

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50054764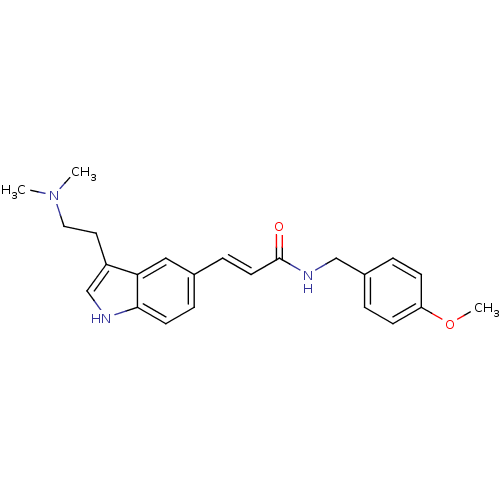 ((E)-3-[3-(2-Dimethylamino-ethyl)-1H-indol-5-yl]-N-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Compound was tested for binding affinity against cloned human 5-hydroxytryptamine 1D receptor beta expressed in CHO-K1 cells. | J Med Chem 39: 4717-26 (1997) Article DOI: 10.1021/jm9604890 BindingDB Entry DOI: 10.7270/Q2R210GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50054764 ((E)-3-[3-(2-Dimethylamino-ethyl)-1H-indol-5-yl]-N-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Compound was tested for binding affinity against cloned human 5-hydroxytryptamine 1D receptor alpha expressed in CHO-K1 cells. | J Med Chem 39: 4717-26 (1997) Article DOI: 10.1021/jm9604890 BindingDB Entry DOI: 10.7270/Q2R210GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50054764 ((E)-3-[3-(2-Dimethylamino-ethyl)-1H-indol-5-yl]-N-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Compound was tested for binding affinity against cloned human 5-hydroxytryptamine 1A receptor expressed in CHO-K1 cells. | J Med Chem 39: 4717-26 (1997) Article DOI: 10.1021/jm9604890 BindingDB Entry DOI: 10.7270/Q2R210GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50054762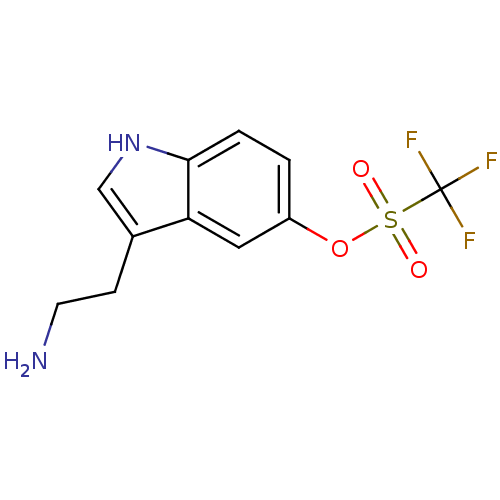 (CHEMBL143512 | Trifluoro-methanesulfonic acid 3-(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Compound was tested for binding affinity against cloned human 5-hydroxytryptamine 1D receptor alpha expressed in CHO-K1 cells. | J Med Chem 39: 4717-26 (1997) Article DOI: 10.1021/jm9604890 BindingDB Entry DOI: 10.7270/Q2R210GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50054761 (CHEMBL143767 | Trifluoro-methanesulfonic acid 3-(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Compound was tested for binding affinity against cloned human 5-hydroxytryptamine 1D receptor alpha expressed in CHO-K1 cells. | J Med Chem 39: 4717-26 (1997) Article DOI: 10.1021/jm9604890 BindingDB Entry DOI: 10.7270/Q2R210GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50005835 ((3-[2-(dimethylamino)ethyl]-1H-indol-5-yl)-N-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | DrugBank Article PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Compound was tested for binding affinity against cloned human 5-hydroxytryptamine 1D receptor alpha expressed in CHO-K1 cells. | J Med Chem 39: 4717-26 (1997) Article DOI: 10.1021/jm9604890 BindingDB Entry DOI: 10.7270/Q2R210GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50054757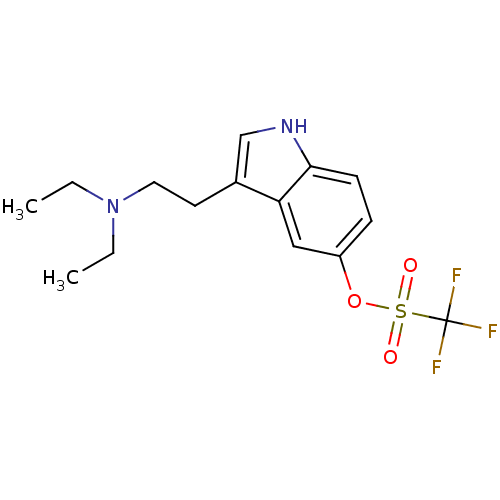 (CHEMBL143510 | Trifluoro-methanesulfonic acid 3-(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Compound was tested for binding affinity against cloned human 5-hydroxytryptamine 1D receptor alpha expressed in CHO-K1 cells. | J Med Chem 39: 4717-26 (1997) Article DOI: 10.1021/jm9604890 BindingDB Entry DOI: 10.7270/Q2R210GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50054762 (CHEMBL143512 | Trifluoro-methanesulfonic acid 3-(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Compound was tested for binding affinity against cloned human 5-hydroxytryptamine 1D receptor beta expressed in CHO-K1 cells. | J Med Chem 39: 4717-26 (1997) Article DOI: 10.1021/jm9604890 BindingDB Entry DOI: 10.7270/Q2R210GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50054762 (CHEMBL143512 | Trifluoro-methanesulfonic acid 3-(2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Compound was tested for binding affinity against cloned human 5-hydroxytryptamine 1A receptor expressed in CHO-K1 cells. | J Med Chem 39: 4717-26 (1997) Article DOI: 10.1021/jm9604890 BindingDB Entry DOI: 10.7270/Q2R210GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50054762 (CHEMBL143512 | Trifluoro-methanesulfonic acid 3-(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Compound was tested for intrinsic efficacy against human 5-hydroxytryptamine 1D receptor beta measured as the reduction of forskolin-stimulated cAMP. | J Med Chem 39: 4717-26 (1997) Article DOI: 10.1021/jm9604890 BindingDB Entry DOI: 10.7270/Q2R210GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50054762 (CHEMBL143512 | Trifluoro-methanesulfonic acid 3-(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Compound was tested for intrinsic efficacy against human 5-hydroxytryptamine 1D receptor alpha measured as the reduction of forskolin-stimulated cAMP... | J Med Chem 39: 4717-26 (1997) Article DOI: 10.1021/jm9604890 BindingDB Entry DOI: 10.7270/Q2R210GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50005835 ((3-[2-(dimethylamino)ethyl]-1H-indol-5-yl)-N-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | DrugBank Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Compound was tested for binding affinity against cloned human 5-hydroxytryptamine 1D receptor beta expressed in CHO-K1 cells. | J Med Chem 39: 4717-26 (1997) Article DOI: 10.1021/jm9604890 BindingDB Entry DOI: 10.7270/Q2R210GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50054760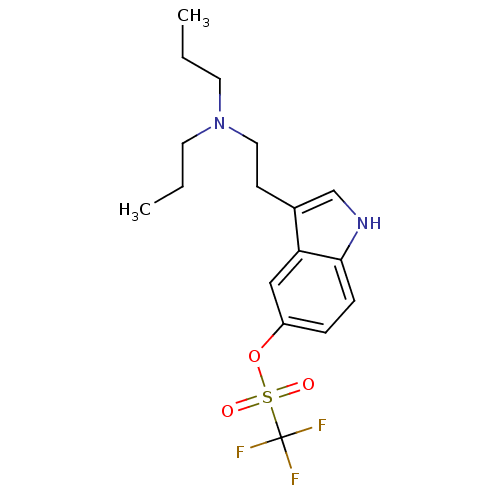 (CHEMBL143967 | Trifluoro-methanesulfonic acid 3-(2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Compound was tested for binding affinity against cloned human 5-hydroxytryptamine 1A receptor expressed in CHO-K1 cells. | J Med Chem 39: 4717-26 (1997) Article DOI: 10.1021/jm9604890 BindingDB Entry DOI: 10.7270/Q2R210GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50005835 ((3-[2-(dimethylamino)ethyl]-1H-indol-5-yl)-N-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | DrugBank Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Compound was tested for intrinsic efficacy against human 5-hydroxytryptamine 1D receptor beta measured as the reduction of forskolin-stimulated cAMP. | J Med Chem 39: 4717-26 (1997) Article DOI: 10.1021/jm9604890 BindingDB Entry DOI: 10.7270/Q2R210GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50054757 (CHEMBL143510 | Trifluoro-methanesulfonic acid 3-(2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Compound was tested for binding affinity against cloned human 5-hydroxytryptamine 1A receptor expressed in CHO-K1 cells. | J Med Chem 39: 4717-26 (1997) Article DOI: 10.1021/jm9604890 BindingDB Entry DOI: 10.7270/Q2R210GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50054761 (CHEMBL143767 | Trifluoro-methanesulfonic acid 3-(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Compound was tested for intrinsic efficacy against human 5-hydroxytryptamine 1D receptor beta measured as the reduction of forskolin-stimulated cAMP. | J Med Chem 39: 4717-26 (1997) Article DOI: 10.1021/jm9604890 BindingDB Entry DOI: 10.7270/Q2R210GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50054757 (CHEMBL143510 | Trifluoro-methanesulfonic acid 3-(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Compound was tested for intrinsic efficacy against human 5-hydroxytryptamine 1D receptor beta measured as the reduction of forskolin-stimulated cAMP. | J Med Chem 39: 4717-26 (1997) Article DOI: 10.1021/jm9604890 BindingDB Entry DOI: 10.7270/Q2R210GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50054761 (CHEMBL143767 | Trifluoro-methanesulfonic acid 3-(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Compound was tested for intrinsic efficacy against human 5-hydroxytryptamine 1D receptor alpha measured as the reduction of forskolin-stimulated cAMP... | J Med Chem 39: 4717-26 (1997) Article DOI: 10.1021/jm9604890 BindingDB Entry DOI: 10.7270/Q2R210GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50005835 ((3-[2-(dimethylamino)ethyl]-1H-indol-5-yl)-N-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | DrugBank Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Compound was tested for intrinsic efficacy against human 5-hydroxytryptamine 1D receptor alpha measured as the reduction of forskolin-stimulated cAMP... | J Med Chem 39: 4717-26 (1997) Article DOI: 10.1021/jm9604890 BindingDB Entry DOI: 10.7270/Q2R210GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50054761 (CHEMBL143767 | Trifluoro-methanesulfonic acid 3-(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Compound was tested for binding affinity against cloned human 5-hydroxytryptamine 1D receptor beta expressed in CHO-K1 cells. | J Med Chem 39: 4717-26 (1997) Article DOI: 10.1021/jm9604890 BindingDB Entry DOI: 10.7270/Q2R210GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50054760 (CHEMBL143967 | Trifluoro-methanesulfonic acid 3-(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Compound was tested for intrinsic efficacy against human 5-hydroxytryptamine 1D receptor alpha measured as the reduction of forskolin-stimulated cAMP... | J Med Chem 39: 4717-26 (1997) Article DOI: 10.1021/jm9604890 BindingDB Entry DOI: 10.7270/Q2R210GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50054757 (CHEMBL143510 | Trifluoro-methanesulfonic acid 3-(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Compound was tested for intrinsic efficacy against human 5-hydroxytryptamine 1D receptor beta measured as the reduction of forskolin-stimulated cAMP. | J Med Chem 39: 4717-26 (1997) Article DOI: 10.1021/jm9604890 BindingDB Entry DOI: 10.7270/Q2R210GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50054761 (CHEMBL143767 | Trifluoro-methanesulfonic acid 3-(2...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Compound was tested for binding affinity against cloned human 5-hydroxytryptamine 1A receptor expressed in CHO-K1 cells. | J Med Chem 39: 4717-26 (1997) Article DOI: 10.1021/jm9604890 BindingDB Entry DOI: 10.7270/Q2R210GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50054760 (CHEMBL143967 | Trifluoro-methanesulfonic acid 3-(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Compound was tested for intrinsic efficacy against human 5-hydroxytryptamine 1D receptor beta measured as the reduction of forskolin-stimulated cAMP. | J Med Chem 39: 4717-26 (1997) Article DOI: 10.1021/jm9604890 BindingDB Entry DOI: 10.7270/Q2R210GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50054764 ((E)-3-[3-(2-Dimethylamino-ethyl)-1H-indol-5-yl]-N-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | 104 