 Found 82 hits with Last Name = 'bertrand' and Initial = 'c'
Found 82 hits with Last Name = 'bertrand' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM86231

(ATR | ATROPINE | Atropine,(-) | CAS_51-55-8 | CHEM...)Show SMILES CN1C2CCC1CC(C2)OC(=O)C(CO)c1ccccc1 |THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Louis Pasteur-Strasbourg
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 250: 309-15 (1989)
BindingDB Entry DOI: 10.7270/Q2KP80NB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(GUINEA PIG) | BDBM86231

(ATR | ATROPINE | Atropine,(-) | CAS_51-55-8 | CHEM...)Show SMILES CN1C2CCC1CC(C2)OC(=O)C(CO)c1ccccc1 |THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Louis Pasteur-Strasbourg
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 250: 309-15 (1989)
BindingDB Entry DOI: 10.7270/Q2KP80NB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(GUINEA PIG) | BDBM86231

(ATR | ATROPINE | Atropine,(-) | CAS_51-55-8 | CHEM...)Show SMILES CN1C2CCC1CC(C2)OC(=O)C(CO)c1ccccc1 |THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Louis Pasteur-Strasbourg
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 250: 309-15 (1989)
BindingDB Entry DOI: 10.7270/Q2KP80NB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM86231

(ATR | ATROPINE | Atropine,(-) | CAS_51-55-8 | CHEM...)Show SMILES CN1C2CCC1CC(C2)OC(=O)C(CO)c1ccccc1 |THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 0.780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Louis Pasteur-Strasbourg
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 250: 309-15 (1989)
BindingDB Entry DOI: 10.7270/Q2KP80NB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50176065
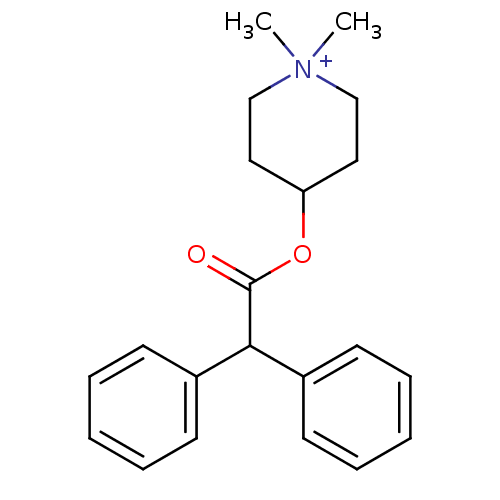
(4-DAMP | 4-Diphenylacetoxy-1,1-dimethyl-piperidini...)Show InChI InChI=1S/C21H26NO2/c1-22(2)15-13-19(14-16-22)24-21(23)20(17-9-5-3-6-10-17)18-11-7-4-8-12-18/h3-12,19-20H,13-16H2,1-2H3/q+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.37 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Louis Pasteur-Strasbourg
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 250: 309-15 (1989)
BindingDB Entry DOI: 10.7270/Q2KP80NB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM86231

(ATR | ATROPINE | Atropine,(-) | CAS_51-55-8 | CHEM...)Show SMILES CN1C2CCC1CC(C2)OC(=O)C(CO)c1ccccc1 |THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1.41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Louis Pasteur-Strasbourg
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 250: 309-15 (1989)
BindingDB Entry DOI: 10.7270/Q2KP80NB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50176065

(4-DAMP | 4-Diphenylacetoxy-1,1-dimethyl-piperidini...)Show InChI InChI=1S/C21H26NO2/c1-22(2)15-13-19(14-16-22)24-21(23)20(17-9-5-3-6-10-17)18-11-7-4-8-12-18/h3-12,19-20H,13-16H2,1-2H3/q+1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Louis Pasteur-Strasbourg
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 250: 309-15 (1989)
BindingDB Entry DOI: 10.7270/Q2KP80NB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50176065

(4-DAMP | 4-Diphenylacetoxy-1,1-dimethyl-piperidini...)Show InChI InChI=1S/C21H26NO2/c1-22(2)15-13-19(14-16-22)24-21(23)20(17-9-5-3-6-10-17)18-11-7-4-8-12-18/h3-12,19-20H,13-16H2,1-2H3/q+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Louis Pasteur-Strasbourg
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 250: 309-15 (1989)
BindingDB Entry DOI: 10.7270/Q2KP80NB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(GUINEA PIG) | BDBM50176065

(4-DAMP | 4-Diphenylacetoxy-1,1-dimethyl-piperidini...)Show InChI InChI=1S/C21H26NO2/c1-22(2)15-13-19(14-16-22)24-21(23)20(17-9-5-3-6-10-17)18-11-7-4-8-12-18/h3-12,19-20H,13-16H2,1-2H3/q+1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 6.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Louis Pasteur-Strasbourg
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 250: 309-15 (1989)
BindingDB Entry DOI: 10.7270/Q2KP80NB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(GUINEA PIG) | BDBM50176065

