 Found 169 hits with Last Name = 'berghuis' and Initial = 'am'
Found 169 hits with Last Name = 'berghuis' and Initial = 'am' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
GNAT family acetyltransferase
(Enterococcus durans) | BDBM50193475
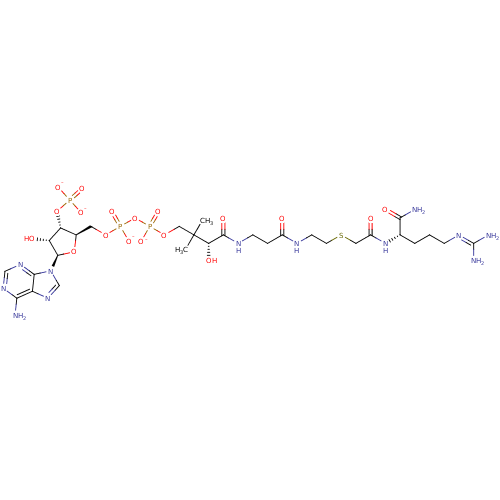
((3R)-3-{[2-({2-[({[(1S)-1-carbamoyl-4-[(diaminomet...)Show SMILES [#6]C([#6])([#6]-[#8]P([#8-])(=O)[#8]P([#8-])(=O)[#8]-[#6]-[#6@H]-1-[#8]-[#6@H](-[#6@H](-[#8])-[#6@@H]-1-[#8]P([#8-])([#8-])=O)-n1cnc2c(-[#7])ncnc12)[#6@@H](-[#8])-[#6](=O)-[#7]-[#6]-[#6]-[#6](=O)-[#7]-[#6]-[#6]-[#16]-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#6]\[#7]=[#6](\[#7])-[#7])-[#6](-[#7])=O Show InChI InChI=1S/C29H51N12O18P3S/c1-29(2,22(45)26(47)35-7-5-17(42)34-8-9-63-11-18(43)40-15(24(31)46)4-3-6-36-28(32)33)12-56-62(53,54)59-61(51,52)55-10-16-21(58-60(48,49)50)20(44)27(57-16)41-14-39-19-23(30)37-13-38-25(19)41/h13-16,20-22,27,44-45H,3-12H2,1-2H3,(H2,31,46)(H,34,42)(H,35,47)(H,40,43)(H,51,52)(H,53,54)(H2,30,37,38)(H4,32,33,36)(H2,48,49,50)/p-4/t15-,16+,20+,21+,22-,27+/m0/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of Enterococcus faecium AAC(6')Ii |
J Med Chem 49: 5273-81 (2006)
Article DOI: 10.1021/jm060732n
BindingDB Entry DOI: 10.7270/Q2183789 |
More data for this
Ligand-Target Pair | |
GNAT family acetyltransferase
(Enterococcus durans) | BDBM50193477
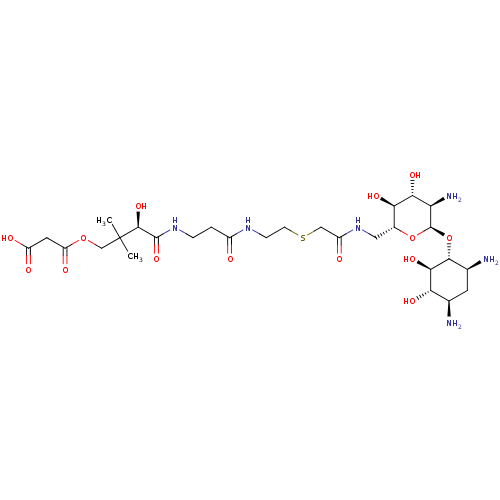
(3-((R)-4-(3-(2-(2-(((2R,3S,4R,5R,6R)-5-amino-6-((1...)Show SMILES CC(C)(COC(=O)CC(O)=O)[C@@H](O)C(=O)NCCC(=O)NCCSCC(=O)NC[C@H]1O[C@H](O[C@@H]2[C@@H](N)C[C@@H](N)[C@H](O)[C@H]2O)[C@H](N)[C@@H](O)[C@@H]1O Show InChI InChI=1S/C28H50N6O14S/c1-28(2,11-46-18(39)8-17(37)38)25(44)26(45)33-4-3-15(35)32-5-6-49-10-16(36)34-9-14-21(41)22(42)19(31)27(47-14)48-24-13(30)7-12(29)20(40)23(24)43/h12-14,19-25,27,40-44H,3-11,29-31H2,1-2H3,(H,32,35)(H,33,45)(H,34,36)(H,37,38)/t12-,13+,14-,19-,20+,21-,22-,23-,24-,25+,27-/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of Enterococcus faecium AAC(6')Ii |
J Med Chem 49: 5273-81 (2006)
Article DOI: 10.1021/jm060732n
BindingDB Entry DOI: 10.7270/Q2183789 |
More data for this
Ligand-Target Pair | |
GNAT family acetyltransferase
(Enterococcus durans) | BDBM50193487
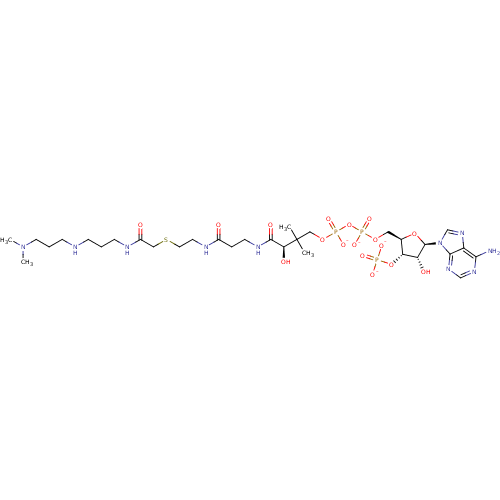
((3R)-3-[(2-{[2-({[(3-{[3-(dimethylamino)propyl]ami...)Show SMILES CN(C)CCCNCCCNC(=O)CSCCNC(=O)CCNC(=O)[C@H](O)C(C)(C)COP([O-])(=O)OP([O-])(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP([O-])([O-])=O)n1cnc2c(N)ncnc12 Show InChI InChI=1S/C31H57N10O17P3S/c1-31(2,26(45)29(46)36-11-7-21(42)35-12-14-62-16-22(43)34-10-5-8-33-9-6-13-40(3)4)17-55-61(52,53)58-60(50,51)54-15-20-25(57-59(47,48)49)24(44)30(56-20)41-19-39-23-27(32)37-18-38-28(23)41/h18-20,24-26,30,33,44-45H,5-17H2,1-4H3,(H,34,43)(H,35,42)(H,36,46)(H,50,51)(H,52,53)(H2,32,37,38)(H2,47,48,49)/p-4/t20-,24-,25-,26+,30-/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of Enterococcus faecium AAC(6')Ii |
J Med Chem 49: 5273-81 (2006)
Article DOI: 10.1021/jm060732n
BindingDB Entry DOI: 10.7270/Q2183789 |
More data for this
Ligand-Target Pair | |
GNAT family acetyltransferase
(Enterococcus durans) | BDBM50193484
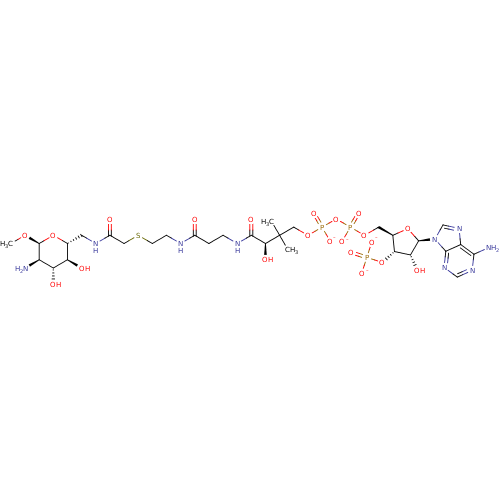
(CID44414951 | [(2R,3S,4R,5R)-5-(6-amino-9H-purin-9...)Show SMILES CO[C@H]1O[C@H](CNC(=O)CSCCNC(=O)CCNC(=O)[C@H](O)C(C)(C)COP([O-])(=O)OP([O-])(=O)OC[C@H]2O[C@H]([C@H](O)[C@@H]2OP([O-])([O-])=O)n2cnc3c(N)ncnc23)[C@@H](O)[C@H](O)[C@H]1N Show InChI InChI=1S/C30H52N9O21P3S/c1-30(2,24(45)27(46)34-5-4-16(40)33-6-7-64-10-17(41)35-8-14-20(42)21(43)18(31)29(54-3)58-14)11-56-63(52,53)60-62(50,51)55-9-15-23(59-61(47,48)49)22(44)28(57-15)39-13-38-19-25(32)36-12-37-26(19)39/h12-15,18,20-24,28-29,42-45H,4-11,31H2,1-3H3,(H,33,40)(H,34,46)(H,35,41)(H,50,51)(H,52,53)(H2,32,36,37)(H2,47,48,49)/p-4/t14-,15-,18-,20-,21-,22-,23-,24+,28-,29+/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of Enterococcus faecium AAC(6')Ii |
J Med Chem 49: 5273-81 (2006)
Article DOI: 10.1021/jm060732n
BindingDB Entry DOI: 10.7270/Q2183789 |
More data for this
Ligand-Target Pair | |
GNAT family acetyltransferase
(Enterococcus durans) | BDBM50193478
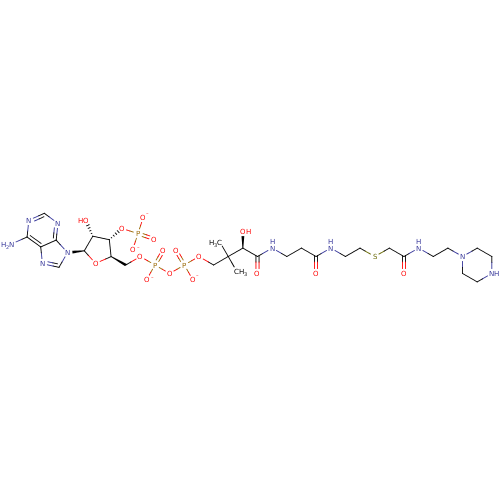
