

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM13390 (4-{[(4-cyanophenyl)(1-methyl-1H-imidazol-5-yl)meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Globe Pharmaceutical R and Abbott Laboratories | Assay Description The in vitro activity of compounds inhibiting FTase or GGTase-I was determined by using scintillation proximity assay (SPA) technology. The assays we... | J Med Chem 47: 612-26 (2004) Article DOI: 10.1021/jm030434f BindingDB Entry DOI: 10.7270/Q2Z60M8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM13389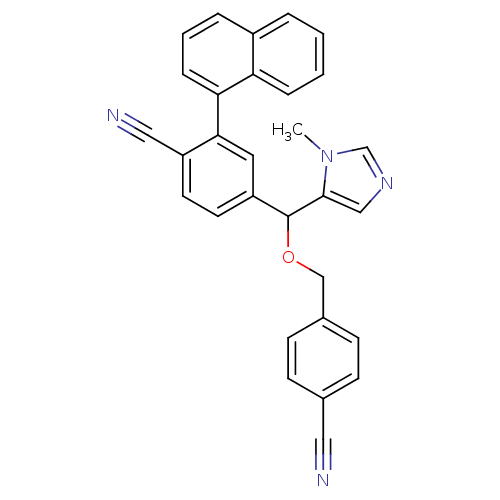 (4-{[(4-cyanophenyl)methoxy](1-methyl-1H-imidazol-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | 7.0 | 37 |
Globe Pharmaceutical R and Abbott Laboratories | Assay Description The in vitro activity of compounds inhibiting FTase or GGTase-I was determined by using scintillation proximity assay (SPA) technology. The assays we... | J Med Chem 47: 612-26 (2004) Article DOI: 10.1021/jm030434f BindingDB Entry DOI: 10.7270/Q2Z60M8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM13384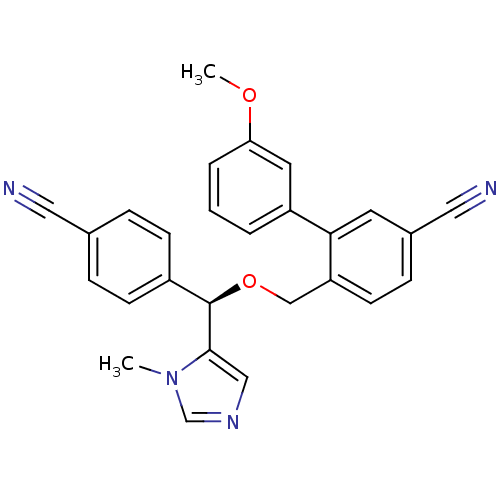 (4-{[(R)-(4-cyanophenyl)(1-methyl-1H-imidazol-5-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | 7.0 | 37 |
Globe Pharmaceutical R and Abbott Laboratories | Assay Description The in vitro activity of compounds inhibiting FTase or GGTase-I was determined by using scintillation proximity assay (SPA) technology. The assays we... | J Med Chem 47: 612-26 (2004) Article DOI: 10.1021/jm030434f BindingDB Entry DOI: 10.7270/Q2Z60M8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM13405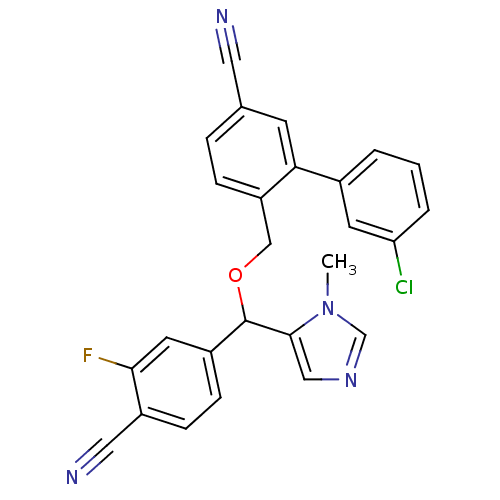 (3-(3-chlorophenyl)-4-{[(4-cyano-3-fluorophenyl)(1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Globe Pharmaceutical R and Abbott Laboratories | Assay Description The in vitro activity of compounds inhibiting FTase or GGTase-I was determined by using scintillation proximity assay (SPA) technology. The assays we... | J Med Chem 47: 612-26 (2004) Article DOI: 10.1021/jm030434f BindingDB Entry DOI: 10.7270/Q2Z60M8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM13415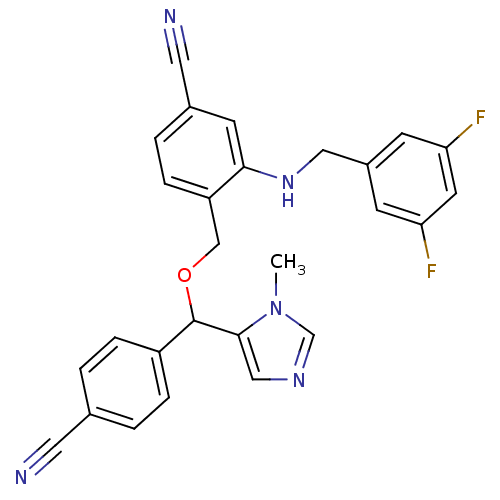 (4-{[(4-cyanophenyl)(1-methyl-1H-imidazol-5-yl)meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
Globe Pharmaceutical R and Abbott Laboratories | Assay Description The in vitro activity of compounds inhibiting FTase or GGTase-I was determined by using scintillation proximity assay (SPA) technology. The assays we... | J Med Chem 47: 612-26 (2004) Article DOI: 10.1021/jm030434f BindingDB Entry DOI: 10.7270/Q2Z60M8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM13388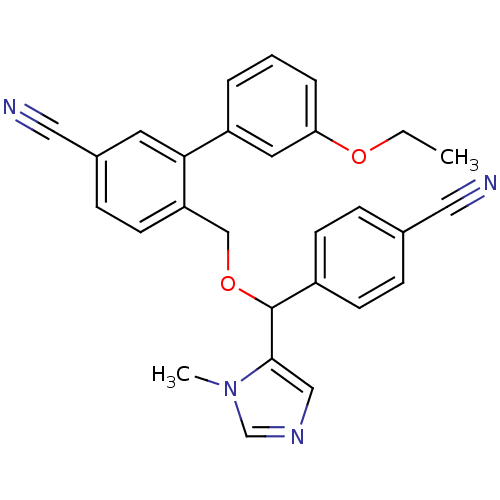 (4-{[(4-cyanophenyl)(1-methyl-1H-imidazol-5-yl)meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.690 | n/a | n/a | n/a | n/a | 7.0 | 37 |