(4-DAMP | 4-Diphenylacetoxy-1,1-dimethyl-piperidini...)Show InChI InChI=1S/C21H26NO2/c1-22(2)15-13-19(14-16-22)24-21(23)20(17-9-5-3-6-10-17)18-11-7-4-8-12-18/h3-12,19-20H,13-16H2,1-2H3/q+1 | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 10.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Louis Pasteur-Strasbourg
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 250: 309-15 (1989)
BindingDB Entry DOI: 10.7270/Q2KP80NB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM39341
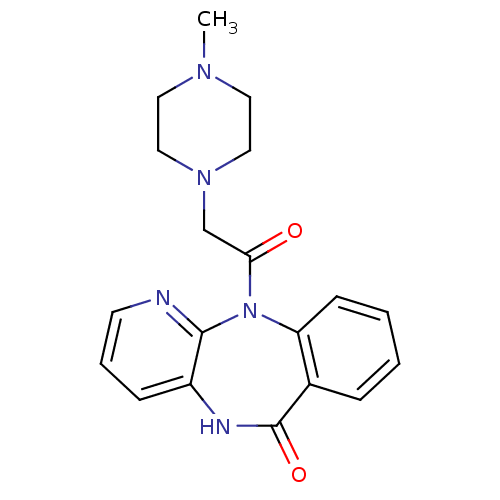
(11-[(4-methylpiperazin-1-yl)acetyl]-5,11-dihydro-6...)Show InChI InChI=1S/C19H21N5O2/c1-22-9-11-23(12-10-22)13-17(25)24-16-7-3-2-5-14(16)19(26)21-15-6-4-8-20-18(15)24/h2-8H,9-13H2,1H3,(H,21,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 17.3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Louis Pasteur-Strasbourg
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 250: 309-15 (1989)
BindingDB Entry DOI: 10.7270/Q2KP80NB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50018056
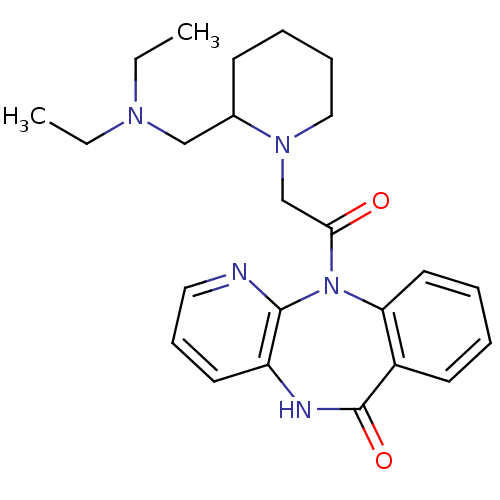
((AF-DX 116) 11-[2-(2-Diethylaminomethyl-piperidin-...)Show SMILES CCN(CC)CC1CCCCN1CC(=O)N1c2ccccc2C(=O)Nc2cccnc12 Show InChI InChI=1S/C24H31N5O2/c1-3-27(4-2)16-18-10-7-8-15-28(18)17-22(30)29-21-13-6-5-11-19(21)24(31)26-20-12-9-14-25-23(20)29/h5-6,9,11-14,18H,3-4,7-8,10,15-17H2,1-2H3,(H,26,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 17.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Louis Pasteur-Strasbourg
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 250: 309-15 (1989)
BindingDB Entry DOI: 10.7270/Q2KP80NB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(GUINEA PIG) | BDBM50018056

((AF-DX 116) 11-[2-(2-Diethylaminomethyl-piperidin-...)Show SMILES CCN(CC)CC1CCCCN1CC(=O)N1c2ccccc2C(=O)Nc2cccnc12 Show InChI InChI=1S/C24H31N5O2/c1-3-27(4-2)16-18-10-7-8-15-28(18)17-22(30)29-21-13-6-5-11-19(21)24(31)26-20-12-9-14-25-23(20)29/h5-6,9,11-14,18H,3-4,7-8,10,15-17H2,1-2H3,(H,26,31) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 21.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Louis Pasteur-Strasbourg
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 250: 309-15 (1989)
BindingDB Entry DOI: 10.7270/Q2KP80NB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50018056

((AF-DX 116) 11-[2-(2-Diethylaminomethyl-piperidin-...)Show SMILES CCN(CC)CC1CCCCN1CC(=O)N1c2ccccc2C(=O)Nc2cccnc12 Show InChI InChI=1S/C24H31N5O2/c1-3-27(4-2)16-18-10-7-8-15-28(18)17-22(30)29-21-13-6-5-11-19(21)24(31)26-20-12-9-14-25-23(20)29/h5-6,9,11-14,18H,3-4,7-8,10,15-17H2,1-2H3,(H,26,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 26.9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Louis Pasteur-Strasbourg
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 250: 309-15 (1989)
BindingDB Entry DOI: 10.7270/Q2KP80NB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(GUINEA PIG) | BDBM50018056

((AF-DX 116) 11-[2-(2-Diethylaminomethyl-piperidin-...)Show SMILES CCN(CC)CC1CCCCN1CC(=O)N1c2ccccc2C(=O)Nc2cccnc12 Show InChI InChI=1S/C24H31N5O2/c1-3-27(4-2)16-18-10-7-8-15-28(18)17-22(30)29-21-13-6-5-11-19(21)24(31)26-20-12-9-14-25-23(20)29/h5-6,9,11-14,18H,3-4,7-8,10,15-17H2,1-2H3,(H,26,31) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 28.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Louis Pasteur-Strasbourg
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 250: 309-15 (1989)
BindingDB Entry DOI: 10.7270/Q2KP80NB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50018056

((AF-DX 116) 11-[2-(2-Diethylaminomethyl-piperidin-...)Show SMILES CCN(CC)CC1CCCCN1CC(=O)N1c2ccccc2C(=O)Nc2cccnc12 Show InChI InChI=1S/C24H31N5O2/c1-3-27(4-2)16-18-10-7-8-15-28(18)17-22(30)29-21-13-6-5-11-19(21)24(31)26-20-12-9-14-25-23(20)29/h5-6,9,11-14,18H,3-4,7-8,10,15-17H2,1-2H3,(H,26,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 31.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Louis Pasteur-Strasbourg
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 250: 309-15 (1989)
BindingDB Entry DOI: 10.7270/Q2KP80NB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(GUINEA PIG) | BDBM39341