(CID44415032 | [(2R,3S,4R,5R)-5-(6-amino-9H-purin-9...)Show SMILES CC(C)(COP([O-])(=O)OP([O-])(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP([O-])([O-])=O)n1cnc2c(N)ncnc12)[C@@H](O)C(=O)NCCC(=O)NCCSCC(=O)NCCN1CCNCC1 Show InChI InChI=1S/C29H51N10O17P3S/c1-29(2,24(43)27(44)34-4-3-19(40)33-8-12-60-14-20(41)32-7-11-38-9-5-31-6-10-38)15-53-59(50,51)56-58(48,49)52-13-18-23(55-57(45,46)47)22(42)28(54-18)39-17-37-21-25(30)35-16-36-26(21)39/h16-18,22-24,28,31,42-43H,3-15H2,1-2H3,(H,32,41)(H,33,40)(H,34,44)(H,48,49)(H,50,51)(H2,30,35,36)(H2,45,46,47)/p-4/t18-,22-,23-,24+,28-/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of Enterococcus faecium AAC(6')Ii |
J Med Chem 49: 5273-81 (2006)
Article DOI: 10.1021/jm060732n
BindingDB Entry DOI: 10.7270/Q2183789 |
More data for this
Ligand-Target Pair | |
GNAT family acetyltransferase
(Enterococcus durans) | BDBM50193488
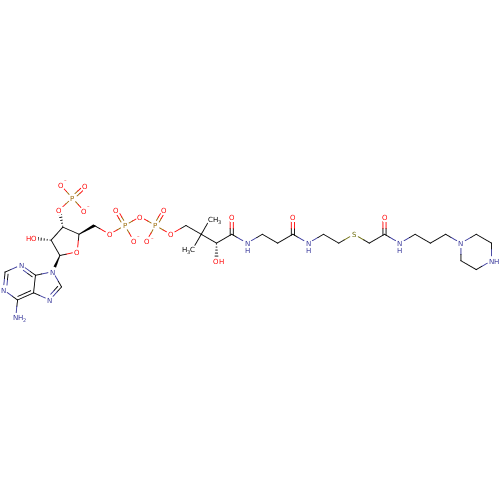
(CID44415033 | [(2R,3S,4R,5R)-5-(6-amino-9H-purin-9...)Show SMILES CC(C)(COP([O-])(=O)OP([O-])(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP([O-])([O-])=O)n1cnc2c(N)ncnc12)[C@@H](O)C(=O)NCCC(=O)NCCSCC(=O)NCCCN1CCNCC1 Show InChI InChI=1S/C30H53N10O17P3S/c1-30(2,25(44)28(45)35-6-4-20(41)34-9-13-61-15-21(42)33-5-3-10-39-11-7-32-8-12-39)16-54-60(51,52)57-59(49,50)53-14-19-24(56-58(46,47)48)23(43)29(55-19)40-18-38-22-26(31)36-17-37-27(22)40/h17-19,23-25,29,32,43-44H,3-16H2,1-2H3,(H,33,42)(H,34,41)(H,35,45)(H,49,50)(H,51,52)(H2,31,36,37)(H2,46,47,48)/p-4/t19-,23-,24-,25+,29-/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of Enterococcus faecium AAC(6')Ii |
J Med Chem 49: 5273-81 (2006)
Article DOI: 10.1021/jm060732n
BindingDB Entry DOI: 10.7270/Q2183789 |
More data for this
Ligand-Target Pair | |
GNAT family acetyltransferase
(Enterococcus durans) | BDBM50193474
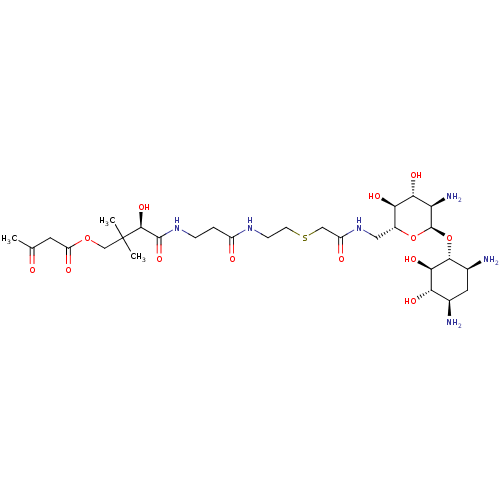
((R)-4-(3-(2-(2-(((2R,3S,4R,5R,6R)-5-amino-6-((1R,2...)Show SMILES CC(=O)CC(=O)OCC(C)(C)[C@@H](O)C(=O)NCCC(=O)NCCSCC(=O)NC[C@H]1O[C@H](O[C@@H]2[C@@H](N)C[C@@H](N)[C@H](O)[C@H]2O)[C@H](N)[C@@H](O)[C@@H]1O Show InChI InChI=1S/C29H52N6O13S/c1-13(36)8-19(39)46-12-29(2,3)26(44)27(45)34-5-4-17(37)33-6-7-49-11-18(38)35-10-16-22(41)23(42)20(32)28(47-16)48-25-15(31)9-14(30)21(40)24(25)43/h14-16,20-26,28,40-44H,4-12,30-32H2,1-3H3,(H,33,37)(H,34,45)(H,35,38)/t14-,15+,16-,20-,21+,22-,23-,24-,25-,26+,28-/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of Enterococcus faecium AAC(6')Ii |
J Med Chem 49: 5273-81 (2006)
Article DOI: 10.1021/jm060732n
BindingDB Entry DOI: 10.7270/Q2183789 |
More data for this
Ligand-Target Pair | |
GNAT family acetyltransferase
(Enterococcus durans) | BDBM50193480
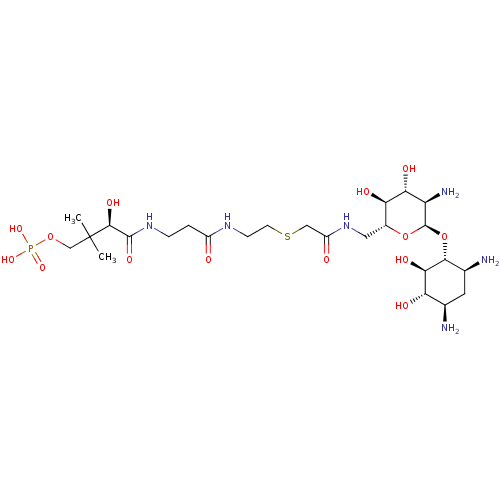
((R)-4-(3-(2-(2-(((2R,3S,4R,5R,6R)-5-amino-6-((1R,2...)Show SMILES CC(C)(COP(O)(O)=O)[C@@H](O)C(=O)NCCC(=O)NCCSCC(=O)NC[C@H]1O[C@H](O[C@@H]2[C@@H](N)C[C@@H](N)[C@H](O)[C@H]2O)[C@H](N)[C@@H](O)[C@@H]1O Show InChI InChI=1S/C25H49N6O14PS/c1-25(2,10-43-46(40,41)42)22(38)23(39)30-4-3-14(32)29-5-6-47-9-15(33)31-8-13-18(35)19(36)16(28)24(44-13)45-21-12(27)7-11(26)17(34)20(21)37/h11-13,16-22,24,34-38H,3-10,26-28H2,1-2H3,(H,29,32)(H,30,39)(H,31,33)(H2,40,41,42)/t11-,12+,13-,16-,17+,18-,19-,20-,21-,22+,24-/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of Enterococcus faecium AAC(6')Ii |
J Med Chem 49: 5273-81 (2006)
Article DOI: 10.1021/jm060732n
BindingDB Entry DOI: 10.7270/Q2183789 |
More data for this
Ligand-Target Pair | |
GNAT family acetyltransferase
(Enterococcus durans) | BDBM50193476
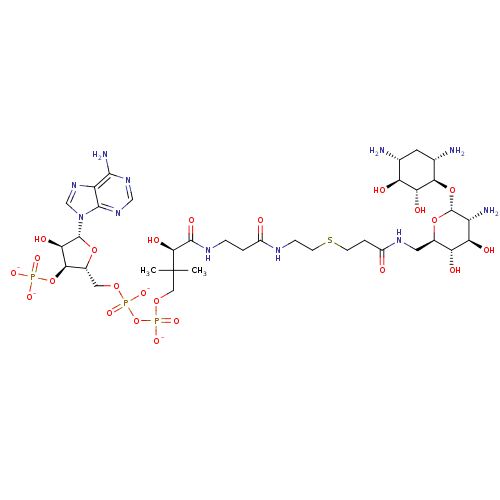
(CID44415023 | [(2R,3S,4R,5R)-5-(6-amino-9H-purin-9...)Show SMILES CC(C)(COP([O-])(=O)OP([O-])(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP([O-])([O-])=O)n1cnc2c(N)ncnc12)[C@@H](O)C(=O)NCCC(=O)NCCSCCC(=O)NC[C@H]1O[C@H](O[C@@H]2[C@@H](N)C[C@@H](N)[C@H](O)[C@H]2O)[C@H](N)[C@@H](O)[C@@H]1O Show InChI InChI=1S/C36H64N11O23P3S/c1-36(2,12-65-73(62,63)70-72(60,61)64-11-18-29(69-71(57,58)59)27(54)34(66-18)47-14-46-22-31(40)44-13-45-32(22)47)30(55)33(56)42-5-3-19(48)41-6-8-74-7-4-20(49)43-10-17-24(51)25(52)21(39)35(67-17)68-28-16(38)9-15(37)23(50)26(28)53/h13-18,21,23-30,34-35,50-55H,3-12,37-39H2,1-2H3,(H,41,48)(H,42,56)(H,43,49)(H,60,61)(H,62,63)(H2,40,44,45)(H2,57,58,59)/p-4/t15-,16+,17-,18-,21-,23+,24-,25-,26-,27-,28-,29-,30+,34-,35-/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of Enterococcus faecium AAC(6')Ii |
J Med Chem 49: 5273-81 (2006)
Article DOI: 10.1021/jm060732n
BindingDB Entry DOI: 10.7270/Q2183789 |
More data for this
Ligand-Target Pair | |
GNAT family acetyltransferase
(Enterococcus durans) | BDBM50193486

