 Found 920 hits with Last Name = 'berry' and Initial = 'cb'
Found 920 hits with Last Name = 'berry' and Initial = 'cb' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50027081
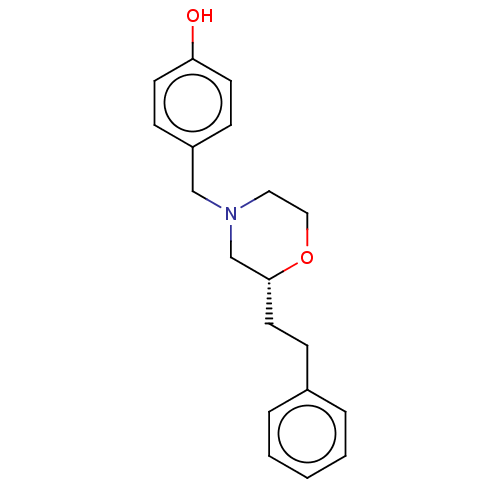
(CHEMBL3335555)Show InChI InChI=1S/C19H23NO2/c21-18-9-6-17(7-10-18)14-20-12-13-22-19(15-20)11-8-16-4-2-1-3-5-16/h1-7,9-10,19,21H,8,11-15H2/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D4 receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50027083
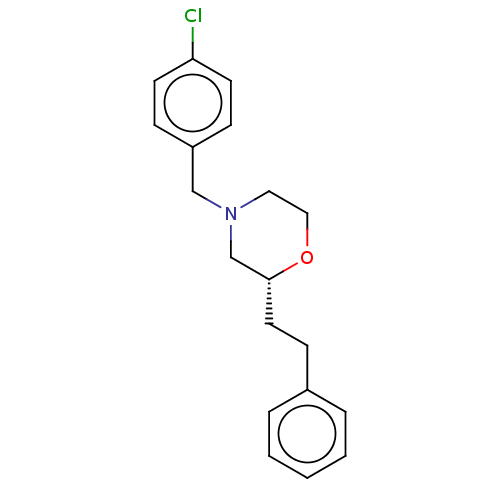
(CHEMBL3335556)Show InChI InChI=1S/C19H22ClNO/c20-18-9-6-17(7-10-18)14-21-12-13-22-19(15-21)11-8-16-4-2-1-3-5-16/h1-7,9-10,19H,8,11-15H2/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D4 receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50027078
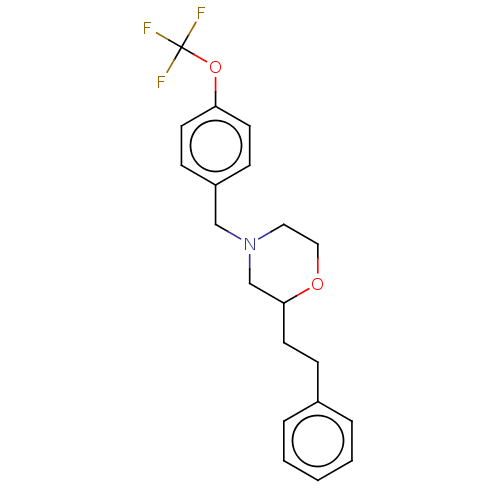
(CHEMBL3335538)Show InChI InChI=1S/C20H22F3NO2/c21-20(22,23)26-18-9-7-17(8-10-18)14-24-12-13-25-19(15-24)11-6-16-4-2-1-3-5-16/h1-5,7-10,19H,6,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D4 receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50027079
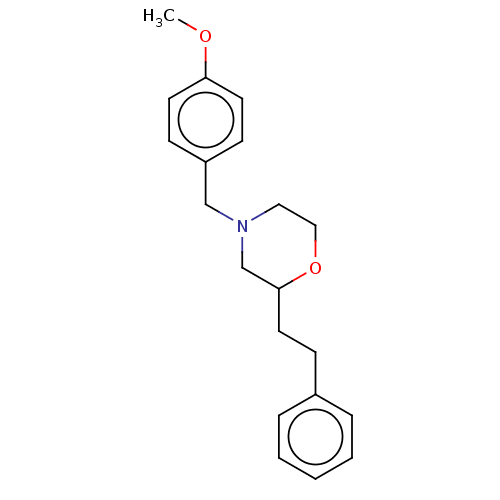
(CHEMBL3335539)Show InChI InChI=1S/C20H25NO2/c1-22-19-10-8-18(9-11-19)15-21-13-14-23-20(16-21)12-7-17-5-3-2-4-6-17/h2-6,8-11,20H,7,12-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D4 receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50027080
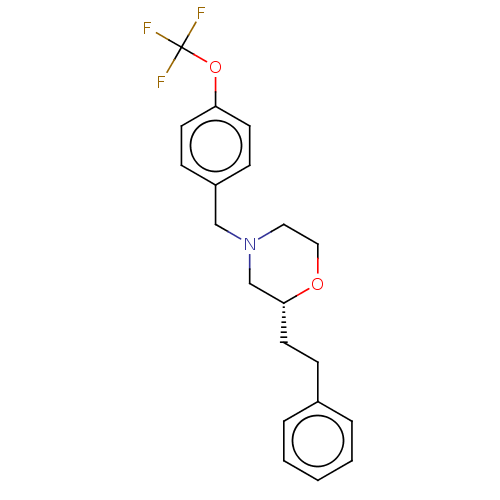
(CHEMBL3335554)Show SMILES FC(F)(F)Oc1ccc(CN2CCO[C@H](CCc3ccccc3)C2)cc1 |r| Show InChI InChI=1S/C20H22F3NO2/c21-20(22,23)26-18-9-7-17(8-10-18)14-24-12-13-25-19(15-24)11-6-16-4-2-1-3-5-16/h1-5,7-10,19H,6,11-15H2/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D4 receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50027074
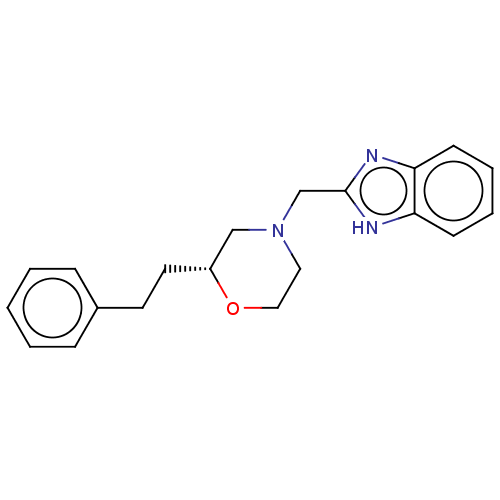
(CHEMBL3335537)Show SMILES C(Cc1ccccc1)[C@@H]1CN(Cc2nc3ccccc3[nH]2)CCO1 |r| Show InChI InChI=1S/C20H23N3O/c1-2-6-16(7-3-1)10-11-17-14-23(12-13-24-17)15-20-21-18-8-4-5-9-19(18)22-20/h1-9,17H,10-15H2,(H,21,22)/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D4 receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50027092
