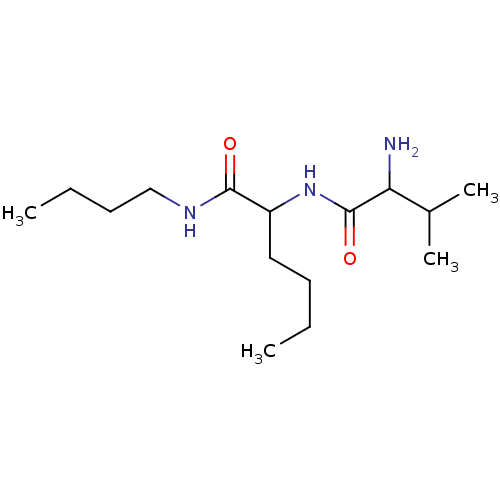 (2-(2-Amino-3-methyl-butyrylamino)-hexanoic acid bu...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition potency against Cholecystokinin-8-Inactivating Peptidase (Serine Peptidase). | J Med Chem 43: 664-74 (2000) BindingDB Entry DOI: 10.7270/Q2RX9CTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tripeptidyl-peptidase 2 (Rattus norvegicus) | BDBM50176915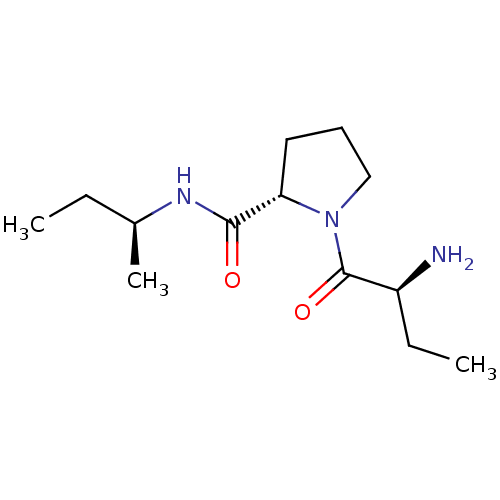 ((2S)-aminobutyryl-L-proline (S)-sec-butylamide | C...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition of TPPII in rat cererbral membrane | J Med Chem 48: 7333-42 (2005) Article DOI: 10.1021/jm0500830 BindingDB Entry DOI: 10.7270/Q2KK9CMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tripeptidyl-peptidase 2 (Rattus norvegicus) | BDBM50176921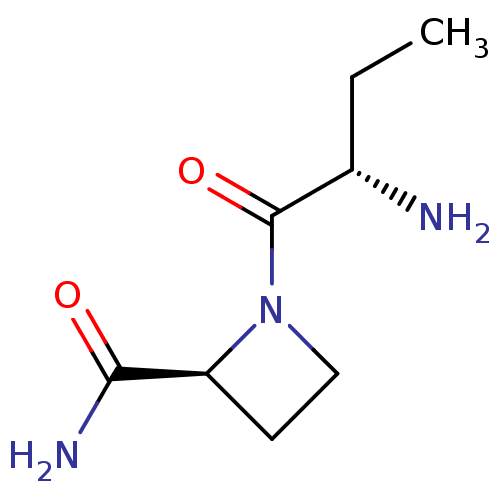 ((2S)-aminobutyryl-(2S)-azetidine carboxamide | CHE...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition of TPPII in rat cererbral membrane | J Med Chem 48: 7333-42 (2005) Article DOI: 10.1021/jm0500830 BindingDB Entry DOI: 10.7270/Q2KK9CMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tripeptidyl-peptidase 2 (Rattus norvegicus) | BDBM50085074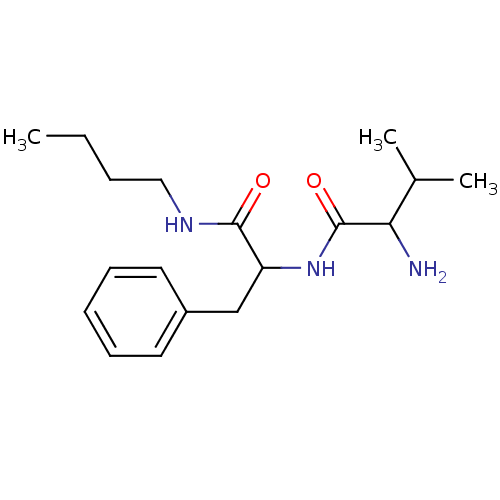 (2-Amino-N-(1-butylcarbamoyl-2-phenyl-ethyl)-3-meth...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition potency against Cholecystokinin-8-Inactivating Peptidase (Serine Peptidase). | J Med Chem 43: 664-74 (2000) BindingDB Entry DOI: 10.7270/Q2RX9CTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tripeptidyl-peptidase 2 (Rattus norvegicus) | BDBM50085106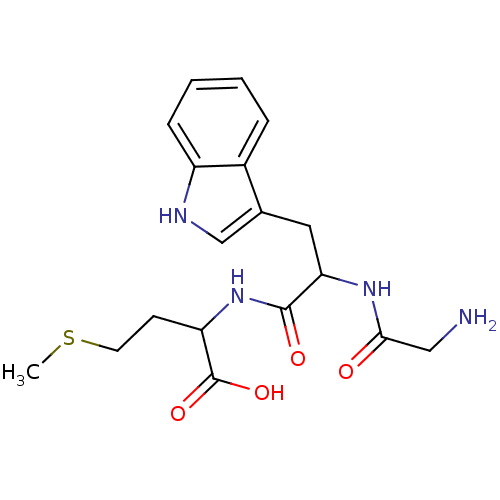 (2-[2-(2-Amino-acetylamino)-3-(1H-indol-3-yl)-propi...