

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alpha-1B adrenergic receptor (Homo sapiens (Human)) | BDBM69602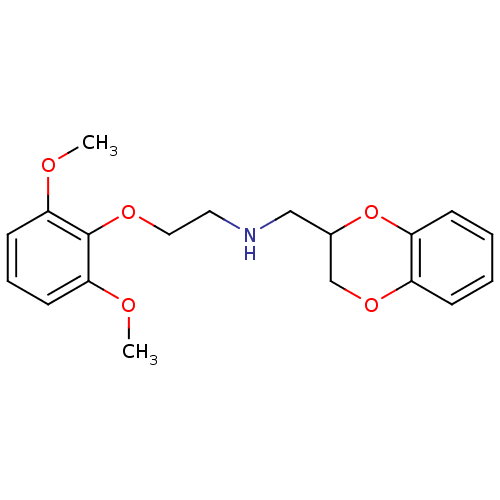 (2,3-dihydro-1,4-benzodioxin-3-ylmethyl-[2-(2,6-dim...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.0380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from human recombinant adrenergic alpha-1B receptor expressed in CHO cell membranes incubated for 60 mins | J Med Chem 63: 433-439 (2020) Article DOI: 10.1021/acs.jmedchem.9b01465 BindingDB Entry DOI: 10.7270/Q2P55RW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM69602 (2,3-dihydro-1,4-benzodioxin-3-ylmethyl-[2-(2,6-dim...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from human recombinant adrenergic alpha-1A receptor expressed in CHO cell membranes incubated for 60 mins | J Med Chem 63: 433-439 (2020) Article DOI: 10.1021/acs.jmedchem.9b01465 BindingDB Entry DOI: 10.7270/Q2P55RW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM29568 (CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from human recombinant adrenergic alpha-1D receptor expressed in CHO cell membranes incubated for 60 mins | J Med Chem 63: 433-439 (2020) Article DOI: 10.1021/acs.jmedchem.9b01465 BindingDB Entry DOI: 10.7270/Q2P55RW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50000296 (CHEMBL441765 | CHEMBL482811 | U-50488H | US1149237...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human recombinant kappa opioid receptor expressed in rat RBL cell membranes incubated for 60 mins | J Med Chem 63: 433-439 (2020) Article DOI: 10.1021/acs.jmedchem.9b01465 BindingDB Entry DOI: 10.7270/Q2P55RW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM21015 ((2S)-2-{2-[(2R)-2-[(2S)-2-amino-3-(4-hydroxyphenyl...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human recombinant mu opioid receptor expressed in HEK293 cell membranes incubated for 60 mins | J Med Chem 63: 433-439 (2020) Article DOI: 10.1021/acs.jmedchem.9b01465 BindingDB Entry DOI: 10.7270/Q2P55RW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50013515 ((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Displacement of [3H]RX 821002 from human recombinant adrenergic alpha-2C receptor expressed in CHO cell membranes incubated for 60 mins | J Med Chem 63: 433-439 (2020) Article DOI: 10.1021/acs.jmedchem.9b01465 BindingDB Entry DOI: 10.7270/Q2P55RW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2B adrenergic receptor (Homo sapiens (Human)) | BDBM50013515 ((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Displacement of [3H]RX 821002 from human recombinant adrenergic alpha-2B receptor expressed in CHO cell membranes incubated for 60 mins | J Med Chem 63: 433-439 (2020) Article DOI: 10.1021/acs.jmedchem.9b01465 BindingDB Entry DOI: 10.7270/Q2P55RW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM21008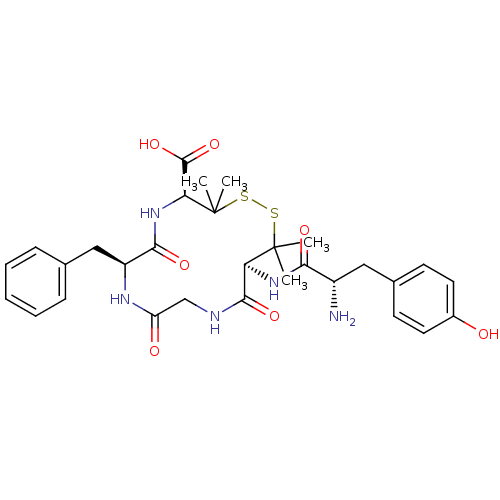 ((4S,7S,13S)-13-[(2S)-2-amino-3-(4-hydroxyphenyl)pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from human recombinant delta opioid receptor expressed in rat Chem-1 (RBL) cell membranes incubated for 60 mins | J Med Chem 63: 433-439 (2020) Article DOI: 10.1021/acs.jmedchem.9b01465 BindingDB Entry DOI: 10.7270/Q2P55RW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens) | BDBM50013515 ((+)-yohimbine | (16alpha,17alpha)-17-hydroxyyohimb...