 Found 48 hits with Last Name = 'bernardi' and Initial = 'a'
Found 48 hits with Last Name = 'bernardi' and Initial = 'a' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50068039
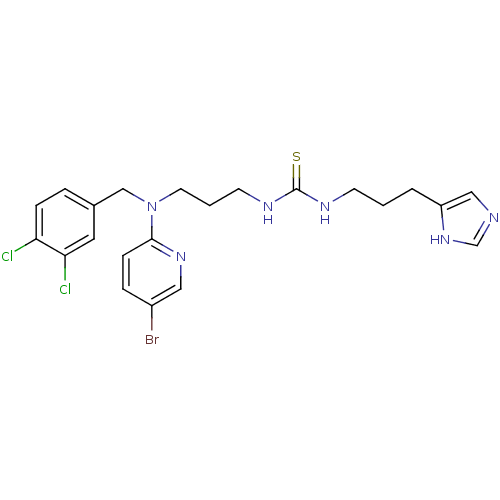
(1-{3-[(5-Bromo-pyridin-2-yl)-(3,4-dichloro-benzyl)...)Show SMILES Clc1ccc(CN(CCCNC(=S)NCCCc2cnc[nH]2)c2ccc(Br)cn2)cc1Cl Show InChI InChI=1S/C22H25BrCl2N6S/c23-17-5-7-21(29-12-17)31(14-16-4-6-19(24)20(25)11-16)10-2-9-28-22(32)27-8-1-3-18-13-26-15-30-18/h4-7,11-13,15H,1-3,8-10,14H2,(H,26,30)(H2,27,28,32) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human SST4 receptor |
Medchemcomm 3: 56-60 (2012)
Article DOI: 10.1039/c1md00200g
BindingDB Entry DOI: 10.7270/Q2XP77XJ |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50496732
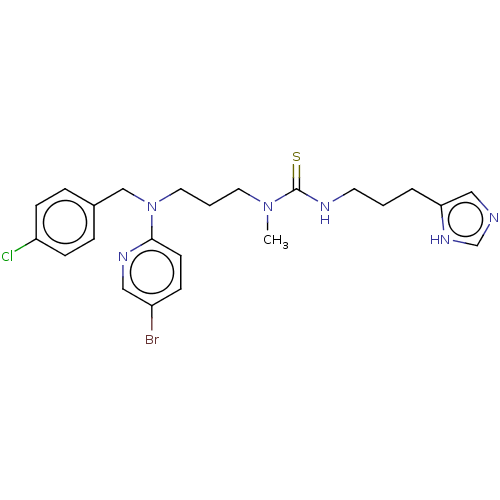
(CHEMBL1591268)Show SMILES CN(CCCN(Cc1ccc(Cl)cc1)c1ccc(Br)cn1)C(=S)NCCCc1cnc[nH]1 Show InChI InChI=1S/C23H28BrClN6S/c1-30(23(32)27-11-2-4-21-15-26-17-29-21)12-3-13-31(22-10-7-19(24)14-28-22)16-18-5-8-20(25)9-6-18/h5-10,14-15,17H,2-4,11-13,16H2,1H3,(H,26,29)(H,27,32) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human SST4 receptor |
Medchemcomm 3: 56-60 (2012)
Article DOI: 10.1039/c1md00200g
BindingDB Entry DOI: 10.7270/Q2XP77XJ |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50496735

(CHEMBL1355048)Show SMILES CN(CCCc1cnc[nH]1)C(=S)NCCCN(Cc1ccc(Cl)cc1)c1ccc(Br)cn1 Show InChI InChI=1S/C23H28BrClN6S/c1-30(12-2-4-21-15-26-17-29-21)23(32)27-11-3-13-31(22-10-7-19(24)14-28-22)16-18-5-8-20(25)9-6-18/h5-10,14-15,17H,2-4,11-13,16H2,1H3,(H,26,29)(H,27,32) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human SST4 receptor |
Medchemcomm 3: 56-60 (2012)
Article DOI: 10.1039/c1md00200g
BindingDB Entry DOI: 10.7270/Q2XP77XJ |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50496733
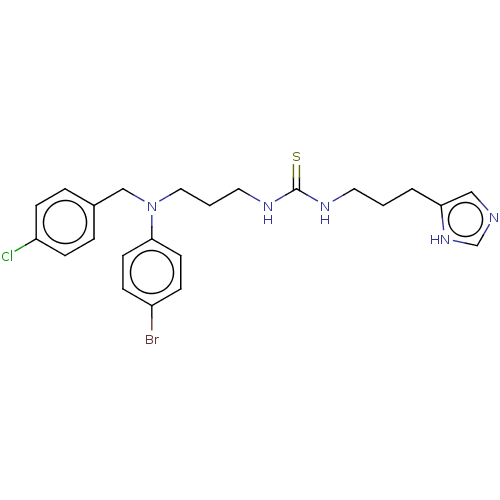
(CHEMBL1591395)Show SMILES Clc1ccc(CN(CCCNC(=S)NCCCc2cnc[nH]2)c2ccc(Br)cc2)cc1 Show InChI InChI=1S/C23H27BrClN5S/c24-19-6-10-22(11-7-19)30(16-18-4-8-20(25)9-5-18)14-2-13-28-23(31)27-12-1-3-21-15-26-17-29-21/h4-11,15,17H,1-3,12-14,16H2,(H,26,29)(H2,27,28,31) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human SST4 receptor |
Medchemcomm 3: 56-60 (2012)
Article DOI: 10.1039/c1md00200g
BindingDB Entry DOI: 10.7270/Q2XP77XJ |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(Homo sapiens (Human)) | BDBM50496734