(CID44415022 | [(2R,3S,4R,5R)-5-(6-amino-9H-purin-9...)Show SMILES CC(C)(COP([O-])(=O)OP([O-])(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP([O-])([O-])=O)n1cnc2c(N)ncnc12)[C@@H](O)C(=O)NCCC(=O)NCCSCC(=O)NC[C@H]1O[C@H](O[C@@H]2[C@@H](N)C[C@@H](N)[C@H](O)[C@H]2O)[C@H](N)[C@@H](O)[C@@H]1O Show InChI InChI=1S/C35H62N11O23P3S/c1-35(2,11-64-72(61,62)69-71(59,60)63-9-17-28(68-70(56,57)58)26(53)33(65-17)46-13-45-21-30(39)43-12-44-31(21)46)29(54)32(55)41-4-3-18(47)40-5-6-73-10-19(48)42-8-16-23(50)24(51)20(38)34(66-16)67-27-15(37)7-14(36)22(49)25(27)52/h12-17,20,22-29,33-34,49-54H,3-11,36-38H2,1-2H3,(H,40,47)(H,41,55)(H,42,48)(H,59,60)(H,61,62)(H2,39,43,44)(H2,56,57,58)/p-4/t14-,15+,16-,17-,20-,22+,23-,24-,25-,26-,27-,28-,29+,33-,34-/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of Enterococcus faecium AAC(6')Ii |
J Med Chem 49: 5273-81 (2006)
Article DOI: 10.1021/jm060732n
BindingDB Entry DOI: 10.7270/Q2183789 |
More data for this
Ligand-Target Pair | |
GNAT family acetyltransferase
(Enterococcus durans) | BDBM50193482
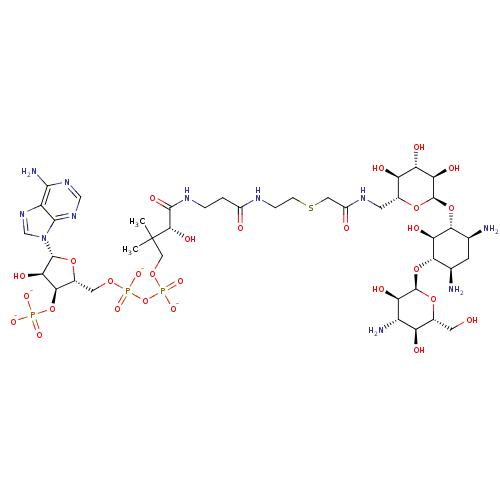
(CID44414946 | [(2R,3S,4R,5R)-5-(6-amino-9H-purin-9...)Show SMILES CC(C)(COP([O-])(=O)OP([O-])(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP([O-])([O-])=O)n1cnc2c(N)ncnc12)[C@@H](O)C(=O)NCCC(=O)NCCSCC(=O)NC[C@H]1O[C@H](O[C@@H]2[C@@H](N)C[C@@H](N)[C@H](O[C@H]3O[C@H](CO)[C@@H](O)[C@H](N)[C@H]3O)[C@H]2O)[C@H](O)[C@@H](O)[C@@H]1O Show InChI InChI=1S/C41H72N11O28P3S/c1-41(2,12-73-83(70,71)80-82(68,69)72-10-19-33(79-81(65,66)67)30(62)38(74-19)52-14-51-23-35(45)49-13-50-36(23)52)34(63)37(64)47-4-3-20(54)46-5-6-84-11-21(55)48-8-17-25(57)27(59)28(60)40(75-17)78-32-16(43)7-15(42)31(29(32)61)77-39-26(58)22(44)24(56)18(9-53)76-39/h13-19,22,24-34,38-40,53,56-63H,3-12,42-44H2,1-2H3,(H,46,54)(H,47,64)(H,48,55)(H,68,69)(H,70,71)(H2,45,49,50)(H2,65,66,67)/p-4/t15-,16+,17-,18-,19-,22+,24-,25-,26-,27+,28-,29-,30-,31+,32-,33-,34+,38-,39-,40-/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of Enterococcus faecium AAC(6')Ii |
J Med Chem 49: 5273-81 (2006)
Article DOI: 10.1021/jm060732n
BindingDB Entry DOI: 10.7270/Q2183789 |
More data for this
Ligand-Target Pair | |
GNAT family acetyltransferase
(Enterococcus durans) | BDBM50193479
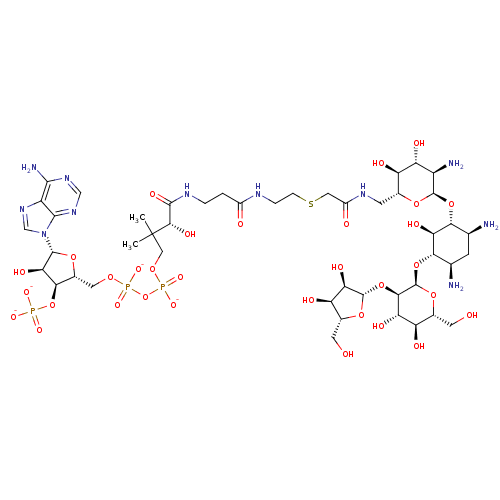
(CID44414949 | [(2R,3S,4R,5R)-5-(6-amino-9H-purin-9...)Show SMILES CC(C)(COP([O-])(=O)OP([O-])(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP([O-])([O-])=O)n1cnc2c(N)ncnc12)[C@@H](O)C(=O)NCCC(=O)NCCSCC(=O)NC[C@H]1O[C@H](O[C@@H]2[C@@H](N)C[C@@H](N)[C@H](O[C@H]3O[C@H](CO)[C@@H](O)[C@H](O)[C@H]3O[C@@H]3O[C@H](CO)[C@@H](O)[C@H]3O)[C@H]2O)[C@H](N)[C@@H](O)[C@@H]1O Show InChI InChI=1S/C46H80N11O32P3S/c1-46(2,13-80-92(77,78)89-91(75,76)79-11-21-36(88-90(72,73)74)33(69)42(81-21)57-15-56-25-39(50)54-14-55-40(25)57)38(70)41(71)52-4-3-22(60)51-5-6-93-12-23(61)53-8-18-26(62)29(65)24(49)43(82-18)85-34-16(47)7-17(48)35(32(34)68)86-45-37(30(66)27(63)19(9-58)84-45)87-44-31(67)28(64)20(10-59)83-44/h14-21,24,26-38,42-45,58-59,62-70H,3-13,47-49H2,1-2H3,(H,51,60)(H,52,71)(H,53,61)(H,75,76)(H,77,78)(H2,50,54,55)(H2,72,73,74)/p-4/t16-,17+,18+,19+,20+,21+,24+,26+,27+,28+,29+,30-,31+,32-,33+,34+,35-,36+,37+,38-,42+,43+,44-,45+/m0/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 119 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of Enterococcus faecium AAC(6')Ii |
J Med Chem 49: 5273-81 (2006)
Article DOI: 10.1021/jm060732n
BindingDB Entry DOI: 10.7270/Q2183789 |
More data for this
Ligand-Target Pair | |
GNAT family acetyltransferase
(Enterococcus durans) | BDBM50193485
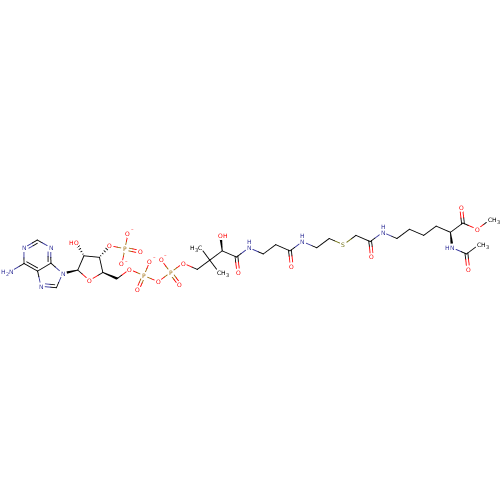
((3R)-3-{[2-({2-[({[(5S)-5-acetamido-6-methoxy-6-ox...)Show SMILES COC(=O)[C@H](CCCCNC(=O)CSCCNC(=O)CCNC(=O)[C@H](O)C(C)(C)COP([O-])(=O)OP([O-])(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP([O-])([O-])=O)n1cnc2c(N)ncnc12)NC(C)=O Show InChI InChI=1S/C32H54N9O20P3S/c1-18(42)40-19(31(48)56-4)7-5-6-9-34-22(44)14-65-12-11-35-21(43)8-10-36-29(47)26(46)32(2,3)15-58-64(54,55)61-63(52,53)57-13-20-25(60-62(49,50)51)24(45)30(59-20)41-17-39-23-27(33)37-16-38-28(23)41/h16-17,19-20,24-26,30,45-46H,5-15H2,1-4H3,(H,34,44)(H,35,43)(H,36,47)(H,40,42)(H,52,53)(H,54,55)(H2,33,37,38)(H2,49,50,51)/p-4/t19-,20+,24+,25+,26-,30+/m0/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of Enterococcus faecium AAC(6')Ii |
J Med Chem 49: 5273-81 (2006)
Article DOI: 10.1021/jm060732n
BindingDB Entry DOI: 10.7270/Q2183789 |
More data for this
Ligand-Target Pair | |
GNAT family acetyltransferase
(Enterococcus durans) | BDBM50193483
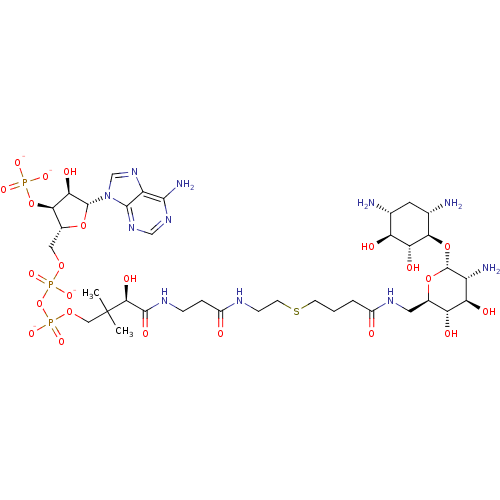
(CID44415024 | [(2R,3S,4R,5R)-5-(6-amino-9H-purin-9...)Show SMILES CC(C)(COP([O-])(=O)OP([O-])(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP([O-])([O-])=O)n1cnc2c(N)ncnc12)[C@@H](O)C(=O)NCCC(=O)NCCSCCCC(=O)NC[C@H]1O[C@H](O[C@@H]2[C@@H](N)C[C@@H](N)[C@H](O)[C@H]2O)[C@H](N)[C@@H](O)[C@@H]1O Show InChI InChI=1S/C37H66N11O23P3S/c1-37(2,13-66-74(63,64)71-73(61,62)65-12-19-30(70-72(58,59)60)28(55)35(67-19)48-15-47-23-32(41)45-14-46-33(23)48)31(56)34(57)43-6-5-21(50)42-7-9-75-8-3-4-20(49)44-11-18-25(52)26(53)22(40)36(68-18)69-29-17(39)10-16(38)24(51)27(29)54/h14-19,22,24-31,35-36,51-56H,3-13,38-40H2,1-2H3,(H,42,50)(H,43,57)(H,44,49)(H,61,62)(H,63,64)(H2,41,45,46)(H2,58,59,60)/p-4/t16-,17+,18-,19-,22-,24+,25-,26-,27-,28-,29-,30-,31+,35-,36-/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 161 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of Enterococcus faecium AAC(6')Ii |
J Med Chem 49: 5273-81 (2006)
Article DOI: 10.1021/jm060732n
BindingDB Entry DOI: 10.7270/Q2183789 |
More data for this
Ligand-Target Pair | |
GNAT family acetyltransferase
(Enterococcus durans) | BDBM50193481
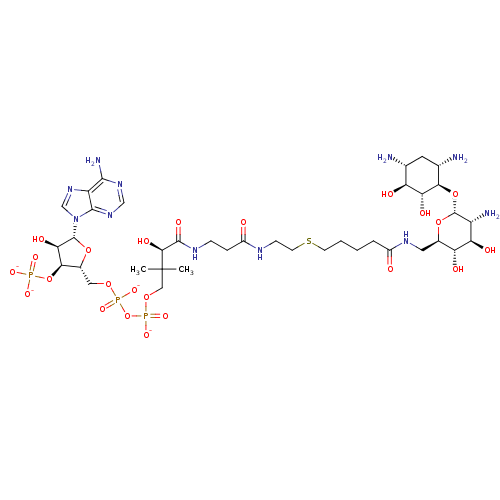
(CID44414945 | [(2R,3S,4R,5R)-5-(6-amino-9H-purin-9...)Show SMILES CC(C)(COP([O-])(=O)OP([O-])(=O)OC[C@H]1O[C@H]([C@H](O)[C@@H]1OP([O-])([O-])=O)n1cnc2c(N)ncnc12)[C@@H](O)C(=O)NCCC(=O)NCCSCCCCC(=O)NC[C@H]1O[C@H](O[C@@H]2[C@@H](N)C[C@@H](N)[C@H](O)[C@H]2O)[C@H](N)[C@@H](O)[C@@H]1O Show InChI InChI=1S/C38H68N11O23P3S/c1-38(2,14-67-75(64,65)72-74(62,63)66-13-20-31(71-73(59,60)61)29(56)36(68-20)49-16-48-24-33(42)46-15-47-34(24)49)32(57)35(58)44-7-6-22(51)43-8-10-76-9-4-3-5-21(50)45-12-19-26(53)27(54)23(41)37(69-19)70-30-18(40)11-17(39)25(52)28(30)55/h15-20,23,25-32,36-37,52-57H,3-14,39-41H2,1-2H3,(H,43,51)(H,44,58)(H,45,50)(H,62,63)(H,64,65)(H2,42,46,47)(H2,59,60,61)/p-4/t17-,18+,19-,20-,23-,25+,26-,27-,28-,29-,30-,31-,32+,36-,37-/m1/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of Enterococcus faecium AAC(6')Ii |
J Med Chem 49: 5273-81 (2006)
Article DOI: 10.1021/jm060732n
BindingDB Entry DOI: 10.7270/Q2183789 |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM25308
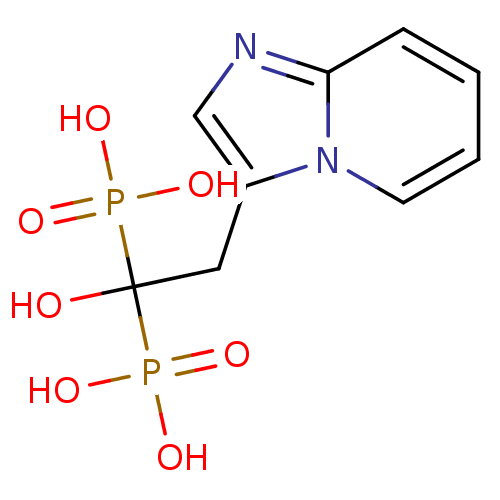
((1-hydroxy-2-{imidazo[1,2-a]pyridin-3-yl}-1-phosph...)Show InChI InChI=1S/C9H12N2O7P2/c12-9(19(13,14)15,20(16,17)18)5-7-6-10-8-3-1-2-4-11(7)8/h1-4,6,12H,5H2,(H2,13,14,15)(H2,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of human FPPS |
Bioorg Med Chem Lett 25: 1117-23 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.089
BindingDB Entry DOI: 10.7270/Q25X2BMF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM12578

