

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50130312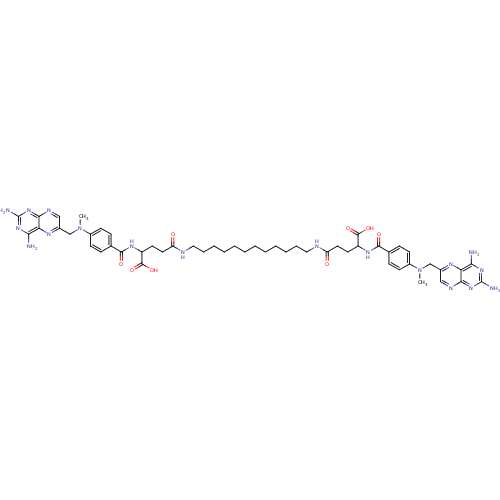 (4-[12-(4-Carboxy-4-{4-[(2,4-diamino-pteridin-6-ylm...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitory constant of the compound for murine Wild-type dihydrofolate reductase (DHFR) | J Med Chem 46: 2816-8 (2003) Article DOI: 10.1021/jm034057i BindingDB Entry DOI: 10.7270/Q2JS9PTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50130313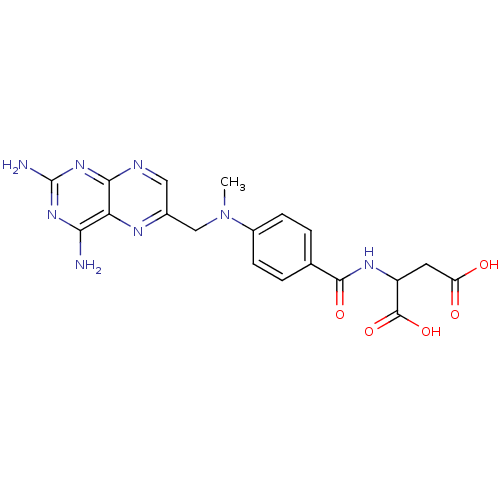 (2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-methyl-ami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitory constant of the compound for murine Wild-type dihydrofolate reductase (DHFR) | J Med Chem 46: 2816-8 (2003) Article DOI: 10.1021/jm034057i BindingDB Entry DOI: 10.7270/Q2JS9PTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50011320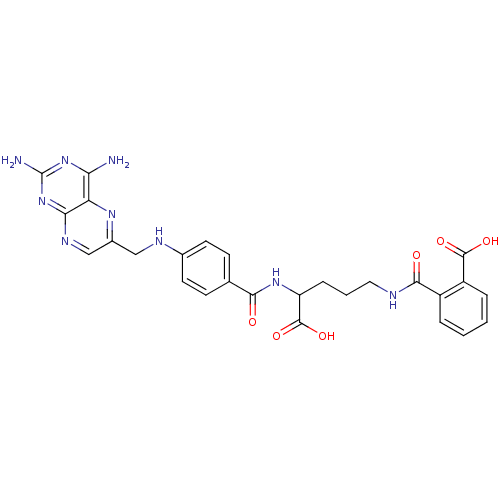 (CHEMBL18155 | N-(4-Carboxy-4-{4-[(2,4-diamino-pter...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitory constant of the compound for murine Wild-type dihydrofolate reductase (DHFR) | J Med Chem 46: 2816-8 (2003) Article DOI: 10.1021/jm034057i BindingDB Entry DOI: 10.7270/Q2JS9PTZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM18268 (5-methyl-6-{[(3,4,5-trimethoxyphenyl)amino]methyl}...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitory constant of the compound for murine Wild-type dihydrofolate reductase (DHFR) | J Med Chem 46: 2816-8 (2003) Article DOI: 10.1021/jm034057i BindingDB Entry DOI: 10.7270/Q2JS9PTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18512 (5-(4-chlorophenyl)-6-ethylpyrimidine-2,4-diamine |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pennsylvania State University Curated by ChEMBL | Assay Description Inhibitor constant of compound for plasmodium falciparum dihydrofolate reductase | J Med Chem 35: 2912-5 (1992) BindingDB Entry DOI: 10.7270/Q20Z728B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bifunctional dihydrofolate reductase-thymidylate synthase (Plasmodium falciparum (isolate K1 / Thailand)) | BDBM18512 (5-(4-chlorophenyl)-6-ethylpyrimidine-2,4-diamine |...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pennsylvania State University Curated by ChEMBL | Assay Description Thermodynamic dissociation constant of compound for mutant T46S E. coli dihydrofolate reductase | J Med Chem 35: 2912-5 (1992) BindingDB Entry DOI: 10.7270/Q20Z728B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM18268 (5-methyl-6-{[(3,4,5-trimethoxyphenyl)amino]methyl}...