

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260806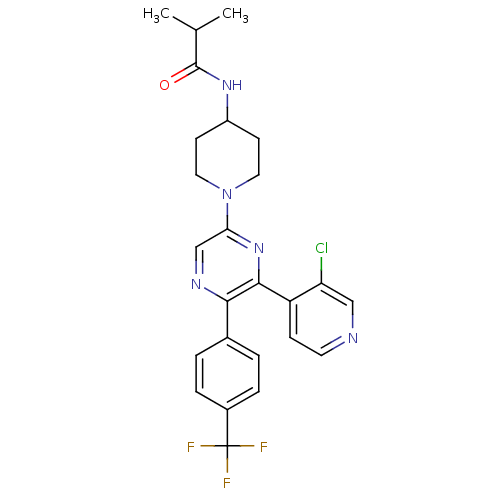 (CHEMBL497557 | N-(1-(6-(3-chloropyridin-4-yl)-5-(4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at rat CB1 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50260806 (CHEMBL497557 | N-(1-(6-(3-chloropyridin-4-yl)-5-(4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at rat CB1 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260767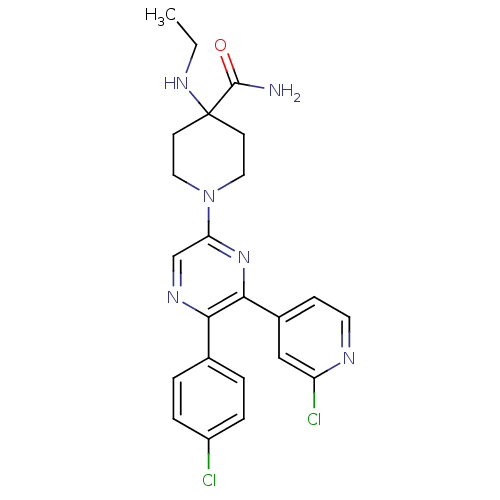 (1-(5-(4-chlorophenyl)-6-(2-chloropyridin-4-yl)pyra...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260805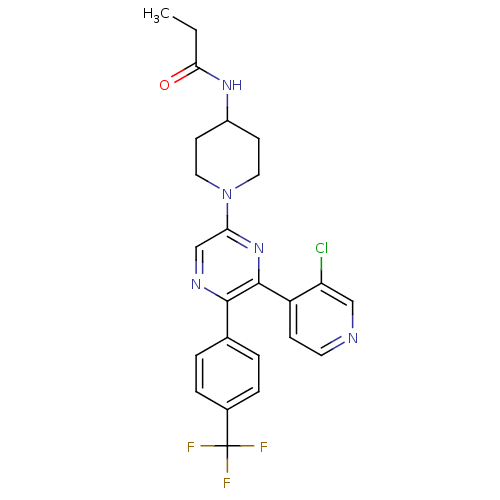 (CHEMBL524804 | N-(1-(6-(3-chloropyridin-4-yl)-5-(4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260681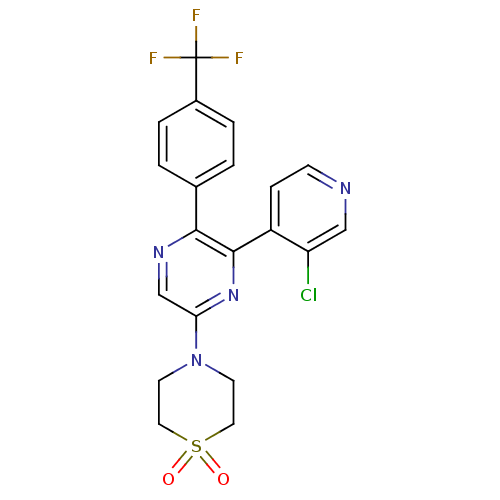 (4-[6-(3-Chloro-pyridin-4-yl)-5-(4-trifluoromethyl-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50260681 (4-[6-(3-Chloro-pyridin-4-yl)-5-(4-trifluoromethyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at rat CB1 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260768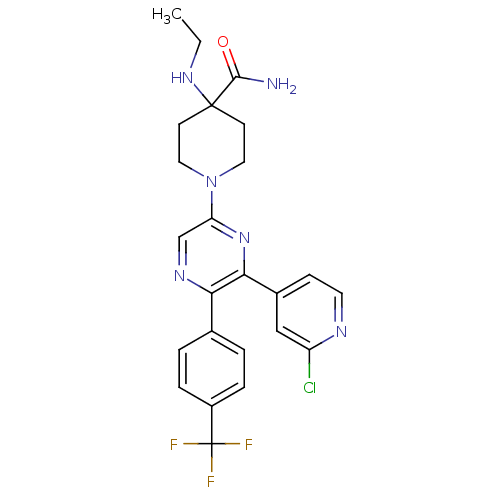 (1-(6-(2-chloropyridin-4-yl)-5-(4-(trifluoromethyl)...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260682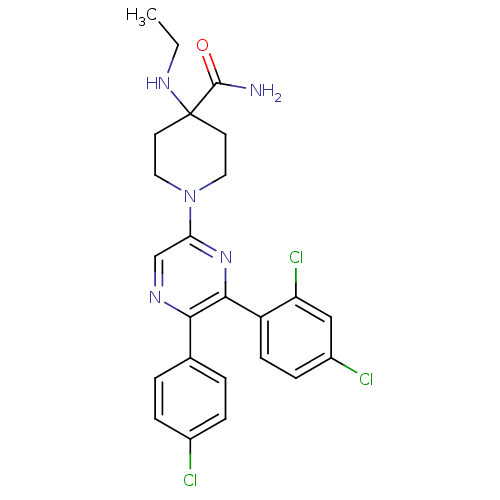 (1-(5-(4-chlorophenyl)-6-(2,4-dichlorophenyl)pyrazi...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260803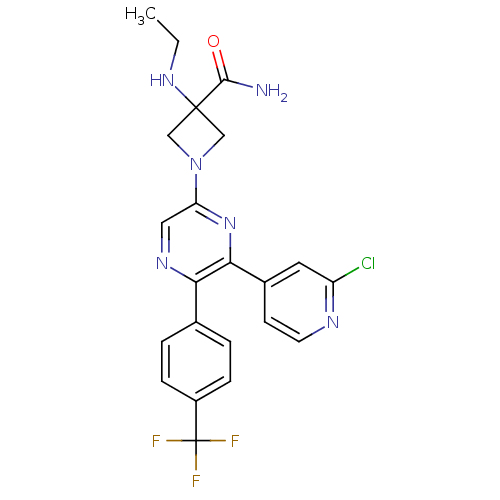 (1-(6-(2-chloropyridin-4-yl)-5-(4-(trifluoromethyl)...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260718 (1-(6-(2-chlorophenyl)-5-(4-chlorophenyl)pyrazin-2-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260717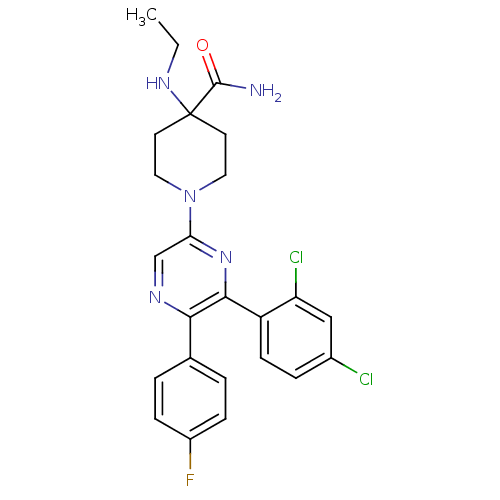 (1-(6-(2,4-dichlorophenyl)-5-(4-fluorophenyl)pyrazi...