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Compound was tested for intrinsic efficacy against human 5-hydroxytryptamine 1D receptor beta measured as the reduction of forskolin-stimulated cAMP. | J Med Chem 39: 4717-26 (1997) Article DOI: 10.1021/jm9604890 BindingDB Entry DOI: 10.7270/Q2R210GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50054757 (CHEMBL143510 | Trifluoro-methanesulfonic acid 3-(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 171 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Compound was tested for binding affinity against cloned human 5-hydroxytryptamine 1D receptor alpha expressed in CHO-K1 cells. | J Med Chem 39: 4717-26 (1997) Article DOI: 10.1021/jm9604890 BindingDB Entry DOI: 10.7270/Q2R210GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50054760 (CHEMBL143967 | Trifluoro-methanesulfonic acid 3-(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Compound was tested for binding affinity against cloned human 5-hydroxytryptamine 1D receptor alpha expressed in CHO-K1 cells. | J Med Chem 39: 4717-26 (1997) Article DOI: 10.1021/jm9604890 BindingDB Entry DOI: 10.7270/Q2R210GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50005835 ((3-[2-(dimethylamino)ethyl]-1H-indol-5-yl)-N-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | Article PubMed | >218 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Compound was tested for binding affinity against cloned human dopamine D2 receptor expressed in CHO-K1 cells. | J Med Chem 39: 4717-26 (1997) Article DOI: 10.1021/jm9604890 BindingDB Entry DOI: 10.7270/Q2R210GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50054760 (CHEMBL143967 | Trifluoro-methanesulfonic acid 3-(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 246 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Compound was tested for binding affinity against cloned human 5-HT1D beta receptor expressed in CHO-K1 cells. | J Med Chem 39: 4717-26 (1997) Article DOI: 10.1021/jm9604890 BindingDB Entry DOI: 10.7270/Q2R210GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50005835 ((3-[2-(dimethylamino)ethyl]-1H-indol-5-yl)-N-methy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents | Article PubMed | 341 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Compound was tested for binding affinity against cloned human 5-hydroxytryptamine 1A receptor expressed in CHO-K1 cells. | J Med Chem 39: 4717-26 (1997) Article DOI: 10.1021/jm9604890 BindingDB Entry DOI: 10.7270/Q2R210GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50054760 (CHEMBL143967 | Trifluoro-methanesulfonic acid 3-(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 502 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Compound was tested for binding affinity against cloned human dopamine D2 receptor expressed in CHO-K1 cells. | J Med Chem 39: 4717-26 (1997) Article DOI: 10.1021/jm9604890 BindingDB Entry DOI: 10.7270/Q2R210GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50054761 (CHEMBL143767 | Trifluoro-methanesulfonic acid 3-(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 538 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Compound was tested for binding affinity against cloned human dopamine D2 receptor expressed in CHO-K1 cells. | J Med Chem 39: 4717-26 (1997) Article DOI: 10.1021/jm9604890 BindingDB Entry DOI: 10.7270/Q2R210GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50054757 (CHEMBL143510 | Trifluoro-methanesulfonic acid 3-(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 637 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Compound was tested for binding affinity against cloned human dopamine D2 receptor expressed in CHO-K1 cells. | J Med Chem 39: 4717-26 (1997) Article DOI: 10.1021/jm9604890 BindingDB Entry DOI: 10.7270/Q2R210GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50054762 (CHEMBL143512 | Trifluoro-methanesulfonic acid 3-(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 658 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Compound was tested for binding affinity against cloned human dopamine D2 receptor expressed in CHO-K1 cells. | J Med Chem 39: 4717-26 (1997) Article DOI: 10.1021/jm9604890 BindingDB Entry DOI: 10.7270/Q2R210GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50054764 ((E)-3-[3-(2-Dimethylamino-ethyl)-1H-indol-5-yl]-N-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Compound was tested for binding affinity against cloned human Dopamine receptor D2 expressed in CHO-K1 cells. | J Med Chem 39: 4717-26 (1997) Article DOI: 10.1021/jm9604890 BindingDB Entry DOI: 10.7270/Q2R210GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50054761 (CHEMBL143767 | Trifluoro-methanesulfonic acid 3-(2...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.40 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Groningen Curated by ChEMBL | Assay Description Compound was tested for binding affinity against human 5-hydroxytryptamine 1D receptor alpha clones expressed in human embryonic kidney (HEK 293) cel... | J Med Chem 39: 4717-26 (1997) Article DOI: 10.1021/jm9604890 BindingDB Entry DOI: 