 Found 337 hits with Last Name = 'berrodin' and Initial = 'tj'
Found 337 hits with Last Name = 'berrodin' and Initial = 'tj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607602

(CHEMBL5220994)Show SMILES F[C@@H]1C[C@@H](N(C1)C(=O)C1(CC(F)(F)C1)c1ccc(Cl)cc1)C(=O)Nc1ccc2[nH]ncc2n1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607596

(CHEMBL5221053)Show SMILES Clc1ccc(cc1)C1(CCC1)C(=O)N1CCC[C@@H]1C(=O)Nc1ccc2[nH]ncc2n1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607597
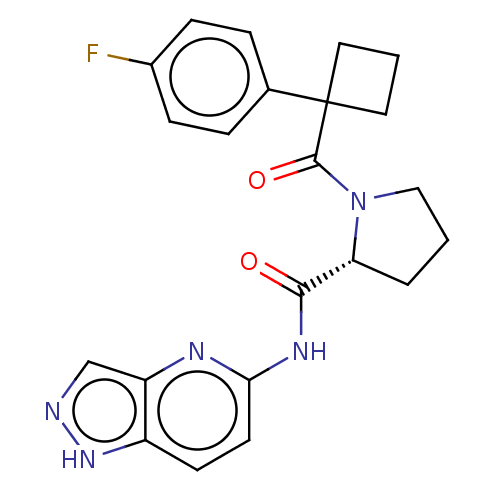
(CHEMBL5219157)Show SMILES Fc1ccc(cc1)C1(CCC1)C(=O)N1CCC[C@@H]1C(=O)Nc1ccc2[nH]ncc2n1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.126 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607593
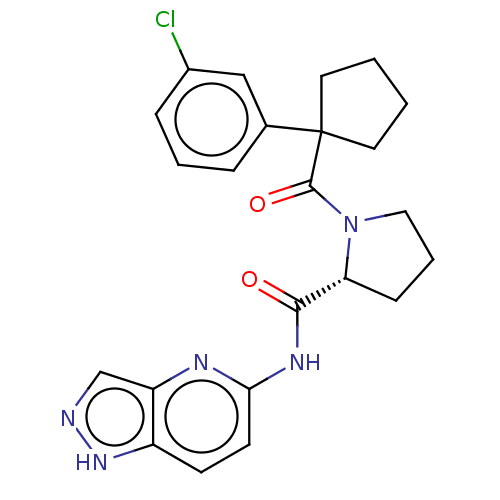
(CHEMBL5219693)Show SMILES Clc1cccc(c1)C1(CCCC1)C(=O)N1CCC[C@@H]1C(=O)Nc1ccc2[nH]ncc2n1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607595

(CHEMBL5219030)Show SMILES Clc1cccc(c1)C1(CCCC1)C(=O)N1CCC[C@@H]1C(=O)Nc1ccc2[nH]ccc2n1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.158 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607601
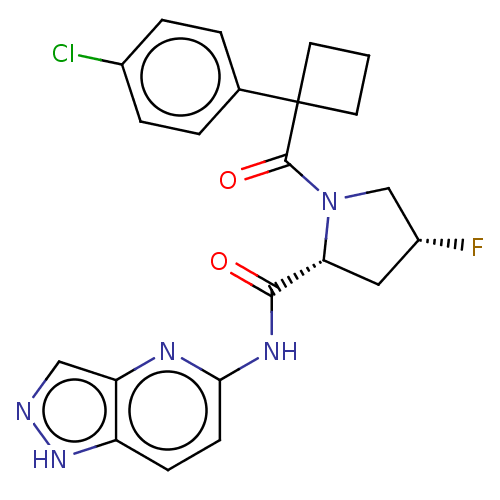
(CHEMBL5219667)Show SMILES F[C@@H]1C[C@@H](N(C1)C(=O)C1(CCC1)c1ccc(Cl)cc1)C(=O)Nc1ccc2[nH]ncc2n1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at progesterone receptor in human T47D cells assessed as inhibition of progesterone-induced alkaline phosphatase activity |
Bioorg Med Chem Lett 20: 4816-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.109
BindingDB Entry DOI: 10.7270/Q2ZW1MX6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Progesterone receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of progesterone receptor mediated progesterone-induced alkaline phosphatase activity in human T47D cells |
Bioorg Med Chem 16: 6589-600 (2008)
Article DOI: 10.1016/j.bmc.2008.05.018
BindingDB Entry DOI: 10.7270/Q2V125QZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Progesterone receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Antagonist activity at human PR expressed in human T47D cells assessed as inhibition of progesterone induced alkaline phosphatase |
J Med Chem 51: 1861-73 (2008)
Article DOI: 10.1021/jm701080t
BindingDB Entry DOI: 10.7270/Q2G44R56 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Progesterone receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Antagonist activity at progesterone receptor assessed as progesterone-induced alkaline phosphatase activity in human T47D cells |
Bioorg Med Chem Lett 18: 5015-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.015
BindingDB Entry DOI: 10.7270/Q25B03D7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607598
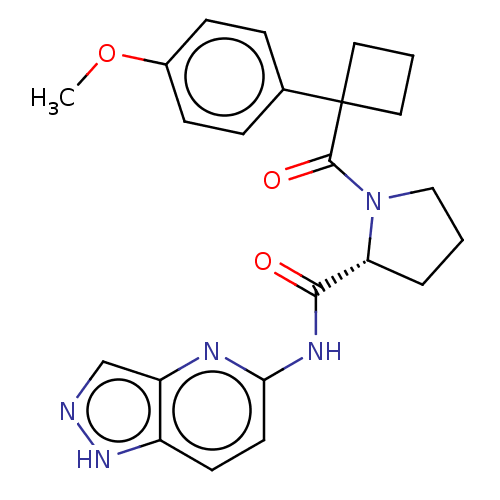
(CHEMBL5220447)Show SMILES COc1ccc(cc1)C1(CCC1)C(=O)N1CCC[C@@H]1C(=O)Nc1ccc2[nH]ncc2n1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607599
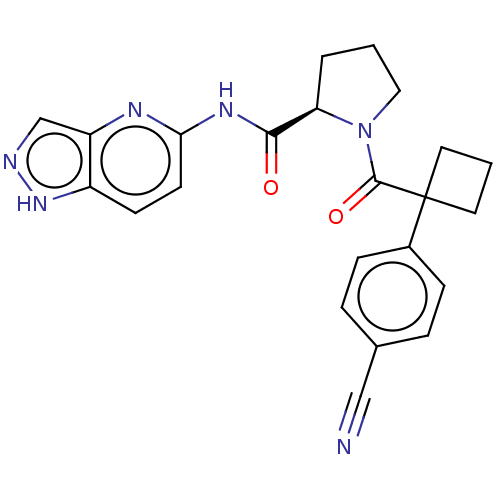
(CHEMBL5220732)Show SMILES O=C(Nc1ccc2[nH]ncc2n1)[C@H]1CCCN1C(=O)C1(CCC1)c1ccc(cc1)C#N |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.251 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607600