(2-(imidazol-1-yl)-1-hydroxyethylidene-1,1-bisphosp...)Show InChI InChI=1S/C5H10N2O7P2/c8-5(15(9,10)11,16(12,13)14)3-7-2-1-6-4-7/h1-2,4,8H,3H2,(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His-tagged human FPPS (1 to 353 residues) expressed in Escherichia coli BL21 (DE3) pre-incubated for 10 mins before addition... |
J Med Chem 62: 9691-9702 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01104
BindingDB Entry DOI: 10.7270/Q23T9MJV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM12578

(2-(imidazol-1-yl)-1-hydroxyethylidene-1,1-bisphosp...)Show InChI InChI=1S/C5H10N2O7P2/c8-5(15(9,10)11,16(12,13)14)3-7-2-1-6-4-7/h1-2,4,8H,3H2,(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Allosteric inhibition of human recombinant FPPS using GPP and [3H]IPP as substrate incubated with enzyme for 10 mins prior to substrate addition by l... |
J Med Chem 57: 5764-76 (2014)
Article DOI: 10.1021/jm500629e
BindingDB Entry DOI: 10.7270/Q2GH9KJR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50098378
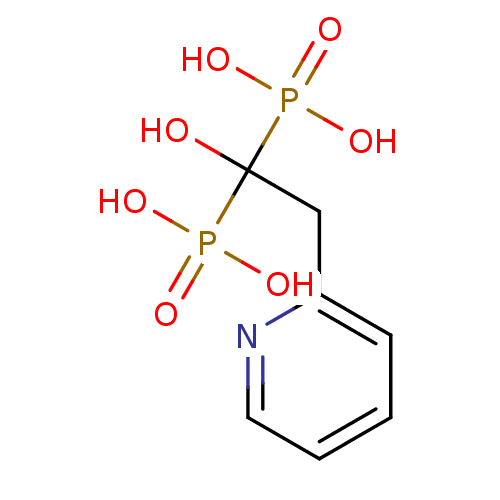
((1-Hydroxy-1-phosphono-2-pyridin-2-yl-ethyl)-phosp...)Show InChI InChI=1S/C7H11NO7P2/c9-7(16(10,11)12,17(13,14)15)5-6-3-1-2-4-8-6/h1-4,9H,5H2,(H2,10,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of human FPPS using [14C]-IPP and FPP as substrates after 10 mins by scintillation counting |
J Med Chem 61: 6904-6917 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00886
BindingDB Entry DOI: 10.7270/Q2CJ8HWF |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM12578