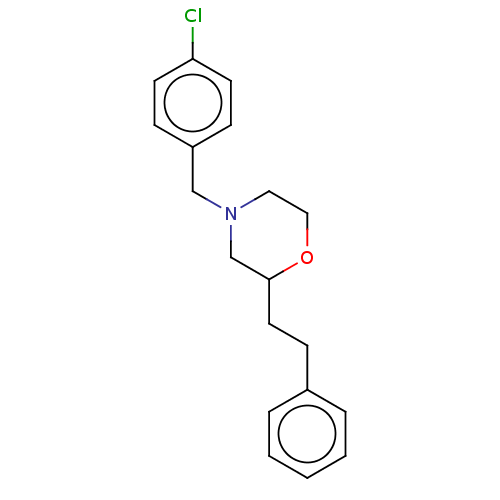
(CHEMBL3335540)Show InChI InChI=1S/C19H22ClNO/c20-18-9-6-17(7-10-18)14-21-12-13-22-19(15-21)11-8-16-4-2-1-3-5-16/h1-7,9-10,19H,8,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 81 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D4 receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50027187
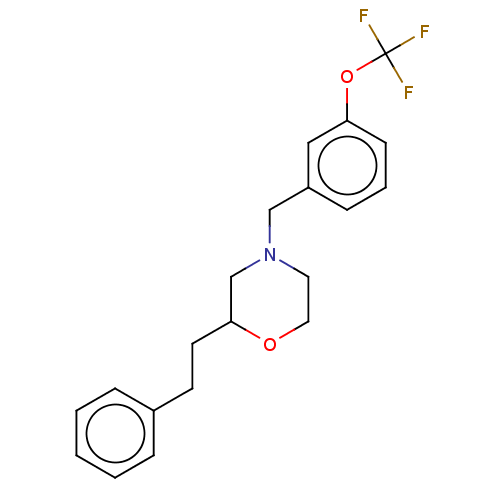
(CHEMBL3335542)Show InChI InChI=1S/C20H22F3NO2/c21-20(22,23)26-18-8-4-7-17(13-18)14-24-11-12-25-19(15-24)10-9-16-5-2-1-3-6-16/h1-8,13,19H,9-12,14-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D4 receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50027072
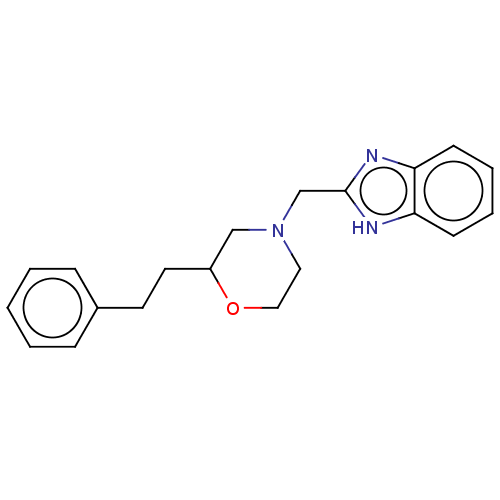
(CHEMBL3335535)Show InChI InChI=1S/C20H23N3O/c1-2-6-16(7-3-1)10-11-17-14-23(12-13-24-17)15-20-21-18-8-4-5-9-19(18)22-20/h1-9,17H,10-15H2,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D4 receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50027257
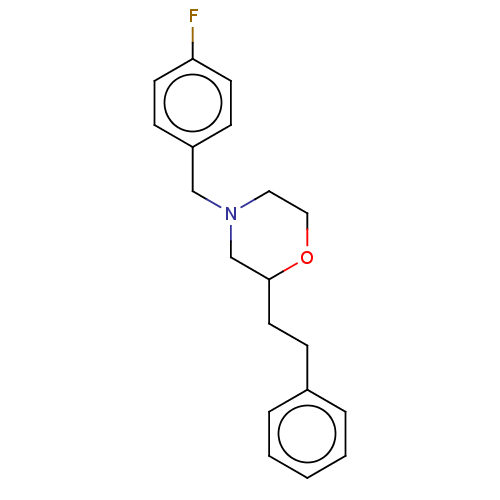
(CHEMBL3335543)Show InChI InChI=1S/C19H22FNO/c20-18-9-6-17(7-10-18)14-21-12-13-22-19(15-21)11-8-16-4-2-1-3-5-16/h1-7,9-10,19H,8,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D4 receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50027258
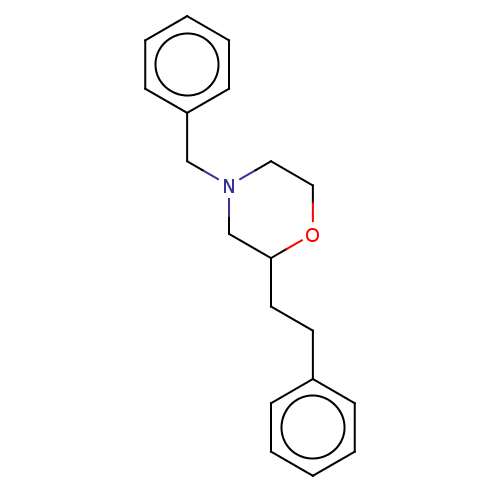
(CHEMBL3335544)Show InChI InChI=1S/C19H23NO/c1-3-7-17(8-4-1)11-12-19-16-20(13-14-21-19)15-18-9-5-2-6-10-18/h1-10,19H,11-16H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D4 receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50027182
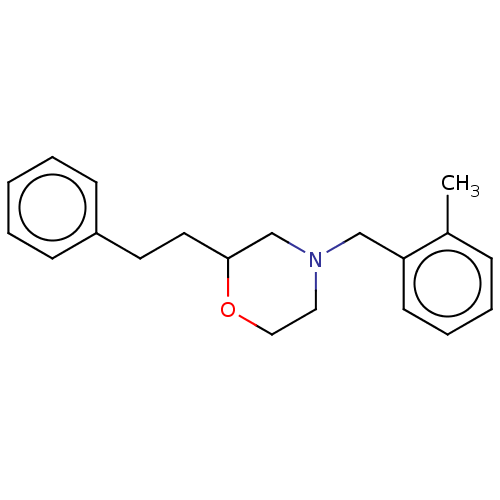
(CHEMBL3335541)Show InChI InChI=1S/C20H25NO/c1-17-7-5-6-10-19(17)15-21-13-14-22-20(16-21)12-11-18-8-3-2-4-9-18/h2-10,20H,11-16H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D4 receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50027261
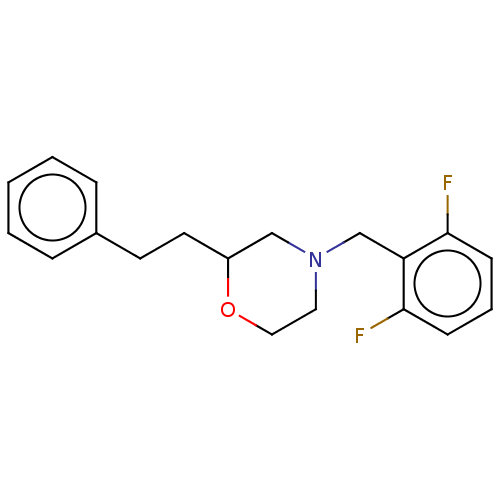
(CHEMBL3335545)Show InChI InChI=1S/C19H21F2NO/c20-18-7-4-8-19(21)17(18)14-22-11-12-23-16(13-22)10-9-15-5-2-1-3-6-15/h1-8,16H,9-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D4 receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50027081