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Displacement of [3H]RX 821002 from human recombinant adrenergic alpha-2A receptor expressed in CHO cell membranes incubated for 60 mins | J Med Chem 63: 433-439 (2020) Article DOI: 10.1021/acs.jmedchem.9b01465 BindingDB Entry DOI: 10.7270/Q2P55RW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM50069984 ((R)-1-((S)-2-((S)-2-(benzyloxycarbonyl)-4-methylpe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ProScript, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against Cathepsin B | Bioorg Med Chem Lett 8: 333-8 (1999) BindingDB Entry DOI: 10.7270/Q2RV0MVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50474153 (CHEMBL61630) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human recombinant mu opioid receptor expressed in HEK293 cell membranes incubated for 60 mins | J Med Chem 63: 433-439 (2020) Article DOI: 10.1021/acs.jmedchem.9b01465 BindingDB Entry DOI: 10.7270/Q2P55RW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM50474149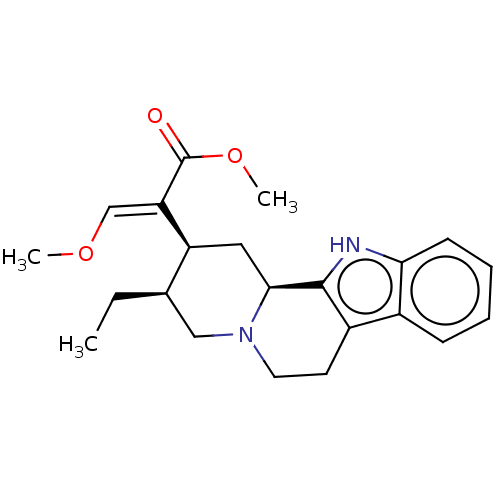 (CHEBI:70073 | Corynanrheidine) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from human recombinant adrenergic alpha-1D receptor expressed in CHO cell membranes incubated for 60 mins | J Med Chem 63: 433-439 (2020) Article DOI: 10.1021/acs.jmedchem.9b01465 BindingDB Entry DOI: 10.7270/Q2P55RW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50519927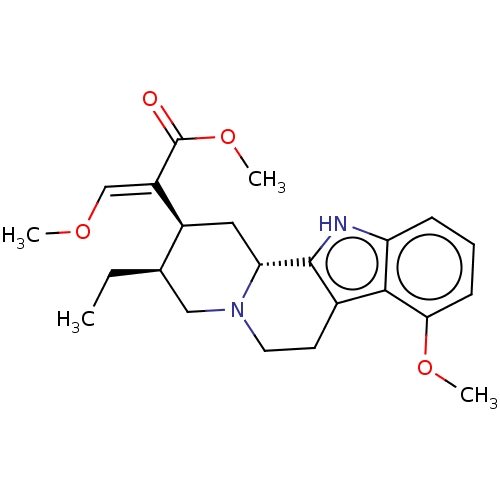 (CHEMBL4546925) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human recombinant mu opioid receptor expressed in HEK293 cell membranes incubated for 60 mins | J Med Chem 63: 433-439 (2020) Article DOI: 10.1021/acs.jmedchem.9b01465 BindingDB Entry DOI: 10.7270/Q2P55RW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50474153 (CHEMBL61630) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human recombinant kappa opioid receptor expressed in rat RBL cell membranes incubated for 60 mins | J Med Chem 63: 433-439 (2020) Article DOI: 10.1021/acs.jmedchem.9b01465 BindingDB Entry DOI: 10.7270/Q2P55RW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50474151 (CHEMBL292521) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human recombinant mu opioid receptor expressed in HEK293 cell membranes incubated for 60 mins | J Med Chem 63: 433-439 (2020) Article DOI: 10.1021/acs.jmedchem.9b01465 BindingDB Entry DOI: 10.7270/Q2P55RW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50519927 (CHEMBL4546925) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 116 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human recombinant kappa opioid receptor expressed in rat RBL cell membranes incubated for 60 mins | J Med Chem 63: 433-439 (2020) Article DOI: 10.1021/acs.jmedchem.9b01465 BindingDB Entry DOI: 