Globe Pharmaceutical R and Abbott Laboratories | Assay Description The in vitro activity of compounds inhibiting FTase or GGTase-I was determined by using scintillation proximity assay (SPA) technology. The assays we... | J Med Chem 47: 612-26 (2004) Article DOI: 10.1021/jm030434f BindingDB Entry DOI: 10.7270/Q2Z60M8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM13383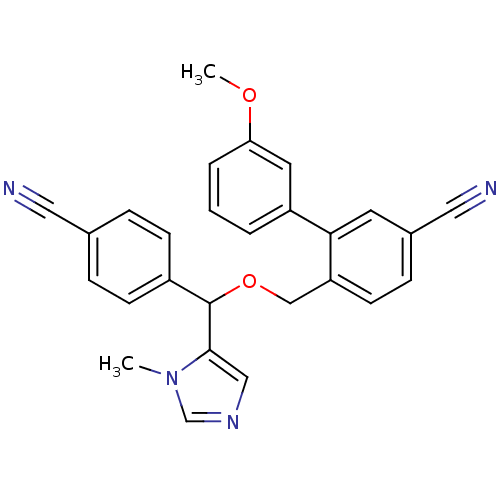 (4-{[(4-cyanophenyl)(1-methyl-1H-imidazol-5-yl)meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | 7.0 | 37 |
Globe Pharmaceutical R and Abbott Laboratories | Assay Description The in vitro activity of compounds inhibiting FTase or GGTase-I was determined by using scintillation proximity assay (SPA) technology. The assays we... | J Med Chem 47: 612-26 (2004) Article DOI: 10.1021/jm030434f BindingDB Entry DOI: 10.7270/Q2Z60M8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM13418 (A313326 Analogue 65 | N-(3-chlorophenyl)-5-cyano-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Globe Pharmaceutical R and Abbott Laboratories | Assay Description The in vitro activity of compounds inhibiting FTase or GGTase-I was determined by using scintillation proximity assay (SPA) technology. The assays we... | J Med Chem 47: 612-26 (2004) Article DOI: 10.1021/jm030434f BindingDB Entry DOI: 10.7270/Q2Z60M8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM13406 (3-(3-chlorophenyl)-4-{[(4-cyano-2-fluorophenyl)(1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
Globe Pharmaceutical R and Abbott Laboratories | Assay Description The in vitro activity of compounds inhibiting FTase or GGTase-I was determined by using scintillation proximity assay (SPA) technology. The assays we... | J Med Chem 47: 612-26 (2004) Article DOI: 10.1021/jm030434f BindingDB Entry DOI: 10.7270/Q2Z60M8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM13375 (4-({[4-chloro-2-(3-chlorophenyl)phenyl]methoxy}(1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | 7.0 | 37 |
Globe Pharmaceutical R and Abbott Laboratories | Assay Description The in vitro activity of compounds inhibiting FTase or GGTase-I was determined by using scintillation proximity assay (SPA) technology. The assays we... | J Med Chem 47: 612-26 (2004) Article DOI: 10.1021/jm030434f BindingDB Entry DOI: 10.7270/Q2Z60M8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM13393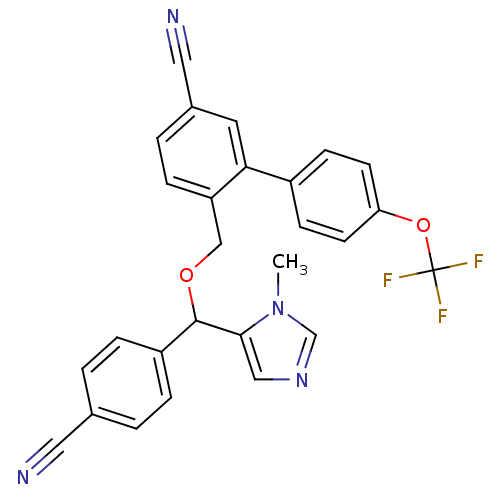 (4-{[(4-cyanophenyl)(1-methyl-1H-imidazol-5-yl)meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.840 | n/a | n/a | n/a | n/a | n/a | n/a |
Globe Pharmaceutical R and Abbott Laboratories | Assay Description The in vitro activity of compounds inhibiting FTase or GGTase-I was determined by using scintillation proximity assay (SPA) technology. The assays we... | J Med Chem 47: 612-26 (2004) Article DOI: 10.1021/jm030434f BindingDB Entry DOI: 10.7270/Q2Z60M8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM13386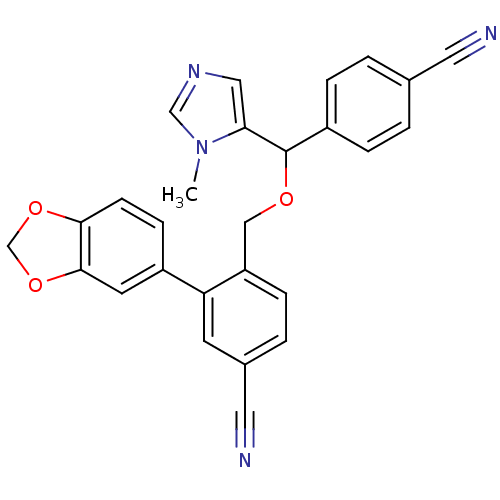 (3-(2H-1,3-benzodioxol-5-yl)-4-{[(4-cyanophenyl)(1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | 7.0 | 37 |