(11-[(4-methylpiperazin-1-yl)acetyl]-5,11-dihydro-6...)Show InChI InChI=1S/C19H21N5O2/c1-22-9-11-23(12-10-22)13-17(25)24-16-7-3-2-5-14(16)19(26)21-15-6-4-8-20-18(15)24/h2-8H,9-13H2,1H3,(H,21,26) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 80.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Louis Pasteur-Strasbourg
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 250: 309-15 (1989)
BindingDB Entry DOI: 10.7270/Q2KP80NB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50018056

((AF-DX 116) 11-[2-(2-Diethylaminomethyl-piperidin-...)Show SMILES CCN(CC)CC1CCCCN1CC(=O)N1c2ccccc2C(=O)Nc2cccnc12 Show InChI InChI=1S/C24H31N5O2/c1-3-27(4-2)16-18-10-7-8-15-28(18)17-22(30)29-21-13-6-5-11-19(21)24(31)26-20-12-9-14-25-23(20)29/h5-6,9,11-14,18H,3-4,7-8,10,15-17H2,1-2H3,(H,26,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 99.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Louis Pasteur-Strasbourg
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 250: 309-15 (1989)
BindingDB Entry DOI: 10.7270/Q2KP80NB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM39341

(11-[(4-methylpiperazin-1-yl)acetyl]-5,11-dihydro-6...)Show InChI InChI=1S/C19H21N5O2/c1-22-9-11-23(12-10-22)13-17(25)24-16-7-3-2-5-14(16)19(26)21-15-6-4-8-20-18(15)24/h2-8H,9-13H2,1H3,(H,21,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 173 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Louis Pasteur-Strasbourg
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 250: 309-15 (1989)
BindingDB Entry DOI: 10.7270/Q2KP80NB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM39341

(11-[(4-methylpiperazin-1-yl)acetyl]-5,11-dihydro-6...)Show InChI InChI=1S/C19H21N5O2/c1-22-9-11-23(12-10-22)13-17(25)24-16-7-3-2-5-14(16)19(26)21-15-6-4-8-20-18(15)24/h2-8H,9-13H2,1H3,(H,21,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 203 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Louis Pasteur-Strasbourg
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 250: 309-15 (1989)
BindingDB Entry DOI: 10.7270/Q2KP80NB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(GUINEA PIG) | BDBM39341

(11-[(4-methylpiperazin-1-yl)acetyl]-5,11-dihydro-6...)Show InChI InChI=1S/C19H21N5O2/c1-22-9-11-23(12-10-22)13-17(25)24-16-7-3-2-5-14(16)19(26)21-15-6-4-8-20-18(15)24/h2-8H,9-13H2,1H3,(H,21,26) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 276 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Louis Pasteur-Strasbourg
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 250: 309-15 (1989)
BindingDB Entry DOI: 10.7270/Q2KP80NB |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM39341

(11-[(4-methylpiperazin-1-yl)acetyl]-5,11-dihydro-6...)Show InChI InChI=1S/C19H21N5O2/c1-22-9-11-23(12-10-22)13-17(25)24-16-7-3-2-5-14(16)19(26)21-15-6-4-8-20-18(15)24/h2-8H,9-13H2,1H3,(H,21,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 459 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université Louis Pasteur-Strasbourg
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 250: 309-15 (1989)
BindingDB Entry DOI: 10.7270/Q2KP80NB |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50106901
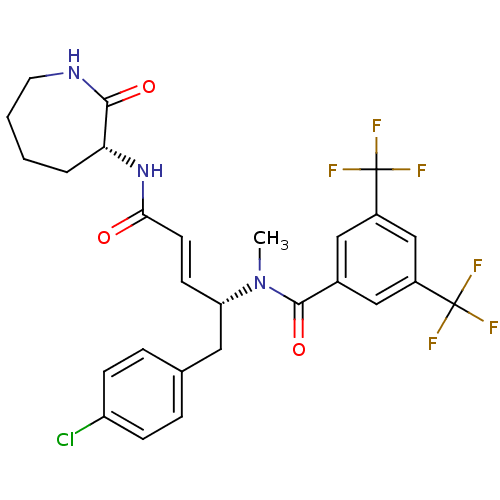
(CHEMBL104954 | N-[(E)-(R)-1-(4-Chloro-benzyl)-3-((...)Show SMILES CN([C@H](Cc1ccc(Cl)cc1)\C=C\C(=O)N[C@@H]1CCCCNC1=O)C(=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C27H26ClF6N3O3/c1-37(25(40)17-13-18(26(29,30)31)15-19(14-17)27(32,33)34)21(12-16-5-7-20(28)8-6-16)9-10-23(38)36-22-4-2-3-11-35-24(22)39/h5-10,13-15,21-22H,2-4,11-12H2,1H3,(H,35,39)(H,36,38)/b10-9+/t21-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
In vitro inhibition of [3H]-Sar9-substance P binding to Tachykinin receptor 1 in bovine retinal membranes |
Bioorg Med Chem Lett 10: 1467-70 (2000)
BindingDB Entry DOI: 10.7270/Q2M907X9 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50115967