(CHEMBL5220956)Show SMILES FC1(F)CC(C1)(C(=O)N1CCC[C@@H]1C(=O)Nc1ccc2[nH]ncc2n1)c1ccc(Cl)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607589
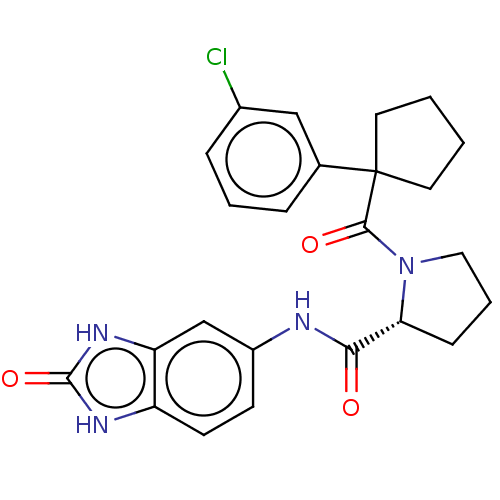
(CHEMBL5219678)Show SMILES Clc1cccc(c1)C1(CCCC1)C(=O)N1CCC[C@@H]1C(=O)Nc1ccc2[nH]c(=O)[nH]c2c1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50375821
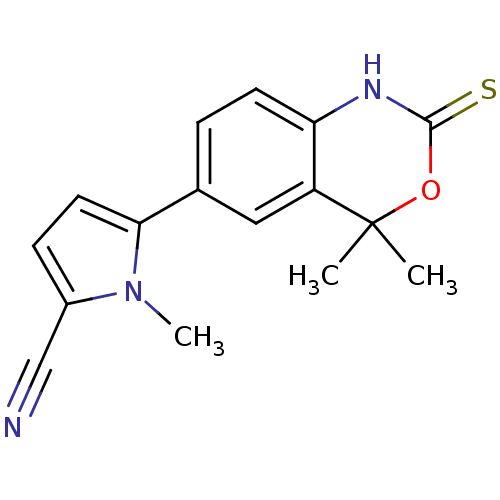
(TANAPROGET)Show InChI InChI=1S/C16H15N3OS/c1-16(2)12-8-10(4-6-13(12)18-15(21)20-16)14-7-5-11(9-17)19(14)3/h4-8H,1-3H3,(H,18,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]R5020 from human PR in human T47D cells by whole cell assay |
J Med Chem 51: 1861-73 (2008)
Article DOI: 10.1021/jm701080t
BindingDB Entry DOI: 10.7270/Q2G44R56 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Antagonist activity at glucocorticoid receptor by Gal4-DNA binding domain-hormone receptor LBD one-hybrid assay |
Bioorg Med Chem Lett 18: 5015-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.015
BindingDB Entry DOI: 10.7270/Q25B03D7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at glucocorticoid receptor ligand binding domain by two hybrid assay |
Bioorg Med Chem Lett 20: 4816-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.109
BindingDB Entry DOI: 10.7270/Q2ZW1MX6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Progesterone receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]R5020 from human PR in human T47D cells by whole cell assay |
J Med Chem 51: 1861-73 (2008)
Article DOI: 10.1021/jm701080t
BindingDB Entry DOI: 10.7270/Q2G44R56 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Antagonist activity at cloned glucocorticoid receptor-ligand binding domain expressed in african green monkey COS7 cells by GAL4 luciferase reporter ... |
Bioorg Med Chem 16: 6589-600 (2008)
Article DOI: 10.1016/j.bmc.2008.05.018
BindingDB Entry DOI: 10.7270/Q2V125QZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Antagonist activity at human GR ligand binding domain expressed in african green monkey COS7 cells in presence of Dexamethasone by Gal4 hybrid assay |
J Med Chem 51: 1861-73 (2008)
Article DOI: 10.1021/jm701080t
BindingDB Entry DOI: 10.7270/Q2G44R56 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607600