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition potency against Cholecystokinin-8-Inactivating Peptidase (Serine Peptidase). | J Med Chem 43: 664-74 (2000) BindingDB Entry DOI: 10.7270/Q2RX9CTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tripeptidyl-peptidase 2 (Rattus norvegicus) | BDBM50085102 (2-Amino-3-methyl-N-(1-methylcarbamoyl-2-phenyl-eth...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition potency against Cholecystokinin-8-Inactivating Peptidase (Serine Peptidase). | J Med Chem 43: 664-74 (2000) BindingDB Entry DOI: 10.7270/Q2RX9CTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tripeptidyl-peptidase 2 (Rattus norvegicus) | BDBM50085093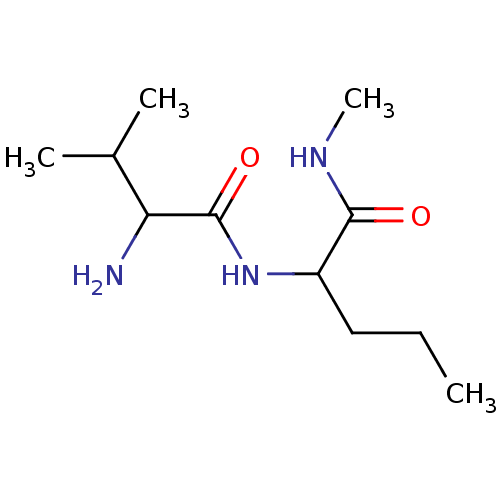 (2-(2-Amino-3-methyl-butyrylamino)-pentanoic acid m...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition potency against Cholecystokinin-8-Inactivating Peptidase (Serine Peptidase). | J Med Chem 43: 664-74 (2000) BindingDB Entry DOI: 10.7270/Q2RX9CTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tripeptidyl-peptidase 2 (Rattus norvegicus) | BDBM50085101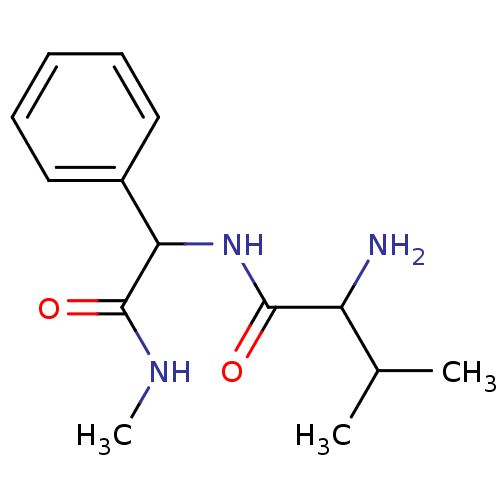 (2-Amino-3-methyl-N-(methylcarbamoyl-phenyl-methyl)...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition potency against Cholecystokinin-8-Inactivating Peptidase (Serine Peptidase). | J Med Chem 43: 664-74 (2000) BindingDB Entry DOI: 10.7270/Q2RX9CTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tripeptidyl-peptidase 2 (Rattus norvegicus) | BDBM50085078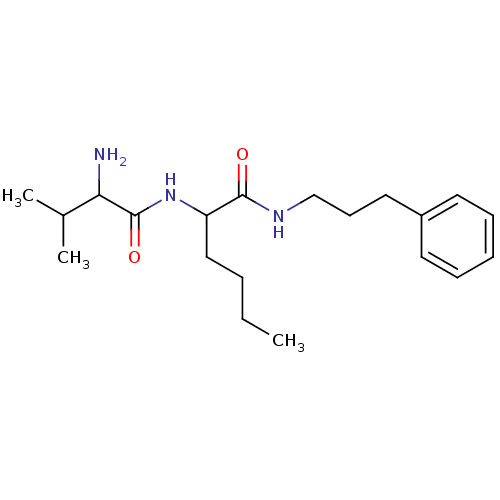 (2-(2-Amino-3-methyl-butyrylamino)-hexanoic acid (3...