

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50553107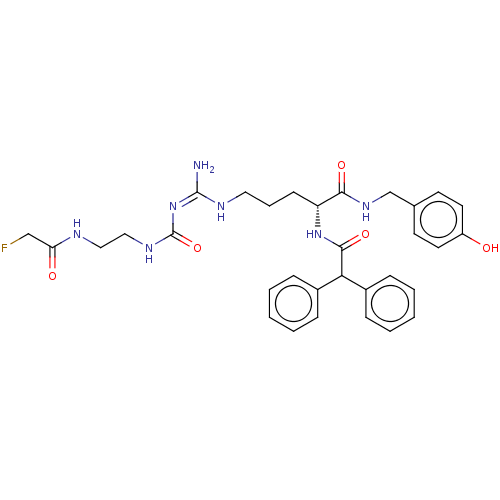 (CHEMBL4746035) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]UR-MK299 from Y1 receptor in human SK-N-MC cells by radioligand binding assay | Citation and Details Article DOI: 10.1039/c9md00538b BindingDB Entry DOI: 10.7270/Q2PZ5DGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50246649 ((R)-ethyl 2-(3-((5-(4-hydroxybenzylamino)-4-(2,2-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale) Curated by ChEMBL | Assay Description Displacement of (R)-Na-Diphenylacetyl-Nomega[2-([2,3-3H]-propionylamino)ethyl]aminocarbonyl (4-hydroxybenzyl)-argininamide from NPY1R in human SK-N-M... | J Med Chem 58: 8834-49 (2015) Article DOI: 10.1021/acs.jmedchem.5b00925 BindingDB Entry DOI: 10.7270/Q2RN3BVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50553110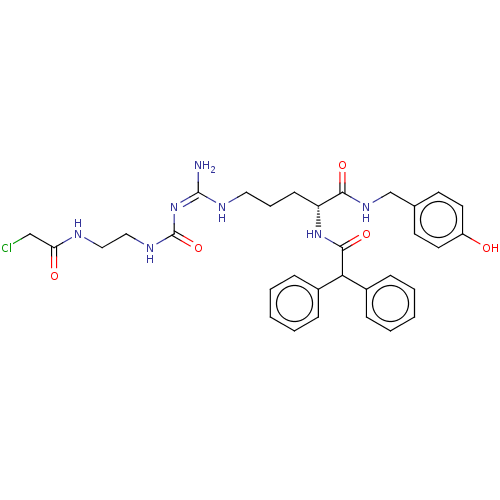 (CHEMBL4794692) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0525 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]UR-MK299 from Y1 receptor in human SK-N-MC cells by radioligand binding assay | Citation and Details Article DOI: 10.1039/c9md00538b BindingDB Entry DOI: 10.7270/Q2PZ5DGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50553104 (CHEMBL4743976) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0589 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]UR-MK299 from Y1 receptor in human SK-N-MC cells by radioligand binding assay | Citation and Details Article DOI: 10.1039/c9md00538b BindingDB Entry DOI: 10.7270/Q2PZ5DGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50246648 ((R)-1-((5-(4-hydroxybenzylamino)-4-(2,2-diphenylac...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale) Curated by ChEMBL | Assay Description Displacement of [3H]propionyl-pNPY from NPY1R (unknown origin) | J Med Chem 58: 8834-49 (2015) Article DOI: 10.1021/acs.jmedchem.5b00925 BindingDB Entry DOI: 10.7270/Q2RN3BVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50246649 ((R)-ethyl 2-(3-((5-(4-hydroxybenzylamino)-4-(2,2-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale) Curated by ChEMBL | Assay Description Displacement of [3H]propionyl-pNPY from NPY1R (unknown origin) | J Med Chem 58: 8834-49 (2015) Article DOI: 10.1021/acs.jmedchem.5b00925 BindingDB Entry DOI: 10.7270/Q2RN3BVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50525130 (CHEMBL4540949) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from human muscarinic M2 receptor expressed in CHO-K9 cells after 3 hrs by microbeta2 scintillation counting method | J Med Chem 62: 5358-5369 (2019) Article DOI: 10.1021/acs.jmedchem.8b01967 BindingDB Entry DOI: 10.7270/Q25H7KQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50241132 (3-Hydroxy-2-phenyl-propionic acid 9-methyl-3-oxa-9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H]UNSW-MK259 from human muscarinic acetylcholine receptor M2 expressed in CHOK9 cells after 3 hrs by liquid scintillation counting ... | J Med Chem 60: 3314-3334 (2017) Article DOI: 10.1021/acs.jmedchem.6b01892 BindingDB Entry DOI: 10.7270/Q29S1THC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50525115 (CHEMBL4582879) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Competitive displacement of [3H]-NMS from human muscarinic