(CHEMBL1590268)Show InChI InChI=1S/C15H21BrN6S/c16-12-4-5-14(21-9-12)18-7-2-8-20-15(23)19-6-1-3-13-10-17-11-22-13/h4-5,9-11H,1-3,6-8H2,(H,17,22)(H,18,21)(H2,19,20,23) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to human SST4 receptor |
Medchemcomm 3: 56-60 (2012)
Article DOI: 10.1039/c1md00200g
BindingDB Entry DOI: 10.7270/Q2XP77XJ |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50042857
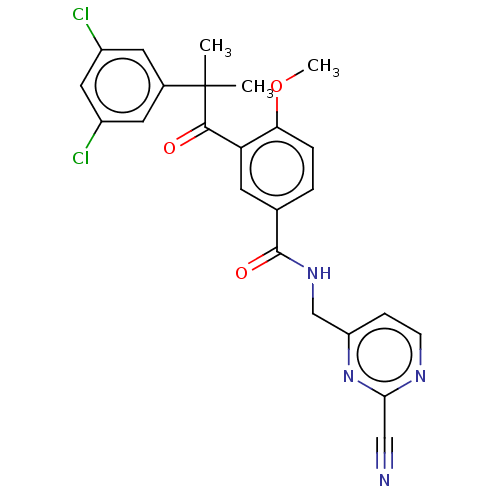
(CHEMBL3354494)Show SMILES COc1ccc(cc1C(=O)C(C)(C)c1cc(Cl)cc(Cl)c1)C(=O)NCc1ccnc(n1)C#N Show InChI InChI=1S/C24H20Cl2N4O3/c1-24(2,15-9-16(25)11-17(26)10-15)22(31)19-8-14(4-5-20(19)33-3)23(32)29-13-18-6-7-28-21(12-27)30-18/h4-11H,13H2,1-3H3,(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Cathepsin S using fluorogenic peptide substrate |
Bioorg Med Chem Lett 25: 438-43 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.057
BindingDB Entry DOI: 10.7270/Q20G3MSB |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50042857

(CHEMBL3354494)Show SMILES COc1ccc(cc1C(=O)C(C)(C)c1cc(Cl)cc(Cl)c1)C(=O)NCc1ccnc(n1)C#N Show InChI InChI=1S/C24H20Cl2N4O3/c1-24(2,15-9-16(25)11-17(26)10-15)22(31)19-8-14(4-5-20(19)33-3)23(32)29-13-18-6-7-28-21(12-27)30-18/h4-11H,13H2,1-3H3,(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Cathepsin L using fluorogenic peptide substrate |
Bioorg Med Chem Lett 25: 438-43 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.057
BindingDB Entry DOI: 10.7270/Q20G3MSB |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50042857

(CHEMBL3354494)Show SMILES COc1ccc(cc1C(=O)C(C)(C)c1cc(Cl)cc(Cl)c1)C(=O)NCc1ccnc(n1)C#N Show InChI InChI=1S/C24H20Cl2N4O3/c1-24(2,15-9-16(25)11-17(26)10-15)22(31)19-8-14(4-5-20(19)33-3)23(32)29-13-18-6-7-28-21(12-27)30-18/h4-11H,13H2,1-3H3,(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Cathepsin B using fluorogenic peptide substrate |
Bioorg Med Chem Lett 25: 438-43 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.057
BindingDB Entry DOI: 10.7270/Q20G3MSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50042857

(CHEMBL3354494)Show SMILES COc1ccc(cc1C(=O)C(C)(C)c1cc(Cl)cc(Cl)c1)C(=O)NCc1ccnc(n1)C#N Show InChI InChI=1S/C24H20Cl2N4O3/c1-24(2,15-9-16(25)11-17(26)10-15)22(31)19-8-14(4-5-20(19)33-3)23(32)29-13-18-6-7-28-21(12-27)30-18/h4-11H,13H2,1-3H3,(H,29,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Cathepsin K using fluorogenic peptide substrate |
Bioorg Med Chem Lett 25: 438-43 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.057
BindingDB Entry DOI: 10.7270/Q20G3MSB |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50042856
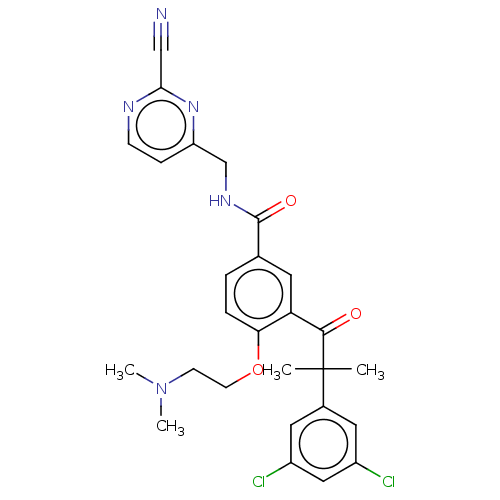
(CHEMBL3354495)Show SMILES CN(C)CCOc1ccc(cc1C(=O)C(C)(C)c1cc(Cl)cc(Cl)c1)C(=O)NCc1ccnc(n1)C#N Show InChI InChI=1S/C27H27Cl2N5O3/c1-27(2,18-12-19(28)14-20(29)13-18)25(35)22-11-17(5-6-23(22)37-10-9-34(3)4)26(36)32-16-21-7-8-31-24(15-30)33-21/h5-8,11-14H,9-10,16H2,1-4H3,(H,32,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Cathepsin L using fluorogenic peptide substrate |
Bioorg Med Chem Lett 25: 438-43 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.057
BindingDB Entry DOI: 10.7270/Q20G3MSB |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM50042856

(CHEMBL3354495)Show SMILES CN(C)CCOc1ccc(cc1C(=O)C(C)(C)c1cc(Cl)cc(Cl)c1)C(=O)NCc1ccnc(n1)C#N Show InChI InChI=1S/C27H27Cl2N5O3/c1-27(2,18-12-19(28)14-20(29)13-18)25(35)22-11-17(5-6-23(22)37-10-9-34(3)4)26(36)32-16-21-7-8-31-24(15-30)33-21/h5-8,11-14H,9-10,16H2,1-4H3,(H,32,36) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Cathepsin C using fluorogenic peptide substrate |
Bioorg Med Chem Lett 25: 438-43 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.057
BindingDB Entry DOI: 10.7270/Q20G3MSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50042856

