

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM190273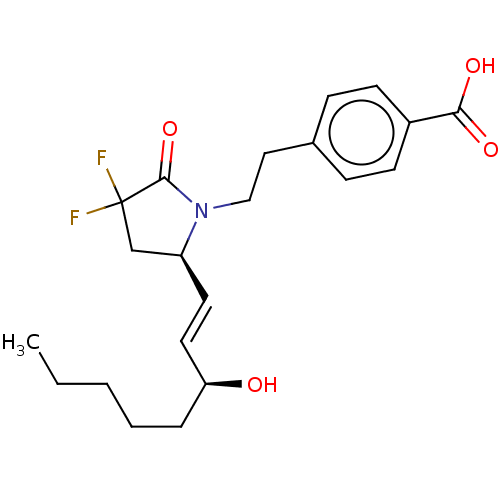 (US9180116, 21C) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0820 | n/a | 0.220 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Cayman Chemical Company, Inc. US Patent | Assay Description Assay Volume and Format:200 μl in 96-well plateCell membrane homogenates (20 μg protein) are incubated for 120 min at 22° C. with 0.5 nM [3... | US Patent US9180116 (2015) BindingDB Entry DOI: 10.7270/Q2QC029S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM190282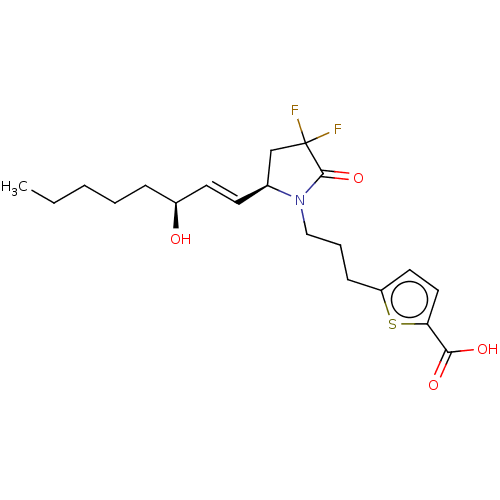 (US9180116, 33C) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.100 | n/a | 0.280 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Cayman Chemical Company, Inc. US Patent | Assay Description Assay Volume and Format:200 μl in 96-well plateCell membrane homogenates (20 μg protein) are incubated for 120 min at 22° C. with 0.5 nM [3... | US Patent US9180116 (2015) BindingDB Entry DOI: 10.7270/Q2QC029S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM35847 ((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human recombinant EP4 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... | J Med Chem 62: 4731-4741 (2019) Article DOI: 10.1021/acs.jmedchem.9b00336 BindingDB Entry DOI: 10.7270/Q22R3W32 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM190272 (US9180116, 12D) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.120 | n/a | 0.320 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Cayman Chemical Company, Inc. US Patent | Assay Description Assay Volume and Format:200 μl in 96-well plateCell membrane homogenates (20 μg protein) are incubated for 120 min at 22° C. with 0.5 nM [3... | US Patent US9180116 (2015) BindingDB Entry DOI: 10.7270/Q2QC029S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50101822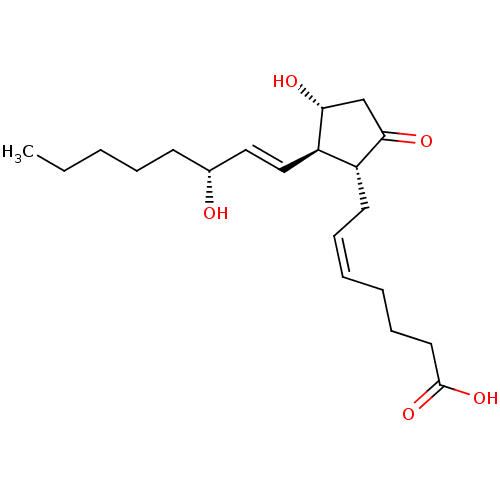 ((Z)-7-[(1R,2R,3R)-3-Hydroxy-2-((E)-(R)-3-hydroxy-o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | 0.140 | n/a | 0.380 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Cayman Chemical Company, Inc. US Patent | Assay Description Assay Volume and Format:200 μl in 96-well plateCell membrane homogenates (20 μg protein) are incubated for 120 min at 22° C. with 0.5 nM [3... | US Patent US9180116 (2015) BindingDB Entry DOI: 10.7270/Q2QC029S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM190270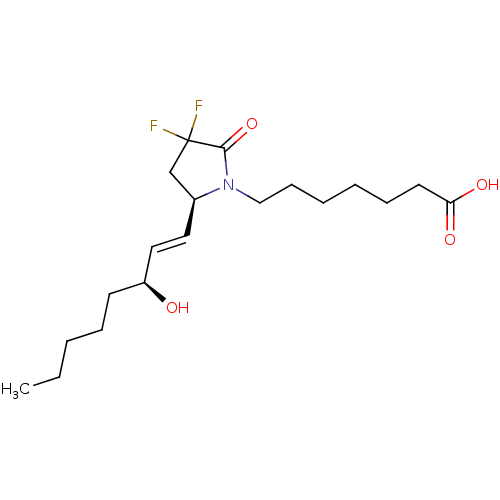 (US9180116, 9C) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human recombinant EP4 