M2 receptor expressed in CHO-K9 cells using 0.2 nM [3H]-NMS after 3 hrs by microbeta2 scin... | J Med Chem 62: 5358-5369 (2019) Article DOI: 10.1021/acs.jmedchem.8b01967 BindingDB Entry DOI: 10.7270/Q25H7KQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50525115 (CHEMBL4582879) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from human muscarinic M2 receptor expressed in CHO-K9 cells after 3 hrs by microbeta2 scintillation counting method | J Med Chem 62: 5358-5369 (2019) Article DOI: 10.1021/acs.jmedchem.8b01967 BindingDB Entry DOI: 10.7270/Q25H7KQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50553105 (CHEMBL4762668) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]UR-MK299 from Y1 receptor in human SK-N-MC cells by radioligand binding assay | Citation and Details Article DOI: 10.1039/c9md00538b BindingDB Entry DOI: 10.7270/Q2PZ5DGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50553108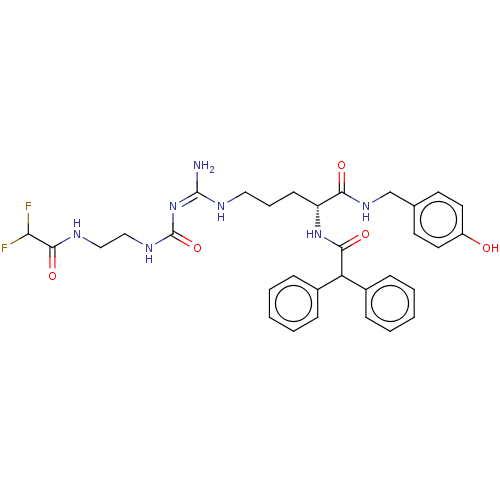 (CHEMBL4743858) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0676 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]UR-MK299 from Y1 receptor in human SK-N-MC cells by radioligand binding assay | Citation and Details Article DOI: 10.1039/c9md00538b BindingDB Entry DOI: 10.7270/Q2PZ5DGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50553109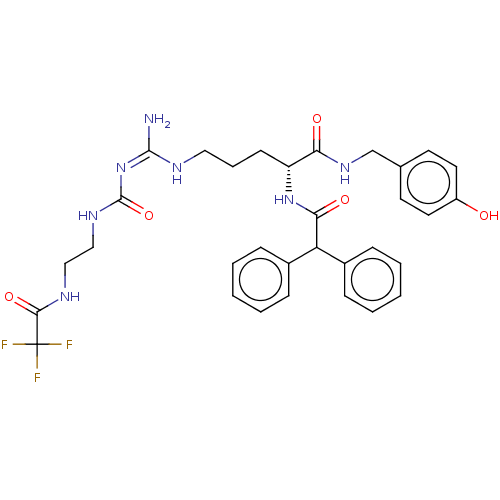 (CHEMBL4751322) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0708 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]UR-MK299 from Y1 receptor in human SK-N-MC cells by radioligand binding assay | Citation and Details Article DOI: 10.1039/c9md00538b BindingDB Entry DOI: 10.7270/Q2PZ5DGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50553106 (CHEMBL4752392) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0741 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]UR-MK299 from Y1 receptor in human SK-N-MC cells by radioligand binding assay | Citation and Details Article DOI: 10.1039/c9md00538b BindingDB Entry DOI: 10.7270/Q2PZ5DGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50500151 (CHEMBL3746386) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale) Curated by ChEMBL | Assay Description Displacement of (R)-Na-Diphenylacetyl-Nomega[2-([2,3-3H]-propionylamino)ethyl]aminocarbonyl (4-hydroxybenzyl)-argininamide from NPY1R in human SK-N-M... | J Med Chem 58: 8834-49 (2015) Article DOI: 10.1021/acs.jmedchem.5b00925 BindingDB Entry DOI: 10.7270/Q2RN3BVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50525122 (CHEMBL4589047) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from human muscarinic M2 receptor expressed in CHO-K9 cells after 3 hrs by microbeta2 scintillation counting method | J Med Chem 62: 5358-5369 (2019) Article DOI: 10.1021/acs.jmedchem.8b01967 BindingDB Entry DOI: 10.7270/Q25H7KQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50525132 (CHEMBL4451383) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from human muscarinic M2 receptor expressed in CHO-K9 cells after 3 hrs by microbeta2 scintillation counting method | J Med Chem 62: 5358-5369 (2019) Article DOI: 10.1021/acs.jmedchem.8b01967 BindingDB Entry DOI: 10.7270/Q25H7KQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50525124 (CHEMBL4569639) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from