

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DNA polymerase catalytic subunit (Human cytomegalovirus (HCMV strain AD169) ) | BDBM222924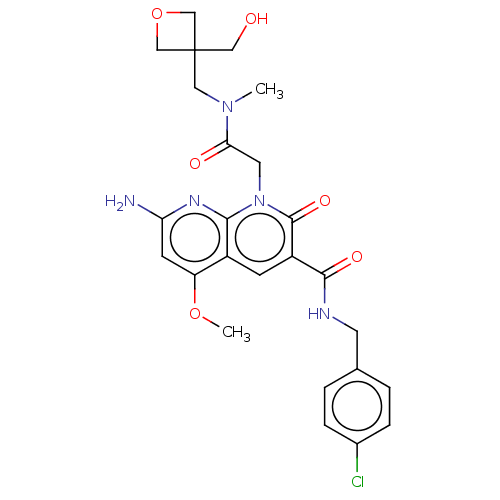 (US9315499, 6011) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 22 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The assay conditions are the following: 10 mM HEPES pH 7.5, 25 mM KCl, 7.5 mM NaCl, 5 mM MgCl2, 0.2 mg BSA/mL, 1 mM TCEP, 1.5% glycerol, 5% DMSO, 235... | US Patent US9315499 (2016) BindingDB Entry DOI: 10.7270/Q2028QCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50011004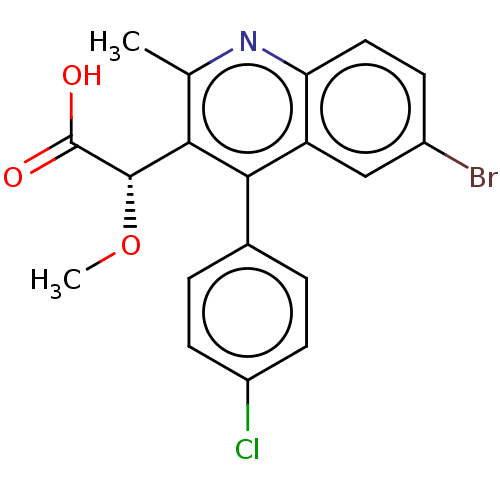 (CHEMBL3259893) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase by LTR cleavage assay | ACS Med Chem Lett 5: 422-7 (2014) Article DOI: 10.1021/ml500002n BindingDB Entry DOI: 10.7270/Q2K64KMK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| DNA polymerase catalytic subunit (Human cytomegalovirus (HCMV strain AD169) ) | BDBM222906 (US9315499, 4012) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The assay conditions are the following: 10 mM HEPES pH 7.5, 25 mM KCl, 7.5 mM NaCl, 5 mM MgCl2, 0.2 mg BSA/mL, 1 mM TCEP, 1.5% glycerol, 5% DMSO, 235... | US Patent US9315499 (2016) BindingDB Entry DOI: 10.7270/Q2028QCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50175297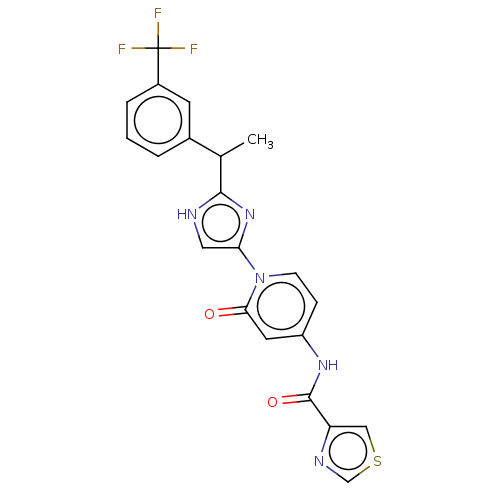 (CHEMBL3810245) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | ACS Med Chem Lett 7: 525-30 (2016) Article DOI: 10.1021/acsmedchemlett.6b00064 BindingDB Entry DOI: 10.7270/Q24B337T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50011132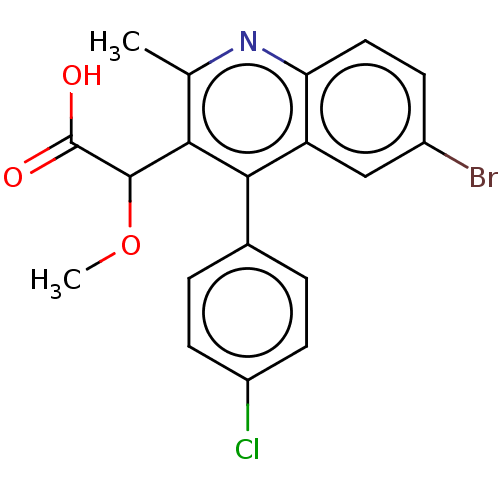 (ALLINI-1 | CHEMBL3259891) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase by LTR cleavage assay | ACS Med Chem Lett 5: 422-7 (2014) Article DOI: 10.1021/ml500002n BindingDB Entry DOI: 10.7270/Q2K64KMK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| DNA polymerase catalytic subunit (Human cytomegalovirus (HCMV strain AD169) ) | BDBM222899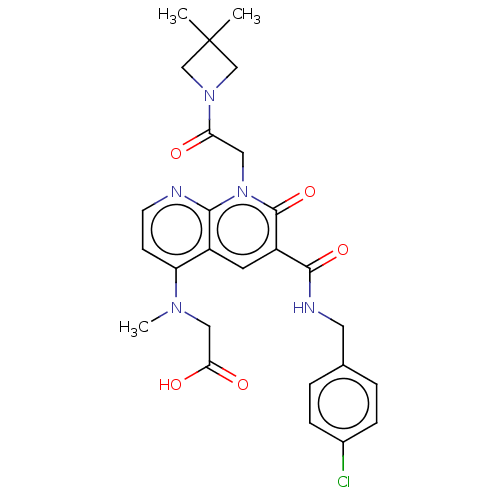 (US9315499, 3019) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 50 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The assay conditions are the following: 10 mM HEPES pH 7.5, 25 mM KCl, 7.5 mM NaCl, 5 mM MgCl2, 0.2 mg BSA/mL, 1 mM TCEP, 1.5% glycerol, 5% DMSO, 235... | US Patent US9315499 (2016) BindingDB Entry DOI: 10.7270/Q2028QCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase catalytic subunit (Human cytomegalovirus (HCMV strain AD169) ) | BDBM222915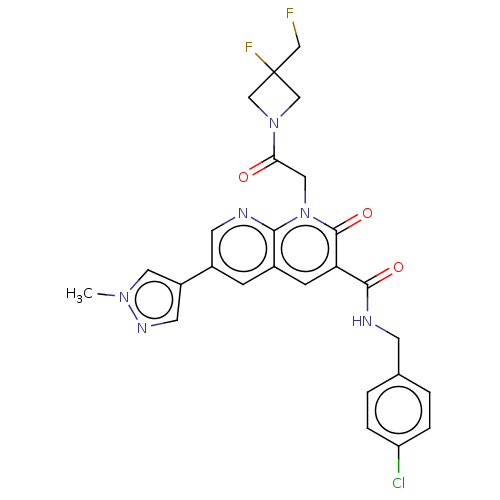 (US9315499, 4062) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 59 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The assay conditions are the following: 10 mM HEPES pH 7.5, 25 mM KCl, 7.5 mM NaCl, 5 mM MgCl2, 0.2 mg BSA/mL, 1 mM TCEP, 1.5% glycerol, 5% DMSO, 235... | US Patent US9315499 (2016) BindingDB Entry DOI: 10.7270/Q2028QCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase catalytic subunit (Human cytomegalovirus (HCMV strain AD169) ) | BDBM222903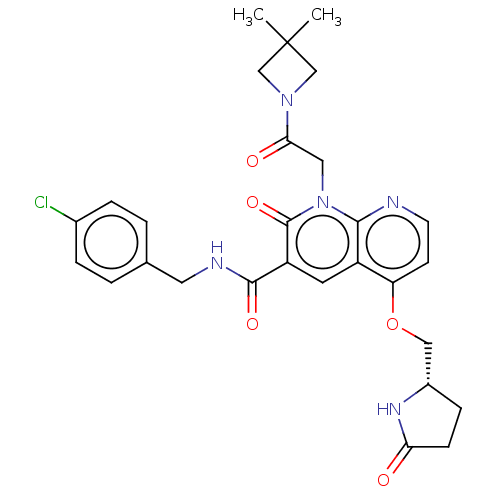 (US9315499, 3038) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 62 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The assay conditions are the following: 10 mM HEPES pH 7.5, 25 mM KCl, 7.5 mM NaCl, 5 mM MgCl2, 0.2 mg BSA/mL, 1 mM TCEP, 1.5% glycerol, 5% DMSO, 235... | US Patent US9315499 (2016) BindingDB Entry DOI: 10.7270/Q2028QCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase catalytic subunit (Human cytomegalovirus (HCMV strain AD169) ) | BDBM222905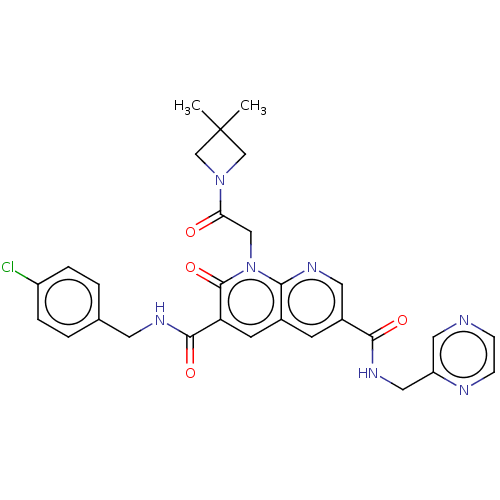 (US9315499, 4005) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 65 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The assay conditions are the following: 10 mM HEPES pH 7.5, 25 mM KCl, 7.5 mM NaCl, 5 mM MgCl2, 0.2 mg BSA/mL, 1 mM TCEP, 1.5% glycerol, 5% DMSO, 235... | US Patent US9315499 (2016) BindingDB Entry DOI: 