Globe Pharmaceutical R and Abbott Laboratories | Assay Description The in vitro activity of compounds inhibiting FTase or GGTase-I was determined by using scintillation proximity assay (SPA) technology. The assays we... | J Med Chem 47: 612-26 (2004) Article DOI: 10.1021/jm030434f BindingDB Entry DOI: 10.7270/Q2Z60M8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM13382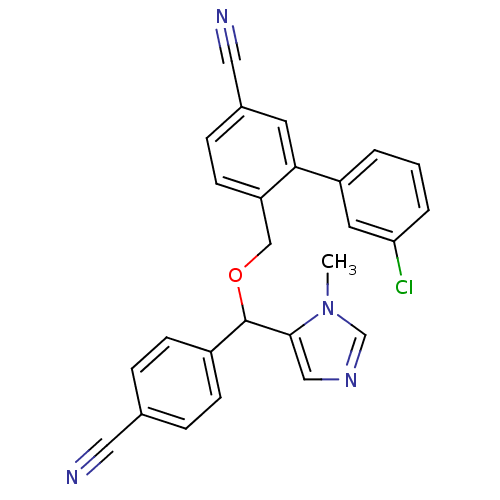 (3-(3-chlorophenyl)-4-{[(4-cyanophenyl)(1-methyl-1H...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | 7.0 | 37 |
Globe Pharmaceutical R and Abbott Laboratories | Assay Description The in vitro activity of compounds inhibiting FTase or GGTase-I was determined by using scintillation proximity assay (SPA) technology. The assays we... | J Med Chem 47: 612-26 (2004) Article DOI: 10.1021/jm030434f BindingDB Entry DOI: 10.7270/Q2Z60M8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM13412 (A313326 Analogue 60a | N-(5-cyano-2-{[(4-cyanophen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a |
Globe Pharmaceutical R and Abbott Laboratories | Assay Description The in vitro activity of compounds inhibiting FTase or GGTase-I was determined by using scintillation proximity assay (SPA) technology. The assays we... | J Med Chem 47: 612-26 (2004) Article DOI: 10.1021/jm030434f BindingDB Entry DOI: 10.7270/Q2Z60M8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM13377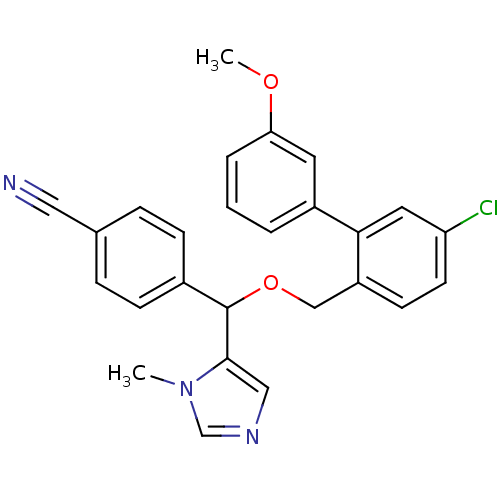 (4-({[4-chloro-2-(3-methoxyphenyl)phenyl]methoxy}(1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.910 | n/a | n/a | n/a | n/a | 7.0 | 37 |
Globe Pharmaceutical R and Abbott Laboratories | Assay Description The in vitro activity of compounds inhibiting FTase or GGTase-I was determined by using scintillation proximity assay (SPA) technology. The assays we... | J Med Chem 47: 612-26 (2004) Article DOI: 10.1021/jm030434f BindingDB Entry DOI: 10.7270/Q2Z60M8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM13380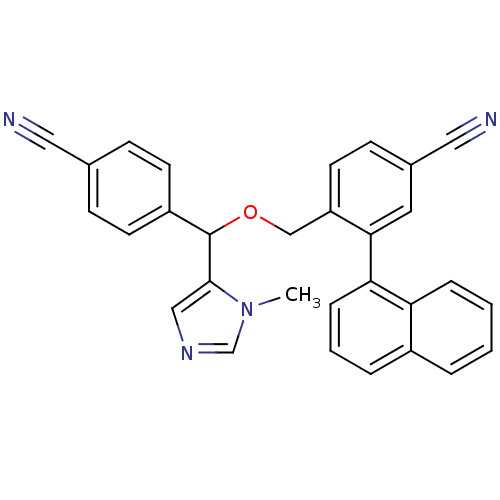 (4-{[(4-cyanophenyl)(1-methyl-1H-imidazol-5-yl)meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | 7.0 | 37 |
Globe Pharmaceutical R and Abbott Laboratories | Assay Description The in vitro activity of compounds inhibiting FTase or GGTase-I was determined by using scintillation proximity assay (SPA) technology. The assays we... | J Med Chem 47: 612-26 (2004) Article DOI: 10.1021/jm030434f BindingDB Entry DOI: 10.7270/Q2Z60M8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM13391 (4-{[(4-cyanophenyl)(1-methyl-1H-imidazol-5-yl)meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.960 | n/a | n/a | n/a | n/a | n/a | n/a |
Globe Pharmaceutical R and Abbott Laboratories | Assay Description The in vitro activity of compounds inhibiting FTase or GGTase-I was determined by using scintillation proximity assay (SPA) technology. The assays we... | J Med Chem 47: 612-26 (2004) Article DOI: 10.1021/jm030434f BindingDB Entry DOI: 10.7270/Q2Z60M8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM13404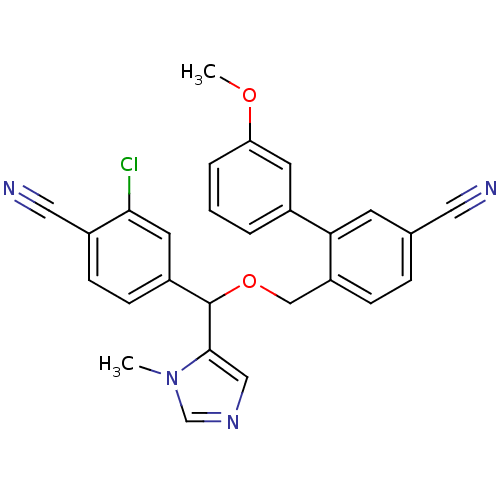 (4-{[(3-chloro-4-cyanophenyl)(1-methyl-1H-imidazol-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a |