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260804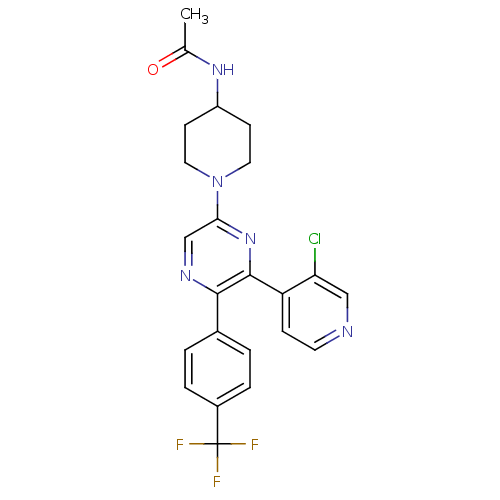 (CHEMBL497556 | N-(1-(6-(3-chloropyridin-4-yl)-5-(4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.97 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Rattus norvegicus (rat)) | BDBM50260803 (1-(6-(2-chloropyridin-4-yl)-5-(4-(trifluoromethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.06 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at rat CB1 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260720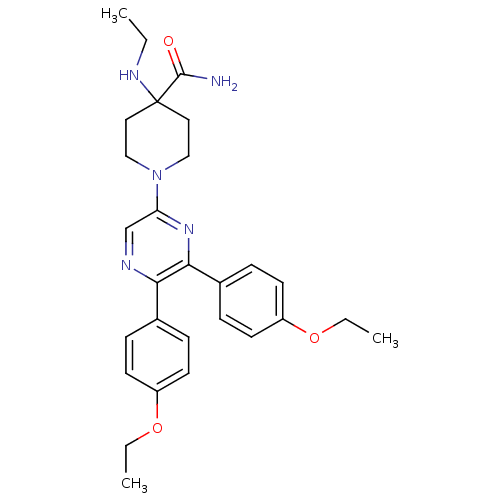 (1-(5,6-bis(4-ethoxyphenyl)pyrazin-2-yl)-4-(ethylam...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260766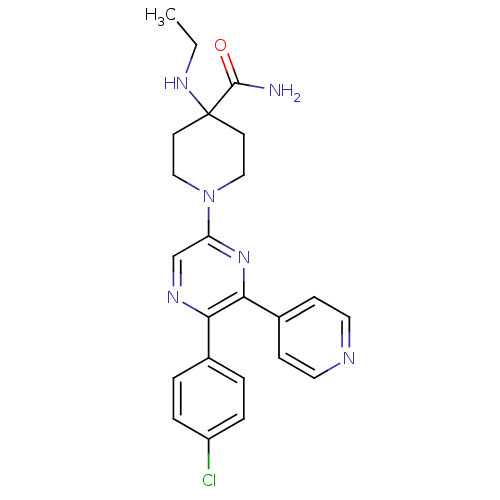 (1-(5-(4-chlorophenyl)-6-(pyridin-4-yl)pyrazin-2-yl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260719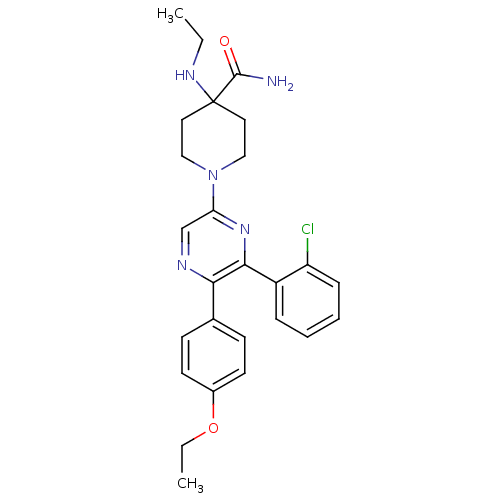 (1-(6-(2-chlorophenyl)-5-(4-ethoxyphenyl)pyrazin-2-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15.0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260765 (1-(5-(4-chlorophenyl)-6-(4-cyanophenyl)pyrazin-2-y...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 32.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB1 receptor expressed in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 815 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB2 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50260681 (4-[6-(3-Chloro-pyridin-4-yl)-5-(4-trifluoromethyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB2 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50260806 (CHEMBL497557 | N-(1-(6-(3-chloropyridin-4-yl)-5-(4...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 4.