(CHEMBL5220956)Show SMILES FC1(F)CC(C1)(C(=O)N1CCC[C@@H]1C(=O)Nc1ccc2[nH]ncc2n1)c1ccc(Cl)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607585
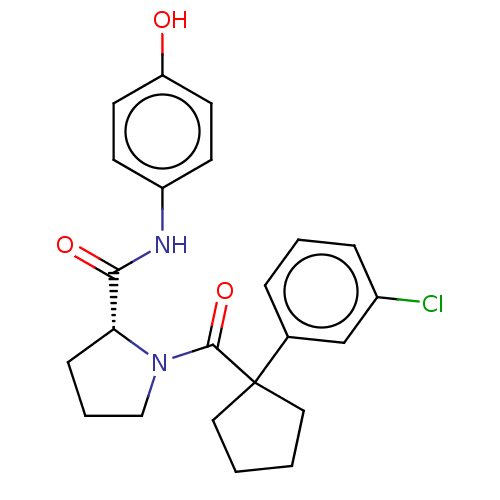
(CHEMBL5219512)Show SMILES Oc1ccc(NC(=O)[C@H]2CCCN2C(=O)C2(CCCC2)c2cccc(Cl)c2)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607586
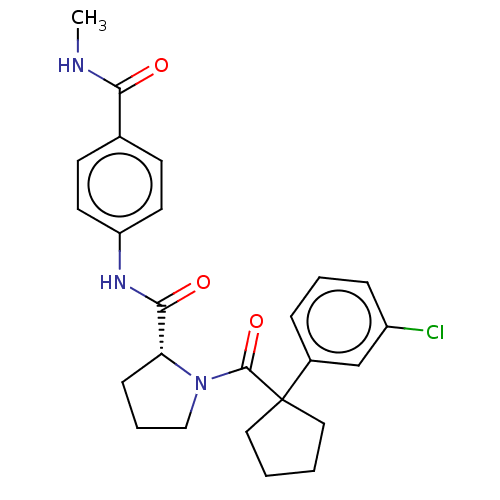
(CHEMBL5221030)Show SMILES CNC(=O)c1ccc(NC(=O)[C@H]2CCCN2C(=O)C2(CCCC2)c2cccc(Cl)c2)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607588
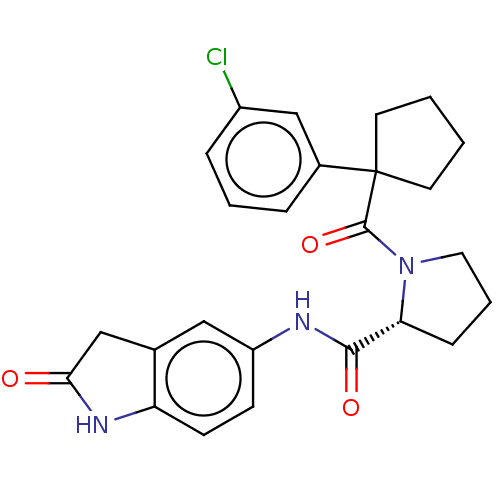
(CHEMBL5220546)Show SMILES Clc1cccc(c1)C1(CCCC1)C(=O)N1CCC[C@@H]1C(=O)Nc1ccc2NC(=O)Cc2c1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.794 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607590
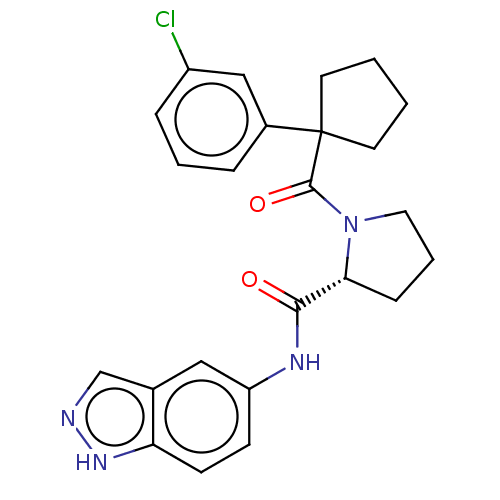
(CHEMBL5220332)Show SMILES Clc1cccc(c1)C1(CCCC1)C(=O)N1CCC[C@@H]1C(=O)Nc1ccc2[nH]ncc2c1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607605
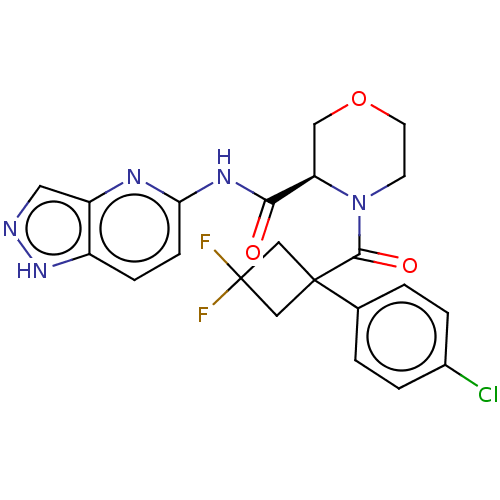
(CHEMBL5219177)Show SMILES FC1(F)CC(C1)(C(=O)N1CCOC[C@@H]1C(=O)Nc1ccc2[nH]ncc2n1)c1ccc(Cl)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Mineralocorticoid receptor
(Homo sapiens (Human)) | BDBM50228080