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitory constant of the compound for F31A/F34A murine dihydrofolate reductase (DHFR) | J Med Chem 46: 2816-8 (2003) Article DOI: 10.1021/jm034057i BindingDB Entry DOI: 10.7270/Q2JS9PTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM18512 (5-(4-chlorophenyl)-6-ethylpyrimidine-2,4-diamine |...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pennsylvania State University Curated by ChEMBL | Assay Description Inhibitor constant of compound for mutant S108 N plasmodium falciparum i dihydrofolate reductase | J Med Chem 35: 2912-5 (1992) BindingDB Entry DOI: 10.7270/Q20Z728B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM18512 (5-(4-chlorophenyl)-6-ethylpyrimidine-2,4-diamine |...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pennsylvania State University Curated by ChEMBL | Assay Description Dissociation rate constant of compound for mutant T46N Escherichia coli dihydrofolate reductase | J Med Chem 35: 2912-5 (1992) BindingDB Entry DOI: 10.7270/Q20Z728B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM18512 (5-(4-chlorophenyl)-6-ethylpyrimidine-2,4-diamine |...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pennsylvania State University Curated by ChEMBL | Assay Description Dissociation rate constant of compound for mutant T46A Escherichia coli dihydrofolate reductase | J Med Chem 35: 2912-5 (1992) BindingDB Entry DOI: 10.7270/Q20Z728B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM18512 (5-(4-chlorophenyl)-6-ethylpyrimidine-2,4-diamine |...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pennsylvania State University Curated by ChEMBL | Assay Description Inhibitor constant of compound for mutant T46S E. coli dihydrofolate reductase | J Med Chem 35: 2912-5 (1992) BindingDB Entry DOI: 10.7270/Q20Z728B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50011320 (CHEMBL18155 | N-(4-Carboxy-4-{4-[(2,4-diamino-pter...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 25.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitory constant of the compound for F31A/F34A murine dihydrofolate reductase (DHFR) | J Med Chem 46: 2816-8 (2003) Article DOI: 10.1021/jm034057i BindingDB Entry DOI: 10.7270/Q2JS9PTZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50022831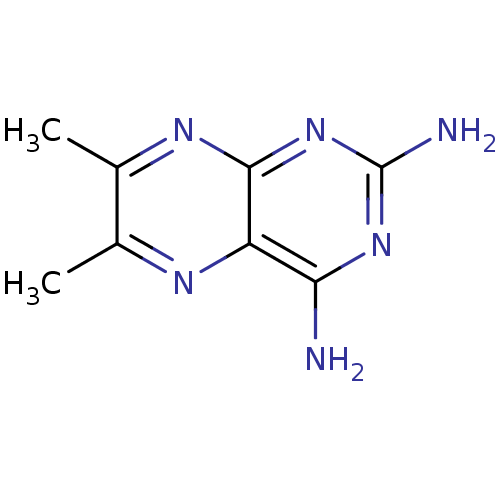 (6,7-Dimethyl-pteridine-2,4-diamine | CHEMBL29143) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pennsylvania State University Curated by ChEMBL | Assay Description Thermodynamic Dissociation Constant for compound-Tyr31-dihydrofolate reductase (DHFR) complex at pH 8 | J Med Chem 31: 129-37 (1988) BindingDB Entry DOI: 10.7270/Q2MP53VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50022831 (6,7-Dimethyl-pteridine-2,4-diamine | CHEMBL29143) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pennsylvania State University Curated by ChEMBL | Assay Description Inhibition constant for Tyr31-dihydrofolate reductase (DHFR)-NADPH-Compound complex | J Med Chem 31: 129-37 (1988) BindingDB Entry DOI: 10.7270/Q2MP53VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM50367875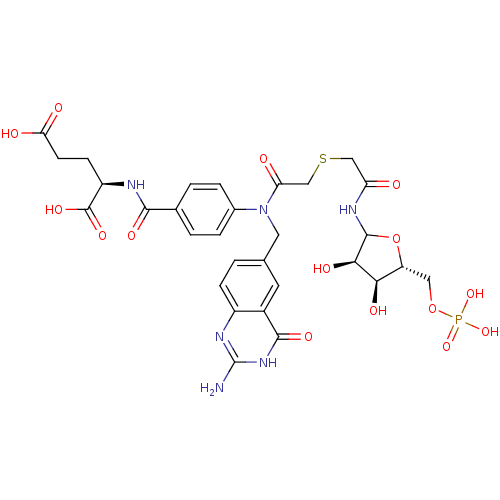 (CHEMBL607957) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | >100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pennsylvania State University Curated by ChEMBL | Assay Description Compound was tested for inhibition constant against GAR TFase in mice | J Med Chem 32: 937-40 (1989) BindingDB Entry DOI: 10.7270/Q2RX9CN7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phenylalanine-4-hydroxylase (Rattus norvegicus) | BDBM50028430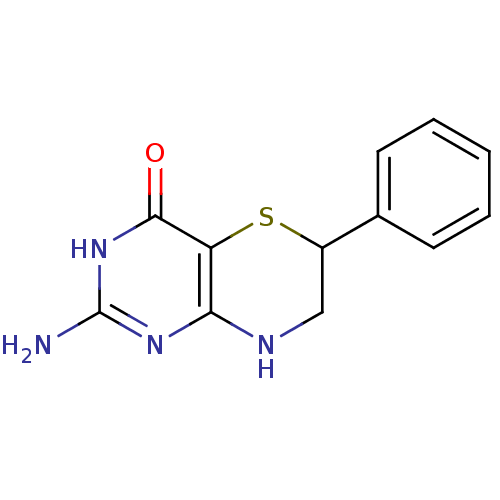 (2-Amino-6-phenyl-7,8-dihydro-3H,6H-pyrimido[5,4-b]...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for competitive binding inhibition against phenylalanine hydroxylase in rat liver | J Med Chem 26: 559-63 (1983) BindingDB Entry DOI: 10.7270/Q2028QKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50130312 (4-[12-(4-Carboxy-4-{4-[(2,4-diamino-pteridin-6-ylm...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 212 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitory constant of the compound for F31A/F34A murine dihydrofolate reductase (DHFR) | J Med Chem 46: 2816-8 (2003) Article DOI: 10.1021/jm034057i BindingDB Entry DOI: 10.7270/Q2JS9PTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pennsylvania State University Curated by ChEMBL | Assay Description Thermodynamic Dissociation Constant for compound-Tyr31-dihydrofolate reductase (DHFR) complex at pH 9.5 | J Med Chem 31: 129-37 (1988) BindingDB Entry DOI: 10.7270/Q2MP53VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Mus musculus (Mouse)) | BDBM50130313 (2-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-methyl-ami...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 333 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Minnesota Curated by ChEMBL | Assay Description Inhibitory constant of the compound for F31A/F34A murine dihydrofolate reductase (DHFR) | J Med Chem 46: 2816-8 (2003) Article DOI: 10.1021/jm034057i BindingDB Entry DOI: 10.7270/Q2JS9PTZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM92374 (RY Analogue, 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 685 | -35.2 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