(CHEMBL3335555)Show InChI InChI=1S/C19H23NO2/c21-18-9-6-17(7-10-18)14-20-12-13-22-19(15-20)11-8-16-4-2-1-3-5-16/h1-7,9-10,19,21H,8,11-15H2/t19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D3 receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50027080

(CHEMBL3335554)Show SMILES FC(F)(F)Oc1ccc(CN2CCO[C@H](CCc3ccccc3)C2)cc1 |r| Show InChI InChI=1S/C20H22F3NO2/c21-20(22,23)26-18-9-7-17(8-10-18)14-24-12-13-25-19(15-24)11-6-16-4-2-1-3-5-16/h1-5,7-10,19H,6,11-15H2/t19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D3 receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50027078

(CHEMBL3335538)Show InChI InChI=1S/C20H22F3NO2/c21-20(22,23)26-18-9-7-17(8-10-18)14-24-12-13-25-19(15-24)11-6-16-4-2-1-3-5-16/h1-5,7-10,19H,6,11-15H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D3 receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50027079

(CHEMBL3335539)Show InChI InChI=1S/C20H25NO2/c1-22-19-10-8-18(9-11-19)15-21-13-14-23-20(16-21)12-7-17-5-3-2-4-6-17/h2-6,8-11,20H,7,12-16H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.77E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D3 receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50027078

(CHEMBL3335538)Show InChI InChI=1S/C20H22F3NO2/c21-20(22,23)26-18-9-7-17(8-10-18)14-24-12-13-25-19(15-24)11-6-16-4-2-1-3-5-16/h1-5,7-10,19H,6,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D2L receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50027080

(CHEMBL3335554)Show SMILES FC(F)(F)Oc1ccc(CN2CCO[C@H](CCc3ccccc3)C2)cc1 |r| Show InChI InChI=1S/C20H22F3NO2/c21-20(22,23)26-18-9-7-17(8-10-18)14-24-12-13-25-19(15-24)11-6-16-4-2-1-3-5-16/h1-5,7-10,19H,6,11-15H2/t19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D2L receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50027072

(CHEMBL3335535)Show InChI InChI=1S/C20H23N3O/c1-2-6-16(7-3-1)10-11-17-14-23(12-13-24-17)15-20-21-18-8-4-5-9-19(18)22-20/h1-9,17H,10-15H2,(H,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D3 receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50027074

(CHEMBL3335537)Show SMILES C(Cc1ccccc1)[C@@H]1CN(Cc2nc3ccccc3[nH]2)CCO1 |r| Show InChI InChI=1S/C20H23N3O/c1-2-6-16(7-3-1)10-11-17-14-23(12-13-24-17)15-20-21-18-8-4-5-9-19(18)22-20/h1-9,17H,10-15H2,(H,21,22)/t17-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D3 receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
D(3) dopamine receptor
(Homo sapiens (Human)) | BDBM50027077
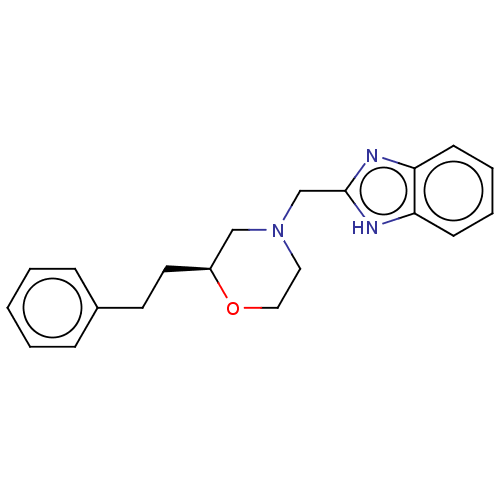
(CHEMBL3335536)Show SMILES C(Cc1ccccc1)[C@H]1CN(Cc2nc3ccccc3[nH]2)CCO1 |r| | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D3 receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
D(4) dopamine receptor
(Homo sapiens (Human)) | BDBM50027077