Globe Pharmaceutical R and Abbott Laboratories | Assay Description The in vitro activity of compounds inhibiting FTase or GGTase-I was determined by using scintillation proximity assay (SPA) technology. The assays we... | J Med Chem 47: 612-26 (2004) Article DOI: 10.1021/jm030434f BindingDB Entry DOI: 10.7270/Q2Z60M8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM13395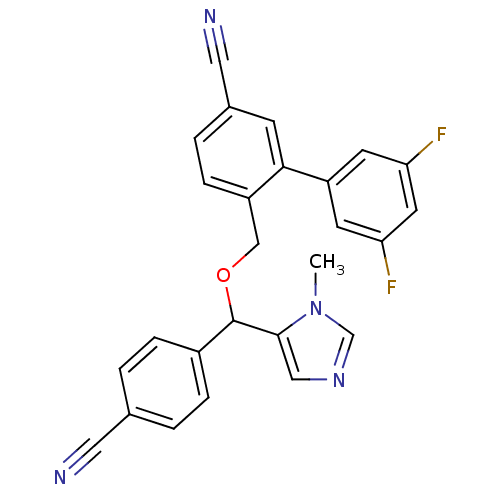 (4-{[(4-cyanophenyl)(1-methyl-1H-imidazol-5-yl)meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
Globe Pharmaceutical R and Abbott Laboratories | Assay Description The in vitro activity of compounds inhibiting FTase or GGTase-I was determined by using scintillation proximity assay (SPA) technology. The assays we... | J Med Chem 47: 612-26 (2004) Article DOI: 10.1021/jm030434f BindingDB Entry DOI: 10.7270/Q2Z60M8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50100934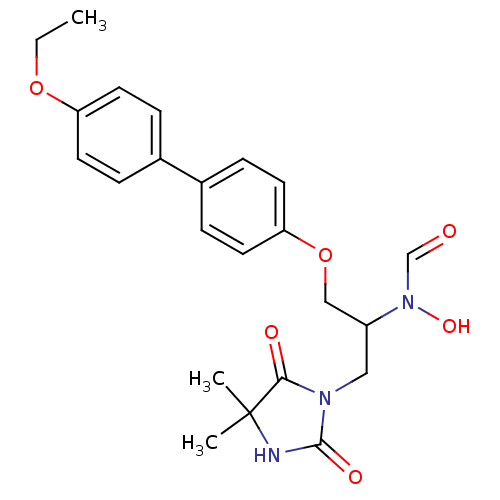 (CHEMBL40151 | N-[2-(4,4-Dimethyl-2,5-dioxo-imidazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibition of human Matrix metalloproteinase-2. | Bioorg Med Chem Lett 11: 1557-60 (2001) BindingDB Entry DOI: 10.7270/Q2CZ36DP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM13413 (A313326 Analogue 60b) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Globe Pharmaceutical R and Abbott Laboratories | Assay Description The in vitro activity of compounds inhibiting FTase or GGTase-I was determined by using scintillation proximity assay (SPA) technology. The assays we... | J Med Chem 47: 612-26 (2004) Article DOI: 10.1021/jm030434f BindingDB Entry DOI: 10.7270/Q2Z60M8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM13394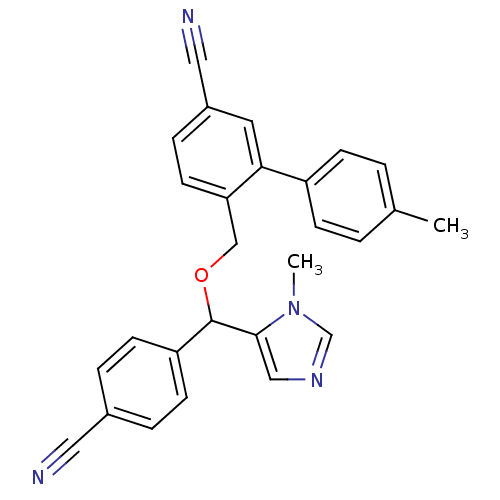 (4-{[(4-cyanophenyl)(1-methyl-1H-imidazol-5-yl)meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Globe Pharmaceutical R and Abbott Laboratories | Assay Description The in vitro activity of compounds inhibiting FTase or GGTase-I was determined by using scintillation proximity assay (SPA) technology. The assays we... | J Med Chem 47: 612-26 (2004) Article DOI: 10.1021/jm030434f BindingDB Entry DOI: 10.7270/Q2Z60M8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM13387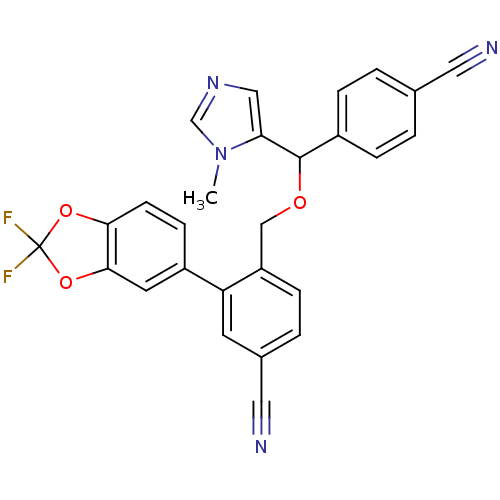 (4-{[(4-cyanophenyl)(1-methyl-1H-imidazol-5-yl)meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.0 | 37 |
Globe Pharmaceutical R and Abbott Laboratories | Assay Description The in vitro activity of compounds inhibiting FTase or GGTase-I was determined by using scintillation proximity assay (SPA) technology. The assays we... | J Med Chem 47: 612-26 (2004) Article DOI: 10.1021/jm030434f BindingDB Entry DOI: 10.7270/Q2Z60M8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM13378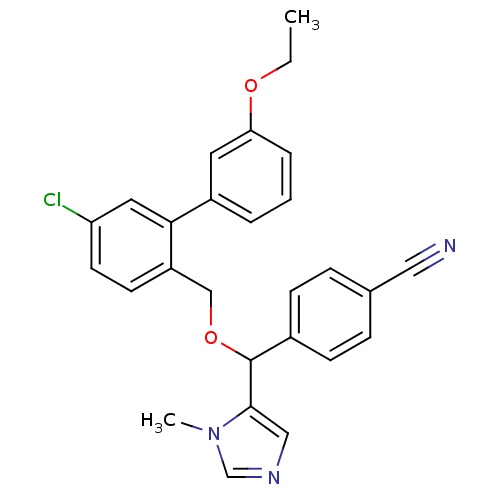 (4-({[4-chloro-2-(3-ethoxyphenyl)phenyl]methoxy}(1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | 7.0 | 37 |