(CHEMBL3354495)Show SMILES CN(C)CCOc1ccc(cc1C(=O)C(C)(C)c1cc(Cl)cc(Cl)c1)C(=O)NCc1ccnc(n1)C#N Show InChI InChI=1S/C27H27Cl2N5O3/c1-27(2,18-12-19(28)14-20(29)13-18)25(35)22-11-17(5-6-23(22)37-10-9-34(3)4)26(36)32-16-21-7-8-31-24(15-30)33-21/h5-8,11-14H,9-10,16H2,1-4H3,(H,32,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Cathepsin K using fluorogenic peptide substrate |
Bioorg Med Chem Lett 25: 438-43 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.057
BindingDB Entry DOI: 10.7270/Q20G3MSB |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50042856

(CHEMBL3354495)Show SMILES CN(C)CCOc1ccc(cc1C(=O)C(C)(C)c1cc(Cl)cc(Cl)c1)C(=O)NCc1ccnc(n1)C#N Show InChI InChI=1S/C27H27Cl2N5O3/c1-27(2,18-12-19(28)14-20(29)13-18)25(35)22-11-17(5-6-23(22)37-10-9-34(3)4)26(36)32-16-21-7-8-31-24(15-30)33-21/h5-8,11-14H,9-10,16H2,1-4H3,(H,32,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Cathepsin S using fluorogenic peptide substrate |
Bioorg Med Chem Lett 25: 438-43 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.057
BindingDB Entry DOI: 10.7270/Q20G3MSB |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM50042857

(CHEMBL3354494)Show SMILES COc1ccc(cc1C(=O)C(C)(C)c1cc(Cl)cc(Cl)c1)C(=O)NCc1ccnc(n1)C#N Show InChI InChI=1S/C24H20Cl2N4O3/c1-24(2,15-9-16(25)11-17(26)10-15)22(31)19-8-14(4-5-20(19)33-3)23(32)29-13-18-6-7-28-21(12-27)30-18/h4-11H,13H2,1-3H3,(H,29,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Cathepsin C using fluorogenic peptide substrate |
Bioorg Med Chem Lett 25: 438-43 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.057
BindingDB Entry DOI: 10.7270/Q20G3MSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50042852
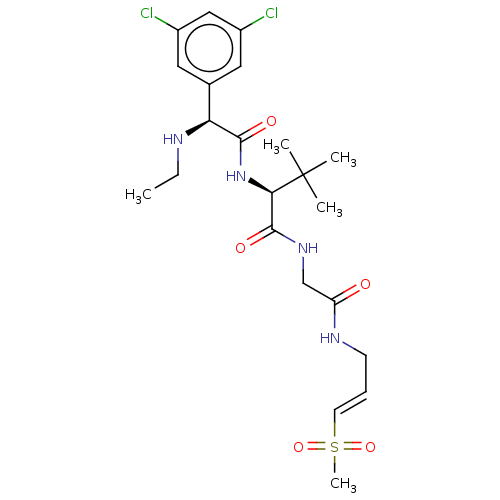
(CHEMBL3354498)Show SMILES CCN[C@H](C(=O)N[C@H](C(=O)NCC(=O)NC\C=C\S(C)(=O)=O)C(C)(C)C)c1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C22H32Cl2N4O5S/c1-6-25-18(14-10-15(23)12-16(24)11-14)20(30)28-19(22(2,3)4)21(31)27-13-17(29)26-8-7-9-34(5,32)33/h7,9-12,18-19,25H,6,8,13H2,1-5H3,(H,26,29)(H,27,31)(H,28,30)/b9-7+/t18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Cathepsin K using fluorogenic peptide substrate |
Bioorg Med Chem Lett 25: 438-43 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.057
BindingDB Entry DOI: 10.7270/Q20G3MSB |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50042856

(CHEMBL3354495)Show SMILES CN(C)CCOc1ccc(cc1C(=O)C(C)(C)c1cc(Cl)cc(Cl)c1)C(=O)NCc1ccnc(n1)C#N Show InChI InChI=1S/C27H27Cl2N5O3/c1-27(2,18-12-19(28)14-20(29)13-18)25(35)22-11-17(5-6-23(22)37-10-9-34(3)4)26(36)32-16-21-7-8-31-24(15-30)33-21/h5-8,11-14H,9-10,16H2,1-4H3,(H,32,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Cathepsin B using fluorogenic peptide substrate |
Bioorg Med Chem Lett 25: 438-43 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.057
BindingDB Entry DOI: 10.7270/Q20G3MSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50042854
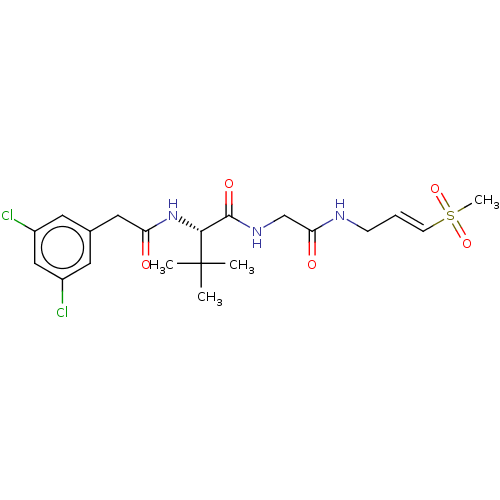
(CHEMBL3354497)Show SMILES CC(C)(C)[C@H](NC(=O)Cc1cc(Cl)cc(Cl)c1)C(=O)NCC(=O)NC\C=C\S(C)(=O)=O |r| Show InChI InChI=1S/C20H27Cl2N3O5S/c1-20(2,3)18(25-16(26)10-13-8-14(21)11-15(22)9-13)19(28)24-12-17(27)23-6-5-7-31(4,29)30/h5,7-9,11,18H,6,10,12H2,1-4H3,(H,23,27)(H,24,28)(H,25,26)/b7-5+/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Cathepsin K using fluorogenic peptide substrate |
Bioorg Med Chem Lett 25: 438-43 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.057
BindingDB Entry DOI: 10.7270/Q20G3MSB |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM50042852