(CHEMBL3335536)Show SMILES C(Cc1ccccc1)[C@H]1CN(Cc2nc3ccccc3[nH]2)CCO1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D4 receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50027074

(CHEMBL3335537)Show SMILES C(Cc1ccccc1)[C@@H]1CN(Cc2nc3ccccc3[nH]2)CCO1 |r| Show InChI InChI=1S/C20H23N3O/c1-2-6-16(7-3-1)10-11-17-14-23(12-13-24-17)15-20-21-18-8-4-5-9-19(18)22-20/h1-9,17H,10-15H2,(H,21,22)/t17-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D2 receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50027077

(CHEMBL3335536)Show SMILES C(Cc1ccccc1)[C@H]1CN(Cc2nc3ccccc3[nH]2)CCO1 |r| | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D2 receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50027072

(CHEMBL3335535)Show InChI InChI=1S/C20H23N3O/c1-2-6-16(7-3-1)10-11-17-14-23(12-13-24-17)15-20-21-18-8-4-5-9-19(18)22-20/h1-9,17H,10-15H2,(H,21,22) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D1 receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50027077

(CHEMBL3335536)Show SMILES C(Cc1ccccc1)[C@H]1CN(Cc2nc3ccccc3[nH]2)CCO1 |r| | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D1 receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM50027072

(CHEMBL3335535)Show InChI InChI=1S/C20H23N3O/c1-2-6-16(7-3-1)10-11-17-14-23(12-13-24-17)15-20-21-18-8-4-5-9-19(18)22-20/h1-9,17H,10-15H2,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D2 receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM50027074

(CHEMBL3335537)Show SMILES C(Cc1ccccc1)[C@@H]1CN(Cc2nc3ccccc3[nH]2)CCO1 |r| Show InChI InChI=1S/C20H23N3O/c1-2-6-16(7-3-1)10-11-17-14-23(12-13-24-17)15-20-21-18-8-4-5-9-19(18)22-20/h1-9,17H,10-15H2,(H,21,22)/t17-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vanderbilt University
Curated by ChEMBL
| Assay Description
Inhibition of human dopamine D1 receptor |
ACS Med Chem Lett 5: 1060-4 (2014)
Article DOI: 10.1021/ml500267c
BindingDB Entry DOI: 10.7270/Q2G44RWM |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50582221

(CHEMBL5090028 | US11708359, Example 60)Show SMILES CC(C)(C)c1cccc(c1)C12CC1CN(CC2)C(=O)[C@H]1C[C@@]2(C1)COC(=O)N2 |r,wU:21.31,19.21,(59.02,-28.21,;59.02,-26.67,;60.35,-25.91,;60.34,-27.44,;57.69,-25.9,;56.35,-26.67,;55.02,-25.89,;55.03,-24.36,;56.37,-23.61,;57.69,-24.37,;56.38,-22.07,;57.7,-21.3,;56.37,-20.54,;55.04,-19.76,;53.72,-20.54,;53.7,-22.08,;55.03,-22.85,;52.38,-19.77,;52.38,-18.23,;51.05,-20.54,;50.65,-22.03,;49.16,-21.63,;49.56,-20.14,;48.84,-23.14,;47.3,-23.3,;46.68,-21.89,;45.17,-21.57,;47.82,-20.86,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2JM2FRS |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM581474

((2s,4s)-2-(2-(3-(tert-Butyl)phenyl)-7-azaspiro[3.5...)Show SMILES CC(C)(C)c1cccc(c1)C1CC2(C1)CCN(CC2)C(=O)[C@H]1C[C@@]2(C1)COC(=O)N2 |r,wU:21.23,wD:23.26,(9.82,-.16,;8.48,-.93,;8.48,.61,;9.82,-1.7,;7.15,-1.7,;7.15,-3.24,;5.82,-4.01,;4.48,-3.24,;4.48,-1.7,;5.82,-.93,;3.15,-.93,;1.66,-1.33,;1.26,.16,;2.75,.56,;-.07,-.61,;-1.41,.16,;-1.41,1.7,;-.07,2.47,;1.26,1.7,;-2.74,2.47,;-2.74,4.01,;-4.07,1.7,;-4.47,.21,;-5.96,.61,;-5.56,2.1,;-5.96,-.93,;-7.42,-1.41,;-8.33,-.16,;-9.82,.24,;-7.42,1.09,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The assay used to measure the in vitro activity of MGL is adapted from the assay used for another serine hydrolase (FAAH) described in Wilson et al.,... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2S186B3 |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM609871

((rac)-(2s,4s)-2-(6-(4-(tert-Butyl)phenyl)-3-azabic...)Show SMILES CC(C)(C)c1ccc(cc1)C12CC1CN(CC2)C(=O)[C@H]1C[C@@]2(C1)COC(=O)N2 |r,wU:19.21,wD:21.24,(5.59,-5.26,;6.36,-3.93,;7.9,-3.93,;7.13,-5.26,;5.59,-2.59,;4.05,-2.59,;3.28,-1.26,;4.05,.08,;5.59,.08,;6.36,-1.26,;3.28,1.41,;4.62,2.18,;3.28,2.95,;1.95,3.72,;.62,2.95,;.62,1.41,;1.95,.64,;-.72,3.72,;-.72,5.26,;-2.05,2.95,;-2.45,1.46,;-3.94,1.86,;-3.54,3.35,;-4.1,.33,;-5.6,.01,;-6.37,1.34,;-7.9,1.5,;-5.34,2.49,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2JM2FRS |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM50582222