human muscarinic M2 receptor expressed in CHO-K9 cells after 3 hrs by microbeta2 scintillation counting method | J Med Chem 62: 5358-5369 (2019) Article DOI: 10.1021/acs.jmedchem.8b01967 BindingDB Entry DOI: 10.7270/Q25H7KQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50525110 (CHEMBL4455570) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from human muscarinic M2 receptor expressed in CHO-K9 cells after 3 hrs by microbeta2 scintillation counting method | J Med Chem 62: 5358-5369 (2019) Article DOI: 10.1021/acs.jmedchem.8b01967 BindingDB Entry DOI: 10.7270/Q25H7KQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50525115 (CHEMBL4582879) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Competitive displacement of [3H]-NMS from human muscarinic M2 receptor expressed in CHO-K9 cells using 2 nM [3H]-NMS after 3 hrs by microbeta2 scinti... | J Med Chem 62: 5358-5369 (2019) Article DOI: 10.1021/acs.jmedchem.8b01967 BindingDB Entry DOI: 10.7270/Q25H7KQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50246648 ((R)-1-((5-(4-hydroxybenzylamino)-4-(2,2-diphenylac...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H]UR-MK114 from neuropeptide Y1 receptor in human SK-N-MC cells | J Med Chem 51: 8168-72 (2008) Article DOI: 10.1021/jm801018u BindingDB Entry DOI: 10.7270/Q2WM1D8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50500151 (CHEMBL3746386) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale) Curated by ChEMBL | Assay Description Displacement of [3H]propionyl-pNPY from NPY1R in human HEL cells preincubated for 30 mins followed by radioligand addition at 60 to 90 mins by flow c... | J Med Chem 58: 8834-49 (2015) Article DOI: 10.1021/acs.jmedchem.5b00925 BindingDB Entry DOI: 10.7270/Q2RN3BVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50246649 ((R)-ethyl 2-(3-((5-(4-hydroxybenzylamino)-4-(2,2-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H]UR-MK114 from neuropeptide Y1 receptor in human SK-N-MC cells | J Med Chem 51: 8168-72 (2008) Article DOI: 10.1021/jm801018u BindingDB Entry DOI: 10.7270/Q2WM1D8T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50265995 (CHEMBL4097258) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H]UNSW-MK259 from human muscarinic acetylcholine receptor M2 expressed in CHOK9 cells after 3 hrs by liquid scintillation counting ... | J Med Chem 60: 3314-3334 (2017) Article DOI: 10.1021/acs.jmedchem.6b01892 BindingDB Entry DOI: 10.7270/Q29S1THC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50553114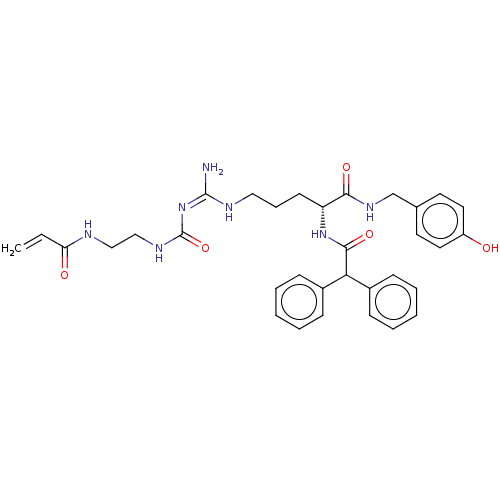 (CHEMBL4753520) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]UR-MK299 from Y1 receptor in human SK-N-MC cells by radioligand binding assay | Citation and Details Article DOI: 10.1039/c9md00538b BindingDB Entry DOI: 10.7270/Q2PZ5DGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50525125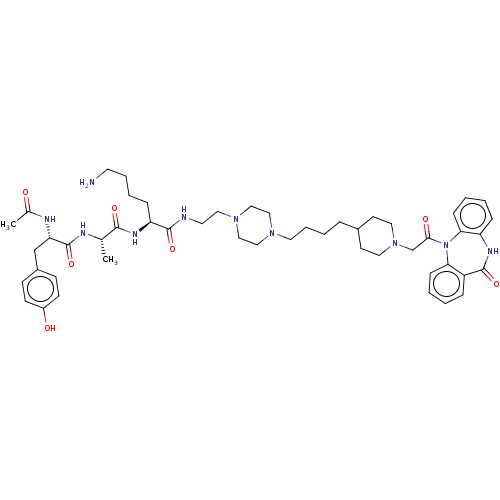 (CHEMBL4515788) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.115 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from human muscarinic M2 receptor expressed in CHO-K9 cells after 3 hrs by microbeta2 scintillation counting method | J Med Chem 62: 5358-5369 (2019) Article DOI: 10.1021/acs.jmedchem.8b01967 BindingDB Entry DOI: 10.7270/Q25H7KQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50246649 ((R)-ethyl 2-(3-((5-(4-hydroxybenzylamino)-4-(2,2-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale) Curated by ChEMBL | Assay Description Displacement of [3H]propionyl-pNPY from NPY1R in human HEL cells preincubated for 30 mins followed by radioligand addition at 60 to 90 mins by flow c... | J Med Chem 58: 8834-49 (2015) Article DOI: 10.1021/acs.jmedchem.5b00925 BindingDB Entry DOI: 10.7270/Q2RN3BVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50553111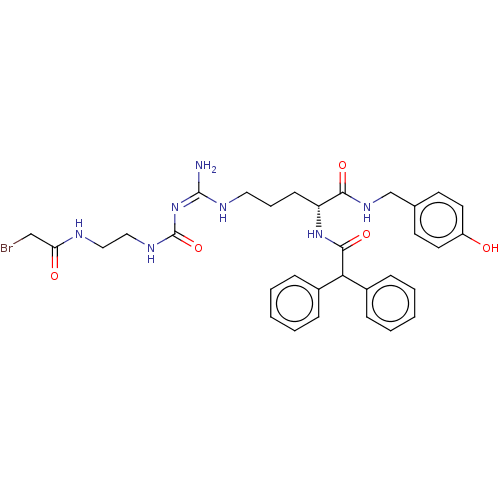 (CHEMBL4786911) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]UR-MK299 from Y1 receptor in human SK-N-MC cells by radioligand binding assay | Citation and Details Article DOI: 10.1039/c9md00538b BindingDB Entry DOI: 10.7270/Q2PZ5DGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50048908 (CHEMBL415788) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H] UR-MK300 from human neurotensin receptor 1 expressed in HT-29 cells incubated in dark after 30 mins by liquid scintillation coun... | ACS Med Chem Lett 11: 16-22 (2020) Article DOI: 10.1021/acsmedchemlett.9b00462 BindingDB Entry DOI: 10.7270/Q2SF30GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50538323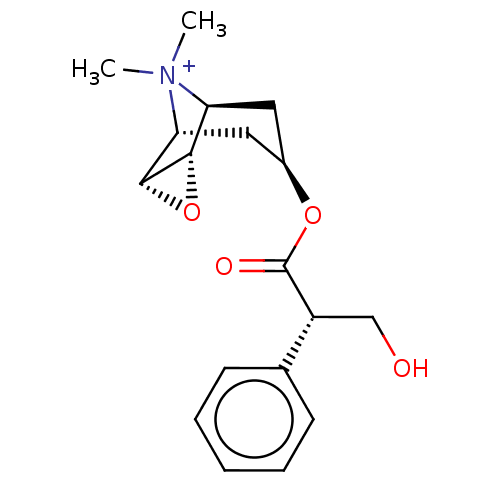 (CHEMBL3140030) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 0.138 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of 4-(2-((1E,3E)-5-((E)-3,3-Dimethyl-1-(6-oxo-6-((2-(3-(1-(4-(1-(2-oxo-2-(11-oxo-10,11-dihydro-5H-dibenzo[b,e][1,4]diazepin-5-yl)ethyl)p... | J Med Chem 63: 4133-4154 (2020) Article DOI: 10.1021/acs.jmedchem.9b02172 BindingDB Entry DOI: 10.7270/Q2R214WT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50048908 (CHEMBL415788) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H]-{Nomega-[N-(4-propanoylaminobutyl)aminocarbonyl]}Arg-Arg-ProTyr-Ile-Leu-OH Tris(hydrotrifluoroacetate) from NTSR1 in human HT-29... | J Med Chem 59: 1925-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01495 BindingDB Entry DOI: 10.7270/Q2V69MGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50553113 (CHEMBL4798118) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.145 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]UR-MK299 from Y1 receptor in human SK-N-MC cells by radioligand binding assay | Citation and Details Article DOI: 10.1039/c9md00538b BindingDB Entry DOI: 10.7270/Q2PZ5DGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50525138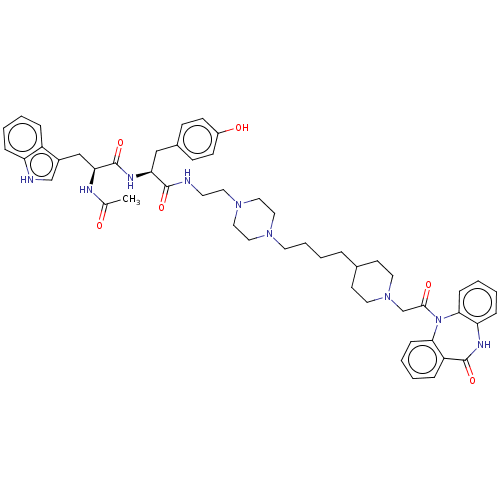 (CHEMBL4454300) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.148 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from human muscarinic M2 receptor expressed in CHO-K9 cells after 3 hrs by microbeta2 scintillation counting method | J Med Chem 62: 5358-5369 (2019) Article DOI: 10.1021/acs.jmedchem.8b01967 BindingDB Entry DOI: 10.7270/Q25H7KQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50236697 (5-L-isoleucineangiotensin II | 5-isoleucine-angiot...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H]-Angiotensin 2 from human placental AT1 receptor expressed in African green monkey COS7 cell membranes after 90 mins by gamma cou... | J Med Chem 59: 1925-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01495 BindingDB Entry DOI: 10.7270/Q2V69MGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50246648 ((R)-1-((5-(4-hydroxybenzylamino)-4-(2,2-diphenylac...