(CHEMBL3354498)Show SMILES CCN[C@H](C(=O)N[C@H](C(=O)NCC(=O)NC\C=C\S(C)(=O)=O)C(C)(C)C)c1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C22H32Cl2N4O5S/c1-6-25-18(14-10-15(23)12-16(24)11-14)20(30)28-19(22(2,3)4)21(31)27-13-17(29)26-8-7-9-34(5,32)33/h7,9-12,18-19,25H,6,8,13H2,1-5H3,(H,26,29)(H,27,31)(H,28,30)/b9-7+/t18-,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Cathepsin C using fluorogenic peptide substrate |
Bioorg Med Chem Lett 25: 438-43 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.057
BindingDB Entry DOI: 10.7270/Q20G3MSB |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM50042854

(CHEMBL3354497)Show SMILES CC(C)(C)[C@H](NC(=O)Cc1cc(Cl)cc(Cl)c1)C(=O)NCC(=O)NC\C=C\S(C)(=O)=O |r| Show InChI InChI=1S/C20H27Cl2N3O5S/c1-20(2,3)18(25-16(26)10-13-8-14(21)11-15(22)9-13)19(28)24-12-17(27)23-6-5-7-31(4,29)30/h5,7-9,11,18H,6,10,12H2,1-4H3,(H,23,27)(H,24,28)(H,25,26)/b7-5+/t18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Cathepsin C using fluorogenic peptide substrate |
Bioorg Med Chem Lett 25: 438-43 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.057
BindingDB Entry DOI: 10.7270/Q20G3MSB |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM50042855
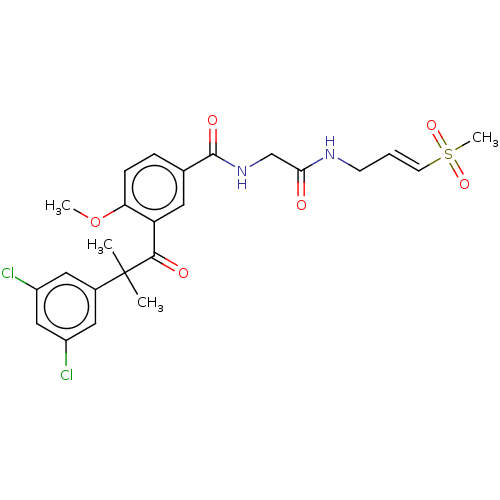
(CHEMBL3354496)Show SMILES COc1ccc(cc1C(=O)C(C)(C)c1cc(Cl)cc(Cl)c1)C(=O)NCC(=O)NC\C=C\S(C)(=O)=O Show InChI InChI=1S/C24H26Cl2N2O6S/c1-24(2,16-11-17(25)13-18(26)12-16)22(30)19-10-15(6-7-20(19)34-3)23(31)28-14-21(29)27-8-5-9-35(4,32)33/h5-7,9-13H,8,14H2,1-4H3,(H,27,29)(H,28,31)/b9-5+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Cathepsin C using fluorogenic peptide substrate |
Bioorg Med Chem Lett 25: 438-43 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.057
BindingDB Entry DOI: 10.7270/Q20G3MSB |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM50042858
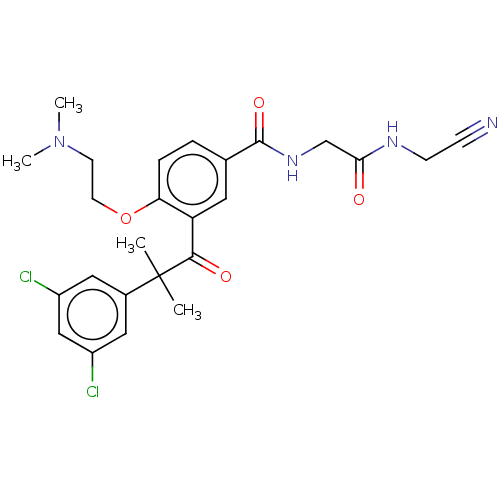
(CHEMBL3354493)Show SMILES CN(C)CCOc1ccc(cc1C(=O)C(C)(C)c1cc(Cl)cc(Cl)c1)C(=O)NCC(=O)NCC#N Show InChI InChI=1S/C25H28Cl2N4O4/c1-25(2,17-12-18(26)14-19(27)13-17)23(33)20-11-16(5-6-21(20)35-10-9-31(3)4)24(34)30-15-22(32)29-8-7-28/h5-6,11-14H,8-10,15H2,1-4H3,(H,29,32)(H,30,34) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Cathepsin C using fluorogenic peptide substrate |
Bioorg Med Chem Lett 25: 438-43 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.057
BindingDB Entry DOI: 10.7270/Q20G3MSB |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM50042850
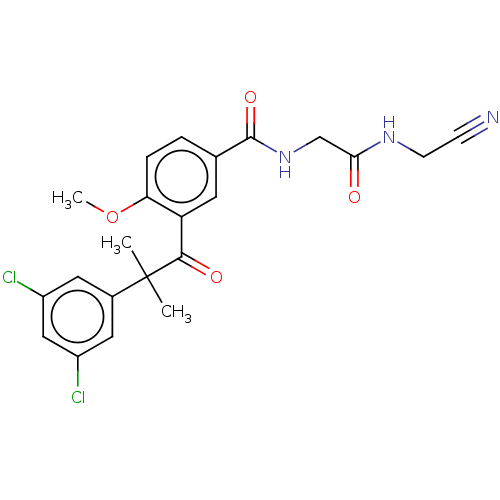
(CHEMBL3354492)Show SMILES COc1ccc(cc1C(=O)C(C)(C)c1cc(Cl)cc(Cl)c1)C(=O)NCC(=O)NCC#N Show InChI InChI=1S/C22H21Cl2N3O4/c1-22(2,14-9-15(23)11-16(24)10-14)20(29)17-8-13(4-5-18(17)31-3)21(30)27-12-19(28)26-7-6-25/h4-5,8-11H,7,12H2,1-3H3,(H,26,28)(H,27,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Cathepsin C using fluorogenic peptide substrate |
Bioorg Med Chem Lett 25: 438-43 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.057
BindingDB Entry DOI: 10.7270/Q20G3MSB |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50042851
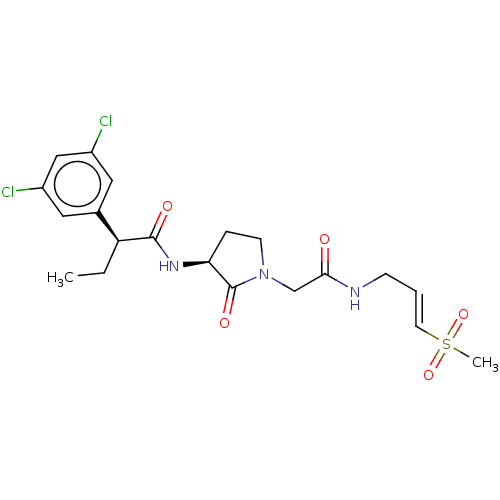
(CHEMBL3354499)Show SMILES CC[C@H](C(=O)N[C@H]1CCN(CC(=O)NC\C=C\S(C)(=O)=O)C1=O)c1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C20H25Cl2N3O5S/c1-3-16(13-9-14(21)11-15(22)10-13)19(27)24-17-5-7-25(20(17)28)12-18(26)23-6-4-8-31(2,29)30/h4,8-11,16-17H,3,5-7,12H2,1-2H3,(H,23,26)(H,24,27)/b8-4+/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Cathepsin B using fluorogenic peptide substrate |
Bioorg Med Chem Lett 25: 438-43 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.057
BindingDB Entry DOI: 10.7270/Q20G3MSB |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50042852