(CHEMBL5074982 | US11708359, Example 71)Show SMILES CC1(CC1)c1cccc(c1)C12CC1CN(CC2)C(=O)[C@H]1C[C@@]2(C1)COC(=O)N2 |r,wU:21.32,19.22,(81.16,-28.5,;81.16,-26.95,;81.56,-25.46,;82.65,-26.55,;79.83,-26.18,;78.5,-26.95,;77.17,-26.18,;77.18,-24.64,;78.52,-23.89,;79.83,-24.65,;78.52,-22.35,;79.85,-21.58,;78.52,-20.82,;77.18,-20.04,;75.86,-20.82,;75.85,-22.36,;77.18,-23.13,;74.53,-20.05,;74.53,-18.51,;73.2,-20.82,;72.8,-22.31,;71.31,-21.91,;71.71,-20.42,;70.99,-23.42,;69.45,-23.58,;68.82,-22.17,;67.31,-21.85,;69.97,-21.14,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2JM2FRS |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM581599

((2r,4s)-2-(2-(5-(tert-Butyl)-2-methylphenyl)-7-aza...)Show SMILES Cc1ccc(cc1C1CC2(C1)CCN(CC2)C(=O)[C@H]1C[C@@]2(C1)COC(=O)N2)C(C)(C)C |r,wU:18.20,wD:20.23,(3.21,-4.01,;4.54,-3.24,;5.88,-4.01,;7.21,-3.24,;7.21,-1.7,;5.88,-.93,;4.54,-1.7,;3.21,-.93,;1.72,-1.33,;1.32,.16,;2.81,.56,;-.01,-.61,;-1.35,.16,;-1.35,1.7,;-.01,2.47,;1.32,1.7,;-2.68,2.47,;-2.68,4.01,;-4.01,1.7,;-4.41,.21,;-5.9,.61,;-5.5,2.1,;-6.06,-.92,;-7.57,-1.24,;-8.34,.09,;-9.88,.09,;-7.31,1.24,;8.54,-.93,;9.88,-.16,;8.54,.61,;9.88,-1.7,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The assay used to measure the in vitro activity of MGL is adapted from the assay used for another serine hydrolase (FAAH) described in Wilson et al.,... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2S186B3 |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM609880

((rac)-(2s,4s)-2-(6-(4-(1-Methylcyclopropyl)phenyl)...)Show SMILES CC1(CC1)c1ccc(cc1)C12CC1CN(CC2)C(=O)[C@H]1C[C@@]2(C1)COC(=O)N2 |r,wU:19.22,wD:21.25,(5.7,-5.26,;6.47,-3.93,;7.8,-4.7,;7.8,-3.16,;5.7,-2.59,;4.16,-2.59,;3.39,-1.26,;4.16,.08,;5.7,.08,;6.47,-1.26,;3.39,1.41,;4.72,2.18,;3.39,2.95,;2.05,3.72,;.72,2.95,;.72,1.41,;2.05,.64,;-.61,3.72,;-.61,5.26,;-1.95,2.95,;-2.35,1.46,;-3.83,1.86,;-3.43,3.35,;-3.99,.33,;-5.5,.01,;-6.27,1.34,;-7.8,1.5,;-5.24,2.49,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2JM2FRS |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM581624
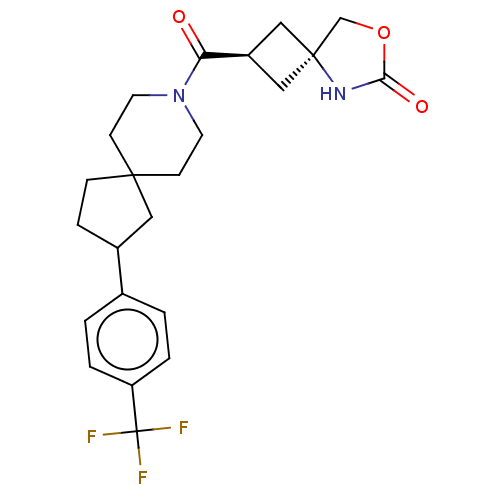
((rac)-(2s,4s)-2-(2-(4-(Trifluoromethyl)phenyl)-8-a...)Show SMILES FC(F)(F)c1ccc(cc1)C1CCC2(C1)CCN(CC2)C(=O)[C@H]1C[C@@]2(C1)COC(=O)N2 |r,wU:22.24,wD:24.27,(10.43,.68,;9.09,-.09,;9.49,1.39,;10.43,-.86,;7.6,-.49,;7.21,-1.98,;5.72,-2.38,;4.63,-1.29,;5.03,.2,;6.52,.6,;3.14,-1.69,;2.24,-2.93,;.77,-2.46,;.77,-.92,;2.24,-.44,;-.56,-1.69,;-1.9,-.92,;-1.9,.62,;-.56,1.39,;.77,.62,;-3.23,1.39,;-3.23,2.93,;-4.56,.62,;-4.96,-.86,;-6.45,-.47,;-6.05,1.02,;-6.61,-2,;-8.12,-2.32,;-8.89,-.98,;-10.43,-.98,;-7.86,.16,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The assay used to measure the in vitro activity of MGL is adapted from the assay used for another serine hydrolase (FAAH) described in Wilson et al.,... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2S186B3 |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM581534
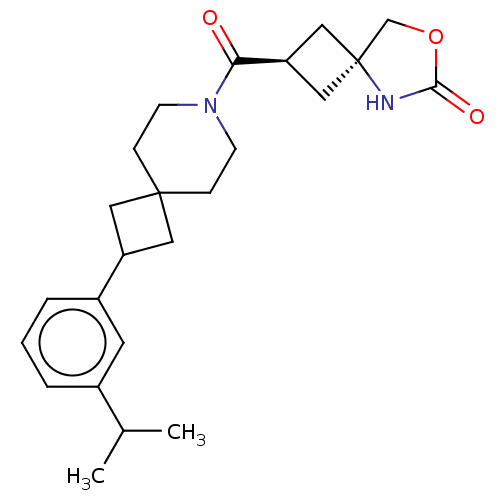
((2s,4s)-2-[2-(3-Isopropylphenyl)-7-azaspiro[3.5]no...)Show SMILES CC(C)c1cccc(c1)C1CC2(C1)CCN(CC2)C(=O)[C@H]1C[C@@]2(C1)COC(=O)N2 |r,wU:20.22,wD:22.25,(9.82,-1.7,;8.48,-.93,;8.48,.61,;7.15,-1.7,;7.15,-3.24,;5.82,-4.01,;4.48,-3.24,;4.48,-1.7,;5.82,-.93,;3.15,-.93,;1.66,-1.33,;1.26,.16,;2.75,.56,;-.07,-.61,;-1.41,.16,;-1.41,1.7,;-.07,2.47,;1.26,1.7,;-2.74,2.47,;-2.74,4.01,;-4.07,1.7,;-4.47,.21,;-5.96,.61,;-5.56,2.1,;-5.96,-.93,;-7.42,-1.41,;-8.33,-.16,;-9.82,.24,;-7.42,1.09,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0410 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The assay used to measure the in vitro activity of MGL is adapted from the assay used for another serine hydrolase (FAAH) described in Wilson et al.,... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2S186B3 |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM581597