10.7270/Q2028QCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase catalytic subunit (Human cytomegalovirus (HCMV strain AD169) ) | BDBM222909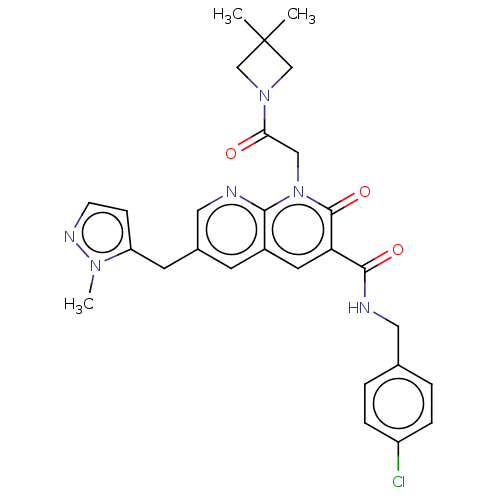 (US9315499, 4020) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 65 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The assay conditions are the following: 10 mM HEPES pH 7.5, 25 mM KCl, 7.5 mM NaCl, 5 mM MgCl2, 0.2 mg BSA/mL, 1 mM TCEP, 1.5% glycerol, 5% DMSO, 235... | US Patent US9315499 (2016) BindingDB Entry DOI: 10.7270/Q2028QCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase catalytic subunit (Human cytomegalovirus (HCMV strain AD169) ) | BDBM222901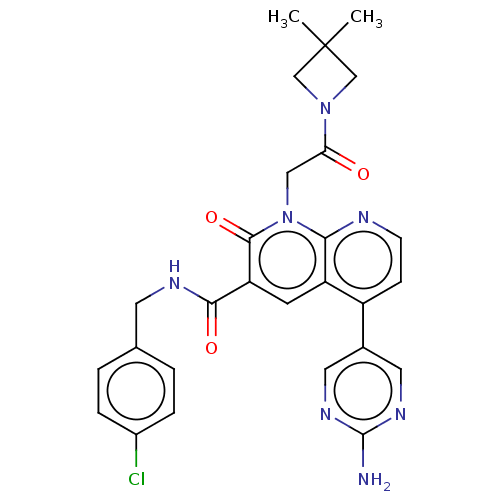 (US9315499, 3027) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 71 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The assay conditions are the following: 10 mM HEPES pH 7.5, 25 mM KCl, 7.5 mM NaCl, 5 mM MgCl2, 0.2 mg BSA/mL, 1 mM TCEP, 1.5% glycerol, 5% DMSO, 235... | US Patent US9315499 (2016) BindingDB Entry DOI: 10.7270/Q2028QCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase catalytic subunit (Human cytomegalovirus (HCMV strain AD169) ) | BDBM222910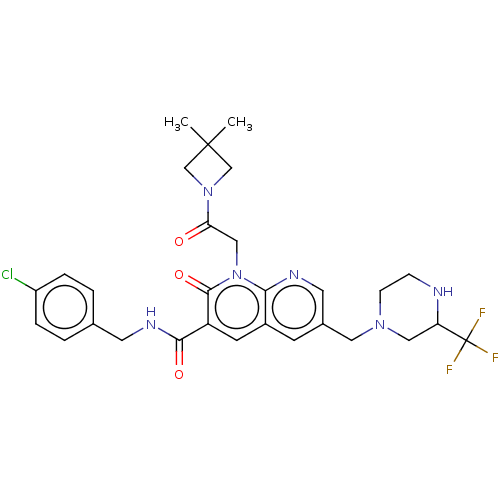 (US9315499, 4024) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 74 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The assay conditions are the following: 10 mM HEPES pH 7.5, 25 mM KCl, 7.5 mM NaCl, 5 mM MgCl2, 0.2 mg BSA/mL, 1 mM TCEP, 1.5% glycerol, 5% DMSO, 235... | US Patent US9315499 (2016) BindingDB Entry DOI: 10.7270/Q2028QCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase catalytic subunit (Human cytomegalovirus (HCMV strain AD169) ) | BDBM222926 (US9315499, 6017) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 77 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The assay conditions are the following: 10 mM HEPES pH 7.5, 25 mM KCl, 7.5 mM NaCl, 5 mM MgCl2, 0.2 mg BSA/mL, 1 mM TCEP, 1.5% glycerol, 5% DMSO, 235... | US