(2-(imidazol-1-yl)-1-hydroxyethylidene-1,1-bisphosp...)Show InChI InChI=1S/C5H10N2O7P2/c8-5(15(9,10)11,16(12,13)14)3-7-2-1-6-4-7/h1-2,4,8H,3H2,(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of human FPPS |
Bioorg Med Chem Lett 25: 1117-23 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.089
BindingDB Entry DOI: 10.7270/Q25X2BMF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM12578

(2-(imidazol-1-yl)-1-hydroxyethylidene-1,1-bisphosp...)Show InChI InChI=1S/C5H10N2O7P2/c8-5(15(9,10)11,16(12,13)14)3-7-2-1-6-4-7/h1-2,4,8H,3H2,(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal-His6 tagged FPPS expressed in Escherichia coli BL21 using [3H]IPP and GPP as substrate incubated for 10 mi... |
J Med Chem 55: 3201-15 (2012)
Article DOI: 10.1021/jm201657x
BindingDB Entry DOI: 10.7270/Q29024V1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM12576

(Bisphosphonate 1 | CHEMBL923 | JMC515594 Compound ...)Show InChI InChI=1S/C7H11NO7P2/c9-7(16(10,11)12,17(13,14)15)4-6-2-1-3-8-5-6/h1-3,5,9H,4H2,(H2,10,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS using GPP/[3H]IPP as substrate incubated for 10 mins prior to substrate addition measured after 8 mins by scinti... |
J Med Chem 56: 7939-50 (2013)
Article DOI: 10.1021/jm400946f
BindingDB Entry DOI: 10.7270/Q20V8F6B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM12576

(Bisphosphonate 1 | CHEMBL923 | JMC515594 Compound ...)Show InChI InChI=1S/C7H11NO7P2/c9-7(16(10,11)12,17(13,14)15)4-6-2-1-3-8-5-6/h1-3,5,9H,4H2,(H2,10,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Allosteric inhibition of human recombinant FPPS using GPP and [3H]IPP as substrate incubated with enzyme for 10 mins prior to substrate addition by l... |
J Med Chem 57: 5764-76 (2014)
Article DOI: 10.1021/jm500629e
BindingDB Entry DOI: 10.7270/Q2GH9KJR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM12576

(Bisphosphonate 1 | CHEMBL923 | JMC515594 Compound ...)Show InChI InChI=1S/C7H11NO7P2/c9-7(16(10,11)12,17(13,14)15)4-6-2-1-3-8-5-6/h1-3,5,9H,4H2,(H2,10,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal-His6 tagged FPPS expressed in Escherichia coli BL21 using [14C]IPP and GPP as substrate incubated for 10 m... |
J Med Chem 55: 3201-15 (2012)
Article DOI: 10.1021/jm201657x
BindingDB Entry DOI: 10.7270/Q29024V1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50443050
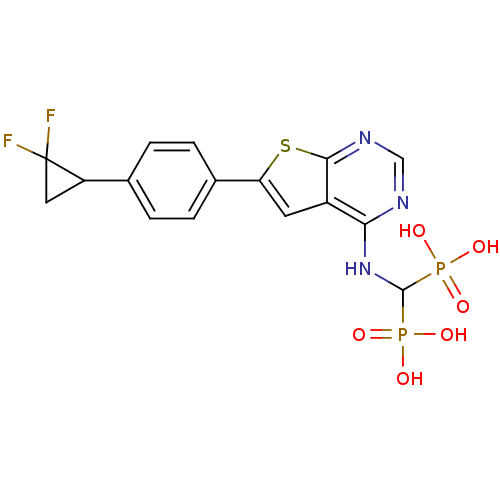
(CHEMBL3087938)Show SMILES OP(O)(=O)C(Nc1ncnc2sc(cc12)-c1ccc(cc1)C1CC1(F)F)P(O)(O)=O Show InChI InChI=1S/C16H15F2N3O6P2S/c17-16(18)6-11(16)8-1-3-9(4-2-8)12-5-10-13(19-7-20-14(10)30-12)21-15(28(22,23)24)29(25,26)27/h1-5,7,11,15H,6H2,(H,19,20,21)(H2,22,23,24)(H2,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS using GPP/[3H]IPP as substrate incubated for 10 mins prior to substrate addition measured after 8 mins by scinti... |
J Med Chem 56: 7939-50 (2013)
Article DOI: 10.1021/jm400946f
BindingDB Entry DOI: 10.7270/Q20V8F6B |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM12576

(Bisphosphonate 1 | CHEMBL923 | JMC515594 Compound ...)Show InChI InChI=1S/C7H11NO7P2/c9-7(16(10,11)12,17(13,14)15)4-6-2-1-3-8-5-6/h1-3,5,9H,4H2,(H2,10,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of human FPPS |
Bioorg Med Chem Lett 25: 1117-23 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.089
BindingDB Entry DOI: 10.7270/Q25X2BMF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM12576