((2s,4s)-2-[2-(2,4-Dimethylphenyl)-7-azaspiro[3.5]n...)Show SMILES Cc1ccc(C2CC3(C2)CCN(CC3)C(=O)[C@H]2C[C@@]3(C2)COC(=O)N3)c(C)c1 |r,wU:16.17,wD:18.20,(9.15,-4.01,;7.82,-3.24,;7.82,-1.7,;6.48,-.93,;5.15,-1.7,;3.81,-.93,;2.33,-1.33,;1.93,.16,;3.42,.56,;.59,-.61,;-.74,.16,;-.74,1.7,;.59,2.47,;1.93,1.7,;-2.07,2.47,;-2.07,4.01,;-3.41,1.7,;-3.8,.21,;-5.29,.61,;-4.89,2.1,;-5.29,-.93,;-6.76,-1.41,;-7.66,-.16,;-9.15,.24,;-6.76,1.09,;5.15,-3.24,;3.81,-4.01,;6.48,-4.01,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0480 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The assay used to measure the in vitro activity of MGL is adapted from the assay used for another serine hydrolase (FAAH) described in Wilson et al.,... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2S186B3 |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM609878

((rac)-(2s,4s)-2-(6-(3-Chloro-4-methylphenyl)-3- az...)Show SMILES Cc1ccc(cc1Cl)C12CC1CN(CC2)C(=O)[C@H]1C[C@@]2(C1)COC(=O)N2 |r,wU:17.19,wD:19.22,(6.36,-4.59,;5.59,-3.26,;4.05,-3.26,;3.28,-1.93,;4.05,-.59,;5.59,-.59,;6.36,-1.93,;7.9,-1.93,;3.28,.74,;4.62,1.51,;3.28,2.28,;1.95,3.05,;.62,2.28,;.62,.74,;1.95,-.03,;-.72,3.05,;-.72,4.59,;-2.05,2.28,;-2.45,.79,;-3.94,1.19,;-3.54,2.68,;-4.1,-.34,;-5.6,-.66,;-6.37,.68,;-7.9,.84,;-5.34,1.82,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.0750 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2JM2FRS |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM581596

((2r,4s)-2-(2-(3-(tert-Butyl)phenyl)-7-azaspiro[3.5...)Show SMILES CC(C)(C)c1cccc(c1)C1CC2(C1)CCN(CC2)C(=O)[C@H]1C[C@@]2(C1)CCC(=O)N2 |r,wU:21.23,wD:23.26,(9.82,-1.7,;8.48,-.93,;8.48,.61,;9.82,-.16,;7.15,-1.7,;7.15,-3.24,;5.82,-4.01,;4.48,-3.24,;4.48,-1.7,;5.82,-.93,;3.15,-.93,;1.66,-1.33,;1.26,.16,;2.75,.56,;-.07,-.61,;-1.41,.16,;-1.41,1.7,;-.07,2.47,;1.26,1.7,;-2.74,2.47,;-2.74,4.01,;-4.07,1.7,;-4.47,.21,;-5.96,.61,;-5.56,2.1,;-5.96,-.93,;-7.42,-1.41,;-8.33,-.16,;-9.82,.24,;-7.42,1.09,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0820 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The assay used to measure the in vitro activity of MGL is adapted from the assay used for another serine hydrolase (FAAH) described in Wilson et al.,... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2S186B3 |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM581635

((rac)-(2s,4s)-2-(6-(3-(tert-Butyl)phenyl)-2-azaspi...)Show SMILES CC(C)(C)c1cccc(c1)C1CCC2(CN(C2)C(=O)[C@H]2C[C@@]3(C2)COC(=O)N3)C1 |r,wU:19.20,wD:21.23,(9.51,-2.32,;8,-2.58,;7.46,-4.03,;8.98,-3.77,;7.01,-1.4,;7.55,.05,;6.56,1.23,;5.04,.97,;4.51,-.47,;5.49,-1.66,;2.97,-.47,;2.07,-1.72,;.6,-1.24,;.6,.3,;.36,1.82,;-1.16,1.58,;-.92,.06,;-2.41,2.49,;-2.41,4.03,;-3.81,1.86,;-4.21,.37,;-5.7,.77,;-5.3,2.26,;-5.62,-.77,;-7.06,-1.32,;-8.03,-.12,;-9.51,.08,;-7.19,1.17,;2.07,.78,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0860 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The assay used to measure the in vitro activity of MGL is adapted from the assay used for another serine hydrolase (FAAH) described in Wilson et al.,... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2S186B3 |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM581518