10.7270/Q2R210GS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267888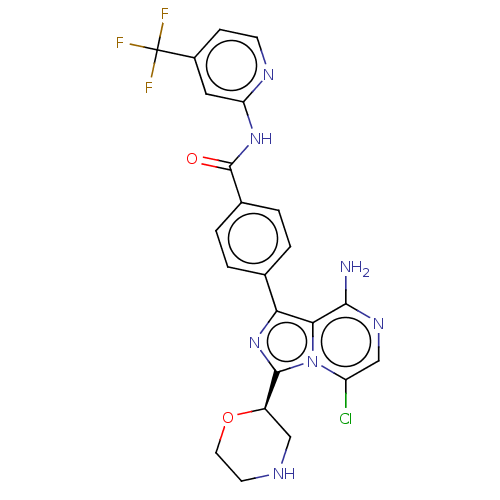 (4-{8-amino-3-[(2R)-4-(3-ethoxypropanoyl)morpholin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267889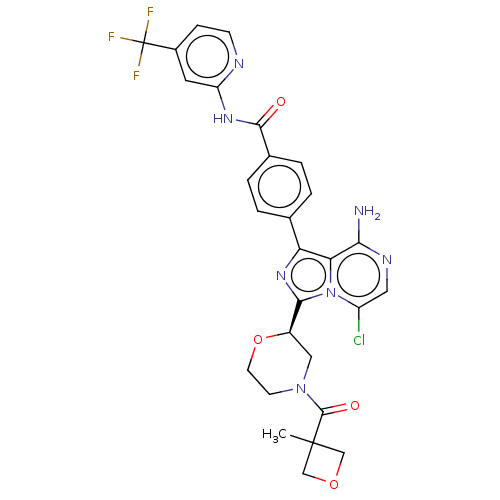 (4-{8-amino-5-chloro-3-[(2R)-morpholin-2-yl]imidazo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267891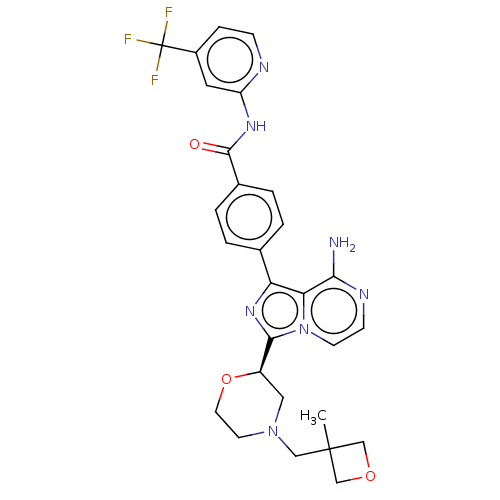 (4-(8-amino-5-methyl-3-{(2R)-4-[(3-methyloxetan-3-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267892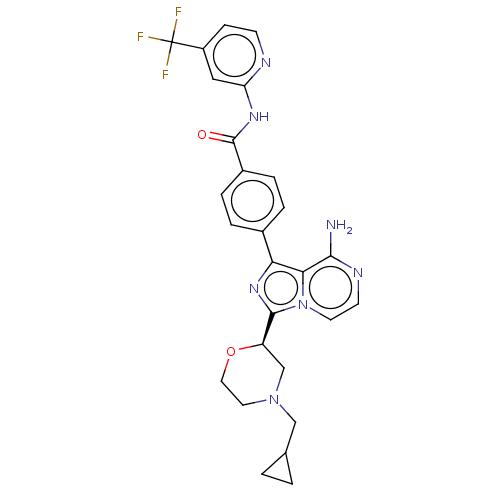 (4-(8-amino-3-{(2R)-4-[(3-methyloxetan-3-yl)methyl]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267893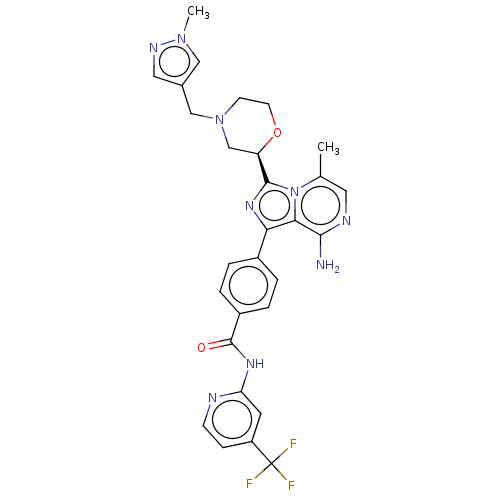 (4-{8-amino-3-[(2R)-4-(cyclopropylmethyl)morpholin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267894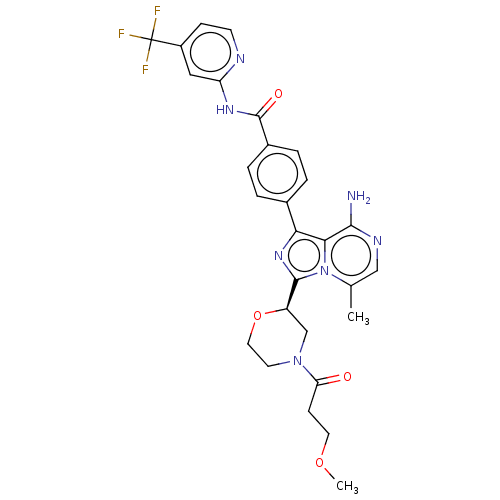 (4-(8-amino-5-methyl-3-{(2R)-4-[(1-methyl-1H-pyrazo...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267895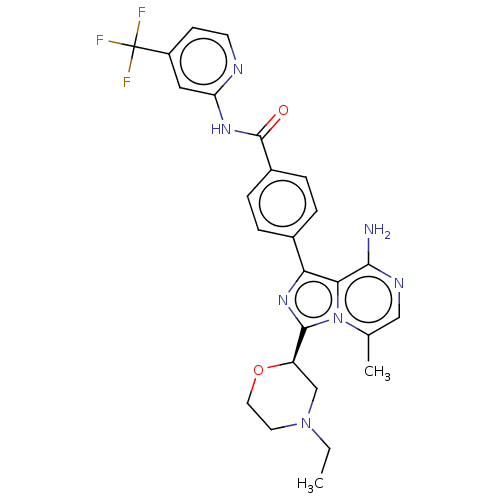 (4-{8-amino-3-[(2R)-4-(3-methoxypropanoyl)morpholin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267896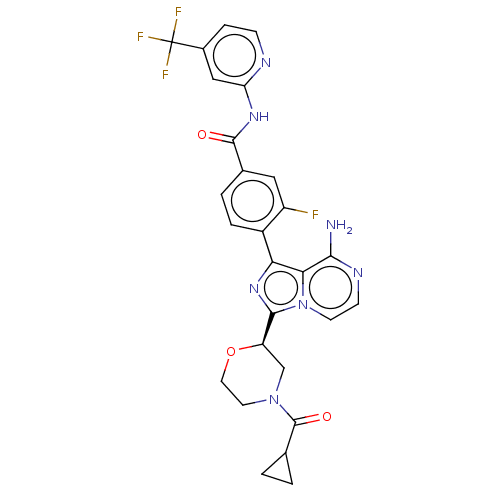 (4-{8-amino-3-[(2R)-4-ethylmorpholin-2-yl]-5-methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267897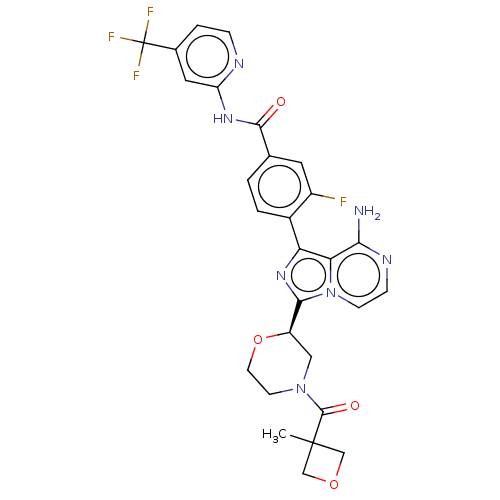 (4-{8-amino-3-[(2R)-4-(cyclopropylcarbonyl)morpholi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267898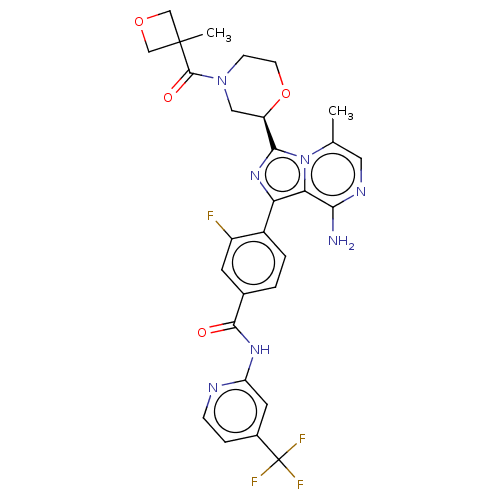 (4-(8-amino-3-{(2R)-4-[(3-methyloxetan-3-yl)carbony...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267899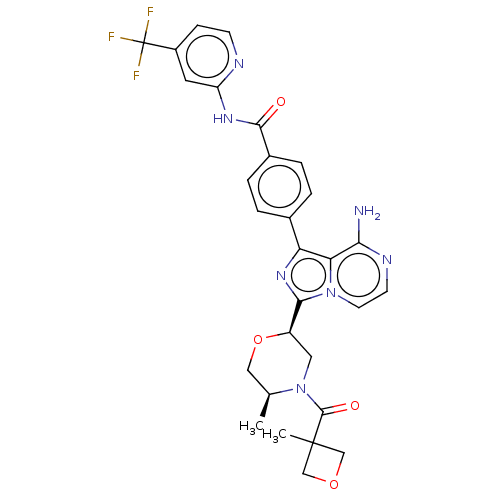 (4-(8-amino-5-methyl-3-{(2R)-4-[(3-methyloxetan-3-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267900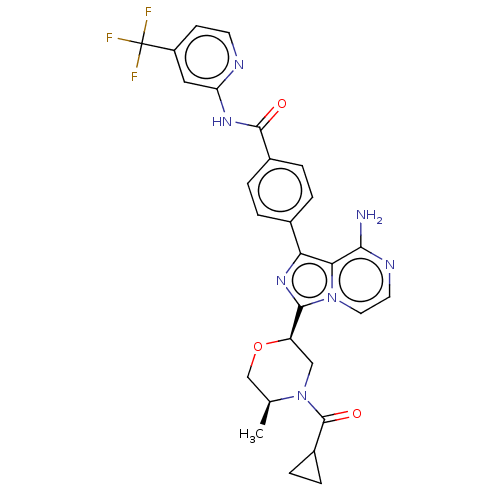 (4-(8-amino-3-{(2R,5S)-5-methyl-4-[(3-methyloxetan-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267901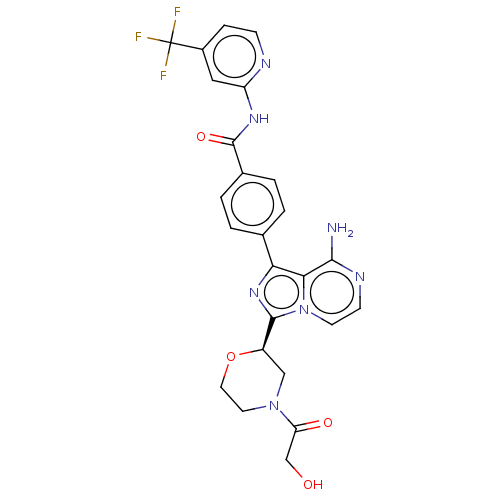 (4-{8-amino-3-[(2R,5S)-4-(cyclopropylcarbonyl)-5-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM267902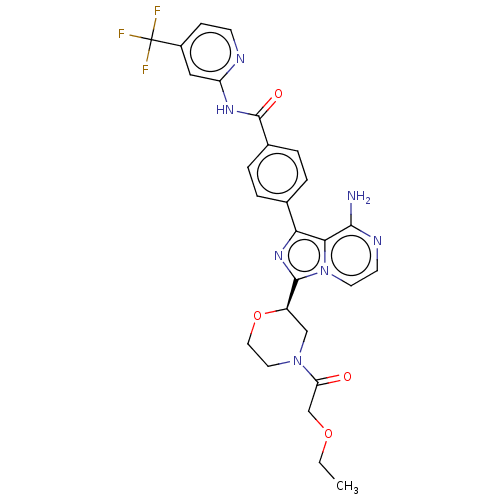 (4-{8-amino-3-[(2R)-4-(hydroxyacetyl)morpholin-2-yl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
MERCK SHARP & DOHME CORP.; MERCK SHARP & DOHME B.V. US Patent | Assay Description BTK enzymatic activity was determined with the LANCE (Lanthanide Chelate Excite) TR-FRET (Time-resolved fluorescence resonance energy transfer) assay... | US Patent US9718828 (2017) BindingDB Entry DOI: 10.7270/Q2H9977T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2534 total ) | Next | Last >> |