(2',15'-dimethyl-5,5'-dioxo-(9'R)-spiro[tetrahydrof...)Show SMILES CC(=O)S[C@@H]1CC2=CC(=O)CC[C@]2(C)[C@H]2CC[C@@]3(C)[C@@H](CC[C@@]33CCC(=O)O3)[C@H]12 |r,t:6| Show InChI InChI=1S/C24H32O4S/c1-14(25)29-19-13-15-12-16(26)4-8-22(15,2)17-5-9-23(3)18(21(17)19)6-10-24(23)11-7-20(27)28-24/h12,17-19,21H,4-11,13H2,1-3H3/t17-,18-,19+,21+,22-,23-,24+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Antagonist activity at mineralocorticoid receptor ligand binding domain expressed in african green monkey COS7 cells co-transfected with Gal4-LBD by ... |
J Nat Prod 72: 1944-8 (2009)
Article DOI: 10.1021/np9004882
BindingDB Entry DOI: 10.7270/Q2R49RQR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607592
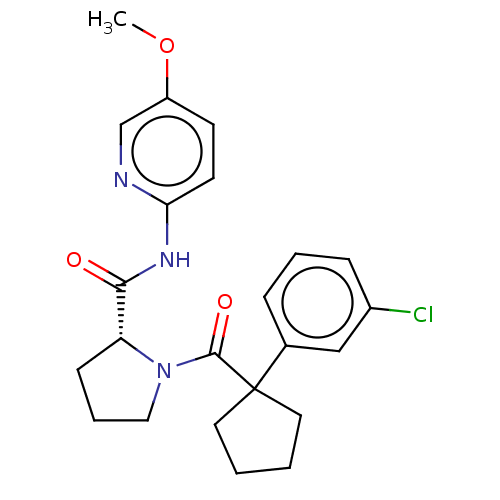
(CHEMBL5219466)Show SMILES COc1ccc(NC(=O)[C@H]2CCCN2C(=O)C2(CCCC2)c2cccc(Cl)c2)nc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50264285

(1-methyl-5-(5-methyl-2-oxo-5-(thiophen-2-yl)-1,2,3...)Show SMILES Cn1c(ccc1-c1ccc2NC(=O)COC(C)(c3cccs3)c2c1)C#N Show InChI InChI=1S/C20H17N3O2S/c1-20(18-4-3-9-26-18)15-10-13(17-8-6-14(11-21)23(17)2)5-7-16(15)22-19(24)12-25-20/h3-10H,12H2,1-2H3,(H,22,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]progesterone from progesterone receptor in human T47D cells |
Bioorg Med Chem Lett 18: 5015-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.015
BindingDB Entry DOI: 10.7270/Q25B03D7 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50299880
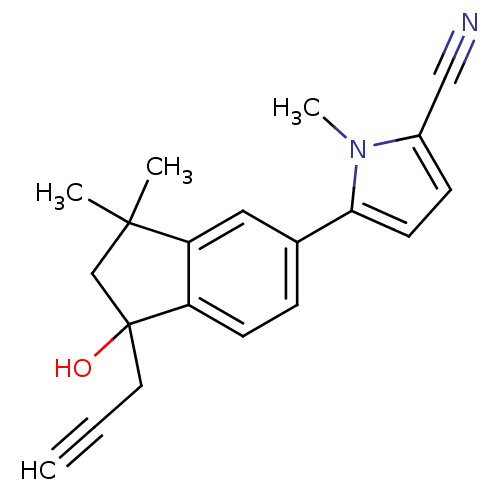
(5-(1-hydroxy-3,3-dimethyl-1-(prop-2-ynyl)-2,3-dihy...)Show InChI InChI=1S/C20H20N2O/c1-5-10-20(23)13-19(2,3)17-11-14(6-8-16(17)20)18-9-7-15(12-21)22(18)4/h1,6-9,11,23H,10,13H2,2-4H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Inhibition of progesterone receptor in human T47D cells assessed as progesteron-induced alkaline phosphatase activity |
Bioorg Med Chem Lett 19: 6666-9 (2009)
Article DOI: 10.1016/j.bmcl.2009.10.008
BindingDB Entry DOI: 10.7270/Q2FJ2GVX |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50323591
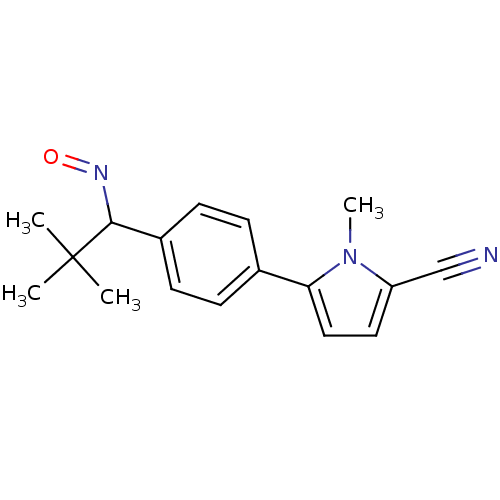
(5-(4-(1-(hydroxyimino)-2,2-dimethylpropyl)phenyl)-...)Show InChI InChI=1S/C17H19N3O/c1-17(2,3)16(19-21)13-7-5-12(6-8-13)15-10-9-14(11-18)20(15)4/h5-10,16H,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at progesterone receptor in human T47D cells assessed as inhibition of progesterone-induced alkaline phosphatase activity |
Bioorg Med Chem Lett 20: 4816-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.109
BindingDB Entry DOI: 10.7270/Q2ZW1MX6 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50323590
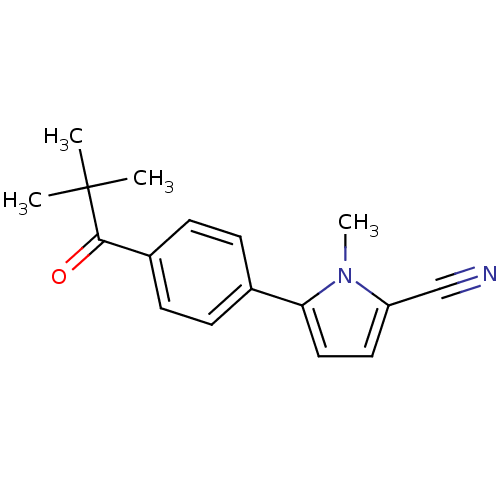
(1-methyl-5-(4-pivaloylphenyl)-1H-pyrrole-2-carboni...)Show InChI InChI=1S/C17H18N2O/c1-17(2,3)16(20)13-7-5-12(6-8-13)15-10-9-14(11-18)19(15)4/h5-10H,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Antagonist activity at progesterone receptor in human T47D cells assessed as inhibition of progesterone-induced alkaline phosphatase activity |
Bioorg Med Chem Lett 20: 4816-8 (2010)
Article DOI: 10.1016/j.bmcl.2010.06.109
BindingDB Entry DOI: 10.7270/Q2ZW1MX6 |
More data for this
Ligand-Target Pair | |
Glucocorticoid receptor
(Homo sapiens (Human)) | BDBM18627