((2s,4s)-2-[2-(3-Cyclopropylphenyl)-7-azaspiro[3.5]...)Show SMILES O=C([C@H]1C[C@@]2(C1)COC(=O)N2)N1CCC2(CC(C2)c2cccc(c2)C2CC2)CC1 |r,wU:2.1,wD:4.4,(-2.84,4.01,;-2.84,2.47,;-4.18,1.7,;-4.57,.21,;-6.06,.61,;-5.66,2.1,;-6.06,-.93,;-7.53,-1.41,;-8.43,-.16,;-9.92,.24,;-7.53,1.09,;-1.51,1.7,;-1.51,.16,;-.18,-.61,;1.16,.16,;1.56,-1.33,;3.04,-.93,;2.65,.56,;4.38,-1.7,;4.38,-3.24,;5.71,-4.01,;7.05,-3.24,;7.05,-1.7,;5.71,-.93,;8.38,-.93,;9.92,-.93,;9.15,.4,;1.16,1.7,;-.18,2.47,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0880 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The assay used to measure the in vitro activity of MGL is adapted from the assay used for another serine hydrolase (FAAH) described in Wilson et al.,... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2S186B3 |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM581594

((2s,4s)-2-[2-[3-(Trifluoromethoxy)phenyl]-7-azaspi...)Show SMILES FC(F)(F)Oc1cccc(c1)C1CC2(C1)CCN(CC2)C(=O)[C@H]1C[C@@]2(C1)COC(=O)N2 |r,wU:22.24,wD:24.27,(8.53,2.18,;8.53,.64,;10.07,.64,;6.99,.64,;8.53,-.9,;7.2,-1.67,;7.15,-3.24,;5.82,-4.01,;4.48,-3.24,;4.48,-1.7,;5.82,-.93,;3.15,-.93,;1.66,-1.33,;1.26,.16,;2.75,.56,;-.07,-.61,;-1.41,.16,;-1.41,1.7,;-.07,2.47,;1.26,1.7,;-2.74,2.47,;-2.74,4.01,;-4.07,1.7,;-4.47,.21,;-5.96,.61,;-5.56,2.1,;-5.96,-.93,;-7.42,-1.41,;-8.33,-.16,;-9.82,.24,;-7.42,1.09,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0940 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The assay used to measure the in vitro activity of MGL is adapted from the assay used for another serine hydrolase (FAAH) described in Wilson et al.,... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2S186B3 |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM581601

((2r,4s)-2-(2-(6-(tert-Butyl)pyridin-2-yl)-7-azaspi...)Show SMILES CC(C)(C)c1cccc(n1)C1CC2(C1)CCN(CC2)C(=O)[C@H]1C[C@@]2(C1)COC(=O)N2 |r,wU:21.23,wD:23.26,(9.88,-.16,;8.54,-.93,;8.54,.61,;9.88,-1.7,;7.21,-1.7,;7.21,-3.24,;5.88,-4.01,;4.54,-3.24,;4.54,-1.7,;5.88,-.93,;3.21,-.93,;1.72,-1.33,;1.32,.16,;2.81,.56,;-.01,-.61,;-1.35,.16,;-1.35,1.7,;-.01,2.47,;1.32,1.7,;-2.68,2.47,;-2.68,4.01,;-4.01,1.7,;-4.41,.21,;-5.9,.61,;-5.5,2.1,;-6.06,-.92,;-7.57,-1.24,;-8.34,.09,;-9.88,.09,;-7.31,1.24,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0950 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The assay used to measure the in vitro activity of MGL is adapted from the assay used for another serine hydrolase (FAAH) described in Wilson et al.,... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2S186B3 |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM581650

((2s,4s)-2-(6-(3-(tert-Butyl)phenyl)-2-azaspiro[3.3...)Show SMILES CC(C)(C)c1cccc(c1)C1CC2(C1)CN(C2)C(=O)[C@H]1C[C@@]2(C1)COC(=O)N2 |r,wU:19.21,wD:21.24,(8.07,.84,;8.07,-.7,;9.4,-1.47,;9.4,.07,;6.74,-1.47,;6.74,-3.01,;5.4,-3.78,;4.07,-3.01,;4.07,-1.47,;5.4,-.7,;2.74,-.7,;1.25,-1.1,;.85,.38,;2.34,.78,;-.64,-.01,;-1.04,1.47,;.45,1.87,;-2.37,2.24,;-2.37,3.78,;-3.7,1.47,;-4.1,-.01,;-5.59,.39,;-5.19,1.87,;-5.51,-1.15,;-6.95,-1.7,;-7.92,-.51,;-9.4,-.43,;-7.08,.78,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The assay used to measure the in vitro activity of MGL is adapted from the assay used for another serine hydrolase (FAAH) described in Wilson et al.,... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2S186B3 |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM581648
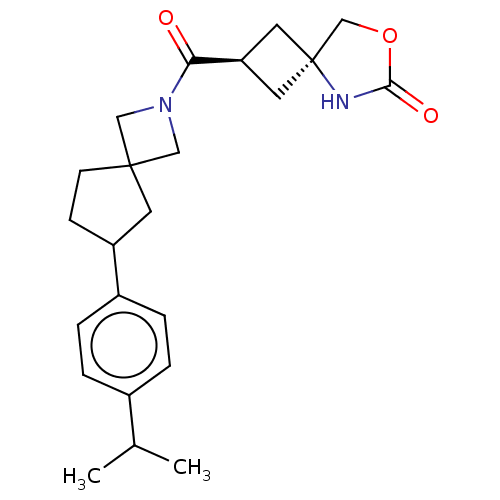
((rac)-(2s,4s)-2-(6-(4-Isopropylphenyl)-2-azaspiro[...)Show SMILES CC(C)c1ccc(cc1)C1CCC2(CN(C2)C(=O)[C@H]2C[C@@]3(C2)COC(=O)N3)C1 |r,wU:18.19,wD:20.22,(9.62,.42,;8.95,-.97,;9.82,-2.24,;7.41,-1.08,;6.74,-2.47,;5.21,-2.58,;4.34,-1.31,;5.01,.08,;6.55,.19,;2.81,-1.42,;1.9,-2.67,;.44,-2.19,;.44,-.65,;-1.05,-1.05,;-1.45,.43,;.04,.83,;-2.78,1.2,;-2.78,2.74,;-4.12,.43,;-4.52,-1.05,;-6,-.65,;-5.6,.83,;-5.92,-2.19,;-7.36,-2.74,;-8.33,-1.55,;-9.82,-1.41,;-7.49,-.26,;1.9,-.18,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The assay used to measure the in vitro activity of MGL is adapted from the assay used for another serine hydrolase (FAAH) described in Wilson et al.,... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2S186B3 |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM581631