(Bisphosphonate 1 | CHEMBL923 | JMC515594 Compound ...)Show InChI InChI=1S/C7H11NO7P2/c9-7(16(10,11)12,17(13,14)15)4-6-2-1-3-8-5-6/h1-3,5,9H,4H2,(H2,10,11,12)(H2,13,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS using GPP/[3H]IPP as substrate incubated for 10 mins prior to substrate addition by scintillation counting analy... |
J Med Chem 56: 7939-50 (2013)
Article DOI: 10.1021/jm400946f
BindingDB Entry DOI: 10.7270/Q20V8F6B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50443052
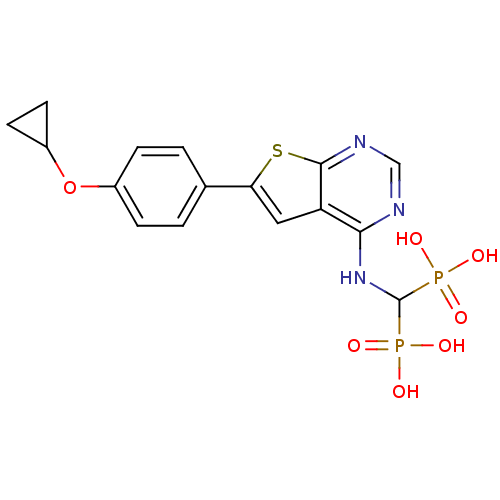
(CHEMBL3087936 | US11279719, Example C-13)Show SMILES OP(O)(=O)C(Nc1ncnc2sc(cc12)-c1ccc(OC2CC2)cc1)P(O)(O)=O Show InChI InChI=1S/C16H17N3O7P2S/c20-27(21,22)16(28(23,24)25)19-14-12-7-13(29-15(12)18-8-17-14)9-1-3-10(4-2-9)26-11-5-6-11/h1-4,7-8,11,16H,5-6H2,(H,17,18,19)(H2,20,21,22)(H2,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS using GPP/[3H]IPP as substrate incubated for 10 mins prior to substrate addition measured after 8 mins by scinti... |
J Med Chem 56: 7939-50 (2013)
Article DOI: 10.1021/jm400946f
BindingDB Entry DOI: 10.7270/Q20V8F6B |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50443052

(CHEMBL3087936 | US11279719, Example C-13)Show SMILES OP(O)(=O)C(Nc1ncnc2sc(cc12)-c1ccc(OC2CC2)cc1)P(O)(O)=O Show InChI InChI=1S/C16H17N3O7P2S/c20-27(21,22)16(28(23,24)25)19-14-12-7-13(29-15(12)18-8-17-14)9-1-3-10(4-2-9)26-11-5-6-11/h1-4,7-8,11,16H,5-6H2,(H,17,18,19)(H2,20,21,22)(H2,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Allosteric inhibition of human recombinant FPPS using GPP and [3H]IPP as substrate incubated with enzyme for 10 mins prior to substrate addition by l... |
J Med Chem 57: 5764-76 (2014)
Article DOI: 10.1021/jm500629e
BindingDB Entry DOI: 10.7270/Q2GH9KJR |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50443055
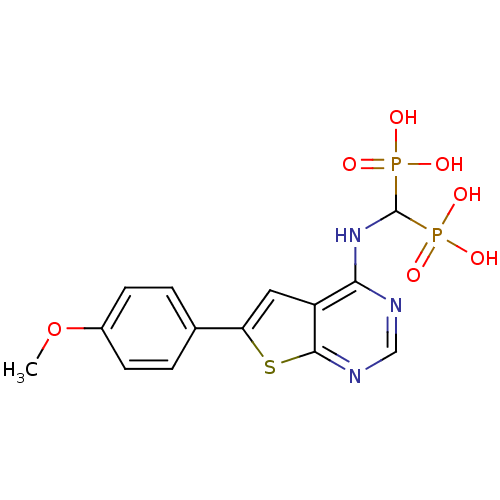
(CHEMBL3087933)Show SMILES COc1ccc(cc1)-c1cc2c(NC(P(O)(O)=O)P(O)(O)=O)ncnc2s1 Show InChI InChI=1S/C14H15N3O7P2S/c1-24-9-4-2-8(3-5-9)11-6-10-12(15-7-16-13(10)27-11)17-14(25(18,19)20)26(21,22)23/h2-7,14H,1H3,(H,15,16,17)(H2,18,19,20)(H2,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS using GPP/[3H]IPP as substrate incubated for 10 mins prior to substrate addition measured after 8 mins by scinti... |
J Med Chem 56: 7939-50 (2013)
Article DOI: 10.1021/jm400946f
BindingDB Entry DOI: 10.7270/Q20V8F6B |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50421094
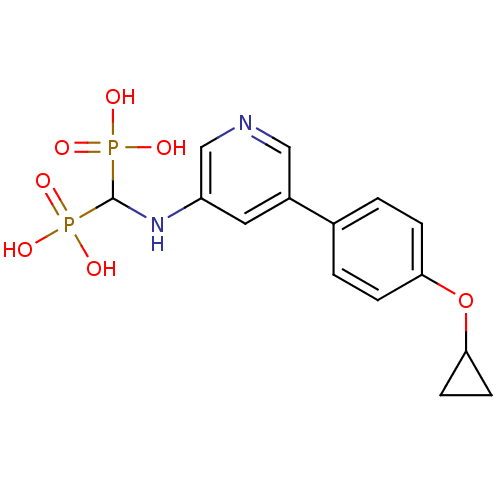
(CHEMBL2088339)Show SMILES OP(O)(=O)C(Nc1cncc(c1)-c1ccc(OC2CC2)cc1)P(O)(O)=O Show InChI InChI=1S/C15H18N2O7P2/c18-25(19,20)15(26(21,22)23)17-12-7-11(8-16-9-12)10-1-3-13(4-2-10)24-14-5-6-14/h1-4,7-9,14-15,17H,5-6H2,(H2,18,19,20)(H2,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS using GPP/[3H]IPP as substrate incubated for 10 mins prior to substrate addition by scintillation counting analy... |
J Med Chem 56: 7939-50 (2013)
Article DOI: 10.1021/jm400946f
BindingDB Entry DOI: 10.7270/Q20V8F6B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50421094

(CHEMBL2088339)Show SMILES OP(O)(=O)C(Nc1cncc(c1)-c1ccc(OC2CC2)cc1)P(O)(O)=O Show InChI InChI=1S/C15H18N2O7P2/c18-25(19,20)15(26(21,22)23)17-12-7-11(8-16-9-12)10-1-3-13(4-2-10)24-14-5-6-14/h1-4,7-9,14-15,17H,5-6H2,(H2,18,19,20)(H2,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Allosteric inhibition of human recombinant FPPS using GPP and [3H]IPP as substrate incubated with enzyme for 10 mins prior to substrate addition by l... |
J Med Chem 57: 5764-76 (2014)
Article DOI: 10.1021/jm500629e
BindingDB Entry DOI: 10.7270/Q2GH9KJR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50421094

(CHEMBL2088339)Show SMILES OP(O)(=O)C(Nc1cncc(c1)-c1ccc(OC2CC2)cc1)P(O)(O)=O Show InChI InChI=1S/C15H18N2O7P2/c18-25(19,20)15(26(21,22)23)17-12-7-11(8-16-9-12)10-1-3-13(4-2-10)24-14-5-6-14/h1-4,7-9,14-15,17H,5-6H2,(H2,18,19,20)(H2,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS using GPP/[3H]IPP as substrate incubated for 10 mins prior to substrate addition measured after 8 mins by scinti... |
J Med Chem 56: 7939-50 (2013)
Article DOI: 10.1021/jm400946f
BindingDB Entry DOI: 10.7270/Q20V8F6B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50443054
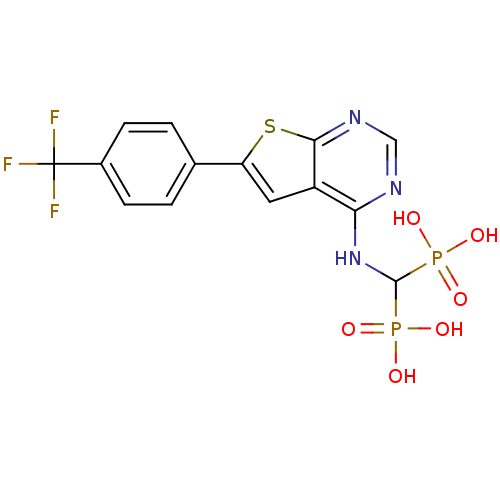
(CHEMBL3087934 | US11279719, Example C-12)Show SMILES OP(O)(=O)C(Nc1ncnc2sc(cc12)-c1ccc(cc1)C(F)(F)F)P(O)(O)=O Show InChI InChI=1S/C14H12F3N3O6P2S/c15-14(16,17)8-3-1-7(2-4-8)10-5-9-11(18-6-19-12(9)29-10)20-13(27(21,22)23)28(24,25)26/h1-6,13H,(H,18,19,20)(H2,21,22,23)(H2,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS using GPP/[3H]IPP as substrate incubated for 10 mins prior to substrate addition measured after 8 mins by scinti... |
J Med Chem 56: 7939-50 (2013)
Article DOI: 10.1021/jm400946f
BindingDB Entry DOI: 10.7270/Q20V8F6B |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50432306
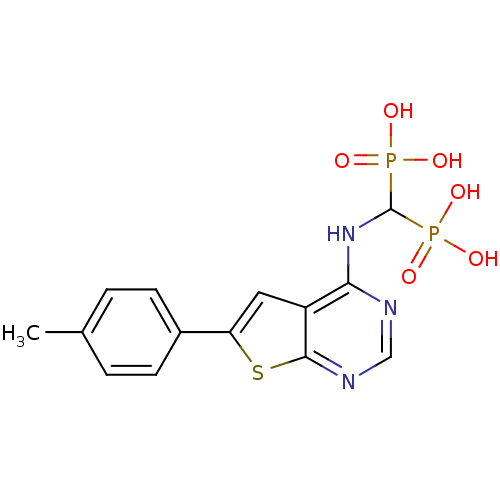
(CHEMBL2347862)Show SMILES Cc1ccc(cc1)-c1cc2c(NC(P(O)(O)=O)P(O)(O)=O)ncnc2s1 Show InChI InChI=1S/C14H15N3O6P2S/c1-8-2-4-9(5-3-8)11-6-10-12(15-7-16-13(10)26-11)17-14(24(18,19)20)25(21,22)23/h2-7,14H,1H3,(H,15,16,17)(H2,18,19,20)(H2,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Allosteric inhibition of human recombinant FPPS using GPP and [3H]IPP as substrate incubated with enzyme for 10 mins prior to substrate addition by l... |
J Med Chem 57: 5764-76 (2014)
Article DOI: 10.1021/jm500629e
BindingDB Entry DOI: 10.7270/Q2GH9KJR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50432306