Globe Pharmaceutical R and Abbott Laboratories | Assay Description The in vitro activity of compounds inhibiting FTase or GGTase-I was determined by using scintillation proximity assay (SPA) technology. The assays we... | J Med Chem 47: 612-26 (2004) Article DOI: 10.1021/jm030434f BindingDB Entry DOI: 10.7270/Q2Z60M8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM13396 (4-({[2-(3-methoxyphenyl)-4-nitrophenyl]methoxy}(1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Globe Pharmaceutical R and Abbott Laboratories | Assay Description The in vitro activity of compounds inhibiting FTase or GGTase-I was determined by using scintillation proximity assay (SPA) technology. The assays we... | J Med Chem 47: 612-26 (2004) Article DOI: 10.1021/jm030434f BindingDB Entry DOI: 10.7270/Q2Z60M8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM13407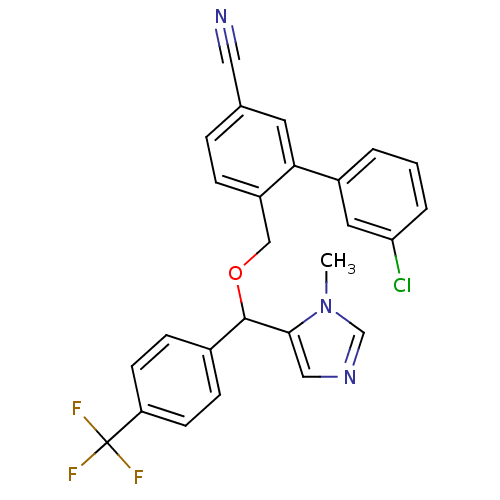 (3-(3-chlorophenyl)-4-{[(1-methyl-1H-imidazol-5-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Globe Pharmaceutical R and Abbott Laboratories | Assay Description The in vitro activity of compounds inhibiting FTase or GGTase-I was determined by using scintillation proximity assay (SPA) technology. The assays we... | J Med Chem 47: 612-26 (2004) Article DOI: 10.1021/jm030434f BindingDB Entry DOI: 10.7270/Q2Z60M8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM13392 (4-{[(4-cyanophenyl)(1-methyl-1H-imidazol-5-yl)meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Globe Pharmaceutical R and Abbott Laboratories | Assay Description The in vitro activity of compounds inhibiting FTase or GGTase-I was determined by using scintillation proximity assay (SPA) technology. The assays we... | J Med Chem 47: 612-26 (2004) Article DOI: 10.1021/jm030434f BindingDB Entry DOI: 10.7270/Q2Z60M8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Bos taurus (bovine)) | BDBM13382 (3-(3-chlorophenyl)-4-{[(4-cyanophenyl)(1-methyl-1H...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against Factor Xa | Bioorg Med Chem Lett 14: 4603-6 (2004) Article DOI: 10.1016/j.bmcl.2004.07.004 BindingDB Entry DOI: 10.7270/Q27080WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM13379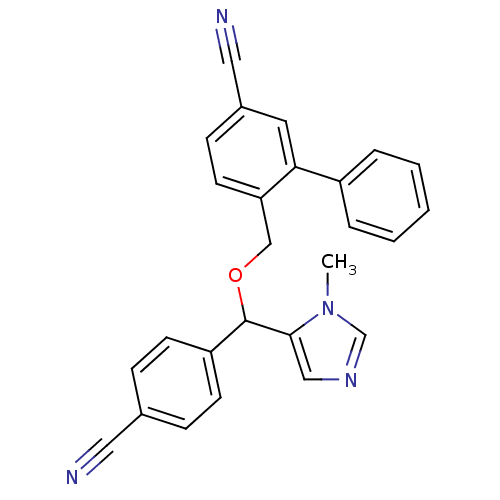 (4-{[(4-cyanophenyl)(1-methyl-1H-imidazol-5-yl)meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | 7.0 | 37 |
Globe Pharmaceutical R and Abbott Laboratories | Assay Description The in vitro activity of compounds inhibiting FTase or GGTase-I was determined by using scintillation proximity assay (SPA) technology. The assays we... | J Med Chem 47: 612-26 (2004) Article DOI: 10.1021/jm030434f BindingDB Entry DOI: 10.7270/Q2Z60M8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM13409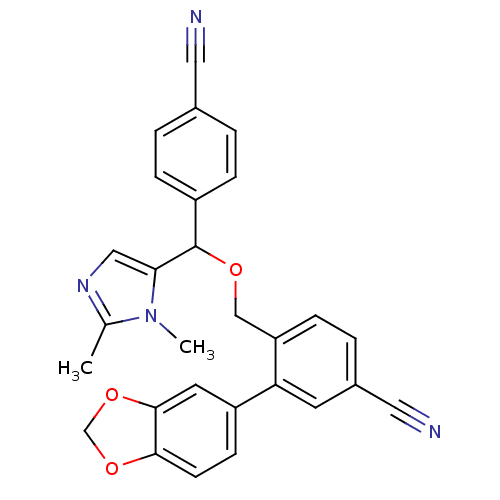 (3-(2H-1,3-benzodioxol-5-yl)-4-{[(4-cyanophenyl)(1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Globe Pharmaceutical R and Abbott Laboratories | Assay Description The in vitro activity of compounds inhibiting FTase or GGTase-I was determined by using scintillation proximity assay (SPA) technology. The assays we... | J Med Chem 47: 612-26 (2004) Article DOI: 10.1021/jm030434f BindingDB Entry DOI: 10.7270/Q2Z60M8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM13402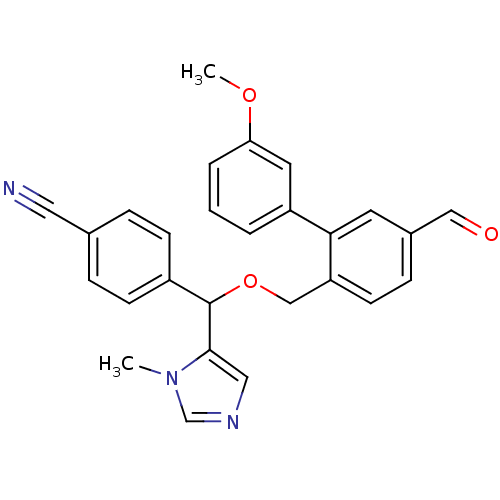 (4-({[4-formyl-2-(3-methoxyphenyl)phenyl]methoxy}(1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Globe Pharmaceutical R and Abbott Laboratories | Assay Description The in vitro activity of compounds inhibiting FTase or GGTase-I was determined by using scintillation proximity assay (SPA) technology. The assays we... | J Med Chem 47: 612-26 (2004) Article DOI: 10.1021/jm030434f BindingDB Entry DOI: 10.7270/Q2Z60M8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50207507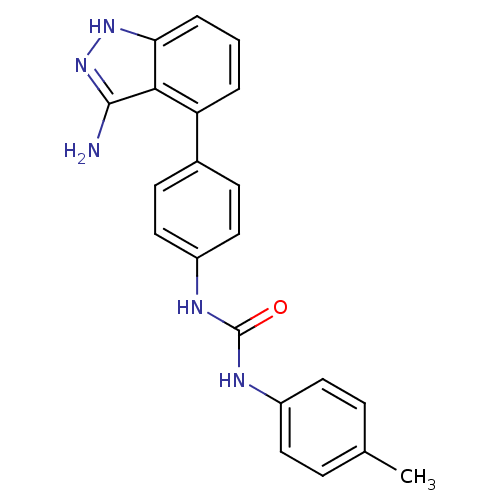 (1-(4-(3-amino-1H-indazol-4-yl)phenyl)-3-p-tolylure...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of FLT3 by HTRF assay | J Med Chem 50: 1584-97 (2007) Article DOI: 10.1021/jm061280h BindingDB Entry DOI: 10.7270/Q2DJ5F95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM13403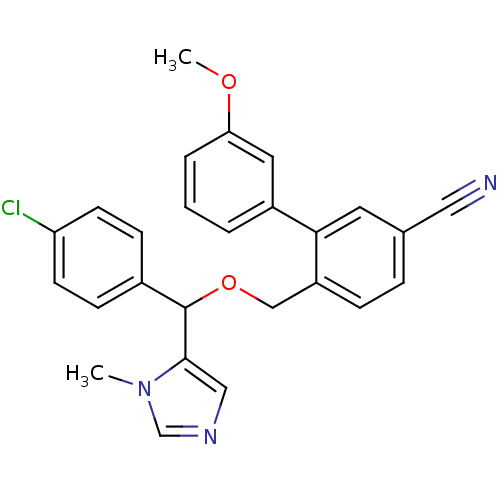 (4-{[(4-chlorophenyl)(1-methyl-1H-imidazol-5-yl)met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Globe Pharmaceutical R and Abbott Laboratories | Assay Description The in vitro activity of compounds inhibiting FTase or GGTase-I was determined by using scintillation proximity assay (SPA) technology. The assays we... | J Med Chem 47: 612-26 (2004) Article DOI: 10.1021/jm030434f BindingDB Entry DOI: 10.7270/Q2Z60M8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM13408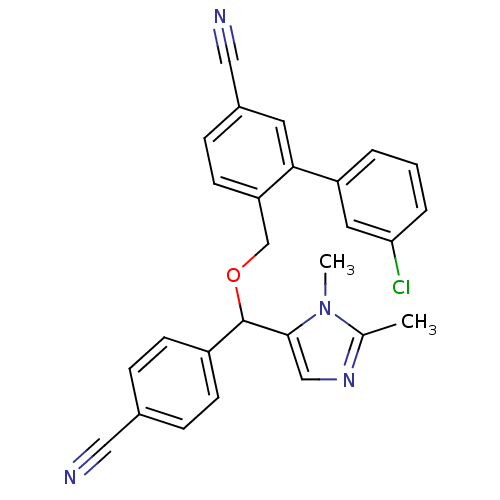 (3-(3-chlorophenyl)-4-{[(4-cyanophenyl)(1,2-dimethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Globe Pharmaceutical R and Abbott Laboratories | Assay Description The in vitro activity of compounds inhibiting FTase or GGTase-I was determined by using scintillation proximity assay (SPA) technology. The assays we... | J Med Chem 47: 612-26 (2004) Article DOI: 10.1021/jm030434f BindingDB Entry DOI: 10.7270/Q2Z60M8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM13417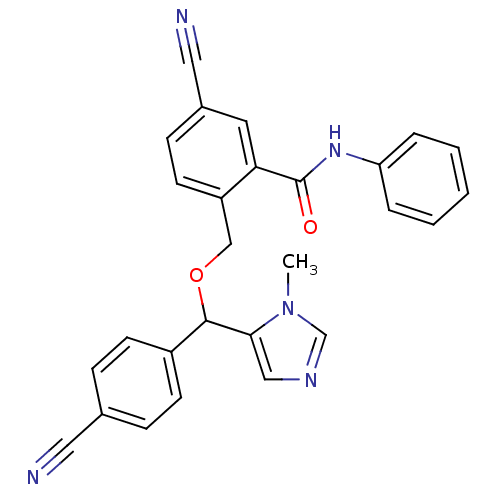 (5-cyano-2-{[(4-cyanophenyl)(1-methyl-1H-imidazol-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Globe Pharmaceutical R and Abbott Laboratories | Assay Description The in vitro activity of compounds inhibiting FTase or GGTase-I was determined by using scintillation proximity assay (SPA) technology. The assays we... | J Med Chem 47: 612-26 (2004) Article DOI: 10.1021/jm030434f BindingDB Entry DOI: 10.7270/Q2Z60M8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM13414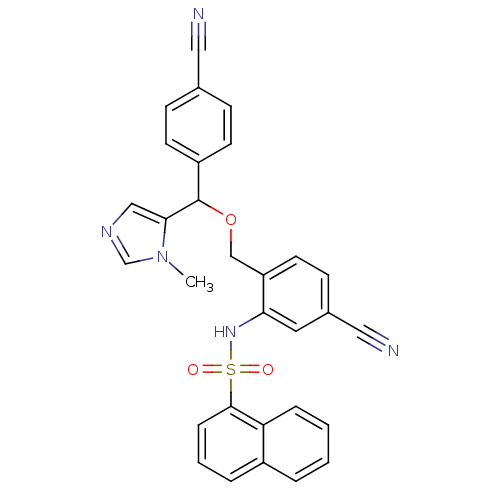 (A313326 Analogue 60c | N-(5-cyano-2-{[(4-cyanophen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Globe Pharmaceutical R and Abbott Laboratories | Assay Description The in vitro activity of compounds inhibiting FTase or GGTase-I was determined by using scintillation proximity assay (SPA) technology. The assays we... | J Med Chem 47: 612-26 (2004) Article DOI: 10.1021/jm030434f BindingDB Entry DOI: 10.7270/Q2Z60M8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Geranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Bos taurus (bovine)) | BDBM50135353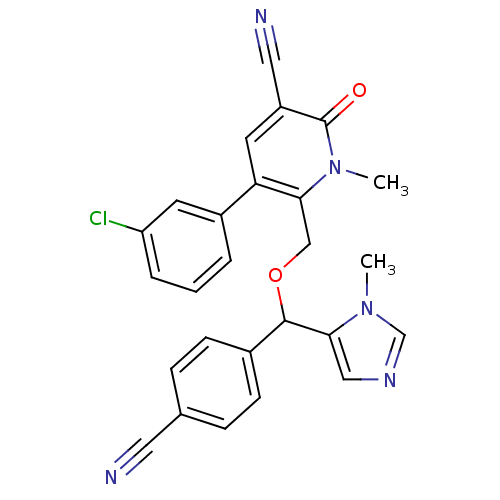 (5-(3-Chloro-phenyl)-6-[(4-cyano-phenyl)-(3-methyl-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description In vitro inhibitory activity against Factor Xa | Bioorg Med Chem Lett 14: 4603-6 (2004) Article DOI: 10.1016/j.bmcl.2004.07.004 BindingDB Entry DOI: 10.7270/Q27080WG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50207484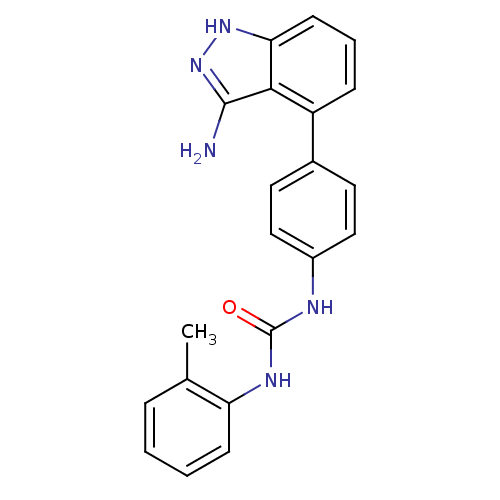 (1-(4-(3-amino-1H-indazol-4-yl)phenyl)-3-o-tolylure...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of FLT3 by HTRF assay | J Med Chem 50: 1584-97 (2007) Article DOI: 10.1021/jm061280h BindingDB Entry DOI: 10.7270/Q2DJ5F95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 1 (Homo sapiens (Human)) | BDBM21079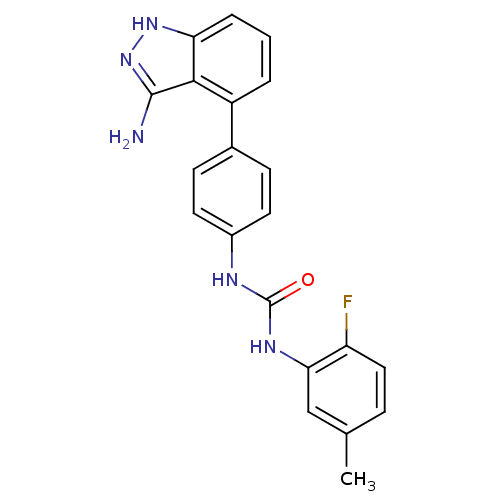 (1-[4-(3-amino-1H-indazol-4-yl)phenyl]-3-(2-fluoro-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of FLT1 by HTRF assay | J Med Chem 50: 1584-97 (2007) Article DOI: 10.1021/jm061280h BindingDB Entry DOI: 10.7270/Q2DJ5F95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage colony-stimulating factor 1 receptor (Homo sapiens (Human)) | BDBM21079 (1-[4-(3-amino-1H-indazol-4-yl)phenyl]-3-(2-fluoro-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of CSF1R by HTRF assay | J Med Chem 50: 1584-97 (2007) Article DOI: 10.1021/jm061280h BindingDB Entry DOI: 10.7270/Q2DJ5F95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50207509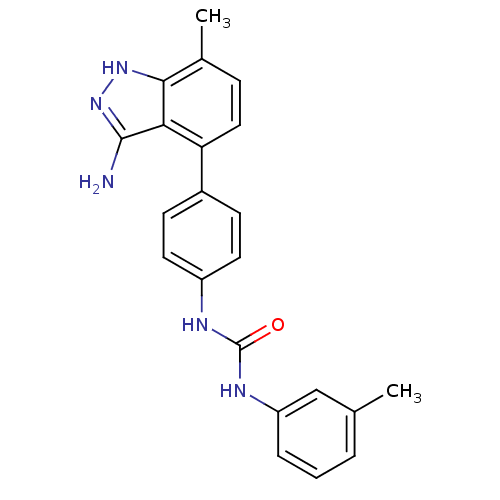 (1-(4-(3-amino-7-methyl-1H-indazol-4-yl)phenyl)-3-m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of VEGF-induced human KDR phosphorylation in mouse 3T3 cells by ELISA | J Med Chem 50: 1584-97 (2007) Article DOI: 10.1021/jm061280h BindingDB Entry DOI: 10.7270/Q2DJ5F95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aromatase (Homo sapiens (Human)) | BDBM8826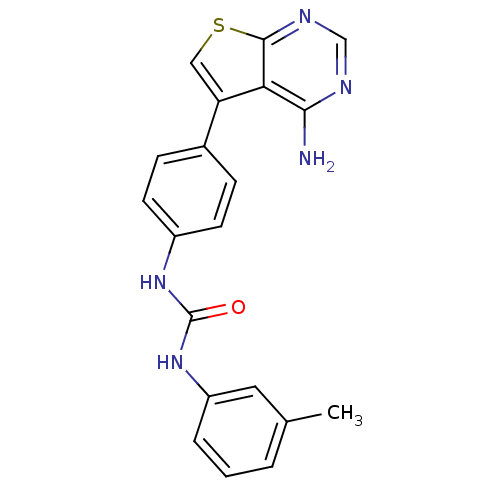 (3-(4-{4-aminothieno[2,3-d]pyrimidin-5-yl}phenyl)-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The assay uses purified enzyme interacting with biotinylated peptide substrate. HTRF is based on the proximity of europium cryptate (donor fluorophor... | J Med Chem 48: 6066-83 (2005) Article DOI: 10.1021/jm050458h BindingDB Entry DOI: 10.7270/Q2WD3XSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Receptor-type tyrosine-protein kinase FLT3 (Homo sapiens (Human)) | BDBM50207473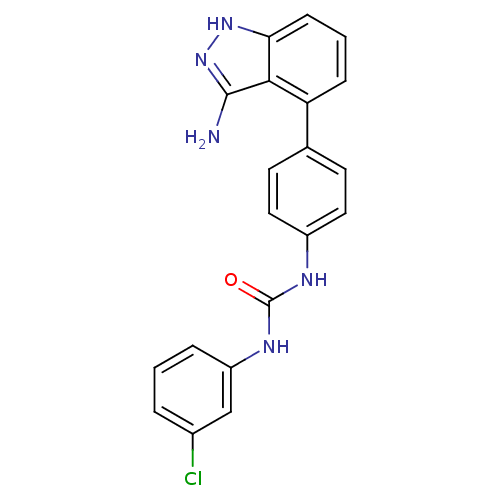 (1-(4-(3-amino-1H-indazol-4-yl)phenyl)-3-(3-chlorop...