((2s,4R*)-2-((S*)-6-(4-(Trifluoromethyl)phenyl)-2-a...)Show SMILES FC(F)(F)c1ccc(cc1)[C@H]1CCC2(CN(C2)C(=O)[C@H]2C[C@@]3(C2)COC(=O)N3)C1 |r,wU:10.10,19.20,wD:21.23,(9.12,.44,;8.54,-.99,;9.48,-2.2,;10.06,-.77,;7.01,-1.2,;6.43,-2.63,;4.91,-2.84,;3.96,-1.62,;4.54,-.2,;6.07,.02,;2.42,-1.62,;1.52,-2.87,;.05,-2.39,;.05,-.85,;-.19,.67,;-1.71,.43,;-1.47,-1.1,;-2.96,1.33,;-2.96,2.87,;-4.36,.7,;-4.76,-.78,;-6.25,-.38,;-5.85,1.1,;-6.17,-1.92,;-7.61,-2.47,;-8.57,-1.28,;-10.06,-1.08,;-7.74,.01,;1.52,-.38,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The assay used to measure the in vitro activity of MGL is adapted from the assay used for another serine hydrolase (FAAH) described in Wilson et al.,... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2S186B3 |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM630876

((7-((3,6-Dimethyl-5-(trifluoromethyl)pyridin-2-yl)...)Show SMILES Cc1cc(c(C)nc1OC1CCC2(CN(C2)C(=O)[C@H]2C[C@@](C)(O)C2)CC1)C(F)(F)F |r,wU:20.23,18.19,wD:20.22,(5.29,-3.62,;5.29,-2.08,;6.62,-1.31,;6.62,.23,;5.29,1,;5.29,2.54,;3.95,.23,;3.95,-1.31,;2.62,-2.08,;1.29,-1.31,;-.05,-2.08,;-1.38,-1.31,;-1.38,.23,;-2.87,-.17,;-3.27,1.31,;-1.78,1.71,;-4.6,2.08,;-4.6,3.62,;-5.93,1.31,;-6.33,-.17,;-7.82,.23,;-8.22,-1.26,;-9.29,.64,;-7.42,1.71,;-.05,1,;1.29,.23,;7.95,1,;7.95,2.54,;9.29,.23,;9.29,1.77,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM581642
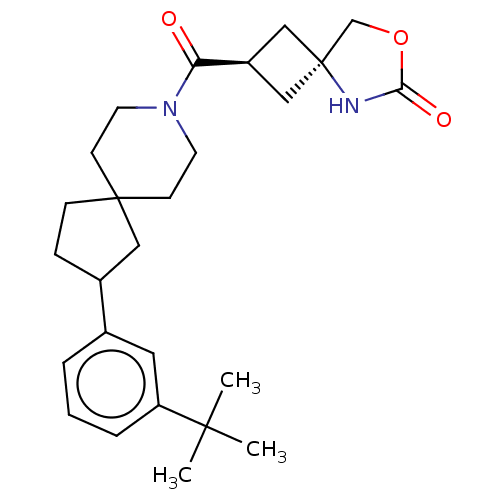
((rac)-(2s,4s)-2-(2-(3-(tert-Butyl)phenyl)-8-azaspi...)Show SMILES CC(C)(C)c1cccc(c1)C1CCC2(C1)CCN(CC2)C(=O)[C@H]1C[C@@]2(C1)COC(=O)N2 |r,wU:22.24,wD:24.27,(7.66,-4.22,;8.36,-2.84,;9.9,-2.76,;9.2,-4.13,;7.52,-1.55,;8.1,-.13,;7.16,1.09,;5.63,.88,;5.05,-.54,;5.99,-1.76,;3.51,-.54,;2.6,-1.79,;1.14,-1.31,;1.14,.23,;2.6,.71,;-.19,-.54,;-1.53,.23,;-1.54,1.77,;-.19,2.54,;1.14,1.77,;-2.79,2.68,;-2.79,4.22,;-4.2,2.05,;-4.6,.56,;-6.08,.96,;-5.68,2.45,;-6,-.58,;-7.44,-1.13,;-8.41,.07,;-9.9,.21,;-7.57,1.36,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The assay used to measure the in vitro activity of MGL is adapted from the assay used for another serine hydrolase (FAAH) described in Wilson et al.,... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2S186B3 |
More data for this
Ligand-Target Pair | |
Monoglyceride lipase
(Homo sapiens (Human)) | BDBM581595

((2s,4s)-2-[2-(2,3-Dimethylphenyl)-7-azaspiro[3.5]n...)Show SMILES Cc1cccc(C2CC3(C2)CCN(CC3)C(=O)[C@H]2C[C@@]3(C2)COC(=O)N3)c1C |r,wU:17.18,wD:19.21,(9.15,-.93,;7.82,-1.7,;7.82,-3.24,;6.48,-4.01,;5.15,-3.24,;5.15,-1.7,;3.81,-.93,;2.33,-1.33,;1.93,.16,;3.42,.56,;.59,-.61,;-.74,.16,;-.74,1.7,;.59,2.47,;1.93,1.7,;-2.07,2.47,;-2.07,4.01,;-3.41,1.7,;-3.8,.21,;-5.29,.61,;-4.89,2.1,;-5.29,-.93,;-6.76,-1.41,;-7.66,-.16,;-9.15,.24,;-6.76,1.09,;6.48,-.93,;6.48,.61,)| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
The assay used to measure the in vitro activity of MGL is adapted from the assay used for another serine hydrolase (FAAH) described in Wilson et al.,... |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2S186B3 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data