(2-[(3,5-Bis-trifluoromethyl-benzoyl)-methyl-amino]...)Show SMILES CN(O)C(=O)c1ccc(-c2ccc(Cl)cc2)c(c1)N(C)C(=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C24H17ClF6N2O3/c1-32(21(34)15-9-16(23(26,27)28)12-17(10-15)24(29,30)31)20-11-14(22(35)33(2)36)5-8-19(20)13-3-6-18(25)7-4-13/h3-12,36H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
inhibition of [3H]-Sar-SP binding to Tachykinin receptor 1 of bovine retina membranes |
Bioorg Med Chem Lett 12: 2065-8 (2002)
BindingDB Entry DOI: 10.7270/Q2K64HD2 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50115966
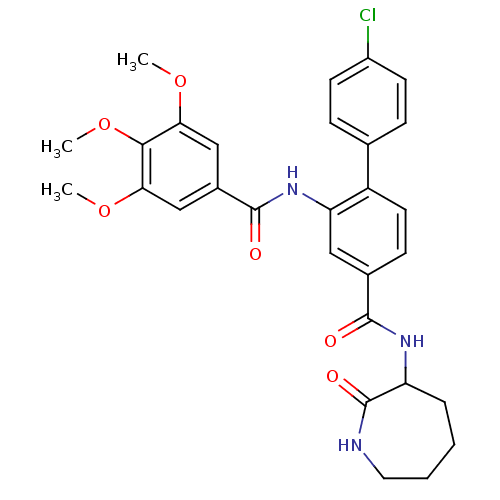
(4'-Chloro-2-(3,4,5-trimethoxy-benzoylamino)-biphen...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)Nc1cc(ccc1-c1ccc(Cl)cc1)C(=O)NC1CCCCNC1=O Show InChI InChI=1S/C29H30ClN3O6/c1-37-24-15-19(16-25(38-2)26(24)39-3)28(35)33-23-14-18(9-12-21(23)17-7-10-20(30)11-8-17)27(34)32-22-6-4-5-13-31-29(22)36/h7-12,14-16,22H,4-6,13H2,1-3H3,(H,31,36)(H,32,34)(H,33,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
inhibition of [3H]-Sar-SP binding to Tachykinin receptor 1 of bovine retina membranes |
Bioorg Med Chem Lett 12: 2065-8 (2002)
BindingDB Entry DOI: 10.7270/Q2K64HD2 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50089567
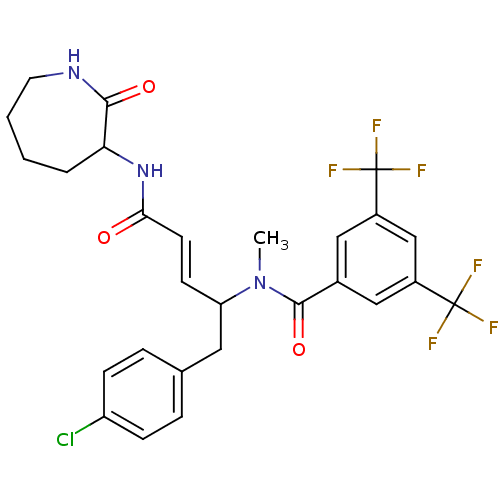
(CHEMBL35857 | N-[(E)-1-(4-Chloro-benzyl)-3-(2-oxo-...)Show SMILES CN(C(Cc1ccc(Cl)cc1)\C=C\C(=O)NC1CCCCNC1=O)C(=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C27H26ClF6N3O3/c1-37(25(40)17-13-18(26(29,30)31)15-19(14-17)27(32,33)34)21(12-16-5-7-20(28)8-6-16)9-10-23(38)36-22-4-2-3-11-35-24(22)39/h5-10,13-15,21-22H,2-4,11-12H2,1H3,(H,35,39)(H,36,38)/b10-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.760 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
In vitro inhibition of [3H]-Sar9-substance P binding to Tachykinin receptor 1 in bovine retinal membranes |
Bioorg Med Chem Lett 10: 1467-70 (2000)
BindingDB Entry DOI: 10.7270/Q2M907X9 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50403920
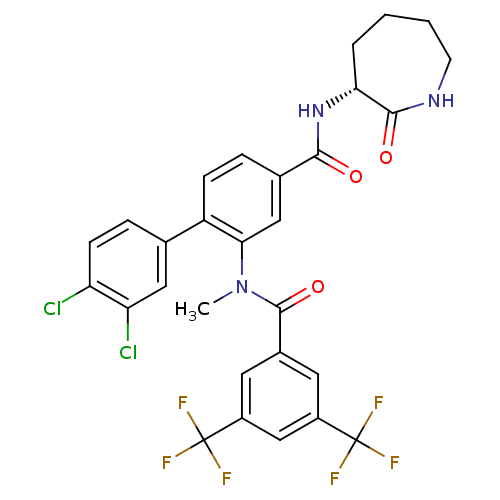
(CHEMBL2112212)Show SMILES CN(C(=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1cc(ccc1-c1ccc(Cl)c(Cl)c1)C(=O)N[C@@H]1CCCCNC1=O |r| Show InChI InChI=1S/C29H23Cl2F6N3O3/c1-40(27(43)17-10-18(28(32,33)34)14-19(11-17)29(35,36)37)24-13-16(25(41)39-23-4-2-3-9-38-26(23)42)5-7-20(24)15-6-8-21(30)22(31)12-15/h5-8,10-14,23H,2-4,9H2,1H3,(H,38,42)(H,39,41)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
inhibition of [3H]-Sar-SP binding to Tachykinin receptor 1 of bovine retina membranes |
Bioorg Med Chem Lett 12: 2065-8 (2002)
BindingDB Entry DOI: 10.7270/Q2K64HD2 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50115965