(CHEMBL3354498)Show SMILES CCN[C@H](C(=O)N[C@H](C(=O)NCC(=O)NC\C=C\S(C)(=O)=O)C(C)(C)C)c1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C22H32Cl2N4O5S/c1-6-25-18(14-10-15(23)12-16(24)11-14)20(30)28-19(22(2,3)4)21(31)27-13-17(29)26-8-7-9-34(5,32)33/h7,9-12,18-19,25H,6,8,13H2,1-5H3,(H,26,29)(H,27,31)(H,28,30)/b9-7+/t18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Cathepsin B using fluorogenic peptide substrate |
Bioorg Med Chem Lett 25: 438-43 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.057
BindingDB Entry DOI: 10.7270/Q20G3MSB |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50042854

(CHEMBL3354497)Show SMILES CC(C)(C)[C@H](NC(=O)Cc1cc(Cl)cc(Cl)c1)C(=O)NCC(=O)NC\C=C\S(C)(=O)=O |r| Show InChI InChI=1S/C20H27Cl2N3O5S/c1-20(2,3)18(25-16(26)10-13-8-14(21)11-15(22)9-13)19(28)24-12-17(27)23-6-5-7-31(4,29)30/h5,7-9,11,18H,6,10,12H2,1-4H3,(H,23,27)(H,24,28)(H,25,26)/b7-5+/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Cathepsin B using fluorogenic peptide substrate |
Bioorg Med Chem Lett 25: 438-43 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.057
BindingDB Entry DOI: 10.7270/Q20G3MSB |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50042855

(CHEMBL3354496)Show SMILES COc1ccc(cc1C(=O)C(C)(C)c1cc(Cl)cc(Cl)c1)C(=O)NCC(=O)NC\C=C\S(C)(=O)=O Show InChI InChI=1S/C24H26Cl2N2O6S/c1-24(2,16-11-17(25)13-18(26)12-16)22(30)19-10-15(6-7-20(19)34-3)23(31)28-14-21(29)27-8-5-9-35(4,32)33/h5-7,9-13H,8,14H2,1-4H3,(H,27,29)(H,28,31)/b9-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Cathepsin B using fluorogenic peptide substrate |
Bioorg Med Chem Lett 25: 438-43 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.057
BindingDB Entry DOI: 10.7270/Q20G3MSB |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50042858

(CHEMBL3354493)Show SMILES CN(C)CCOc1ccc(cc1C(=O)C(C)(C)c1cc(Cl)cc(Cl)c1)C(=O)NCC(=O)NCC#N Show InChI InChI=1S/C25H28Cl2N4O4/c1-25(2,17-12-18(26)14-19(27)13-17)23(33)20-11-16(5-6-21(20)35-10-9-31(3)4)24(34)30-15-22(32)29-8-7-28/h5-6,11-14H,8-10,15H2,1-4H3,(H,29,32)(H,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Cathepsin B using fluorogenic peptide substrate |
Bioorg Med Chem Lett 25: 438-43 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.057
BindingDB Entry DOI: 10.7270/Q20G3MSB |
More data for this
Ligand-Target Pair | |
Dipeptidyl peptidase 1
(Homo sapiens (Human)) | BDBM50042851

(CHEMBL3354499)Show SMILES CC[C@H](C(=O)N[C@H]1CCN(CC(=O)NC\C=C\S(C)(=O)=O)C1=O)c1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C20H25Cl2N3O5S/c1-3-16(13-9-14(21)11-15(22)10-13)19(27)24-17-5-7-25(20(17)28)12-18(26)23-6-4-8-31(2,29)30/h4,8-11,16-17H,3,5-7,12H2,1-2H3,(H,23,26)(H,24,27)/b8-4+/t16-,17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Cathepsin C using fluorogenic peptide substrate |
Bioorg Med Chem Lett 25: 438-43 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.057
BindingDB Entry DOI: 10.7270/Q20G3MSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50042850