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of FLT3 by HTRF assay | J Med Chem 50: 1584-97 (2007) Article DOI: 10.1021/jm061280h BindingDB Entry DOI: 10.7270/Q2DJ5F95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM8826 (3-(4-{4-aminothieno[2,3-d]pyrimidin-5-yl}phenyl)-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The assay uses purified enzyme interacting with biotinylated peptide substrate. HTRF is based on the proximity of europium cryptate (donor fluorophor... | J Med Chem 48: 6066-83 (2005) Article DOI: 10.1021/jm050458h BindingDB Entry DOI: 10.7270/Q2WD3XSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM8870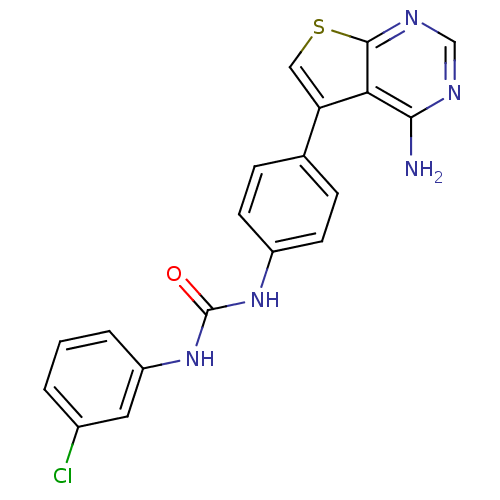 (3-(4-{4-aminothieno[2,3-d]pyrimidin-5-yl}phenyl)-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories | Assay Description The assay uses purified enzyme interacting with biotinylated peptide substrate. HTRF is based on the proximity of europium cryptate (donor fluorophor... | J Med Chem 48: 6066-83 (2005) Article DOI: 10.1021/jm050458h BindingDB Entry DOI: 10.7270/Q2WD3XSF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50207499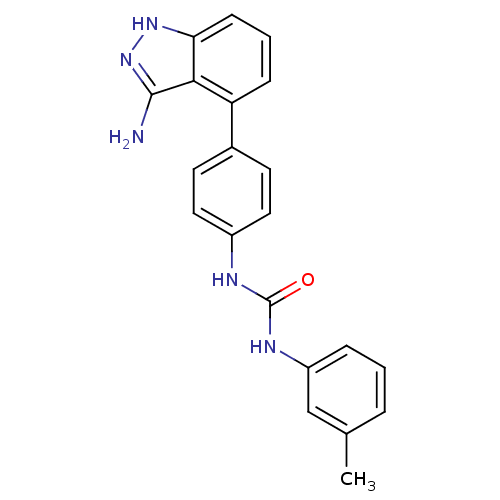 (1-(4-(3-amino-1H-indazol-4-yl)phenyl)-3-m-tolylure...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of KDR by HTRF assay | J Med Chem 50: 1584-97 (2007) Article DOI: 10.1021/jm061280h BindingDB Entry DOI: 10.7270/Q2DJ5F95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM21079 (1-[4-(3-amino-1H-indazol-4-yl)phenyl]-3-(2-fluoro-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of VEGF-induced human KDR phosphorylation in mouse 3T3 cells by ELISA | J Med Chem 50: 1584-97 (2007) Article DOI: 10.1021/jm061280h BindingDB Entry DOI: 10.7270/Q2DJ5F95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha (Homo sapiens (Human)) | BDBM13410 (3-(3-chlorophenyl)-4-{[(4-cyanophenyl)(1,3-thiazol...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Globe Pharmaceutical R and Abbott Laboratories | Assay Description The in vitro activity of compounds inhibiting FTase or GGTase-I was determined by using scintillation proximity assay (SPA) technology. The assays we... | J Med Chem 47: 612-26 (2004) Article DOI: 10.1021/jm030434f BindingDB Entry DOI: 10.7270/Q2Z60M8D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM50207474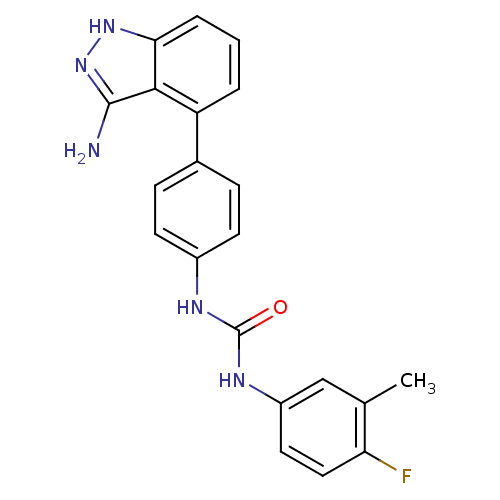 (1-(4-(3-amino-1H-indazol-4-yl)phenyl)-3-(4-fluoro-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of KDR by HTRF assay | J Med Chem 50: 1584-97 (2007) Article DOI: 10.1021/jm061280h BindingDB Entry DOI: 10.7270/Q2DJ5F95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM21079 (1-[4-(3-amino-1H-indazol-4-yl)phenyl]-3-(2-fluoro-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of KDR by HTRF assay | J Med Chem 50: 1584-97 (2007) Article DOI: 10.1021/jm061280h BindingDB Entry DOI: 10.7270/Q2DJ5F95 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 501 total ) | Next | Last >> |