University of Southampton | Assay Description Enzyme inhibition using aminoimidazole carboxamide ribonucleotide transformylase/inosine monophosphate cyclohydrolase (ATIC). | Chembiochem 13: 1628-34 (2012) Article DOI: 10.1002/cbic.201200279 BindingDB Entry DOI: 10.7270/Q2P26WQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine--tRNA ligase, cytoplasmic (Saccharomyces cerevisiae S288c) | BDBM50370987 (TAVABOROLE) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem | Article PubMed | 1.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of Saccharomyces cerevisiae cytoplasmic leucyl-tRNA synthetase after 20 mins | Science 316: 1759-1761 (2007) Article DOI: 10.1126/science.1142189 BindingDB Entry DOI: 10.7270/Q2P84CQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 2.40E+3 | -32.1 | n/a | n/a | n/a | n/a | n/a | 5.5 | 25 |
Pennsylvania State University Curated by ChEMBL | Assay Description Thermodynamic Dissociation Constant for compound-Val31-dihydrofolate reductase (DHFR) complex at pH 7 | J Med Chem 31: 129-37 (1988) BindingDB Entry DOI: 10.7270/Q2MP53VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM92376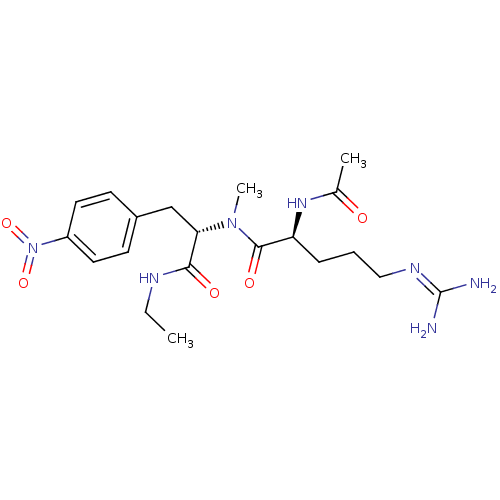 (RY Analogue, 17) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.50E+3 | -32.0 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
University of Southampton | Assay Description Enzyme inhibition using aminoimidazole carboxamide ribonucleotide transformylase/inosine monophosphate cyclohydrolase (ATIC). | Chembiochem 13: 1628-34 (2012) Article DOI: 10.1002/cbic.201200279 BindingDB Entry DOI: 10.7270/Q2P26WQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phenylalanine-4-hydroxylase (Rattus norvegicus) | BDBM50028428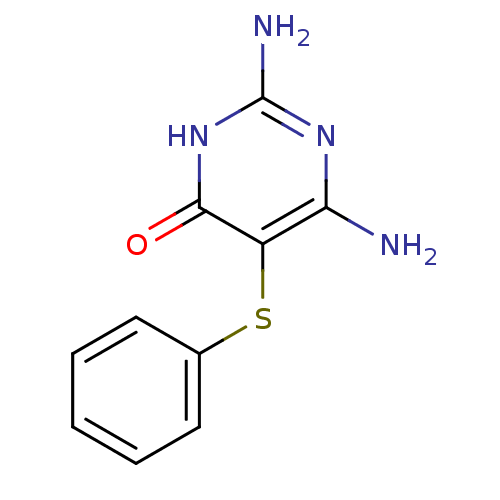 (2,6-Diamino-5-phenylsulfanyl-3H-pyrimidin-4-one | ...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for competitive binding inhibition against phenylalanine hydroxylase in rat liver | J Med Chem 26: 559-63 (1983) BindingDB Entry DOI: 10.7270/Q2028QKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phenylalanine-4-hydroxylase (Rattus norvegicus) | BDBM50028426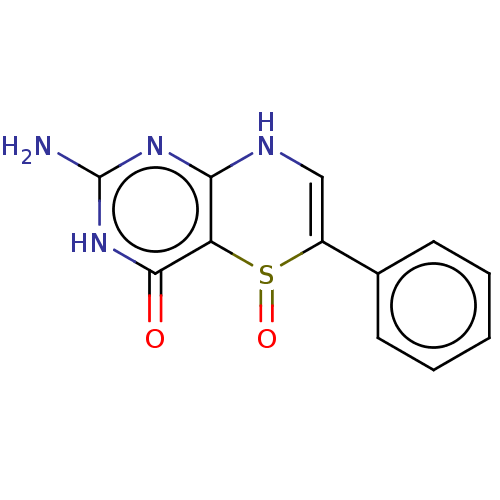 (2-Amino-5-oxo-6-phenyl-5,6,7,8-tetrahydro-3H-5lamb...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for competitive binding inhibition against phenylalanine hydroxylase in rat liver | J Med Chem 26: 559-63 (1983) BindingDB Entry DOI: 10.7270/Q2028QKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM50022831 (6,7-Dimethyl-pteridine-2,4-diamine | CHEMBL29143) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pennsylvania State University Curated by ChEMBL | Assay Description Dissociation constant at(Koff) Tyr-31 of dihydrofolate reductase (DHFR) | J Med Chem 31: 129-37 (1988) BindingDB Entry DOI: 10.7270/Q2MP53VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50089572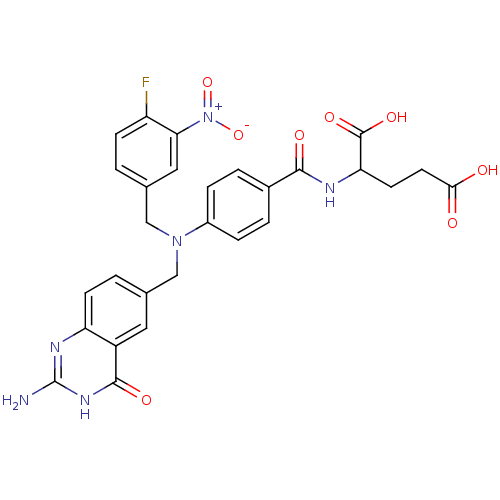 (2-{4-[(2-Amino-4-oxo-3,4-dihydro-quinazolin-6-ylme...