10.7270/Q2P55RW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50474149 (CHEBI:70073 | Corynanrheidine) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 118 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human recombinant mu opioid receptor expressed in HEK293 cell membranes incubated for 60 mins | J Med Chem 63: 433-439 (2020) Article DOI: 10.1021/acs.jmedchem.9b01465 BindingDB Entry DOI: 10.7270/Q2P55RW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50474152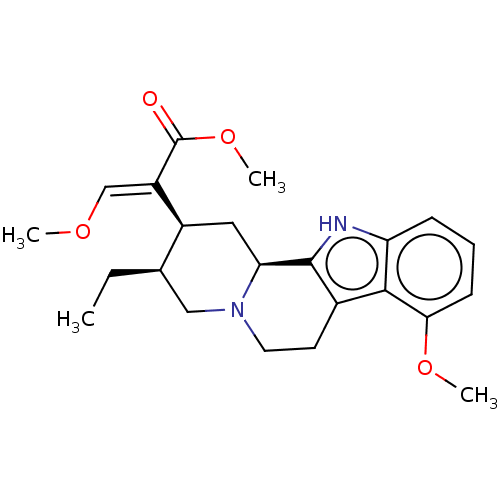 (CHEBI:6956 | CHEMBL299031) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem | Article PubMed | 161 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human recombinant mu opioid receptor expressed in HEK293 cell membranes incubated for 60 mins | J Med Chem 63: 433-439 (2020) Article DOI: 10.1021/acs.jmedchem.9b01465 BindingDB Entry DOI: 10.7270/Q2P55RW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50474152 (CHEBI:6956 | CHEMBL299031) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem | Article PubMed | 198 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human recombinant kappa opioid receptor expressed in rat RBL cell membranes incubated for 60 mins | J Med Chem 63: 433-439 (2020) Article DOI: 10.1021/acs.jmedchem.9b01465 BindingDB Entry DOI: 10.7270/Q2P55RW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50474153 (CHEMBL61630) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 236 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Displacement of [3H]DADLE from human recombinant delta opioid receptor expressed in rat Chem-1 (RBL) cell membranes incubated for 60 mins | J Med Chem 63: 433-439 (2020) Article DOI: 10.1021/acs.jmedchem.9b01465 BindingDB Entry DOI: 10.7270/Q2P55RW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen B (Homo sapiens (Human)) | BDBM50069989 ((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ProScript, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against human Chymotrypsinogen | Bioorg Med Chem Lett 8: 333-8 (1999) BindingDB Entry DOI: 10.7270/Q2RV0MVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin G (Homo sapiens (Human)) | BDBM50069989 ((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ProScript, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against human cathepsin G | Bioorg Med Chem Lett 8: 333-8 (1999) BindingDB Entry DOI: 10.7270/Q2RV0MVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1A adrenergic receptor (Homo sapiens (Human)) | BDBM50474152 (CHEBI:6956 | CHEMBL299031) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem | Article PubMed | 1.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from human recombinant adrenergic alpha-1A receptor expressed in CHO cell membranes incubated for 60 mins | J Med Chem 63: 433-439 (2020) Article DOI: 10.1021/acs.jmedchem.9b01465 BindingDB Entry DOI: 10.7270/Q2P55RW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50474149 (CHEBI:70073 | Corynanrheidine) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 1.