((10S,11S,14S,15S,17R)-17-[4-(dimethylamino)phenyl]...)Show SMILES [H][C@@]12CC[C@@](O)(C#CC)[C@@]1(C)C[C@@H](C1=C3CCC(=O)C=C3CC[C@@]21[H])c1ccc(cc1)N(C)C |r,c:14,20| Show InChI InChI=1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Antagonist activity at glucocorticoid receptor ligand binding domain expressed in african green monkey COS7 cells co-transfected with Gal4-LBD by luc... |
J Nat Prod 72: 1944-8 (2009)
Article DOI: 10.1021/np9004882
BindingDB Entry DOI: 10.7270/Q2R49RQR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50375823
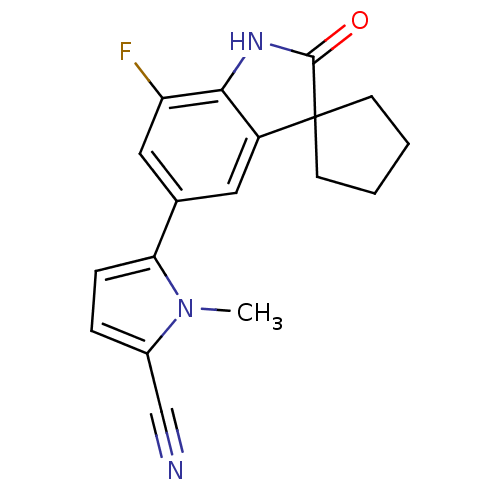
(CHEMBL407847)Show InChI InChI=1S/C18H16FN3O/c1-22-12(10-20)4-5-15(22)11-8-13-16(14(19)9-11)21-17(23)18(13)6-2-3-7-18/h4-5,8-9H,2-3,6-7H2,1H3,(H,21,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Antagonist activity at human PR expressed in human T47D cells assessed as inhibition of progesterone induced alkaline phosphatase |
J Med Chem 51: 1861-73 (2008)
Article DOI: 10.1021/jm701080t
BindingDB Entry DOI: 10.7270/Q2G44R56 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50144435

(1-methyl-5-[2'-oxospiro[cyclohexane-1,3'-(2',3'-di...)Show InChI InChI=1S/C19H19N3O/c1-22-14(12-20)6-8-17(22)13-5-7-16-15(11-13)19(18(23)21-16)9-3-2-4-10-19/h5-8,11H,2-4,9-10H2,1H3,(H,21,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]R5020 from human PR in human T47D cells by whole cell assay |
J Med Chem 51: 1861-73 (2008)
Article DOI: 10.1021/jm701080t
BindingDB Entry DOI: 10.7270/Q2G44R56 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50375817