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... | J Med Chem 62: 4731-4741 (2019) Article DOI: 10.1021/acs.jmedchem.9b00336 BindingDB Entry DOI: 10.7270/Q22R3W32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM190270 (US9180116, 9C) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.210 | n/a | 0.570 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Cayman Chemical Company, Inc. US Patent | Assay Description Assay Volume and Format:200 μl in 96-well plateCell membrane homogenates (20 μg protein) are incubated for 120 min at 22° C. with 0.5 nM [3... | US Patent US9180116 (2015) BindingDB Entry DOI: 10.7270/Q2QC029S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM190275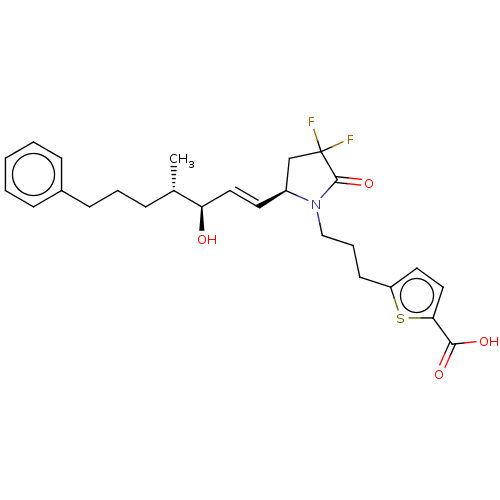 (US9180116, 28C | US9180116, 28H) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.280 | n/a | 0.740 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Cayman Chemical Company, Inc. US Patent | Assay Description Assay Volume and Format:200 μl in 96-well plateCell membrane homogenates (20 μg protein) are incubated for 120 min at 22° C. with 0.5 nM [3... | US Patent US9180116 (2015) BindingDB Entry DOI: 10.7270/Q2QC029S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM190268 (US9180116, 2C) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.378 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human recombinant EP4 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... | J Med Chem 62: 4731-4741 (2019) Article DOI: 10.1021/acs.jmedchem.9b00336 BindingDB Entry DOI: 10.7270/Q22R3W32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50101858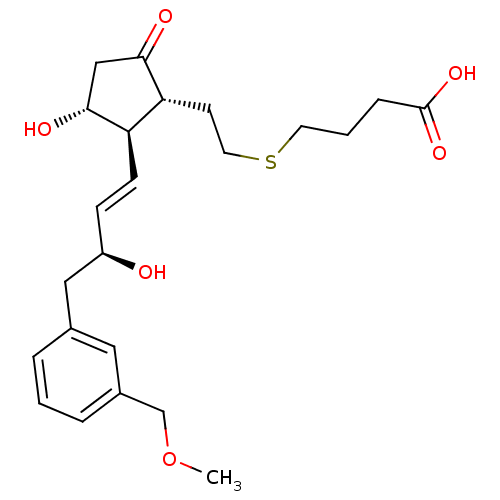 (4-(2-((1R,2R,3R)-3-hydroxy-2-((S,E)-3-hydroxy-4-(3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.396 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human recombinant EP4 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... | J Med Chem 62: 4731-4741 (2019) Article DOI: 10.1021/acs.jmedchem.9b00336 BindingDB Entry DOI: 10.7270/Q22R3W32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM190267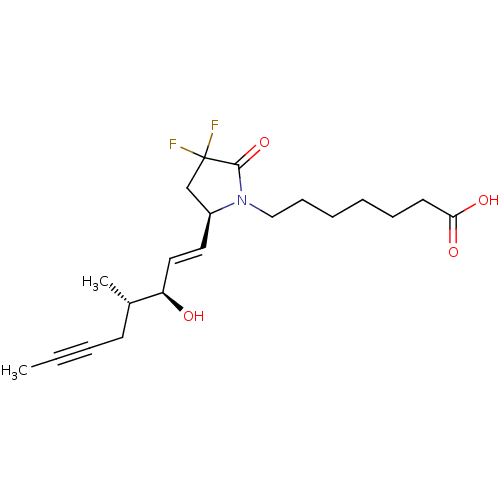 (US9180116, 1F) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.440 | n/a | 1.20 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Cayman Chemical Company, Inc. US Patent | Assay Description Assay Volume and Format:200 μl in 96-well plateCell membrane homogenates (20 μg protein) are incubated for 120 min at 22° C. with 0.5 nM [3... | US Patent US9180116 (2015) BindingDB Entry DOI: 10.7270/Q2QC029S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM190268 (US9180116, 