(CHEMBL2347862)Show SMILES Cc1ccc(cc1)-c1cc2c(NC(P(O)(O)=O)P(O)(O)=O)ncnc2s1 Show InChI InChI=1S/C14H15N3O6P2S/c1-8-2-4-9(5-3-8)11-6-10-12(15-7-16-13(10)26-11)17-14(24(18,19)20)25(21,22)23/h2-7,14H,1H3,(H,15,16,17)(H2,18,19,20)(H2,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS using GPP/[3H]IPP as substrate incubated for 10 mins prior to substrate addition measured after 8 mins by scinti... |
J Med Chem 56: 7939-50 (2013)
Article DOI: 10.1021/jm400946f
BindingDB Entry DOI: 10.7270/Q20V8F6B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50443054

(CHEMBL3087934 | US11279719, Example C-12)Show SMILES OP(O)(=O)C(Nc1ncnc2sc(cc12)-c1ccc(cc1)C(F)(F)F)P(O)(O)=O Show InChI InChI=1S/C14H12F3N3O6P2S/c15-14(16,17)8-3-1-7(2-4-8)10-5-9-11(18-6-19-12(9)29-10)20-13(27(21,22)23)28(24,25)26/h1-6,13H,(H,18,19,20)(H2,21,22,23)(H2,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of human FPPS using [14C]-IPP and FPP as substrates after 10 mins by scintillation counting |
J Med Chem 61: 6904-6917 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00886
BindingDB Entry DOI: 10.7270/Q2CJ8HWF |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50386555
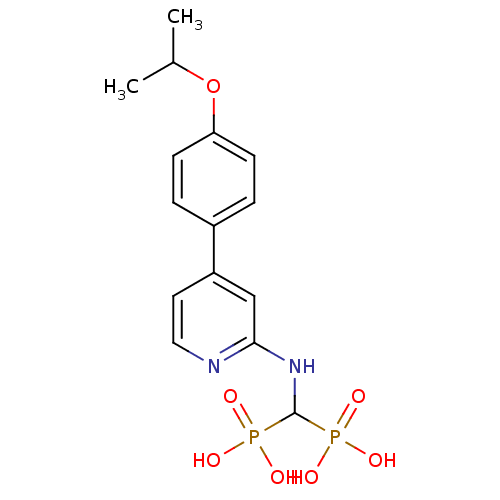
(CHEMBL2048241)Show SMILES CC(C)Oc1ccc(cc1)-c1ccnc(NC(P(O)(O)=O)P(O)(O)=O)c1 Show InChI InChI=1S/C15H20N2O7P2/c1-10(2)24-13-5-3-11(4-6-13)12-7-8-16-14(9-12)17-15(25(18,19)20)26(21,22)23/h3-10,15H,1-2H3,(H,16,17)(H2,18,19,20)(H2,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal-His6 tagged FPPS expressed in Escherichia coli BL21 using [14C]IPP and GPP as substrate incubated for 10 m... |
J Med Chem 55: 3201-15 (2012)
Article DOI: 10.1021/jm201657x
BindingDB Entry DOI: 10.7270/Q29024V1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50386558
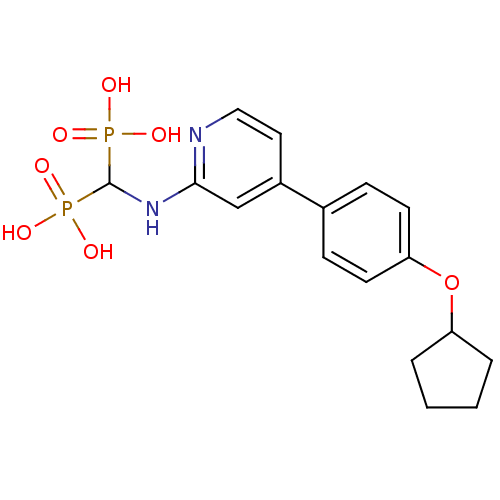
(CHEMBL2048244)Show SMILES OP(O)(=O)C(Nc1cc(ccn1)-c1ccc(OC2CCCC2)cc1)P(O)(O)=O Show InChI InChI=1S/C17H22N2O7P2/c20-27(21,22)17(28(23,24)25)19-16-11-13(9-10-18-16)12-5-7-15(8-6-12)26-14-3-1-2-4-14/h5-11,14,17H,1-4H2,(H,18,19)(H2,20,21,22)(H2,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal-His6 tagged FPPS expressed in Escherichia coli BL21 using [14C]IPP and GPP as substrate incubated for 10 m... |
J Med Chem 55: 3201-15 (2012)
Article DOI: 10.1021/jm201657x
BindingDB Entry DOI: 10.7270/Q29024V1 |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50138725

((1-phosphono-2-pyridin-3-yl-ethyl)-phosphonic acid...)Show InChI InChI=1S/C7H11NO6P2/c9-15(10,11)7(16(12,13)14)4-6-2-1-3-8-5-6/h1-3,5,7H,4H2,(H2,9,10,11)(H2,12,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Allosteric inhibition of human recombinant FPPS using GPP and [3H]IPP as substrate incubated with enzyme for 10 mins prior to substrate addition by l... |
J Med Chem 57: 5764-76 (2014)
Article DOI: 10.1021/jm500629e
BindingDB Entry DOI: 10.7270/Q2GH9KJR |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50386557

(CHEMBL2048243)Show SMILES Cc1ccc(cc1)-c1ccnc(NC(P(O)(O)=O)P(O)(O)=O)c1 Show InChI InChI=1S/C13H16N2O6P2/c1-9-2-4-10(5-3-9)11-6-7-14-12(8-11)15-13(22(16,17)18)23(19,20)21/h2-8,13H,1H3,(H,14,15)(H2,16,17,18)(H2,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal-His6 tagged FPPS expressed in Escherichia coli BL21 using [14C]IPP and GPP as substrate incubated for 10 m... |
J Med Chem 55: 3201-15 (2012)
Article DOI: 10.1021/jm201657x
BindingDB Entry DOI: 10.7270/Q29024V1 |
More data for this
Ligand-Target Pair | |
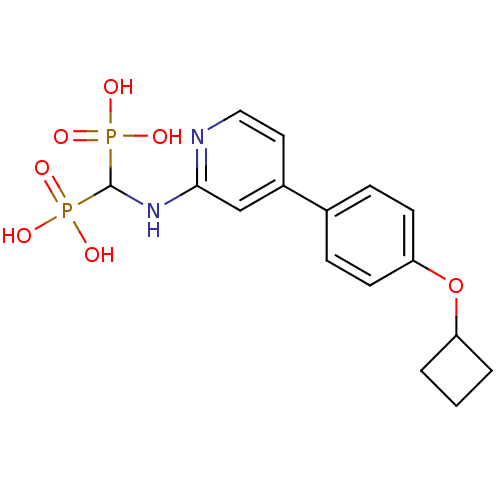
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50386556
(CHEMBL2048242)Show SMILES OP(O)(=O)C(Nc1cc(ccn1)-c1ccc(OC2CCC2)cc1)P(O)(O)=O Show InChI InChI=1S/C16H20N2O7P2/c19-26(20,21)16(27(22,23)24)18-15-10-12(8-9-17-15)11-4-6-14(7-5-11)25-13-2-1-3-13/h4-10,13,16H,1-3H2,(H,17,18)(H2,19,20,21)(H2,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal-His6 tagged FPPS expressed in Escherichia coli BL21 using [14C]IPP and GPP as substrate incubated for 10 m... |
J Med Chem 55: 3201-15 (2012)
Article DOI: 10.1021/jm201657x
BindingDB Entry DOI: 10.7270/Q29024V1 |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50520638