(CHEMBL272663)Show InChI InChI=1S/C18H19N3O/c1-4-18(5-2)14-10-12(6-8-15(14)20-17(18)22)16-9-7-13(11-19)21(16)3/h6-10H,4-5H2,1-3H3,(H,20,22) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Antagonist activity at human PR expressed in human T47D cells assessed as inhibition of progesterone induced alkaline phosphatase |
J Med Chem 51: 1861-73 (2008)
Article DOI: 10.1021/jm701080t
BindingDB Entry DOI: 10.7270/Q2G44R56 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50264310
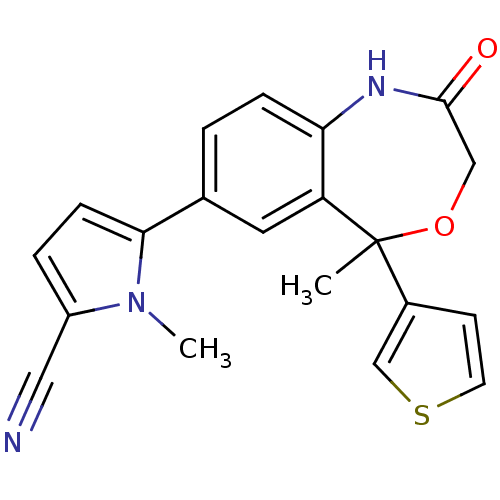
(1-methyl-5-(5-methyl-2-oxo-5-(thiophen-3-yl)-1,2,3...)Show SMILES Cn1c(ccc1-c1ccc2NC(=O)COC(C)(c3ccsc3)c2c1)C#N Show InChI InChI=1S/C20H17N3O2S/c1-20(14-7-8-26-12-14)16-9-13(18-6-4-15(10-21)23(18)2)3-5-17(16)22-19(24)11-25-20/h3-9,12H,11H2,1-2H3,(H,22,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]progesterone from progesterone receptor in human T47D cells |
Bioorg Med Chem Lett 18: 5015-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.015
BindingDB Entry DOI: 10.7270/Q25B03D7 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50375822
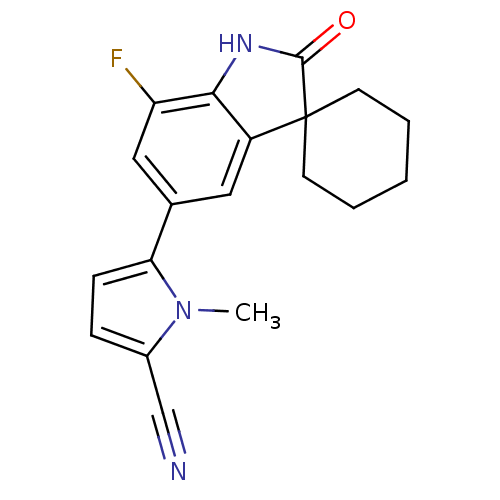
(CHEMBL408648)Show SMILES Cn1c(ccc1-c1cc2c(NC(=O)C22CCCCC2)c(F)c1)C#N Show InChI InChI=1S/C19H18FN3O/c1-23-13(11-21)5-6-16(23)12-9-14-17(15(20)10-12)22-18(24)19(14)7-3-2-4-8-19/h5-6,9-10H,2-4,7-8H2,1H3,(H,22,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]R5020 from human PR in human T47D cells by whole cell assay |
J Med Chem 51: 1861-73 (2008)
Article DOI: 10.1021/jm701080t
BindingDB Entry DOI: 10.7270/Q2G44R56 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50264284
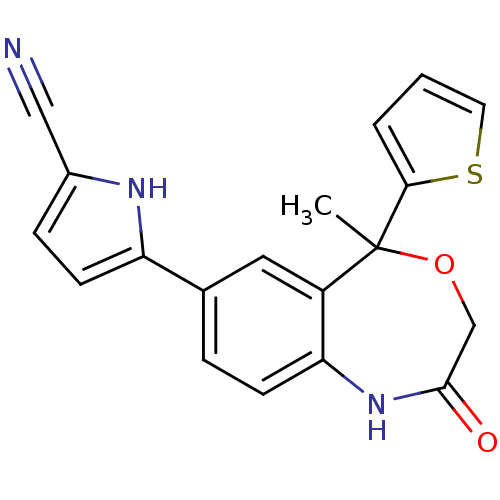
(5-(5-methyl-2-oxo-5-(thiophen-2-yl)-1,2,3,5-tetrah...)Show SMILES CC1(OCC(=O)Nc2ccc(cc12)-c1ccc([nH]1)C#N)c1cccs1 Show InChI InChI=1S/C19H15N3O2S/c1-19(17-3-2-8-25-17)14-9-12(15-7-5-13(10-20)21-15)4-6-16(14)22-18(23)11-24-19/h2-9,21H,11H2,1H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]progesterone from progesterone receptor in human T47D cells |
Bioorg Med Chem Lett 18: 5015-7 (2008)
Article DOI: 10.1016/j.bmcl.2008.08.015
BindingDB Entry DOI: 10.7270/Q25B03D7 |
More data for this
Ligand-Target Pair | |
Estrogen receptor beta
(Homo sapiens (Human)) | BDBM50169743

((13S,17S)-13-Methyl-7-[9-(4,4,5,5,5-pentafluoro-pe...)Show SMILES C[C@]12CC[C@H]3[C@H]([C@@H]1CC[C@@H]2O)[C@H](CCCCCCCCCS(=O)CCCC(F)(F)C(F)(F)F)Cc1cc(O)ccc31 |r| Show InChI InChI=1S/C32H47F5O3S/c1-30-17-15-26-25-12-11-24(38)21-23(25)20-22(29(26)27(30)13-14-28(30)39)10-7-5-3-2-4-6-8-18-41(40)19-9-16-31(33,34)32(35,36)37/h11-12,21-22,26-29,38-39H,2-10,13-20H2,1H3/t22-,26-,27+,28+,29-,30+,41?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Antagonist activity at estrogen receptor beta ligand binding domain expressed in african green monkey COS7 cells co-transfected with Gal4-LBD by luci... |
J Nat Prod 72: 1944-8 (2009)
Article DOI: 10.1021/np9004882
BindingDB Entry DOI: 10.7270/Q2R49RQR |
More data for this
Ligand-Target Pair | |
Estrogen receptor
(Homo sapiens (Human)) | BDBM50169743