2C) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.490 | n/a | 1.30 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Cayman Chemical Company, Inc. US Patent | Assay Description Assay Volume and Format:200 μl in 96-well plateCell membrane homogenates (20 μg protein) are incubated for 120 min at 22° C. with 0.5 nM [3... | US Patent US9180116 (2015) BindingDB Entry DOI: 10.7270/Q2QC029S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM190269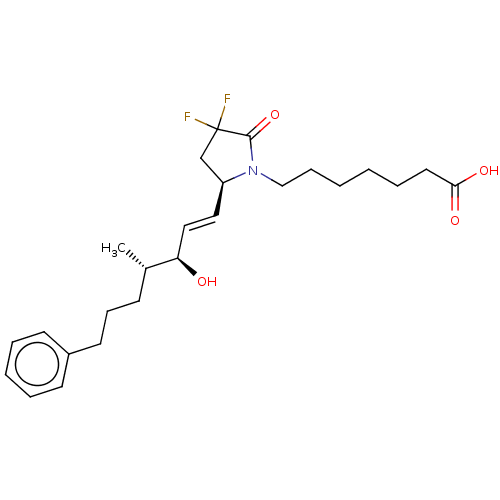 (US9180116, 6D) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.890 | n/a | 2.40 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Cayman Chemical Company, Inc. US Patent | Assay Description Assay Volume and Format:200 μl in 96-well plateCell membrane homogenates (20 μg protein) are incubated for 120 min at 22° C. with 0.5 nM [3... | US Patent US9180116 (2015) BindingDB Entry DOI: 10.7270/Q2QC029S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM35847 ((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human recombinant EP2 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... | J Med Chem 62: 4731-4741 (2019) Article DOI: 10.1021/acs.jmedchem.9b00336 BindingDB Entry DOI: 10.7270/Q22R3W32 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM190274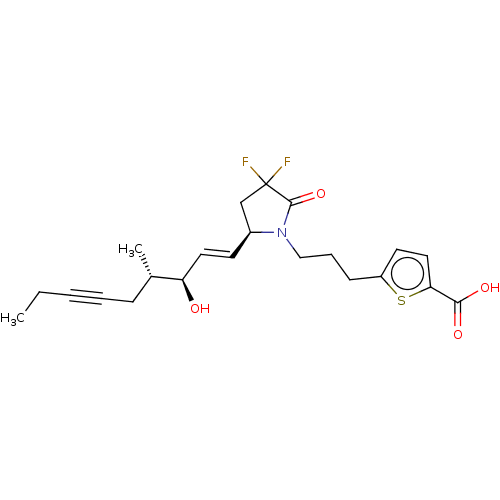 (US9180116, 24D) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.20 | n/a | 3.30 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Cayman Chemical Company, Inc. US Patent | Assay Description Assay Volume and Format:200 μl in 96-well plateCell membrane homogenates (20 μg protein) are incubated for 120 min at 22° C. with 0.5 nM [3... | US Patent US9180116 (2015) BindingDB Entry DOI: 10.7270/Q2QC029S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50142481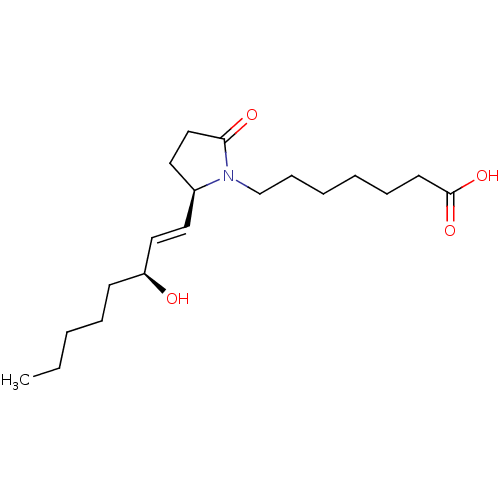 (7-[(R)-2-((E)-(S)-3-Hydroxy-oct-1-enyl)-5-oxo-pyrr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human recombinant EP4 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... | J Med Chem 62: 4731-4741 (2019) Article DOI: 10.1021/acs.jmedchem.9b00336 BindingDB Entry DOI: 10.7270/Q22R3W32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM190271 (US9180116, 10C) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.80 | n/a | 4.90 | n/a | n/a | n/a | n/a | 6.0 | n/a |
Cayman Chemical Company, Inc. US Patent | Assay Description Assay Volume and Format:200 μl in 96-well plateCell membrane homogenates (20 μg protein) are incubated for 120 min at 22° C. with 0.5 nM [3... | US Patent US9180116 (2015) BindingDB Entry DOI: 10.7270/Q2QC029S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM179834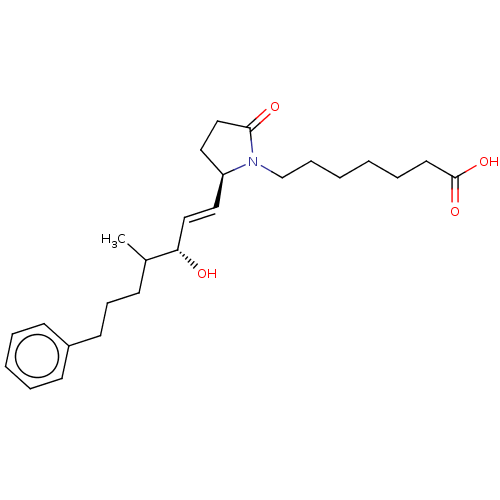 (US9676712, 2C) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.10 | n/a | 5.70 | n/a | n/a | n/a | n/a | 6.0 | n/a |