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human recombinant kappa opioid receptor expressed in rat RBL cell membranes incubated for 60 mins | J Med Chem 63: 433-439 (2020) Article DOI: 10.1021/acs.jmedchem.9b01465 BindingDB Entry DOI: 10.7270/Q2P55RW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50069989 ((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ProScript, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against human leukocyte elastase | Bioorg Med Chem Lett 8: 333-8 (1999) BindingDB Entry DOI: 10.7270/Q2RV0MVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2C adrenergic receptor (Homo sapiens (Human)) | BDBM50474152 (CHEBI:6956 | CHEMBL299031) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem | Article PubMed | 2.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Displacement of [3H]RX 821002 from human recombinant adrenergic alpha-2C receptor expressed in CHO cell membranes incubated for 60 mins | J Med Chem 63: 433-439 (2020) Article DOI: 10.1021/acs.jmedchem.9b01465 BindingDB Entry DOI: 10.7270/Q2P55RW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor (Homo sapiens) | BDBM50474152 (CHEBI:6956 | CHEMBL299031) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem | Article PubMed | 4.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Displacement of [3H]RX 821002 from human recombinant adrenergic alpha-2A receptor expressed in CHO cell membranes incubated for 60 mins | J Med Chem 63: 433-439 (2020) Article DOI: 10.1021/acs.jmedchem.9b01465 BindingDB Entry DOI: 10.7270/Q2P55RW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1B adrenergic receptor (Homo sapiens (Human)) | BDBM50474152 (CHEBI:6956 | CHEMBL299031) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem | Article PubMed | 4.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from human recombinant adrenergic alpha-1B receptor expressed in CHO cell membranes incubated for 60 mins | J Med Chem 63: 433-439 (2020) Article DOI: 10.1021/acs.jmedchem.9b01465 BindingDB Entry DOI: 10.7270/Q2P55RW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-1D adrenergic receptor (Homo sapiens (Human)) | BDBM50474152 (CHEBI:6956 | CHEMBL299031) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem | Article PubMed | 5.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Displacement of [3H]prazosin from human recombinant adrenergic alpha-1D receptor expressed in CHO cell membranes incubated for 60 mins | J Med Chem 63: 433-439 (2020) Article DOI: 10.1021/acs.jmedchem.9b01465 BindingDB Entry DOI: 10.7270/Q2P55RW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2B adrenergic receptor (Homo sapiens (Human)) | BDBM50474152 (CHEBI:6956 | CHEMBL299031) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase KEGG PC cid PC sid UniChem | Article PubMed | 9.