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition potency against Cholecystokinin-8-Inactivating Peptidase (Serine Peptidase). | J Med Chem 43: 664-74 (2000) BindingDB Entry DOI: 10.7270/Q2RX9CTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tripeptidyl-peptidase 2 (Rattus norvegicus) | BDBM50085129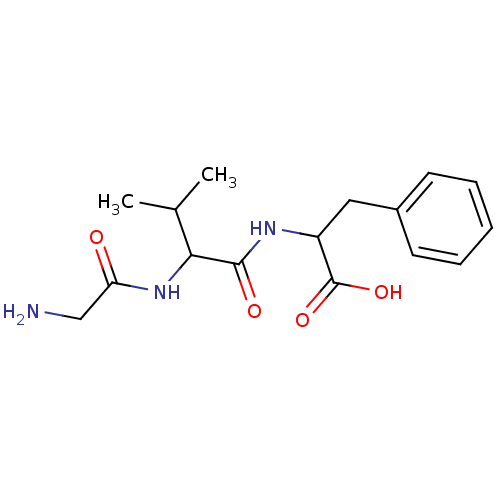 (2-[2-(2-Amino-acetylamino)-3-methyl-butyrylamino]-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition potency against Cholecystokinin-8-Inactivating Peptidase (Serine Peptidase). | J Med Chem 43: 664-74 (2000) BindingDB Entry DOI: 10.7270/Q2RX9CTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tripeptidyl-peptidase 2 (Rattus norvegicus) | BDBM50085128 (2-Amino-3-methyl-N-(4-phenyl-butyl)-butyramide | C...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition potency against Cholecystokinin-8-Inactivating Peptidase (Serine Peptidase). | J Med Chem 43: 664-74 (2000) BindingDB Entry DOI: 10.7270/Q2RX9CTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tripeptidyl-peptidase 2 (Rattus norvegicus) | BDBM50176912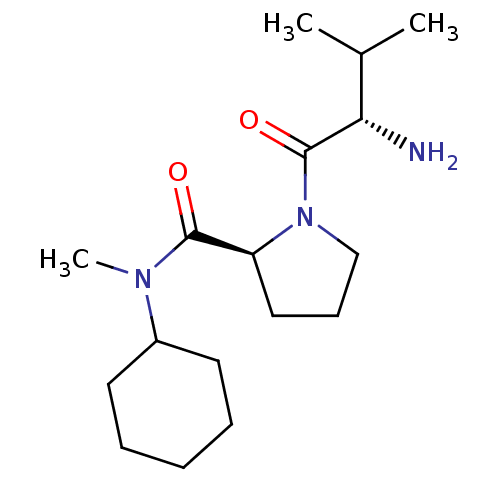 (CHEMBL225596 | L-valyl-L-proline cyclohexylmethyla...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition of TPPII in rat cererbral membrane | J Med Chem 48: 7333-42 (2005) Article DOI: 10.1021/jm0500830 BindingDB Entry DOI: 10.7270/Q2KK9CMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tripeptidyl-peptidase 2 (Rattus norvegicus) | BDBM50176911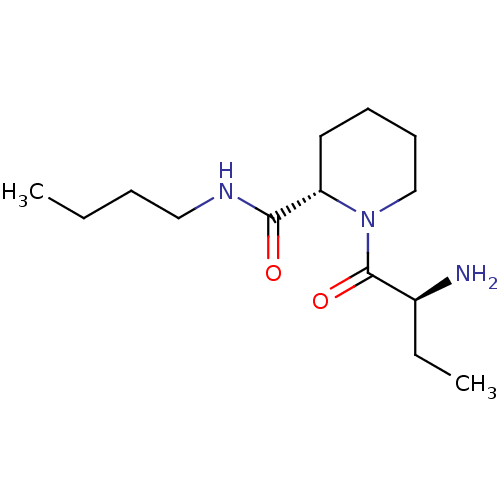 ((2S)-aminobutyryl-L-pipecolinic acid n-butylamide ...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition of TPPII in rat cererbral membrane | J Med Chem 48: 7333-42 (2005) Article DOI: 10.1021/jm0500830 BindingDB Entry DOI: 10.7270/Q2KK9CMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tripeptidyl-peptidase 2 (Rattus norvegicus) | BDBM50176942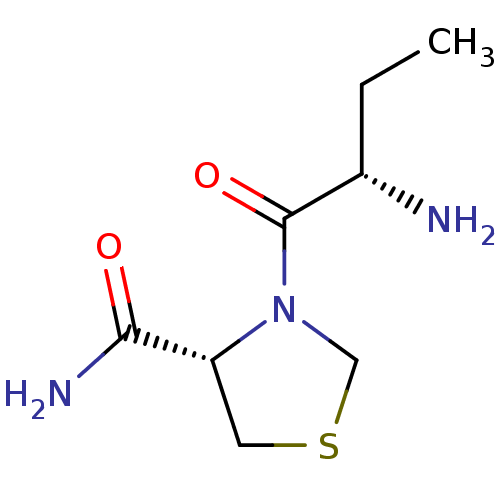 ((2S)-aminobutyryl-4-thia-L-prolinamide | CHEMBL375...