(2-[(3,5-Bis-trifluoromethyl-benzoyl)-methyl-amino]...)Show SMILES CN(C)CCCNC(=O)c1ccc(-c2ccc(Cl)cc2)c(c1)N(C)C(=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C28H26ClF6N3O2/c1-37(2)12-4-11-36-25(39)18-7-10-23(17-5-8-22(29)9-6-17)24(15-18)38(3)26(40)19-13-20(27(30,31)32)16-21(14-19)28(33,34)35/h5-10,13-16H,4,11-12H2,1-3H3,(H,36,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
inhibition of [3H]-Sar-SP binding to Tachykinin receptor 1 of bovine retina membranes |
Bioorg Med Chem Lett 12: 2065-8 (2002)
BindingDB Entry DOI: 10.7270/Q2K64HD2 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50403918
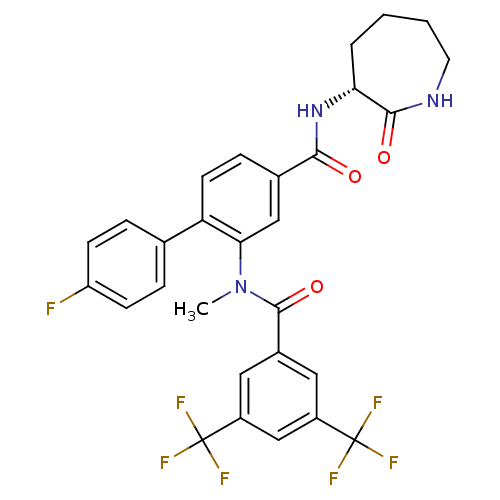
(CHEMBL2113739)Show SMILES CN(C(=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1cc(ccc1-c1ccc(F)cc1)C(=O)N[C@@H]1CCCCNC1=O |r| Show InChI InChI=1S/C29H24F7N3O3/c1-39(27(42)18-12-19(28(31,32)33)15-20(13-18)29(34,35)36)24-14-17(7-10-22(24)16-5-8-21(30)9-6-16)25(40)38-23-4-2-3-11-37-26(23)41/h5-10,12-15,23H,2-4,11H2,1H3,(H,37,41)(H,38,40)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
inhibition of [3H]-Sar-SP binding to Tachykinin receptor 1 of bovine retina membranes |
Bioorg Med Chem Lett 12: 2065-8 (2002)
BindingDB Entry DOI: 10.7270/Q2K64HD2 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50115959
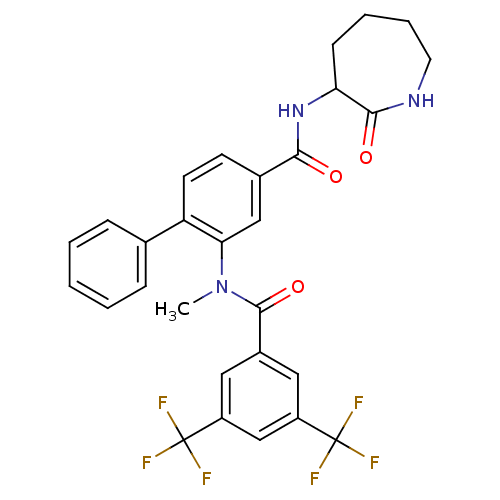
(2-[(3,5-Bis-trifluoromethyl-benzoyl)-methyl-amino]...)Show SMILES CN(C(=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1cc(ccc1-c1ccccc1)C(=O)NC1CCCCNC1=O Show InChI InChI=1S/C29H25F6N3O3/c1-38(27(41)19-13-20(28(30,31)32)16-21(14-19)29(33,34)35)24-15-18(10-11-22(24)17-7-3-2-4-8-17)25(39)37-23-9-5-6-12-36-26(23)40/h2-4,7-8,10-11,13-16,23H,5-6,9,12H2,1H3,(H,36,40)(H,37,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
inhibition of [3H]-Sar-SP binding to Tachykinin receptor 1 of bovine retina membranes |
Bioorg Med Chem Lett 12: 2065-8 (2002)
BindingDB Entry DOI: 10.7270/Q2K64HD2 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50403916

(CHEMBL2113740)Show SMILES CN(C(=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1cc(ccc1-c1ccc(Cl)cc1)C(=O)N[C@@H]1CCCCNC1=O |r| Show InChI InChI=1S/C29H24ClF6N3O3/c1-39(27(42)18-12-19(28(31,32)33)15-20(13-18)29(34,35)36)24-14-17(7-10-22(24)16-5-8-21(30)9-6-16)25(40)38-23-4-2-3-11-37-26(23)41/h5-10,12-15,23H,2-4,11H2,1H3,(H,37,41)(H,38,40)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
inhibition of [3H]-Sar-SP binding to Tachykinin receptor 1 of bovine retina membranes |
Bioorg Med Chem Lett 12: 2065-8 (2002)
BindingDB Entry DOI: 10.7270/Q2K64HD2 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50115957

(2-[(3,5-Bis-trifluoromethyl-benzoyl)-methyl-amino]...)Show SMILES CN(C(=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1cc(ccc1-c1ccc(Cl)cc1)C(=O)NC1CCCCC1 Show InChI InChI=1S/C29H25ClF6N2O2/c1-38(27(40)19-13-20(28(31,32)33)16-21(14-19)29(34,35)36)25-15-18(26(39)37-23-5-3-2-4-6-23)9-12-24(25)17-7-10-22(30)11-8-17/h7-16,23H,2-6H2,1H3,(H,37,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
inhibition of [3H]-Sar-SP binding to Tachykinin receptor 1 of bovine retina membranes |
Bioorg Med Chem Lett 12: 2065-8 (2002)
BindingDB Entry DOI: 10.7270/Q2K64HD2 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50115969
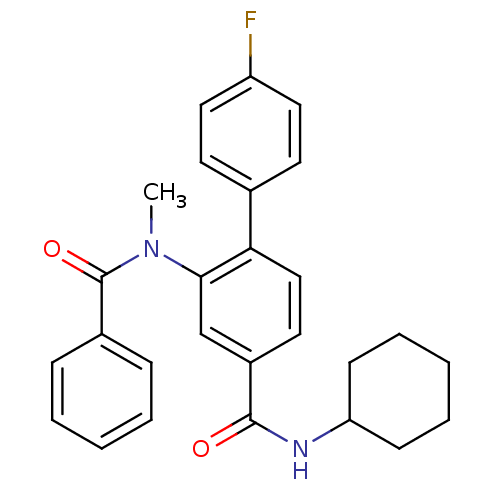
(CHEMBL305375 | N-[4-(N-Cyclohexyl-hydrazinocarbony...)Show SMILES CN(C(=O)c1ccccc1)c1cc(ccc1-c1ccc(F)cc1)C(=O)NC1CCCCC1 Show InChI InChI=1S/C27H27FN2O2/c1-30(27(32)20-8-4-2-5-9-20)25-18-21(26(31)29-23-10-6-3-7-11-23)14-17-24(25)19-12-15-22(28)16-13-19/h2,4-5,8-9,12-18,23H,3,6-7,10-11H2,1H3,(H,29,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
inhibition of [3H]-Sar-SP binding to Tachykinin receptor 1 of bovine retina membranes |
Bioorg Med Chem Lett 12: 2065-8 (2002)
BindingDB Entry DOI: 10.7270/Q2K64HD2 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50403789