CAYMAN CHEMICAL COMPANY, INC. US Patent | Assay Description 200 μl in 96-well plate. Cell membrane homogenates (20 μg protein) are incubated for 120 min at 22° C. with 0.5 nM [3H]PGE2 in the absence ... | US Patent US9676712 (2017) BindingDB Entry DOI: 10.7270/Q23X84T9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM35847 ((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human recombinant EP3 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... | J Med Chem 62: 4731-4741 (2019) Article DOI: 10.1021/acs.jmedchem.9b00336 BindingDB Entry DOI: 10.7270/Q22R3W32 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50521600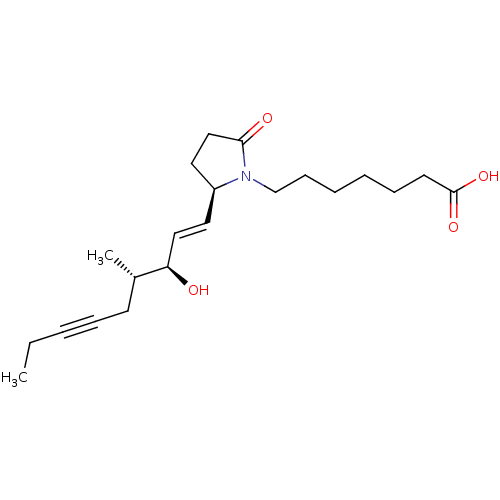 (CHEMBL4558749) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human recombinant EP4 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... | J Med Chem 62: 4731-4741 (2019) Article DOI: 10.1021/acs.jmedchem.9b00336 BindingDB Entry DOI: 10.7270/Q22R3W32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM179827 (US9676712, 1C) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.80 | n/a | 10 | n/a | n/a | n/a | n/a | 6.0 | n/a |
CAYMAN CHEMICAL COMPANY, INC. US Patent | Assay Description 200 μl in 96-well plate. Cell membrane homogenates (20 μg protein) are incubated for 120 min at 22° C. with 0.5 nM [3H]PGE2 in the absence ... | US Patent US9676712 (2017) BindingDB Entry DOI: 10.7270/Q23X84T9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM190268 (US9180116, 2C) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 258 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human recombinant EP3 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... | J Med Chem 62: 4731-4741 (2019) Article DOI: 10.1021/acs.jmedchem.9b00336 BindingDB Entry DOI: 10.7270/Q22R3W32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM190270 (US9180116, 9C) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 329 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human recombinant EP2 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... | J Med Chem 62: 4731-4741 (2019) Article DOI: 10.1021/acs.jmedchem.9b00336 BindingDB Entry DOI: 10.7270/Q22R3W32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM190270 (US9180116, 9C) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human recombinant EP3 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... | J