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against Aminoimidazole carboxamide ribonucleotide transformylase | Bioorg Med Chem Lett 10: 1471-5 (2000) BindingDB Entry DOI: 10.7270/Q2GM86HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine--tRNA ligase, cytoplasmic (Saccharomyces cerevisiae S288c) | BDBM50370987 (TAVABOROLE) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem | Article PubMed | 3.14E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of Saccharomyces cerevisiae cytoplasmic leucyl-tRNA synthetase after 2 mins | Science 316: 1759-1761 (2007) Article DOI: 10.1126/science.1142189 BindingDB Entry DOI: 10.7270/Q2P84CQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50089585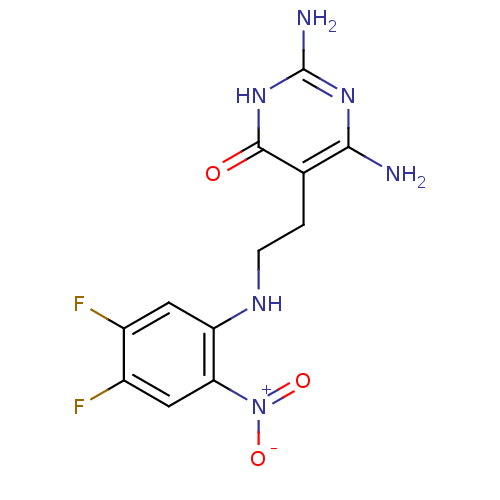 (2,6-Diamino-5-[2-(4,5-difluoro-2-nitro-phenylamino...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against Glycinamide ribonucleotide transformylase (GAR Tfase) after 3 min at 250 uM | Bioorg Med Chem Lett 10: 1471-5 (2000) BindingDB Entry DOI: 10.7270/Q2GM86HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phenylalanine-4-hydroxylase (Rattus norvegicus) | BDBM50028425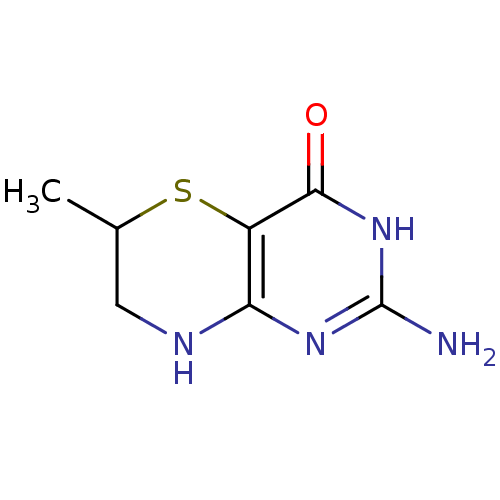 (2-Amino-6-methyl-7,8-dihydro-3H,6H-pyrimido[5,4-b]...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for competitive binding inhibition against phenylalanine hydroxylase in rat liver | J Med Chem 26: 559-63 (1983) BindingDB Entry DOI: 10.7270/Q2028QKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-1-tetrahydrofolate synthase, cytoplasmic (Homo sapiens (Human)) | BDBM50028981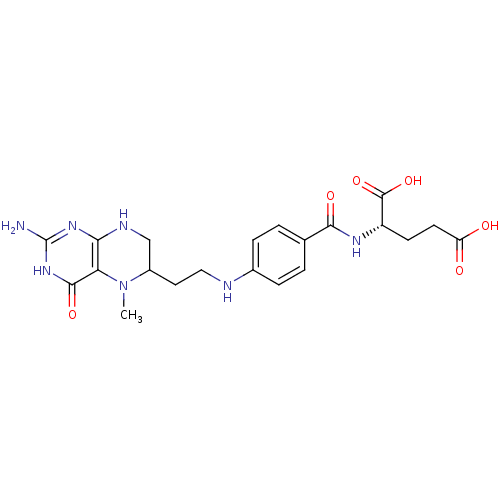 (2-{4-[2-(2-amino-5-methyl-4-oxo-1,4,5,6,7,8-hexahy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for in vitro inhibition of 5,10-Methylene Tetrahydrofolate Cyclohydrolase ,competitive against (+)-L-5,10-methenyltetrahydrofolat... | J Med Chem 24: 1086-8 (1981) BindingDB Entry DOI: 10.7270/Q2JS9PGG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50089594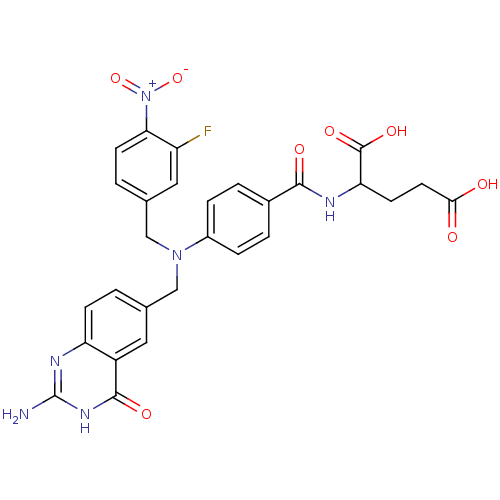 (2-{4-[(2-Amino-4-oxo-3,4-dihydro-quinazolin-6-ylme...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against Glycinamide ribonucleotide transformylase (GAR Tfase) | Bioorg Med Chem Lett 10: 1471-5 (2000) BindingDB Entry DOI: 10.7270/Q2GM86HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50089594 (2-{4-[(2-Amino-4-oxo-3,4-dihydro-quinazolin-6-ylme...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against Aminoimidazole carboxamide ribonucleotide transformylase | Bioorg Med Chem Lett 10: 1471-5 (2000) BindingDB Entry DOI: 10.7270/Q2GM86HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM92372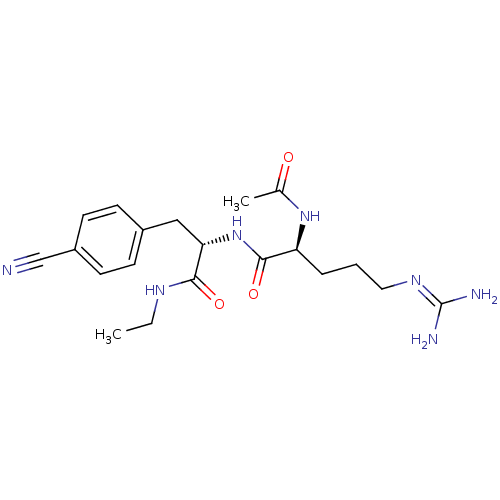 (RY Analogue, 12) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.60E+4 | -24.3 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