(CHEMBL2115343)Show SMILES CN([C@H](Cc1ccc(Cl)cc1)\C=C\C(=O)N[C@H]1CCCCNC1=O)C(=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C27H26ClF6N3O3/c1-37(25(40)17-13-18(26(29,30)31)15-19(14-17)27(32,33)34)21(12-16-5-7-20(28)8-6-16)9-10-23(38)36-22-4-2-3-11-35-24(22)39/h5-10,13-15,21-22H,2-4,11-12H2,1H3,(H,35,39)(H,36,38)/b10-9+/t21-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
In vitro inhibition of [3H]-Sar9-substance P binding to Tachykinin receptor 1 in bovine retinal membranes |
Bioorg Med Chem Lett 10: 1467-70 (2000)
BindingDB Entry DOI: 10.7270/Q2M907X9 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50115964

(2-[(3,5-Bis-trifluoromethyl-benzoyl)-methyl-amino]...)Show SMILES CN(C)C(=O)c1ccc(-c2ccc(Cl)cc2)c(c1)N(C)C(=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C25H19ClF6N2O2/c1-33(2)22(35)15-6-9-20(14-4-7-19(26)8-5-14)21(12-15)34(3)23(36)16-10-17(24(27,28)29)13-18(11-16)25(30,31)32/h4-13H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
inhibition of [3H]-Sar-SP binding to Tachykinin receptor 1 of bovine retina membranes |
Bioorg Med Chem Lett 12: 2065-8 (2002)
BindingDB Entry DOI: 10.7270/Q2K64HD2 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50403919

(CHEMBL2112211)Show SMILES CN(C(=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1cc(ccc1-c1ccc(C)cc1)C(=O)N[C@@H]1CCCCNC1=O |r| Show InChI InChI=1S/C30H27F6N3O3/c1-17-6-8-18(9-7-17)23-11-10-19(26(40)38-24-5-3-4-12-37-27(24)41)15-25(23)39(2)28(42)20-13-21(29(31,32)33)16-22(14-20)30(34,35)36/h6-11,13-16,24H,3-5,12H2,1-2H3,(H,37,41)(H,38,40)/t24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
inhibition of [3H]-Sar-SP binding to Tachykinin receptor 1 of bovine retina membranes |
Bioorg Med Chem Lett 12: 2065-8 (2002)
BindingDB Entry DOI: 10.7270/Q2K64HD2 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50115970

(4'-Chloro-2-(3,4,5-trimethoxy-benzoylamino)-biphen...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)Nc1cc(ccc1-c1ccc(Cl)cc1)C(=O)NC1CCCCC1 Show InChI InChI=1S/C29H31ClN2O5/c1-35-25-16-20(17-26(36-2)27(25)37-3)29(34)32-24-15-19(28(33)31-22-7-5-4-6-8-22)11-14-23(24)18-9-12-21(30)13-10-18/h9-17,22H,4-8H2,1-3H3,(H,31,33)(H,32,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
inhibition of [3H]-Sar-SP binding to Tachykinin receptor 1 of bovine retina membranes |
Bioorg Med Chem Lett 12: 2065-8 (2002)
BindingDB Entry DOI: 10.7270/Q2K64HD2 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50403917

(CHEMBL2112209)Show SMILES COc1cc(cc(OC)c1OC)C(=O)N(C)c1cc(ccc1-c1ccc(Cl)cc1)C(=O)N[C@@H]1CCCCNC1=O |r| Show InChI InChI=1S/C30H32ClN3O6/c1-34(30(37)20-16-25(38-2)27(40-4)26(17-20)39-3)24-15-19(10-13-22(24)18-8-11-21(31)12-9-18)28(35)33-23-7-5-6-14-32-29(23)36/h8-13,15-17,23H,5-7,14H2,1-4H3,(H,32,36)(H,33,35)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
inhibition of [3H]-Sar-SP binding to Tachykinin receptor 1 of bovine retina membranes |
Bioorg Med Chem Lett 12: 2065-8 (2002)
BindingDB Entry DOI: 10.7270/Q2K64HD2 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50403787
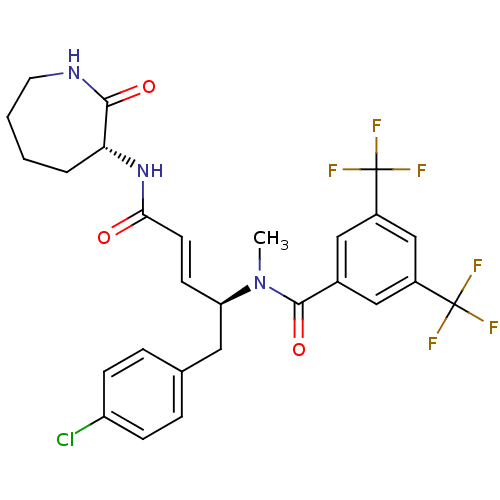
(CHEMBL2112070)Show SMILES CN([C@@H](Cc1ccc(Cl)cc1)\C=C\C(=O)N[C@@H]1CCCCNC1=O)C(=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C27H26ClF6N3O3/c1-37(25(40)17-13-18(26(29,30)31)15-19(14-17)27(32,33)34)21(12-16-5-7-20(28)8-6-16)9-10-23(38)36-22-4-2-3-11-35-24(22)39/h5-10,13-15,21-22H,2-4,11-12H2,1H3,(H,35,39)(H,36,38)/b10-9+/t21-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
In vitro inhibition of [3H]-Sar9-substance P binding to Tachykinin receptor 1 in bovine retinal membranes |
Bioorg Med Chem Lett 10: 1467-70 (2000)
BindingDB Entry DOI: 10.7270/Q2M907X9 |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50106901