Med Chem 62: 4731-4741 (2019) Article DOI: 10.1021/acs.jmedchem.9b00336 BindingDB Entry DOI: 10.7270/Q22R3W32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50521600 (CHEMBL4558749) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human recombinant EP3 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... | J Med Chem 62: 4731-4741 (2019) Article DOI: 10.1021/acs.jmedchem.9b00336 BindingDB Entry DOI: 10.7270/Q22R3W32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50142481 (7-[(R)-2-((E)-(S)-3-Hydroxy-oct-1-enyl)-5-oxo-pyrr...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human recombinant EP3 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... | J Med Chem 62: 4731-4741 (2019) Article DOI: 10.1021/acs.jmedchem.9b00336 BindingDB Entry DOI: 10.7270/Q22R3W32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50142481 (7-[(R)-2-((E)-(S)-3-Hydroxy-oct-1-enyl)-5-oxo-pyrr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.46E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human recombinant EP2 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... | J Med Chem 62: 4731-4741 (2019) Article DOI: 10.1021/acs.jmedchem.9b00336 BindingDB Entry DOI: 10.7270/Q22R3W32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM190268 (US9180116, 2C) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human recombinant EP2 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... | J Med Chem 62: 4731-4741 (2019) Article DOI: 10.1021/acs.jmedchem.9b00336 BindingDB Entry DOI: 10.7270/Q22R3W32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM50300128 (1-(3-(1H-tetrazol-5-yl)propyl)-4-(benzhydryloxy)pi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents | MMDB PDB Article PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Competitive inhibition of human HPGDS using PGH2 as substrate | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127759 BindingDB Entry DOI: 10.7270/Q2VD7346 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin E2 receptor EP2 subtype (Homo sapiens (Human)) | BDBM50521600 (CHEMBL4558749) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 1.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Cayman Chemical Company, Inc. Curated by ChEMBL | Assay Description Displacement of [3H]PGE2 from human recombinant EP2 receptor expressed in HEK293 cell membranes after 120 mins by liquid scintillation counting metho... | J Med Chem 62: 4731-4741 (2019) Article DOI: 10.1021/acs.jmedchem.9b00336 BindingDB Entry DOI: 10.7270/Q22R3W32 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50382321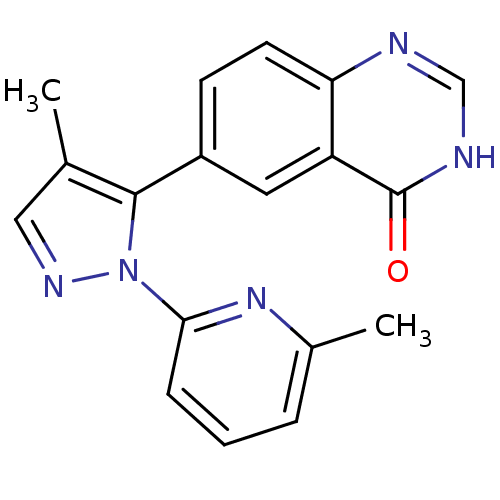 (CHEMBL2024688) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of ALK5 | Bioorg Med Chem Lett 22: 3392-7 (2012) Article DOI: 10.1016/j.bmcl.2012.04.013 BindingDB Entry DOI: 10.7270/Q2QC04H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50382322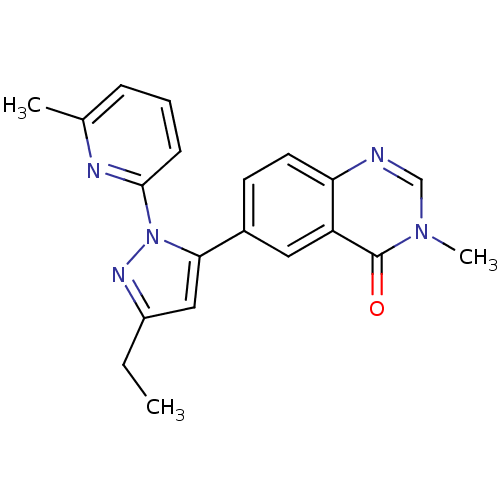 (CHEMBL2024689) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of ALK5 | Bioorg Med Chem Lett 22: 3392-7 (2012) Article DOI: 10.1016/j.bmcl.2012.04.013 BindingDB Entry DOI: 10.7270/Q2QC04H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity mitogen-activated protein kinase kinase 1/2 (Homo sapiens (Human)) | BDBM50222709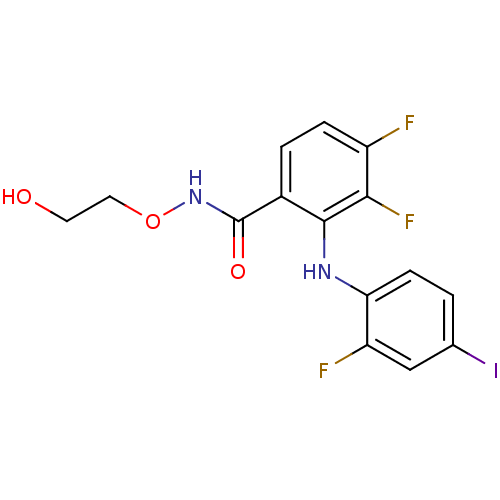 (3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-N-(2-h...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of MEK assessed as inhibition of ERK phosphorylation by Raf-MEK-ERK cascade assay | J Med Chem 50: 5090-102 (2007) Article DOI: 10.1021/jm0704548 BindingDB Entry DOI: 10.7270/Q2474DMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM172452 (US9090625, 4 | US9260450, 4 | US9938289, 4) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.92 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company | Assay Description ALK-5 kinase assay methods have been described in the art (see e.g., Laping et al. (2002) Mol. Pharmacol. 2002; 62: 58-62). The compounds named in th... | Bioorg Med Chem 17: 284-94 (2009) BindingDB Entry DOI: 10.7270/Q25M682V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity mitogen-activated protein kinase kinase 1/2 (Homo sapiens (Human)) | BDBM50222709 (3,4-difluoro-2-(2-fluoro-4-iodophenylamino)-N-(2-h...) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of MEK in mouse colon 26 carcinoma cells assessed as inhibition of ERK phosphorylation by ELISA | J Med Chem 50: 5090-102 (2007) Article DOI: 10.1021/jm0704548 BindingDB Entry DOI: 10.7270/Q2474DMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50382324 (CHEMBL2024691) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of ALK5 | Bioorg Med Chem Lett 22: 3392-7 (2012) Article DOI: 10.1016/j.bmcl.2012.04.013 BindingDB Entry DOI: 10.7270/Q2QC04H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50382320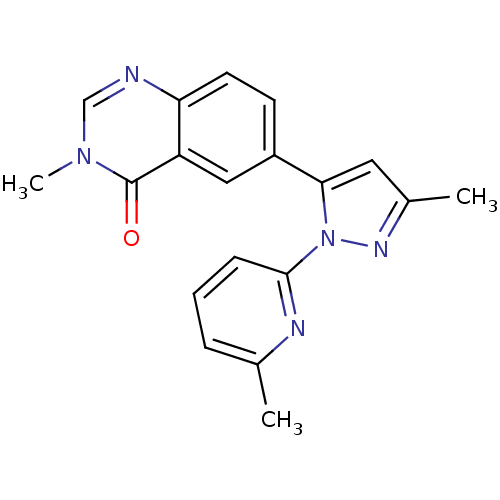 (CHEMBL2024687) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of ALK5 | Bioorg Med Chem Lett 22: 3392-7 (2012) Article DOI: 10.1016/j.bmcl.2012.04.013 BindingDB Entry DOI: 10.7270/Q2QC04H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM179403 (US9126973, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