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale) Curated by ChEMBL | Assay Description Displacement of [3H]UR-MK114 from NPY1R in human SK-N-MC cells by radioligand binding assay | J Med Chem 58: 8834-49 (2015) Article DOI: 10.1021/acs.jmedchem.5b00925 BindingDB Entry DOI: 10.7270/Q2RN3BVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50525139 (CHEMBL4554682) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.174 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from human muscarinic M2 receptor expressed in CHO-K9 cells after 3 hrs by microbeta2 scintillation counting method | J Med Chem 62: 5358-5369 (2019) Article DOI: 10.1021/acs.jmedchem.8b01967 BindingDB Entry DOI: 10.7270/Q25H7KQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 4 (Homo sapiens (Human)) | BDBM50532410 (CHEMBL4456247) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H]18 from human NPY Y4 receptor expressed in CHO cells co-expressing Gqi5-mtAEQ after 90 mins by liquid scintillation counting | J Med Chem 59: 6045-58 (2016) Article DOI: 10.1021/acs.jmedchem.6b00309 BindingDB Entry DOI: 10.7270/Q25T3Q08 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50538317 (CHEMBL4636083) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.182 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from human muscarinic M2 receptor stably expressed in CHO-K9 cells by radioligand competitive binding assay | J Med Chem 63: 4133-4154 (2020) Article DOI: 10.1021/acs.jmedchem.9b02172 BindingDB Entry DOI: 10.7270/Q2R214WT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50538315 (CHEMBL4641328) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.195 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from human muscarinic M2 receptor stably expressed in CHO-K9 cells by radioligand competitive binding assay | J Med Chem 63: 4133-4154 (2020) Article DOI: 10.1021/acs.jmedchem.9b02172 BindingDB Entry DOI: 10.7270/Q2R214WT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50525144 (CHEMBL4448933) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H]-QNB from human muscarinic M2 receptor expressed in stable CHO-K1 cells incubated for 120 mins by radioligand competition binding... | J Med Chem 62: 5358-5369 (2019) Article DOI: 10.1021/acs.jmedchem.8b01967 BindingDB Entry DOI: 10.7270/Q25H7KQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50246649 ((R)-ethyl 2-(3-((5-(4-hydroxybenzylamino)-4-(2,2-d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale) Curated by ChEMBL | Assay Description Displacement of [3H]UR-MK114 from NPY1R in human SK-N-MC cells by radioligand binding assay | J Med Chem 58: 8834-49 (2015) Article DOI: 10.1021/acs.jmedchem.5b00925 BindingDB Entry DOI: 10.7270/Q2RN3BVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50502374 (CHEMBL4593174) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.223 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H]UR-MK300 from human NSTR1 in HT-29 cells incubated for 2 hrs by liquid scintillation counter | ACS Med Chem Lett 10: 960-965 (2019) Article DOI: 10.1021/acsmedchemlett.9b00122 BindingDB Entry DOI: 10.7270/Q2BV7KWP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50500151 (CHEMBL3746386) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale) Curated by ChEMBL | Assay Description Displacement of [3H]propionyl-pNPY from NPY1R in human SK-N-MC cells compound treated immediately post radioligand treatment measured after 2 hrs by ... | J Med Chem 58: 8834-49 (2015) Article DOI: 10.1021/acs.jmedchem.5b00925 BindingDB Entry DOI: 10.7270/Q2RN3BVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50553112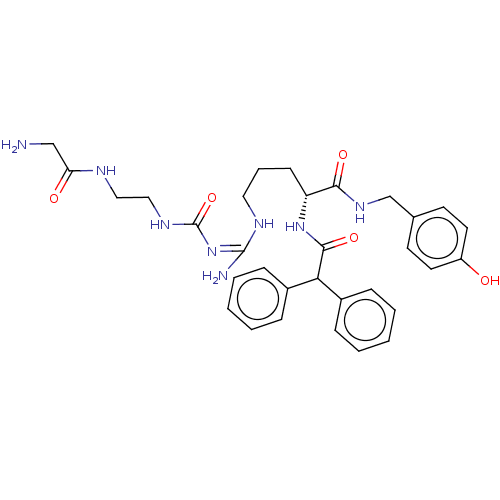 (CHEMBL4752935) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of [3H]UR-MK299 from Y1 receptor in human SK-N-MC cells by radioligand binding assay | Citation and Details Article DOI: 10.1039/c9md00538b BindingDB Entry DOI: 10.7270/Q2PZ5DGM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50525123 (CHEMBL4568151) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H]-NMS from human muscarinic M2 receptor expressed in CHO-K9 cells after 3 hrs by microbeta2 scintillation counting method | J Med Chem 62: 5358-5369 (2019) Article DOI: 10.1021/acs.jmedchem.8b01967 BindingDB Entry DOI: 10.7270/Q25H7KQF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50048908 (CHEMBL415788) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Binding affinity to human NTS1R | ACS Med Chem Lett 11: 334-339 (2020) Article DOI: 10.1021/acsmedchemlett.9b00388 BindingDB Entry DOI: 10.7270/Q2348PQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50048908 (CHEMBL415788) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Binding affinity to human neurotensin receptor 1 | ACS Med Chem Lett 11: 16-22 (2020) Article DOI: 10.1021/acsmedchemlett.9b00462 BindingDB Entry DOI: 10.7270/Q2SF30GN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50246648 ((R)-1-((5-(4-hydroxybenzylamino)-4-(2,2-diphenylac...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS/IPBS (Institut de Pharmacologie et Biologie Structurale) Curated by ChEMBL | Assay Description Displacement of [3H]propionyl-pNPY from NPY1R in human HEL cells preincubated for 30 mins followed by radioligand addition at 60 to 90 mins by flow c... | J Med Chem 58: 8834-49 (2015) Article DOI: 10.1021/acs.jmedchem.5b00925 BindingDB Entry DOI: 10.7270/Q2RN3BVG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Type-1 angiotensin II receptor (Homo sapiens (Human)) | BDBM50236697 (5-L-isoleucineangiotensin II | 5-isoleucine-angiot...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Similars | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Regensburg Curated by ChEMBL | Assay Description Displacement of [3H]-Asp-{Nomega-[N-(4-propanoylaminobutyl)aminocarbonyl]}Arg-ValTyr-Ile-His-Pro-Phe-OH Tris(hydrotrifluoroacetate) from human AT1 re... | J Med Chem 59: 1925-45 (2016) Article DOI: 10.1021/acs.jmedchem.5b01495 BindingDB Entry DOI: 10.7270/Q2V69MGK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuropeptide Y receptor type 1 (Homo sapiens (Human)) | BDBM50343731 ((R)-N-alpha-(2,2-Diphenylacetyl)-N-(4-ureidomethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£t Regensburg Curated by ChEMBL | Assay Description Displacement of [3H]-UR-MK114 from Y1R in human SK-N-MC cells | Bioorg Med Chem 19: 2859-78 (2011) Article DOI: 10.1016/j.bmc.2011.03.045 BindingDB Entry DOI: 10.7270/Q2F47PG7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2575 total ) | Next | Last >> |