(CHEMBL104954 | N-[(E)-(R)-1-(4-Chloro-benzyl)-3-((...)Show SMILES CN([C@H](Cc1ccc(Cl)cc1)\C=C\C(=O)N[C@@H]1CCCCNC1=O)C(=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C27H26ClF6N3O3/c1-37(25(40)17-13-18(26(29,30)31)15-19(14-17)27(32,33)34)21(12-16-5-7-20(28)8-6-16)9-10-23(38)36-22-4-2-3-11-35-24(22)39/h5-10,13-15,21-22H,2-4,11-12H2,1H3,(H,35,39)(H,36,38)/b10-9+/t21-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
In vitro inhibition of [125I]-NKA binding to transfected Chinese hamster ovary cells (CHO cells) expressing recombinant human Tachykinin receptor 2 |
Bioorg Med Chem Lett 10: 1467-70 (2000)
BindingDB Entry DOI: 10.7270/Q2M907X9 |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50403918

(CHEMBL2113739)Show SMILES CN(C(=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1cc(ccc1-c1ccc(F)cc1)C(=O)N[C@@H]1CCCCNC1=O |r| Show InChI InChI=1S/C29H24F7N3O3/c1-39(27(42)18-12-19(28(31,32)33)15-20(13-18)29(34,35)36)24-14-17(7-10-22(24)16-5-8-21(30)9-6-16)25(40)38-23-4-2-3-11-37-26(23)41/h5-10,12-15,23H,2-4,11H2,1H3,(H,37,41)(H,38,40)/t23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Binding affinity of the compound was determined by measuring the inhibition of 125 I-NKA binding to transfected CHO-cells expressing human recombinan... |
Bioorg Med Chem Lett 12: 2065-8 (2002)
BindingDB Entry DOI: 10.7270/Q2K64HD2 |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50115970

(4'-Chloro-2-(3,4,5-trimethoxy-benzoylamino)-biphen...)Show SMILES COc1cc(cc(OC)c1OC)C(=O)Nc1cc(ccc1-c1ccc(Cl)cc1)C(=O)NC1CCCCC1 Show InChI InChI=1S/C29H31ClN2O5/c1-35-25-16-20(17-26(36-2)27(25)37-3)29(34)32-24-15-19(28(33)31-22-7-5-4-6-8-22)11-14-23(24)18-9-12-21(30)13-10-18/h9-17,22H,4-8H2,1-3H3,(H,31,33)(H,32,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Binding affinity was determined by measuring the inhibition of 125 I-NKA binding to transfected CHO-cells expressing human recombinant Tachykinin rec... |
Bioorg Med Chem Lett 12: 2065-8 (2002)
BindingDB Entry DOI: 10.7270/Q2K64HD2 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50115963

(2-[(3,5-Bis-trifluoromethyl-benzoyl)-methyl-amino]...)Show SMILES CN(C(=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1cc(ccc1-c1ccc(F)cc1)C(=O)NC1CCCCC1 Show InChI InChI=1S/C29H25F7N2O2/c1-38(27(40)19-13-20(28(31,32)33)16-21(14-19)29(34,35)36)25-15-18(26(39)37-23-5-3-2-4-6-23)9-12-24(25)17-7-10-22(30)11-8-17/h7-16,23H,2-6H2,1H3,(H,37,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
inhibition of [3H]-Sar-SP binding to Tachykinin receptor 1 of bovine retina membranes |
Bioorg Med Chem Lett 12: 2065-8 (2002)
BindingDB Entry DOI: 10.7270/Q2K64HD2 |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50403920

(CHEMBL2112212)Show SMILES CN(C(=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1cc(ccc1-c1ccc(Cl)c(Cl)c1)C(=O)N[C@@H]1CCCCNC1=O |r| Show InChI InChI=1S/C29H23Cl2F6N3O3/c1-40(27(43)17-10-18(28(32,33)34)14-19(11-17)29(35,36)37)24-13-16(25(41)39-23-4-2-3-9-38-26(23)42)5-7-20(24)15-6-8-21(30)22(31)12-15/h5-8,10-14,23H,2-4,9H2,1H3,(H,38,42)(H,39,41)/t23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Binding affinity of the compound was determined by measuring the inhibition of 125 I-NKA binding to transfected CHO-cells expressing human recombinan... |
Bioorg Med Chem Lett 12: 2065-8 (2002)
BindingDB Entry DOI: 10.7270/Q2K64HD2 |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50115957