(CHEMBL3354492)Show SMILES COc1ccc(cc1C(=O)C(C)(C)c1cc(Cl)cc(Cl)c1)C(=O)NCC(=O)NCC#N Show InChI InChI=1S/C22H21Cl2N3O4/c1-22(2,14-9-15(23)11-16(24)10-14)20(29)17-8-13(4-5-18(17)31-3)21(30)27-12-19(28)26-7-6-25/h4-5,8-11H,7,12H2,1-3H3,(H,26,28)(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Cathepsin K using fluorogenic peptide substrate |
Bioorg Med Chem Lett 25: 438-43 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.057
BindingDB Entry DOI: 10.7270/Q20G3MSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50042858

(CHEMBL3354493)Show SMILES CN(C)CCOc1ccc(cc1C(=O)C(C)(C)c1cc(Cl)cc(Cl)c1)C(=O)NCC(=O)NCC#N Show InChI InChI=1S/C25H28Cl2N4O4/c1-25(2,17-12-18(26)14-19(27)13-17)23(33)20-11-16(5-6-21(20)35-10-9-31(3)4)24(34)30-15-22(32)29-8-7-28/h5-6,11-14H,8-10,15H2,1-4H3,(H,29,32)(H,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Cathepsin K using fluorogenic peptide substrate |
Bioorg Med Chem Lett 25: 438-43 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.057
BindingDB Entry DOI: 10.7270/Q20G3MSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50042855

(CHEMBL3354496)Show SMILES COc1ccc(cc1C(=O)C(C)(C)c1cc(Cl)cc(Cl)c1)C(=O)NCC(=O)NC\C=C\S(C)(=O)=O Show InChI InChI=1S/C24H26Cl2N2O6S/c1-24(2,16-11-17(25)13-18(26)12-16)22(30)19-10-15(6-7-20(19)34-3)23(31)28-14-21(29)27-8-5-9-35(4,32)33/h5-7,9-13H,8,14H2,1-4H3,(H,27,29)(H,28,31)/b9-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Cathepsin K using fluorogenic peptide substrate |
Bioorg Med Chem Lett 25: 438-43 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.057
BindingDB Entry DOI: 10.7270/Q20G3MSB |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM50042851

(CHEMBL3354499)Show SMILES CC[C@H](C(=O)N[C@H]1CCN(CC(=O)NC\C=C\S(C)(=O)=O)C1=O)c1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C20H25Cl2N3O5S/c1-3-16(13-9-14(21)11-15(22)10-13)19(27)24-17-5-7-25(20(17)28)12-18(26)23-6-4-8-31(2,29)30/h4,8-11,16-17H,3,5-7,12H2,1-2H3,(H,23,26)(H,24,27)/b8-4+/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Cathepsin K using fluorogenic peptide substrate |
Bioorg Med Chem Lett 25: 438-43 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.057
BindingDB Entry DOI: 10.7270/Q20G3MSB |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50042850

(CHEMBL3354492)Show SMILES COc1ccc(cc1C(=O)C(C)(C)c1cc(Cl)cc(Cl)c1)C(=O)NCC(=O)NCC#N Show InChI InChI=1S/C22H21Cl2N3O4/c1-22(2,14-9-15(23)11-16(24)10-14)20(29)17-8-13(4-5-18(17)31-3)21(30)27-12-19(28)26-7-6-25/h4-5,8-11H,7,12H2,1-3H3,(H,26,28)(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Cathepsin L using fluorogenic peptide substrate |
Bioorg Med Chem Lett 25: 438-43 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.057
BindingDB Entry DOI: 10.7270/Q20G3MSB |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50042858

(CHEMBL3354493)Show SMILES CN(C)CCOc1ccc(cc1C(=O)C(C)(C)c1cc(Cl)cc(Cl)c1)C(=O)NCC(=O)NCC#N Show InChI InChI=1S/C25H28Cl2N4O4/c1-25(2,17-12-18(26)14-19(27)13-17)23(33)20-11-16(5-6-21(20)35-10-9-31(3)4)24(34)30-15-22(32)29-8-7-28/h5-6,11-14H,8-10,15H2,1-4H3,(H,29,32)(H,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Cathepsin L using fluorogenic peptide substrate |
Bioorg Med Chem Lett 25: 438-43 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.057
BindingDB Entry DOI: 10.7270/Q20G3MSB |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50042855

(CHEMBL3354496)Show SMILES COc1ccc(cc1C(=O)C(C)(C)c1cc(Cl)cc(Cl)c1)C(=O)NCC(=O)NC\C=C\S(C)(=O)=O Show InChI InChI=1S/C24H26Cl2N2O6S/c1-24(2,16-11-17(25)13-18(26)12-16)22(30)19-10-15(6-7-20(19)34-3)23(31)28-14-21(29)27-8-5-9-35(4,32)33/h5-7,9-13H,8,14H2,1-4H3,(H,27,29)(H,28,31)/b9-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Cathepsin L using fluorogenic peptide substrate |
Bioorg Med Chem Lett 25: 438-43 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.057
BindingDB Entry DOI: 10.7270/Q20G3MSB |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50042854

(CHEMBL3354497)Show SMILES CC(C)(C)[C@H](NC(=O)Cc1cc(Cl)cc(Cl)c1)C(=O)NCC(=O)NC\C=C\S(C)(=O)=O |r| Show InChI InChI=1S/C20H27Cl2N3O5S/c1-20(2,3)18(25-16(26)10-13-8-14(21)11-15(22)9-13)19(28)24-12-17(27)23-6-5-7-31(4,29)30/h5,7-9,11,18H,6,10,12H2,1-4H3,(H,23,27)(H,24,28)(H,25,26)/b7-5+/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Cathepsin L using fluorogenic peptide substrate |
Bioorg Med Chem Lett 25: 438-43 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.057
BindingDB Entry DOI: 10.7270/Q20G3MSB |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50042852