University of Southampton | Assay Description Enzyme inhibition using aminoimidazole carboxamide ribonucleotide transformylase/inosine monophosphate cyclohydrolase (ATIC). | Chembiochem 13: 1628-34 (2012) Article DOI: 10.1002/cbic.201200279 BindingDB Entry DOI: 10.7270/Q2P26WQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM92373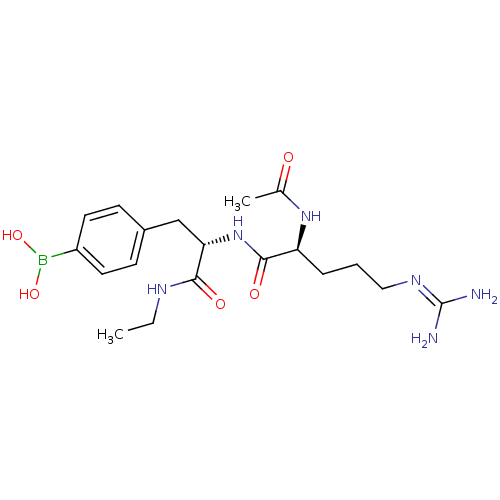 (RY Analogue, 13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 5.90E+4 | -24.1 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
University of Southampton | Assay Description Enzyme inhibition using aminoimidazole carboxamide ribonucleotide transformylase/inosine monophosphate cyclohydrolase (ATIC). | Chembiochem 13: 1628-34 (2012) Article DOI: 10.1002/cbic.201200279 BindingDB Entry DOI: 10.7270/Q2P26WQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM92368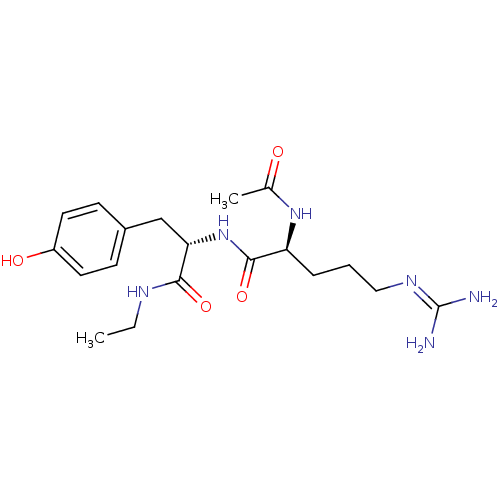 (RY Analogue, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.40E+4 | -23.3 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
University of Southampton | Assay Description Enzyme inhibition using aminoimidazole carboxamide ribonucleotide transformylase/inosine monophosphate cyclohydrolase (ATIC). | Chembiochem 13: 1628-34 (2012) Article DOI: 10.1002/cbic.201200279 BindingDB Entry DOI: 10.7270/Q2P26WQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phenylalanine-4-hydroxylase (Rattus norvegicus) | BDBM50028424 (2-Amino-7,8-dihydro-3H,6H-pyrimido[5,4-b][1,4]thia...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for competitive binding inhibition against phenylalanine hydroxylase in rat liver | J Med Chem 26: 559-63 (1983) BindingDB Entry DOI: 10.7270/Q2028QKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM92371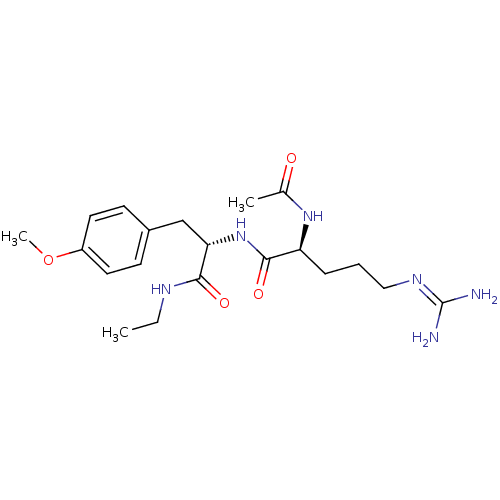 (RY Analogue, 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.70E+4 | -23.2 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
University of Southampton | Assay Description Enzyme inhibition using aminoimidazole carboxamide ribonucleotide transformylase/inosine monophosphate cyclohydrolase (ATIC). | Chembiochem 13: 1628-34 (2012) Article DOI: 10.1002/cbic.201200279 BindingDB Entry DOI: 10.7270/Q2P26WQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Homo sapiens (Human)) | BDBM50089572 (2-{4-[(2-Amino-4-oxo-3,4-dihydro-quinazolin-6-ylme...