((13S,17S)-13-Methyl-7-[9-(4,4,5,5,5-pentafluoro-pe...)Show SMILES C[C@]12CC[C@H]3[C@H]([C@@H]1CC[C@@H]2O)[C@H](CCCCCCCCCS(=O)CCCC(F)(F)C(F)(F)F)Cc1cc(O)ccc31 |r| Show InChI InChI=1S/C32H47F5O3S/c1-30-17-15-26-25-12-11-24(38)21-23(25)20-22(29(26)27(30)13-14-28(30)39)10-7-5-3-2-4-6-8-18-41(40)19-9-16-31(33,34)32(35,36)37/h11-12,21-22,26-29,38-39H,2-10,13-20H2,1H3/t22-,26-,27+,28+,29-,30+,41?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Antagonist activity at estrogen receptor alpha ligand binding domain expressed in african green monkey COS7 cells co-transfected with Gal4-LBD by luc... |
J Nat Prod 72: 1944-8 (2009)
Article DOI: 10.1021/np9004882
BindingDB Entry DOI: 10.7270/Q2R49RQR |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50375827

(CHEMBL407848 | WAY-255348)Show InChI InChI=1S/C16H14FN3O/c1-16(2)11-6-9(7-12(17)14(11)19-15(16)21)13-5-4-10(8-18)20(13)3/h4-7H,1-3H3,(H,19,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]R5020 from human PR in human T47D cells by whole cell assay |
J Med Chem 51: 1861-73 (2008)
Article DOI: 10.1021/jm701080t
BindingDB Entry DOI: 10.7270/Q2G44R56 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50375831
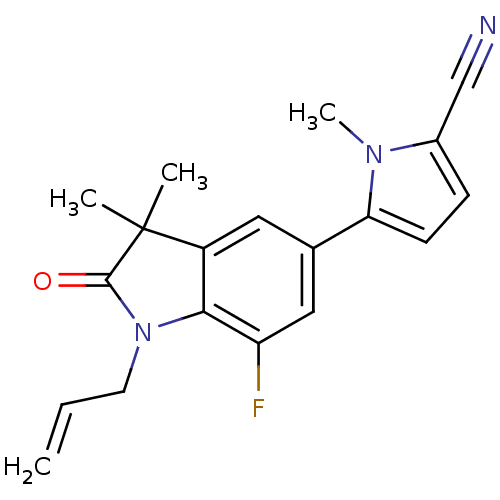
(CHEMBL429572)Show SMILES Cn1c(ccc1-c1cc2c(N(CC=C)C(=O)C2(C)C)c(F)c1)C#N Show InChI InChI=1S/C19H18FN3O/c1-5-8-23-17-14(19(2,3)18(23)24)9-12(10-15(17)20)16-7-6-13(11-21)22(16)4/h5-7,9-10H,1,8H2,2-4H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Antagonist activity at human PR expressed in human T47D cells assessed as inhibition of progesterone induced alkaline phosphatase |
J Med Chem 51: 1861-73 (2008)
Article DOI: 10.1021/jm701080t
BindingDB Entry DOI: 10.7270/Q2G44R56 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50144438

(5-(4,4-Dimethyl-2-oxo-1,4-dihydro-2H-benzo[d][1,3]...)Show InChI InChI=1S/C16H15N3O2/c1-16(2)12-8-10(4-6-13(12)18-15(20)21-16)14-7-5-11(9-17)19(14)3/h4-8H,1-3H3,(H,18,20) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]R5020 from human PR in human T47D cells by whole cell assay |
J Med Chem 51: 1861-73 (2008)
Article DOI: 10.1021/jm701080t
BindingDB Entry DOI: 10.7270/Q2G44R56 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50375824
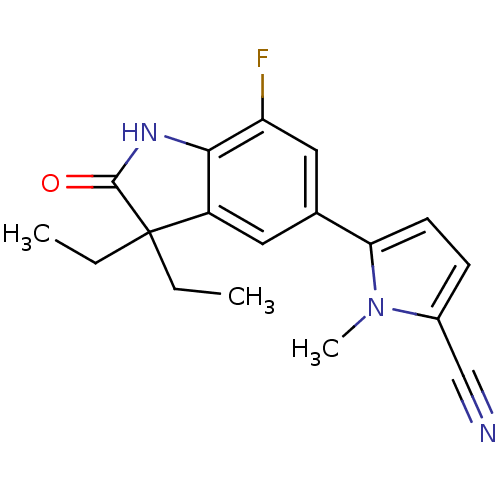
(CHEMBL429928)Show InChI InChI=1S/C18H18FN3O/c1-4-18(5-2)13-8-11(9-14(19)16(13)21-17(18)23)15-7-6-12(10-20)22(15)3/h6-9H,4-5H2,1-3H3,(H,21,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Antagonist activity at human PR expressed in human T47D cells assessed as inhibition of progesterone induced alkaline phosphatase |
J Med Chem 51: 1861-73 (2008)
Article DOI: 10.1021/jm701080t
BindingDB Entry DOI: 10.7270/Q2G44R56 |
More data for this
Ligand-Target Pair | |
Histone acetyltransferase p300
(Homo sapiens (Human)) | BDBM50607584
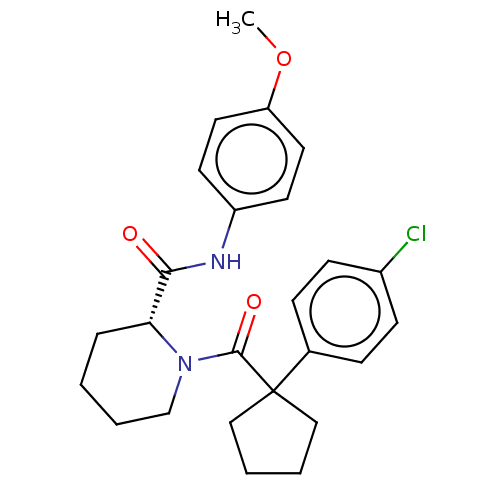
(CHEMBL5221088)Show SMILES COc1ccc(NC(=O)[C@H]2CCCCN2C(=O)C2(CCCC2)c2ccc(Cl)cc2)cc1 |r| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c00670
BindingDB Entry DOI: 10.7270/Q27P93H2 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50375827