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Florida Curated by ChEMBL | Assay Description Displacement of [3H]RX 821002 from human recombinant adrenergic alpha-2B receptor expressed in CHO cell membranes incubated for 60 mins | J Med Chem 63: 433-439 (2020) Article DOI: 10.1021/acs.jmedchem.9b01465 BindingDB Entry DOI: 10.7270/Q2P55RW6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50069989 ((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ProScript, Inc. Curated by ChEMBL | Assay Description Inhibitory activity against thrombin | Bioorg Med Chem Lett 8: 333-8 (1999) BindingDB Entry DOI: 10.7270/Q2RV0MVQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM30142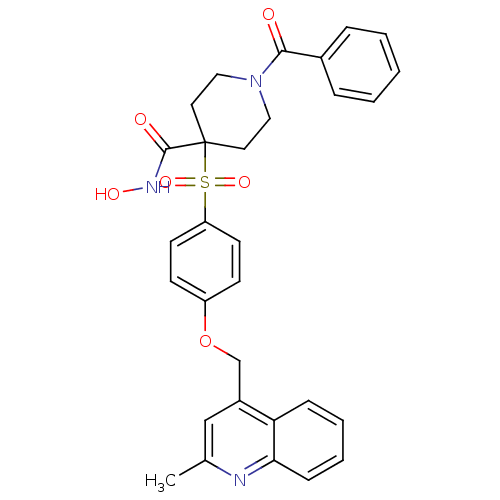 (alpha-sulfone piperidine hydroxamate, 11e) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description Compounds were tested for their ability to inhibit the cleavage of the substrate by the purified enzyme in a fluorescence-based fluorescence resonanc... | Bioorg Med Chem Lett 19: 3445-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.020 BindingDB Entry DOI: 10.7270/Q2GT5KHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM30138 (alpha-sulfone piperidine hydroxamate, 11c) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description Compounds were tested for their ability to inhibit the cleavage of the substrate by the purified enzyme in a fluorescence-based fluorescence resonanc... | Bioorg Med Chem Lett 19: 3445-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.020 BindingDB Entry DOI: 10.7270/Q2GT5KHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM30136 (CHEMBL385821 | beta-sulfone piperidine hydroxamate...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description Compounds were tested for their ability to inhibit the cleavage of the substrate by the purified enzyme in a fluorescence-based fluorescence resonanc... | Bioorg Med Chem Lett 19: 3445-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.020 BindingDB Entry DOI: 10.7270/Q2GT5KHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM30146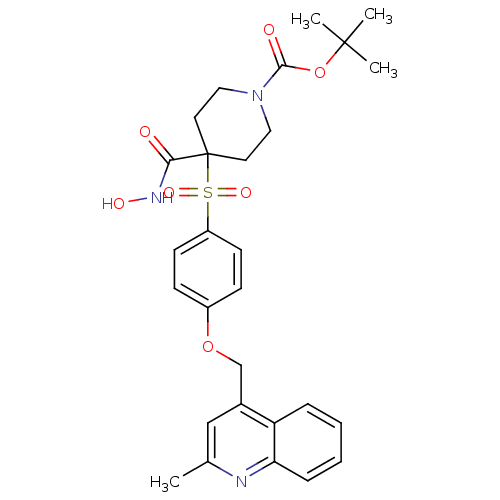 (alpha-sulfone piperidine hydroxamate, 11g) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description Compounds were tested for their ability to inhibit the cleavage of the substrate by the purified enzyme in a fluorescence-based fluorescence resonanc... | Bioorg Med Chem Lett 19: 3445-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.020 BindingDB Entry DOI: 10.7270/Q2GT5KHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM30144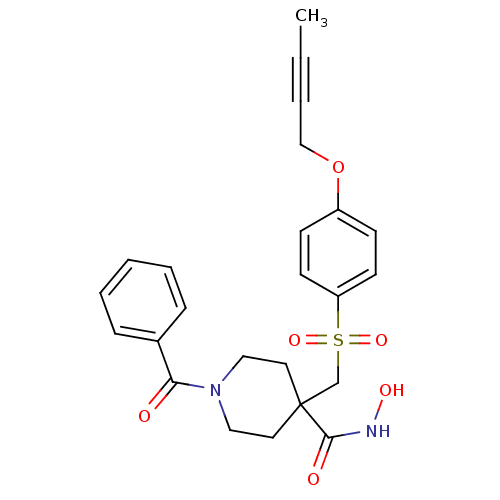 (CHEMBL212481 | beta-sulfone piperidine hydroxamate...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description Compounds were tested for their ability to inhibit the cleavage of the substrate by the purified enzyme in a fluorescence-based fluorescence resonanc... | Bioorg Med Chem Lett 19: 3445-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.020 BindingDB Entry DOI: 10.7270/Q2GT5KHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM30154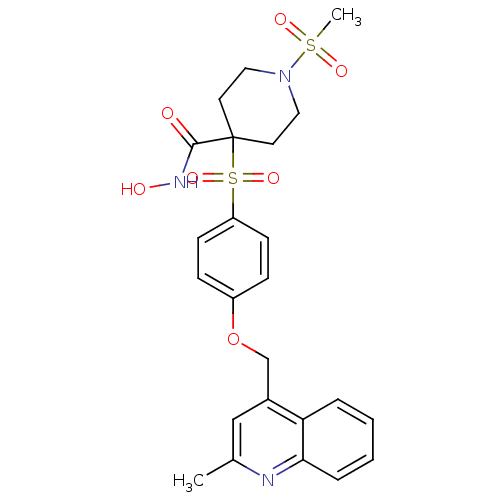 (alpha-sulfone piperidine hydroxamate, 11m) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description Compounds were tested for their ability to inhibit the cleavage of the substrate by the purified enzyme in a fluorescence-based fluorescence resonanc... | Bioorg Med Chem Lett 19: 3445-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.020 BindingDB Entry DOI: 10.7270/Q2GT5KHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Smoothened homolog (Homo sapiens (Human)) | BDBM50293788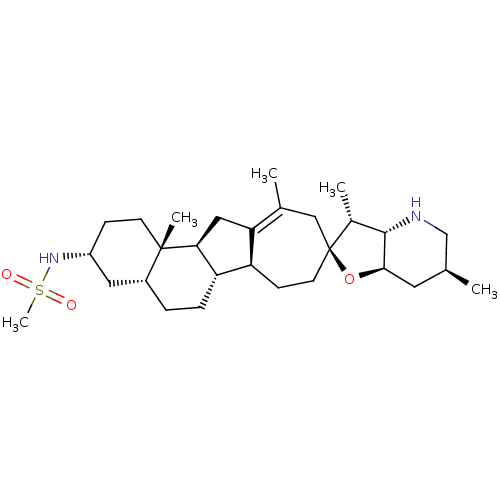 (CHEMBL538867 | N-((2S,3R,3aS,3'R,4a'R,6S,6a'R,6b'S...