(CHEMBL4471037 | US11279719, Example I-18)Show SMILES Cc1ccc(cc1)C(=O)Nc1cccc(c1)-c1nc(NC(P(O)(O)=O)P(O)(O)=O)c2ccsc2n1 Show InChI InChI=1S/C21H20N4O7P2S/c1-12-5-7-13(8-6-12)19(26)22-15-4-2-3-14(11-15)17-23-18(16-9-10-35-20(16)24-17)25-21(33(27,28)29)34(30,31)32/h2-11,21H,1H3,(H,22,26)(H,23,24,25)(H2,27,28,29)(H2,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His6-tagged GGPPS Y246D mutant expressed in Escherichia coli BL21(DE3) using [14C]-IPP and FPP as substrates after 10 ... |
J Med Chem 61: 6904-6917 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00886
BindingDB Entry DOI: 10.7270/Q2CJ8HWF |
More data for this
Ligand-Target Pair | |
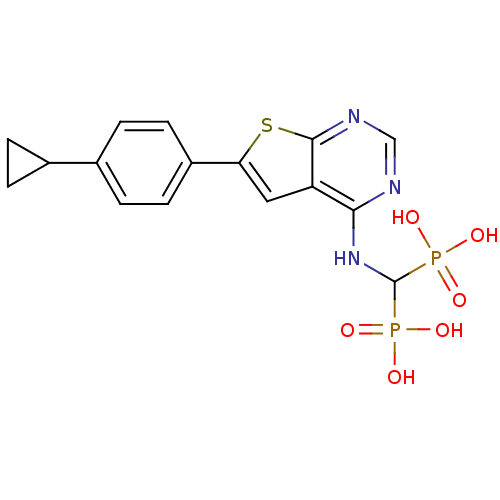
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50443051
(CHEMBL3087937)Show SMILES OP(O)(=O)C(Nc1ncnc2sc(cc12)-c1ccc(cc1)C1CC1)P(O)(O)=O Show InChI InChI=1S/C16H17N3O6P2S/c20-26(21,22)16(27(23,24)25)19-14-12-7-13(28-15(12)18-8-17-14)11-5-3-10(4-6-11)9-1-2-9/h3-9,16H,1-2H2,(H,17,18,19)(H2,20,21,22)(H2,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS using GPP/[3H]IPP as substrate incubated for 10 mins prior to substrate addition measured after 8 mins by scinti... |
J Med Chem 56: 7939-50 (2013)
Article DOI: 10.1021/jm400946f
BindingDB Entry DOI: 10.7270/Q20V8F6B |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50520641

(CHEMBL4455060 | US11279719, Example I-13)Show SMILES OP(O)(=O)C(Nc1nc(nc2sccc12)-c1cccc(NC(=O)c2ccccc2)c1)P(O)(O)=O Show InChI InChI=1S/C20H18N4O7P2S/c25-18(12-5-2-1-3-6-12)21-14-8-4-7-13(11-14)16-22-17(15-9-10-34-19(15)23-16)24-20(32(26,27)28)33(29,30)31/h1-11,20H,(H,21,25)(H,22,23,24)(H2,26,27,28)(H2,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His6-tagged GGPPS Y246D mutant expressed in Escherichia coli BL21(DE3) using [14C]-IPP and FPP as substrates after 10 ... |
J Med Chem 61: 6904-6917 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00886
BindingDB Entry DOI: 10.7270/Q2CJ8HWF |
More data for this
Ligand-Target Pair | |
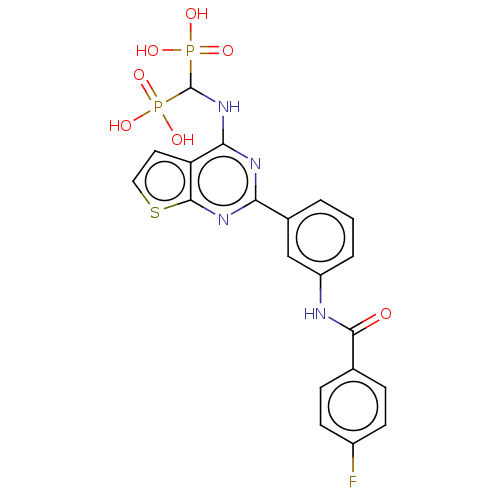
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50520640
(CHEMBL4472025 | US11279719, Example I-34)Show SMILES OP(O)(=O)C(Nc1nc(nc2sccc12)-c1cccc(NC(=O)c2ccc(F)cc2)c1)P(O)(O)=O Show InChI InChI=1S/C20H17FN4O7P2S/c21-13-6-4-11(5-7-13)18(26)22-14-3-1-2-12(10-14)16-23-17(15-8-9-35-19(15)24-16)25-20(33(27,28)29)34(30,31)32/h1-10,20H,(H,22,26)(H,23,24,25)(H2,27,28,29)(H2,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His6-tagged GGPPS expressed in Escherichia coli BL21(DE3) using [14C]-IPP and FPP as substrates after 10 mins by scint... |
J Med Chem 61: 6904-6917 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00886
BindingDB Entry DOI: 10.7270/Q2CJ8HWF |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
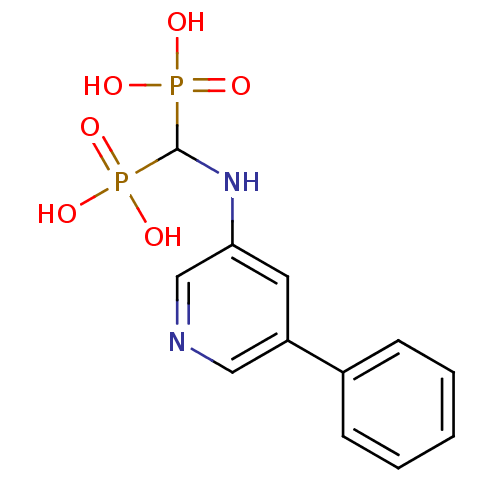
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50386552
(CHEMBL2048238)Show InChI InChI=1S/C12H14N2O6P2/c15-21(16,17)12(22(18,19)20)14-11-6-10(7-13-8-11)9-4-2-1-3-5-9/h1-8,12,14H,(H2,15,16,17)(H2,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant N-terminal-His6 tagged FPPS expressed in Escherichia coli BL21 using [14C]IPP and GPP as substrate incubated for 10 m... |
J Med Chem 55: 3201-15 (2012)
Article DOI: 10.1021/jm201657x
BindingDB Entry DOI: 10.7270/Q29024V1 |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50520639
(CHEMBL4465832 | US11279719, Example I-6)Show SMILES OP(O)(=O)C(Nc1nc(nc2sccc12)-c1cccc(c1)C(=O)Nc1ccccc1)P(O)(O)=O Show InChI InChI=1S/C20H18N4O7P2S/c25-18(21-14-7-2-1-3-8-14)13-6-4-5-12(11-13)16-22-17(15-9-10-34-19(15)23-16)24-20(32(26,27)28)33(29,30)31/h1-11,20H,(H,21,25)(H,22,23,24)(H2,26,27,28)(H2,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His6-tagged GGPPS expressed in Escherichia coli BL21(DE3) using [14C]-IPP and FPP as substrates after 10 mins by scint... |
J Med Chem 61: 6904-6917 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00886
BindingDB Entry DOI: 10.7270/Q2CJ8HWF |
More data for this
Ligand-Target Pair | |
Geranylgeranyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50520639

(CHEMBL4465832 | US11279719, Example I-6)Show SMILES OP(O)(=O)C(Nc1nc(nc2sccc12)-c1cccc(c1)C(=O)Nc1ccccc1)P(O)(O)=O Show InChI InChI=1S/C20H18N4O7P2S/c25-18(21-14-7-2-1-3-8-14)13-6-4-5-12(11-13)16-22-17(15-9-10-34-19(15)23-16)24-20(32(26,27)28)33(29,30)31/h1-11,20H,(H,21,25)(H,22,23,24)(H2,26,27,28)(H2,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His6-tagged GGPPS Y246D mutant expressed in Escherichia coli BL21(DE3) using [14C]-IPP and FPP as substrates after 10 ... |
J Med Chem 61: 6904-6917 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00886
BindingDB Entry DOI: 10.7270/Q2CJ8HWF |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50432305
(CHEMBL2347863)Show SMILES OP(O)(=O)C(Nc1ncnc2sc(cc12)-c1ccc2ccccc2c1)P(O)(O)=O Show InChI InChI=1S/C17H15N3O6P2S/c21-27(22,23)17(28(24,25)26)20-15-13-8-14(29-16(13)19-9-18-15)12-6-5-10-3-1-2-4-11(10)7-12/h1-9,17H,(H,18,19,20)(H2,21,22,23)(H2,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 63 | n/a | n/a | n/a | n/a | n/a | n/a |
McGill University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant FPPS using GPP/[3H]IPP as substrate incubated for 10 mins prior to substrate addition measured after 8 mins by scinti... |
J Med Chem 56: 7939-50 (2013)
Article DOI: 10.1021/jm400946f
BindingDB Entry DOI: 10.7270/Q20V8F6B |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data