CAYMAN CHEMICAL COMPANY, INCORPORATED US Patent | Assay Description The assay is carried out by the following steps: 1. Inhibitor screening is performed in 100 mM Tris-HCl, pH 8.0 containing 1 mM GSH, 1 mM MgCl.sub.2,... | US Patent US9126973 (2015) BindingDB Entry DOI: 10.7270/Q2930RZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity mitogen-activated protein kinase kinase 1/2 (Homo sapiens (Human)) | BDBM50476830 (CHEMBL442235) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of MEK in mouse colon 26 carcinoma cells assessed as inhibition of ERK phosphorylation by ELISA | J Med Chem 50: 5090-102 (2007) Article DOI: 10.1021/jm0704548 BindingDB Entry DOI: 10.7270/Q2474DMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50382319 (CHEMBL2024686) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of ALK5 | Bioorg Med Chem Lett 22: 3392-7 (2012) Article DOI: 10.1016/j.bmcl.2012.04.013 BindingDB Entry DOI: 10.7270/Q2QC04H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50382318 (CHEMBL2024685) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of ALK5 | Bioorg Med Chem Lett 22: 3392-7 (2012) Article DOI: 10.1016/j.bmcl.2012.04.013 BindingDB Entry DOI: 10.7270/Q2QC04H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM172452 (US9090625, 4 | US9260450, 4 | US9938289, 4) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.33 | n/a | n/a | n/a | n/a | n/a | n/a |
THESAN PHARMACEUTICALS, INC. US Patent | Assay Description Pharmacol 2002, 62 58-62) The compounds named in the specified Examples were tested as follows for inhibition of ALK-5 autophosphorylation activity a... | US Patent US9090625 (2015) BindingDB Entry DOI: 10.7270/Q2BG2MRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM172452 (US9090625, 4 | US9260450, 4 | US9938289, 4) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4.33 | n/a | n/a | n/a | n/a | 7.6 | 4 |
THESAN PHARMACEUTICALS, INC. US Patent | Assay Description In a 96 well filter-bottom plate (Millipore, #MSDV N6B 50), 58 ul Assay Buffer (50 mM HEPES, pH 7.6, with 10 mM NaCl, 10 mM MgCl2, and 1 mM DTT ) is ... | US Patent US9260450 (2016) BindingDB Entry DOI: 10.7270/Q2M32TM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dual specificity mitogen-activated protein kinase kinase 1/2 (Homo sapiens (Human)) | BDBM50476834 (CHEMBL234887) | PDB KEGG UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Auckland Curated by ChEMBL | Assay Description Inhibition of MEK in mouse colon 26 carcinoma cells assessed as inhibition of ERK phosphorylation by ELISA | J Med Chem 50: 5090-102 (2007) Article DOI: 10.1021/jm0704548 BindingDB Entry DOI: 10.7270/Q2474DMT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50382325 (CHEMBL2024693) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of ALK5 | Bioorg Med Chem Lett 22: 3392-7 (2012) Article DOI: 10.1016/j.bmcl.2012.04.013 BindingDB Entry DOI: 10.7270/Q2QC04H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM50559393 (CHEMBL4752221) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of HPGDS (unknown origin) by fluorescence polarization assay | Citation and Details Article DOI: 10.1016/j.bmcl.2020.127759 BindingDB Entry DOI: 10.7270/Q2VD7346 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM50382323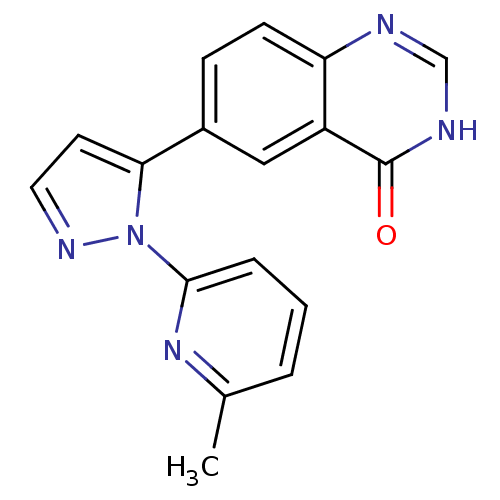 (CHEMBL2024690) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of ALK5 | Bioorg Med Chem Lett 22: 3392-7 (2012) Article DOI: 10.1016/j.bmcl.2012.04.013 BindingDB Entry DOI: 10.7270/Q2QC04H9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Hematopoietic prostaglandin D synthase (Homo sapiens (Human)) | BDBM179422 (US9126973, 19) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 8.0 | 25 |
CAYMAN CHEMICAL COMPANY, INCORPORATED US Patent | Assay Description The assay is carried out by the following steps: 1. Inhibitor screening is performed in 100 mM Tris-HCl, pH 8.0 containing 1 mM GSH, 1 mM MgCl.sub.2,... | US Patent US9126973 (2015) BindingDB Entry DOI: 10.7270/Q2930RZ3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM172455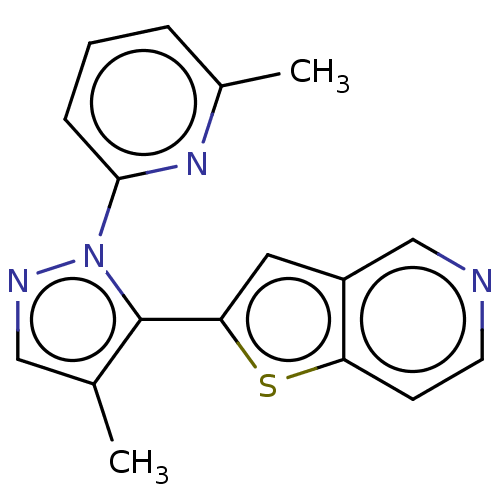 (US9090625, 7 | US9260450, 7 | US9938289, 7) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.65 | n/a | n/a | n/a | n/a | 7.6 | 4 |
THESAN PHARMACEUTICALS, INC. US Patent | Assay Description In a 96 well filter-bottom plate (Millipore, #MSDV N6B 50), 58 ul Assay Buffer (50 mM HEPES, pH 7.6, with 10 mM NaCl, 10 mM MgCl2, and 1 mM DTT ) is ... | US Patent US9260450 (2016) BindingDB Entry DOI: 10.7270/Q2M32TM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| TGF-beta receptor type-1 (Homo sapiens (Human)) | BDBM172455 (US9090625, 7 | US9260450, 7 | US9938289, 7) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.65 | n/a | n/a | n/a | n/a | n/a | n/a |
Kissei Pharmaceutical Company | Assay Description ALK-5 kinase assay methods have been described in the art (see e.g., Laping et al. (2002) Mol. Pharmacol. 2002; 62: 58-62). The compounds named in th... | Bioorg Med Chem 17: 284-94 (2009) BindingDB Entry DOI: 10.7270/Q25M682V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 407 total ) | Next | Last >> |