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Infinity Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of human recombinant SMO expressed in mouse C3H10T1/2 cells assessed as inhibition of association of BODIPY-cyclopamine | J Med Chem 52: 4400-18 (2009) Article DOI: 10.1021/jm900305z BindingDB Entry DOI: 10.7270/Q2CC10QM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM23501 (BMCL193445 Compound 2d | beta-sulfonyl hydroxamate...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description Compounds were tested for their ability to inhibit the cleavage of the substrate by the purified enzyme in a fluorescence-based fluorescence resonanc... | Bioorg Med Chem Lett 19: 3445-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.020 BindingDB Entry DOI: 10.7270/Q2GT5KHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM30141 (alpha-sulfone piperidine hydroxamate, 11d) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description Compounds were tested for their ability to inhibit the cleavage of the substrate by the purified enzyme in a fluorescence-based fluorescence resonanc... | Bioorg Med Chem Lett 19: 3445-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.020 BindingDB Entry DOI: 10.7270/Q2GT5KHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM30140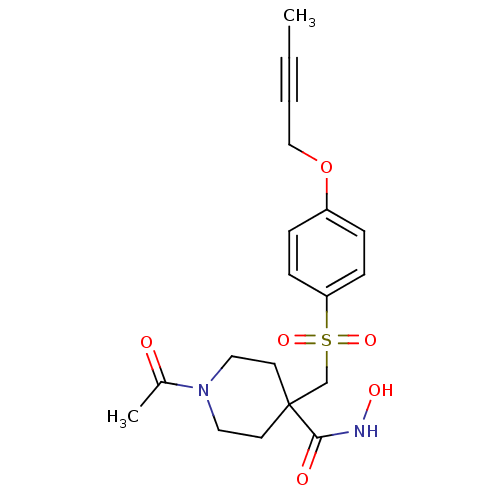 (CHEMBL380049 | beta-sulfone piperidine hydroxamate...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description Compounds were tested for their ability to inhibit the cleavage of the substrate by the purified enzyme in a fluorescence-based fluorescence resonanc... | Bioorg Med Chem Lett 19: 3445-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.020 BindingDB Entry DOI: 10.7270/Q2GT5KHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM30145 (alpha-sulfone piperidine hydroxamate, 11f) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description Compounds were tested for their ability to inhibit the cleavage of the substrate by the purified enzyme in a fluorescence-based fluorescence resonanc... | Bioorg Med Chem Lett 19: 3445-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.020 BindingDB Entry DOI: 10.7270/Q2GT5KHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM30137 (alpha-sulfone piperidine hydroxamate, 11b) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description Compounds were tested for their ability to inhibit the cleavage of the substrate by the purified enzyme in a fluorescence-based fluorescence resonanc... | Bioorg Med Chem Lett 19: 3445-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.020 BindingDB Entry DOI: 10.7270/Q2GT5KHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM30148 (alpha-sulfone piperidine hydroxamate, 11h) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description