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Curated by ChEMBL | Assay Description In vitro inhibitory activity against Glycinamide ribonucleotide transformylase (GAR Tfase) | Bioorg Med Chem Lett 10: 1471-5 (2000) BindingDB Entry DOI: 10.7270/Q2GM86HD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phenylalanine-4-hydroxylase (Rattus norvegicus) | BDBM50028427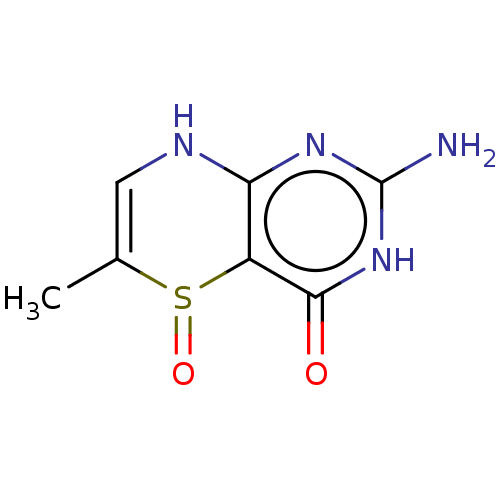 (2-Amino-6-methyl-5-oxo-5,6,7,8-tetrahydro-3H-5lamb...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for competitive binding inhibition against phenylalanine hydroxylase in rat liver | J Med Chem 26: 559-63 (1983) BindingDB Entry DOI: 10.7270/Q2028QKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM92370 (RY Analogue, 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.35E+5 | -22.1 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
University of Southampton | Assay Description Enzyme inhibition using aminoimidazole carboxamide ribonucleotide transformylase/inosine monophosphate cyclohydrolase (ATIC). | Chembiochem 13: 1628-34 (2012) Article DOI: 10.1002/cbic.201200279 BindingDB Entry DOI: 10.7270/Q2P26WQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM92375 (RY Analogue, 16) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.45E+5 | -21.9 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
University of Southampton | Assay Description Enzyme inhibition using aminoimidazole carboxamide ribonucleotide transformylase/inosine monophosphate cyclohydrolase (ATIC). | Chembiochem 13: 1628-34 (2012) Article DOI: 10.1002/cbic.201200279 BindingDB Entry DOI: 10.7270/Q2P26WQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Escherichia coli) | BDBM18050 (2-[(4-{[(2,4-diaminopteridin-6-yl)methyl](methyl)a...) | MMDB NCI pathway Reactome pathway KEGG UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 1.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pennsylvania State University Curated by ChEMBL | Assay Description Thermodynamic Dissociation Constant for compound-Phe31-dihydrofolate reductase (DHFR) complex at pH 8.5 | J Med Chem 31: 129-37 (1988) BindingDB Entry DOI: 10.7270/Q2MP53VF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM92369 (RY Analogue, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 3.39E+5 | -19.8 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