(2-[(3,5-Bis-trifluoromethyl-benzoyl)-methyl-amino]...)Show SMILES CN(C(=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1cc(ccc1-c1ccc(Cl)cc1)C(=O)NC1CCCCC1 Show InChI InChI=1S/C29H25ClF6N2O2/c1-38(27(40)19-13-20(28(31,32)33)16-21(14-19)29(34,35)36)25-15-18(26(39)37-23-5-3-2-4-6-23)9-12-24(25)17-7-10-22(30)11-8-17/h7-16,23H,2-6H2,1H3,(H,37,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Binding affinity was determined by measuring the inhibition of 125 I-NKA binding to transfected CHO-cells expressing human recombinant Tachykinin rec... |
Bioorg Med Chem Lett 12: 2065-8 (2002)
BindingDB Entry DOI: 10.7270/Q2K64HD2 |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50089567

(CHEMBL35857 | N-[(E)-1-(4-Chloro-benzyl)-3-(2-oxo-...)Show SMILES CN(C(Cc1ccc(Cl)cc1)\C=C\C(=O)NC1CCCCNC1=O)C(=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C27H26ClF6N3O3/c1-37(25(40)17-13-18(26(29,30)31)15-19(14-17)27(32,33)34)21(12-16-5-7-20(28)8-6-16)9-10-23(38)36-22-4-2-3-11-35-24(22)39/h5-10,13-15,21-22H,2-4,11-12H2,1H3,(H,35,39)(H,36,38)/b10-9+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
In vitro inhibition of [125I]-NKA binding to transfected Chinese hamster ovary cells (CHO cells) expressing recombinant human Tachykinin receptor 2 |
Bioorg Med Chem Lett 10: 1467-70 (2000)
BindingDB Entry DOI: 10.7270/Q2M907X9 |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50403916

(CHEMBL2113740)Show SMILES CN(C(=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F)c1cc(ccc1-c1ccc(Cl)cc1)C(=O)N[C@@H]1CCCCNC1=O |r| Show InChI InChI=1S/C29H24ClF6N3O3/c1-39(27(42)18-12-19(28(31,32)33)15-20(13-18)29(34,35)36)24-14-17(7-10-22(24)16-5-8-21(30)9-6-16)25(40)38-23-4-2-3-11-37-26(23)41/h5-10,12-15,23H,2-4,11H2,1H3,(H,37,41)(H,38,40)/t23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Binding affinity of the compound was determined by measuring the inhibition of 125 I-NKA binding to transfected CHO-cells expressing human recombinan... |
Bioorg Med Chem Lett 12: 2065-8 (2002)
BindingDB Entry DOI: 10.7270/Q2K64HD2 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50403788
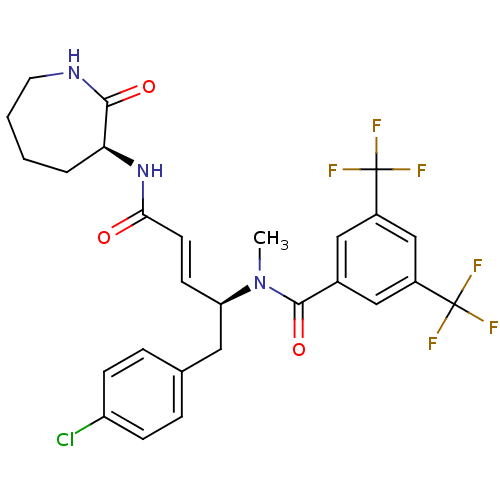
(CHEMBL2114398)Show SMILES CN([C@@H](Cc1ccc(Cl)cc1)\C=C\C(=O)N[C@H]1CCCCNC1=O)C(=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C27H26ClF6N3O3/c1-37(25(40)17-13-18(26(29,30)31)15-19(14-17)27(32,33)34)21(12-16-5-7-20(28)8-6-16)9-10-23(38)36-22-4-2-3-11-35-24(22)39/h5-10,13-15,21-22H,2-4,11-12H2,1H3,(H,35,39)(H,36,38)/b10-9+/t21-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
In vitro inhibition of [3H]-Sar9-substance P binding to Tachykinin receptor 1 in bovine retinal membranes |
Bioorg Med Chem Lett 10: 1467-70 (2000)
BindingDB Entry DOI: 10.7270/Q2M907X9 |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50115965

(2-[(3,5-Bis-trifluoromethyl-benzoyl)-methyl-amino]...)Show SMILES CN(C)CCCNC(=O)c1ccc(-c2ccc(Cl)cc2)c(c1)N(C)C(=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C28H26ClF6N3O2/c1-37(2)12-4-11-36-25(39)18-7-10-23(17-5-8-22(29)9-6-17)24(15-18)38(3)26(40)19-13-20(27(30,31)32)16-21(14-19)28(33,34)35/h5-10,13-16H,4,11-12H2,1-3H3,(H,36,39) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 108 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
Binding affinity was determined by measuring the inhibition of 125 I-NKA binding to transfected CHO-cells expressing human recombinant Tachykinin rec... |
Bioorg Med Chem Lett 12: 2065-8 (2002)
BindingDB Entry DOI: 10.7270/Q2K64HD2 |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50403789

(CHEMBL2115343)Show SMILES CN([C@H](Cc1ccc(Cl)cc1)\C=C\C(=O)N[C@H]1CCCCNC1=O)C(=O)c1cc(cc(c1)C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C27H26ClF6N3O3/c1-37(25(40)17-13-18(26(29,30)31)15-19(14-17)27(32,33)34)21(12-16-5-7-20(28)8-6-16)9-10-23(38)36-22-4-2-3-11-35-24(22)39/h5-10,13-15,21-22H,2-4,11-12H2,1H3,(H,35,39)(H,36,38)/b10-9+/t21-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharma AG
Curated by ChEMBL
| Assay Description
In vitro inhibition of [125I]-NKA binding to transfected Chinese hamster ovary cells (CHO cells) expressing recombinant human Tachykinin receptor 2 |
Bioorg Med Chem Lett 10: 1467-70 (2000)
BindingDB Entry DOI: 10.7270/Q2M907X9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data