(CHEMBL3354498)Show SMILES CCN[C@H](C(=O)N[C@H](C(=O)NCC(=O)NC\C=C\S(C)(=O)=O)C(C)(C)C)c1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C22H32Cl2N4O5S/c1-6-25-18(14-10-15(23)12-16(24)11-14)20(30)28-19(22(2,3)4)21(31)27-13-17(29)26-8-7-9-34(5,32)33/h7,9-12,18-19,25H,6,8,13H2,1-5H3,(H,26,29)(H,27,31)(H,28,30)/b9-7+/t18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Cathepsin L using fluorogenic peptide substrate |
Bioorg Med Chem Lett 25: 438-43 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.057
BindingDB Entry DOI: 10.7270/Q20G3MSB |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM50042851

(CHEMBL3354499)Show SMILES CC[C@H](C(=O)N[C@H]1CCN(CC(=O)NC\C=C\S(C)(=O)=O)C1=O)c1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C20H25Cl2N3O5S/c1-3-16(13-9-14(21)11-15(22)10-13)19(27)24-17-5-7-25(20(17)28)12-18(26)23-6-4-8-31(2,29)30/h4,8-11,16-17H,3,5-7,12H2,1-2H3,(H,23,26)(H,24,27)/b8-4+/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Cathepsin L using fluorogenic peptide substrate |
Bioorg Med Chem Lett 25: 438-43 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.057
BindingDB Entry DOI: 10.7270/Q20G3MSB |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50042850

(CHEMBL3354492)Show SMILES COc1ccc(cc1C(=O)C(C)(C)c1cc(Cl)cc(Cl)c1)C(=O)NCC(=O)NCC#N Show InChI InChI=1S/C22H21Cl2N3O4/c1-22(2,14-9-15(23)11-16(24)10-14)20(29)17-8-13(4-5-18(17)31-3)21(30)27-12-19(28)26-7-6-25/h4-5,8-11H,7,12H2,1-3H3,(H,26,28)(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Cathepsin S using fluorogenic peptide substrate |
Bioorg Med Chem Lett 25: 438-43 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.057
BindingDB Entry DOI: 10.7270/Q20G3MSB |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50042858

(CHEMBL3354493)Show SMILES CN(C)CCOc1ccc(cc1C(=O)C(C)(C)c1cc(Cl)cc(Cl)c1)C(=O)NCC(=O)NCC#N Show InChI InChI=1S/C25H28Cl2N4O4/c1-25(2,17-12-18(26)14-19(27)13-17)23(33)20-11-16(5-6-21(20)35-10-9-31(3)4)24(34)30-15-22(32)29-8-7-28/h5-6,11-14H,8-10,15H2,1-4H3,(H,29,32)(H,30,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Cathepsin S using fluorogenic peptide substrate |
Bioorg Med Chem Lett 25: 438-43 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.057
BindingDB Entry DOI: 10.7270/Q20G3MSB |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50042855

(CHEMBL3354496)Show SMILES COc1ccc(cc1C(=O)C(C)(C)c1cc(Cl)cc(Cl)c1)C(=O)NCC(=O)NC\C=C\S(C)(=O)=O Show InChI InChI=1S/C24H26Cl2N2O6S/c1-24(2,16-11-17(25)13-18(26)12-16)22(30)19-10-15(6-7-20(19)34-3)23(31)28-14-21(29)27-8-5-9-35(4,32)33/h5-7,9-13H,8,14H2,1-4H3,(H,27,29)(H,28,31)/b9-5+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Cathepsin S using fluorogenic peptide substrate |
Bioorg Med Chem Lett 25: 438-43 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.057
BindingDB Entry DOI: 10.7270/Q20G3MSB |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50042854

(CHEMBL3354497)Show SMILES CC(C)(C)[C@H](NC(=O)Cc1cc(Cl)cc(Cl)c1)C(=O)NCC(=O)NC\C=C\S(C)(=O)=O |r| Show InChI InChI=1S/C20H27Cl2N3O5S/c1-20(2,3)18(25-16(26)10-13-8-14(21)11-15(22)9-13)19(28)24-12-17(27)23-6-5-7-31(4,29)30/h5,7-9,11,18H,6,10,12H2,1-4H3,(H,23,27)(H,24,28)(H,25,26)/b7-5+/t18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Cathepsin S using fluorogenic peptide substrate |
Bioorg Med Chem Lett 25: 438-43 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.057
BindingDB Entry DOI: 10.7270/Q20G3MSB |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50042852

(CHEMBL3354498)Show SMILES CCN[C@H](C(=O)N[C@H](C(=O)NCC(=O)NC\C=C\S(C)(=O)=O)C(C)(C)C)c1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C22H32Cl2N4O5S/c1-6-25-18(14-10-15(23)12-16(24)11-14)20(30)28-19(22(2,3)4)21(31)27-13-17(29)26-8-7-9-34(5,32)33/h7,9-12,18-19,25H,6,8,13H2,1-5H3,(H,26,29)(H,27,31)(H,28,30)/b9-7+/t18-,19+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Cathepsin S using fluorogenic peptide substrate |
Bioorg Med Chem Lett 25: 438-43 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.057
BindingDB Entry DOI: 10.7270/Q20G3MSB |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM50042850

(CHEMBL3354492)Show SMILES COc1ccc(cc1C(=O)C(C)(C)c1cc(Cl)cc(Cl)c1)C(=O)NCC(=O)NCC#N Show InChI InChI=1S/C22H21Cl2N3O4/c1-22(2,14-9-15(23)11-16(24)10-14)20(29)17-8-13(4-5-18(17)31-3)21(30)27-12-19(28)26-7-6-25/h4-5,8-11H,7,12H2,1-3H3,(H,26,28)(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Cathepsin B using fluorogenic peptide substrate |
Bioorg Med Chem Lett 25: 438-43 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.057
BindingDB Entry DOI: 10.7270/Q20G3MSB |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM50042851