(CHEMBL407848 | WAY-255348)Show InChI InChI=1S/C16H14FN3O/c1-16(2)11-6-9(7-12(17)14(11)19-15(16)21)13-5-4-10(8-18)20(13)3/h4-7H,1-3H3,(H,19,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Antagonist activity at human PR expressed in human T47D cells assessed as inhibition of progesterone induced alkaline phosphatase |
J Med Chem 51: 1861-73 (2008)
Article DOI: 10.1021/jm701080t
BindingDB Entry DOI: 10.7270/Q2G44R56 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50375826
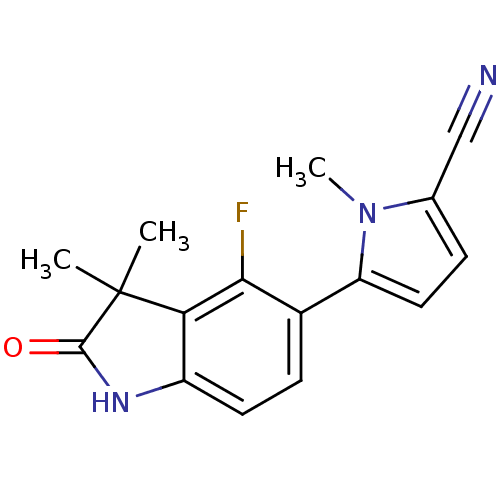
(CHEMBL270714)Show InChI InChI=1S/C16H14FN3O/c1-16(2)13-11(19-15(16)21)6-5-10(14(13)17)12-7-4-9(8-18)20(12)3/h4-7H,1-3H3,(H,19,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of [3H]R5020 from human PR in human T47D cells by whole cell assay |
J Med Chem 51: 1861-73 (2008)
Article DOI: 10.1021/jm701080t
BindingDB Entry DOI: 10.7270/Q2G44R56 |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50312006
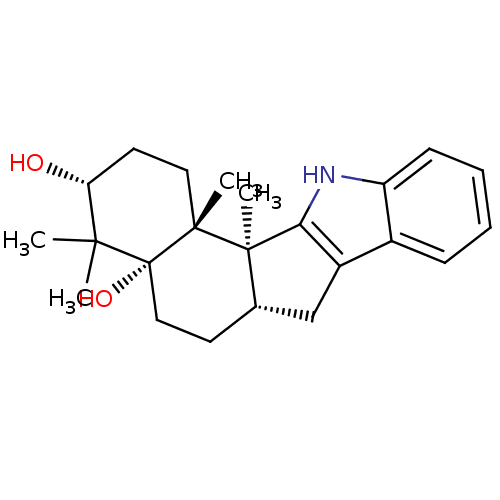
(CHEMBL1076438 | Lecanindole D)Show SMILES C[C@@]12[C@H](Cc3c1[nH]c1ccccc31)CC[C@@]1(O)C(C)(C)[C@H](O)CC[C@]21C |r| Show InChI InChI=1S/C23H31NO2/c1-20(2)18(25)10-11-21(3)22(4)14(9-12-23(20,21)26)13-16-15-7-5-6-8-17(15)24-19(16)22/h5-8,14,18,24-26H,9-13H2,1-4H3/t14-,18+,21+,22+,23+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Displacement of progesterone from progesterone receptor in human T47D cells after 3 hrs |
J Nat Prod 72: 1944-8 (2009)
Article DOI: 10.1021/np9004882
BindingDB Entry DOI: 10.7270/Q2R49RQR |
More data for this
Ligand-Target Pair | |
Progesterone receptor
(Homo sapiens (Human)) | BDBM50375826

(CHEMBL270714)Show InChI InChI=1S/C16H14FN3O/c1-16(2)13-11(19-15(16)21)6-5-10(14(13)17)12-7-4-9(8-18)20(12)3/h4-7H,1-3H3,(H,19,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
Curated by ChEMBL
| Assay Description
Antagonist activity at human PR expressed in human T47D cells assessed as inhibition of progesterone induced alkaline phosphatase |
J Med Chem 51: 1861-73 (2008)
Article DOI: 10.1021/jm701080t
BindingDB Entry DOI: 10.7270/Q2G44R56 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data