University of Southampton | Assay Description Enzyme inhibition using aminoimidazole carboxamide ribonucleotide transformylase/inosine monophosphate cyclohydrolase (ATIC). | Chembiochem 13: 1628-34 (2012) Article DOI: 10.1002/cbic.201200279 BindingDB Entry DOI: 10.7270/Q2P26WQZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phenylalanine-4-hydroxylase (Rattus norvegicus) | BDBM50028431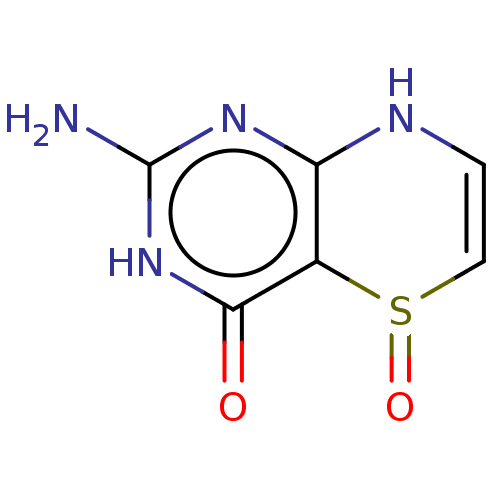 (2-Amino-5-oxo-5,6,7,8-tetrahydro-3H-5lambda*4*-pyr...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for competitive binding inhibition against phenylalanine hydroxylase in rat liver | J Med Chem 26: 559-63 (1983) BindingDB Entry DOI: 10.7270/Q2028QKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phenylalanine-4-hydroxylase (Rattus norvegicus) | BDBM50028429 (2-Amino-3,5,6,7-tetrahydro-pyrimido[4,5-b][1,4]thi...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Tested for competitive binding inhibition against phenylalanine hydroxylase in rat liver | J Med Chem 26: 559-63 (1983) BindingDB Entry DOI: 10.7270/Q2028QKN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50369444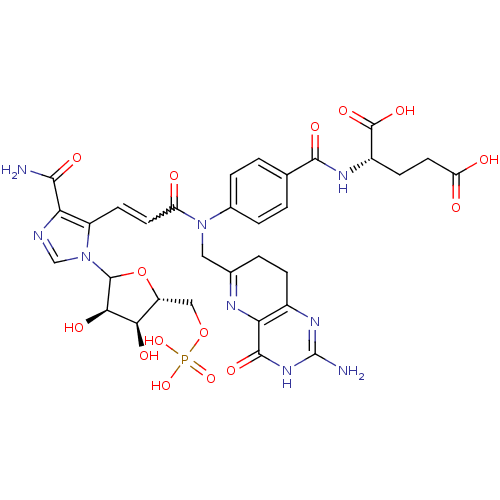 (CHEMBL608337) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
The Pennsylvania State University Curated by ChEMBL | Assay Description Inhibitory activity against AICAR formyltransferase | J Med Chem 42: 3421-4 (1999) Article DOI: 10.1021/jm990323+ BindingDB Entry DOI: 10.7270/Q23X879T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leucine--tRNA ligase, cytoplasmic (Saccharomyces cerevisiae S288c) | BDBM50370987 (TAVABOROLE) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Anacor Pharmaceuticals, Inc Curated by ChEMBL | Assay Description Inhibition of Saccharomyces cerevisiae cytoplasmic leucyl-tRNA synthetase assessed as tRNA amino-acylation | Science 316: 1759-1761 (2007) Article DOI: 10.1126/science.1142189 BindingDB Entry DOI: 10.7270/Q2P84CQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM50080319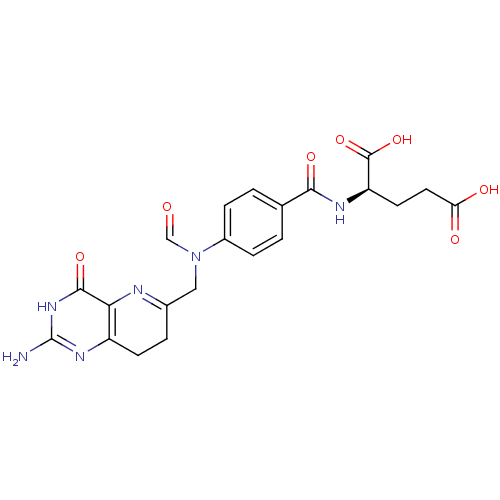 (10-Formyl-8-deazafolic acid | CHEMBL114215) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Pennsylvania State University Curated by ChEMBL | Assay Description Inhibitory activity against AICAR formyltransferase | J Med Chem 42: 3421-4 (1999) Article DOI: 10.1021/jm990323+ BindingDB Entry DOI: 10.7270/Q23X879T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional purine biosynthesis protein ATIC (Homo sapiens (Human)) | BDBM22579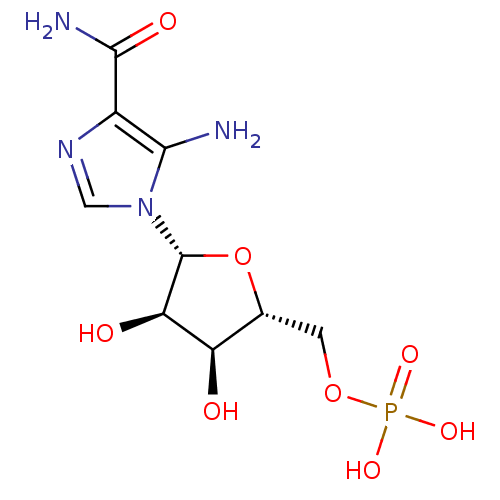 (AICAR | Aminoimidazole-4-carboxamide ribonucleotid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Pennsylvania State University Curated by ChEMBL | Assay Description Inhibitory activity against AICAR formyltransferase | J Med Chem 42: 3421-4 (1999) Article DOI: 10.1021/jm990323+ BindingDB Entry DOI: 10.7270/Q23X879T | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 107 total ) | Next | Last >> |