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB2 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50260803 (1-(6-(2-chloropyridin-4-yl)-5-(4-(trifluoromethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Antagonist activity at human CB2 receptor in SF9 cells assessed as inhibition of CP-55940-stimulated GTPgammaS binding | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260806 (CHEMBL497557 | N-(1-(6-(3-chloropyridin-4-yl)-5-(4...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Inverse agonist at human CB1 receptor expressed in SF9 cells assessed as decrease in GTPgammaS level | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM21278 (5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 1.35 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Inverse agonist at human CB1 receptor expressed in SF9 cells assessed as decrease in GTPgammaS level | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260803 (1-(6-(2-chloropyridin-4-yl)-5-(4-(trifluoromethyl)...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.07 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Inverse agonist at human CB1 receptor expressed in SF9 cells assessed as decrease in GTPgammaS level | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50260681 (4-[6-(3-Chloro-pyridin-4-yl)-5-(4-trifluoromethyl-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Neurogen Corporation Curated by ChEMBL | Assay Description Inverse agonist at human CB1 receptor expressed in SF9 cells assessed as decrease in GTPgammaS level | Bioorg Med Chem Lett 18: 3376-81 (2008) Article DOI: 10.1016/j.bmcl.2008.04.022 BindingDB Entry DOI: 10.7270/Q2QR4Z0T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||