Compounds were tested for their ability to inhibit the cleavage of the substrate by the purified enzyme in a fluorescence-based fluorescence resonanc... | Bioorg Med Chem Lett 19: 3445-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.020 BindingDB Entry DOI: 10.7270/Q2GT5KHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM30149 (alpha-sulfone piperidine hydroxamate, 11i) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description Compounds were tested for their ability to inhibit the cleavage of the substrate by the purified enzyme in a fluorescence-based fluorescence resonanc... | Bioorg Med Chem Lett 19: 3445-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.020 BindingDB Entry DOI: 10.7270/Q2GT5KHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM30151 (alpha-sulfone piperidine hydroxamate, 11k) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description Compounds were tested for their ability to inhibit the cleavage of the substrate by the purified enzyme in a fluorescence-based fluorescence resonanc... | Bioorg Med Chem Lett 19: 3445-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.020 BindingDB Entry DOI: 10.7270/Q2GT5KHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM30155 (alpha-sulfone piperidine hydroxamate, 11n) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description Compounds were tested for their ability to inhibit the cleavage of the substrate by the purified enzyme in a fluorescence-based fluorescence resonanc... | Bioorg Med Chem Lett 19: 3445-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.020 BindingDB Entry DOI: 10.7270/Q2GT5KHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Disintegrin and metalloproteinase domain-containing protein 17 (Homo sapiens (Human)) | BDBM30150 (alpha-sulfone piperidine hydroxamate, 11j) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.4 | 22 |
Wyeth Research | Assay Description Compounds were tested for their ability to inhibit the cleavage of the substrate by the purified enzyme in a fluorescence-based fluorescence resonanc... | Bioorg Med Chem Lett 19: 3445-8 (2009) Article DOI: 10.1016/j.bmcl.2009.05.020 BindingDB Entry DOI: 10.7270/Q2GT5KHW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM2008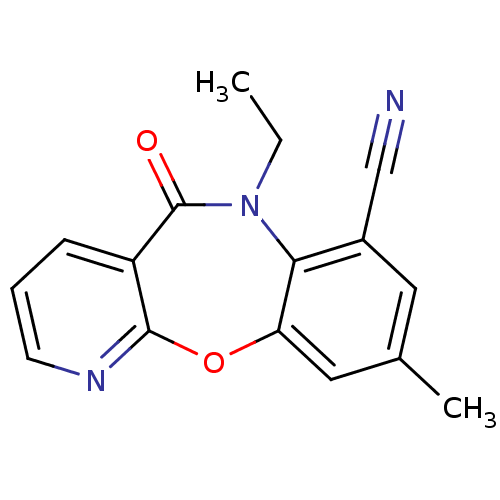 (10-ethyl-14-methyl-9-oxo-2-oxa-4,10-diazatricyclo[...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 35: 1887-97 (1992) Article DOI: 10.1021/jm00088a027 BindingDB Entry DOI: 10.7270/Q2ST7N0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [600-1027,R876K]/[600-1155] (Human immunodeficiency virus type 1) | BDBM1987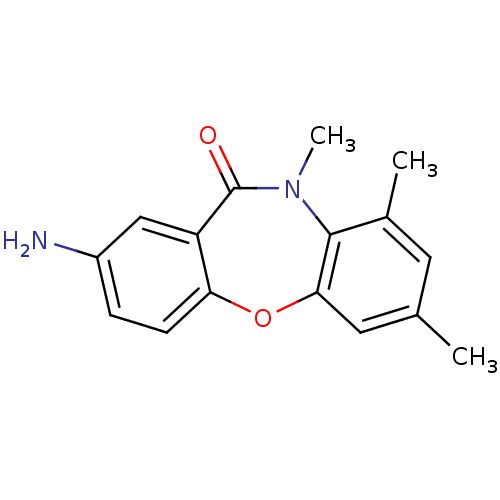 (13-amino-5,7,9-trimethyl-2-oxa-9-azatricyclo[9.4.0...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.8 | 22 |
Boehringer Ingelheim Pharmaceuticals Inc. | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 35: 1887-97 (1992) Article DOI: 10.1021/jm00088a027 BindingDB Entry DOI: 10.7270/Q2ST7N0J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 269 total ) | Next | Last >> |