(CHEMBL3354499)Show SMILES CC[C@H](C(=O)N[C@H]1CCN(CC(=O)NC\C=C\S(C)(=O)=O)C1=O)c1cc(Cl)cc(Cl)c1 |r| Show InChI InChI=1S/C20H25Cl2N3O5S/c1-3-16(13-9-14(21)11-15(22)10-13)19(27)24-17-5-7-25(20(17)28)12-18(26)23-6-4-8-31(2,29)30/h4,8-11,16-17H,3,5-7,12H2,1-2H3,(H,23,26)(H,24,27)/b8-4+/t16-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Cathepsin S using fluorogenic peptide substrate |
Bioorg Med Chem Lett 25: 438-43 (2015)
Article DOI: 10.1016/j.bmcl.2014.12.057
BindingDB Entry DOI: 10.7270/Q20G3MSB |
More data for this
Ligand-Target Pair | |
CD209 antigen
(Homo sapiens (Human)) | BDBM50260173
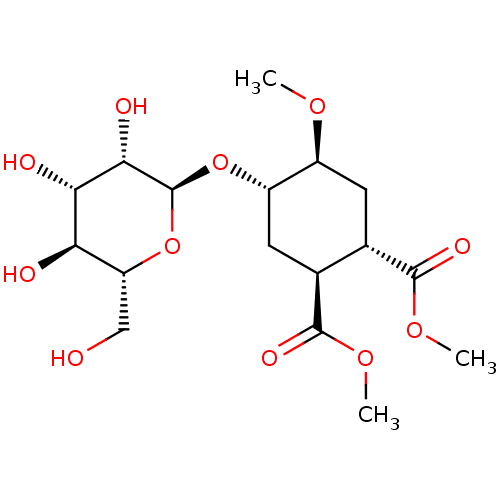
(CHEMBL4095576)Show SMILES [H][C@@]1(C[C@@H]([C@H](C[C@@H]1OC)C(=O)OC)C(=O)OC)O[C@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@@H]1O |r| Show InChI InChI=1S/C17H28O11/c1-24-9-4-7(15(22)25-2)8(16(23)26-3)5-10(9)27-17-14(21)13(20)12(19)11(6-18)28-17/h7-14,17-21H,4-6H2,1-3H3/t7-,8-,9-,10-,11+,12+,13-,14-,17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.20E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Milano
Curated by ChEMBL
| Assay Description
Displacement of [3H]R-1881 from Androgen receptor of PC3-AR cells |
Bioorg Med Chem 25: 5142-5147 (2017)
Article DOI: 10.1016/j.bmc.2017.03.046
BindingDB Entry DOI: 10.7270/Q2319ZBC |
More data for this
Ligand-Target Pair | |
CD209 antigen
(Homo sapiens (Human)) | BDBM50260172
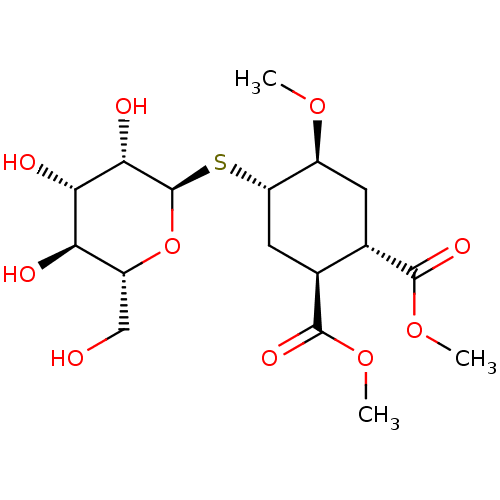
(CHEMBL4068200)Show SMILES [H][C@@]1(C[C@@H]([C@H](C[C@@H]1OC)C(=O)OC)C(=O)OC)S[C@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@@H]1O |r| Show InChI InChI=1S/C17H28O10S/c1-24-9-4-7(15(22)25-2)8(16(23)26-3)5-11(9)28-17-14(21)13(20)12(19)10(6-18)27-17/h7-14,17-21H,4-6H2,1-3H3/t7-,8-,9-,10+,11-,12+,13-,14-,17+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.76E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Milano
Curated by ChEMBL
| Assay Description
Inhibition of binding of immobilized mannosylated BSA to DC-SIGN extracellular domain (66 to 404 residues) (unknown origin) expressed in Escherichia ... |
Bioorg Med Chem 25: 5142-5147 (2017)
Article DOI: 10.1016/j.bmc.2017.03.046
BindingDB Entry DOI: 10.7270/Q2319ZBC |
More data for this
Ligand-Target Pair | |
CD209 antigen
(Homo sapiens (Human)) | BDBM50260174
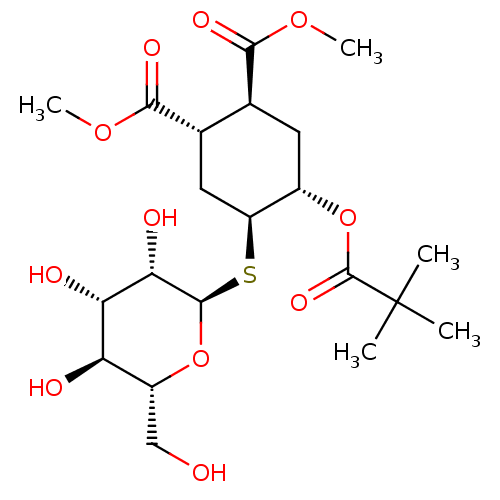
(CHEMBL4061670)Show SMILES [H][C@@]1(C[C@@H]([C@H](C[C@@H]1OC(=O)C(C)(C)C)C(=O)OC)C(=O)OC)S[C@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@@H]1O |r| Show InChI InChI=1S/C21H34O11S/c1-21(2,3)20(28)32-11-6-9(17(26)29-4)10(18(27)30-5)7-13(11)33-19-16(25)15(24)14(23)12(8-22)31-19/h9-16,19,22-25H,6-8H2,1-5H3/t9-,10-,11-,12+,13-,14+,15-,16-,19+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.76E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Universita' degli Studi di Milano
Curated by ChEMBL
| Assay Description
Displacement of [3H]R-1881 from Androgen receptor of LNCaP cells |
Bioorg Med Chem 25: 5142-5147 (2017)
Article DOI: 10.1016/j.bmc.2017.03.046
BindingDB Entry DOI: 10.7270/Q2319ZBC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data