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition of TPPII in rat cererbral membrane | J Med Chem 48: 7333-42 (2005) Article DOI: 10.1021/jm0500830 BindingDB Entry DOI: 10.7270/Q2KK9CMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tripeptidyl-peptidase 2 (Rattus norvegicus) | BDBM50176916 ((2S)-aminobutyryl-(S)-nipecotamide | CHEMBL223665) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition of TPPII in rat cererbral membrane | J Med Chem 48: 7333-42 (2005) Article DOI: 10.1021/jm0500830 BindingDB Entry DOI: 10.7270/Q2KK9CMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tripeptidyl-peptidase 2 (Rattus norvegicus) | BDBM50085099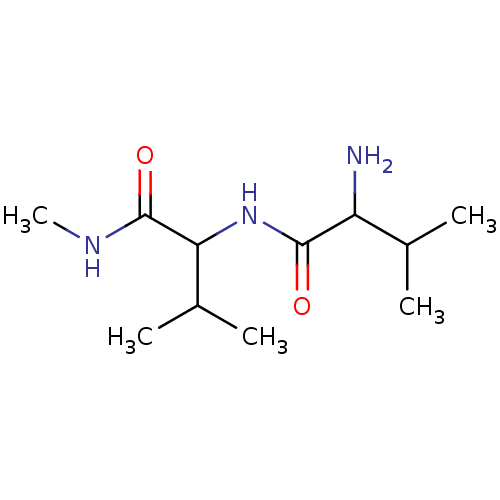 (2-Amino-3-methyl-N-(2-methyl-1-methylcarbamoyl-pro...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition potency against Cholecystokinin-8-Inactivating Peptidase (Serine Peptidase). | J Med Chem 43: 664-74 (2000) BindingDB Entry DOI: 10.7270/Q2RX9CTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tripeptidyl-peptidase 2 (Rattus norvegicus) | BDBM50085073 (3-Amino-N-[1-(1-carboxy-3-methylsulfanyl-propylcar...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition potency against Cholecystokinin-8-Inactivating Peptidase (Serine Peptidase). | J Med Chem 43: 664-74 (2000) BindingDB Entry DOI: 10.7270/Q2RX9CTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tripeptidyl-peptidase 2 (Rattus norvegicus) | BDBM50085082 (2-Amino-N-(1-butylcarbamoyl-2-cyclohexyl-ethyl)-3-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University College London Curated by ChEMBL | Assay Description Inhibition potency against Cholecystokinin-8-Inactivating Peptidase (Serine Peptidase). | J Med Chem 43: 664-74 (2000) BindingDB Entry DOI: 10.7270/Q2RX9CTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 420 total ) | Next | Last >> |