Patent US9315499 (2016) BindingDB Entry DOI: 10.7270/Q2028QCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50011131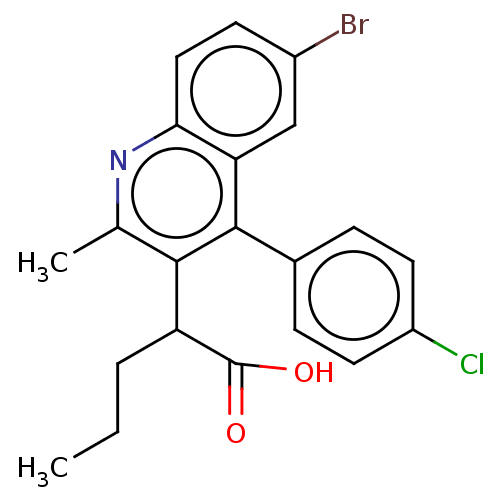 (CHEMBL3259890) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase by LTR cleavage assay | ACS Med Chem Lett 5: 422-7 (2014) Article DOI: 10.1021/ml500002n BindingDB Entry DOI: 10.7270/Q2K64KMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase catalytic subunit (Human cytomegalovirus (HCMV strain AD169) ) | BDBM222928 (US9315499, 6022) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 83 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The assay conditions are the following: 10 mM HEPES pH 7.5, 25 mM KCl, 7.5 mM NaCl, 5 mM MgCl2, 0.2 mg BSA/mL, 1 mM TCEP, 1.5% glycerol, 5% DMSO, 235... | US Patent US9315499 (2016) BindingDB Entry DOI: 10.7270/Q2028QCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase catalytic subunit (Human cytomegalovirus (HCMV strain AD169) ) | BDBM222925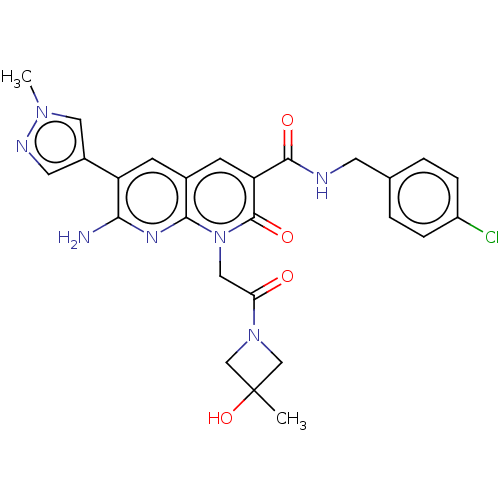 (US9315499, 6012) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 110 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The assay conditions are the following: 10 mM HEPES pH 7.5, 25 mM KCl, 7.5 mM NaCl, 5 mM MgCl2, 0.2 mg BSA/mL, 1 mM TCEP, 1.5% glycerol, 5% DMSO, 235... | US Patent US9315499 (2016) BindingDB Entry DOI: 10.7270/Q2028QCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase catalytic subunit (Human cytomegalovirus (HCMV strain AD169) ) | BDBM222911 (US9315499, 4034) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 120 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The assay conditions are the following: 10 mM HEPES pH 7.5, 25 mM KCl, 7.5 mM NaCl, 5 mM MgCl2, 0.2 mg BSA/mL, 1 mM TCEP, 1.5% glycerol, 5% DMSO, 235... | US Patent US9315499 (2016) BindingDB Entry DOI: 10.7270/Q2028QCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase catalytic subunit (Human cytomegalovirus (HCMV strain AD169) ) | BDBM222922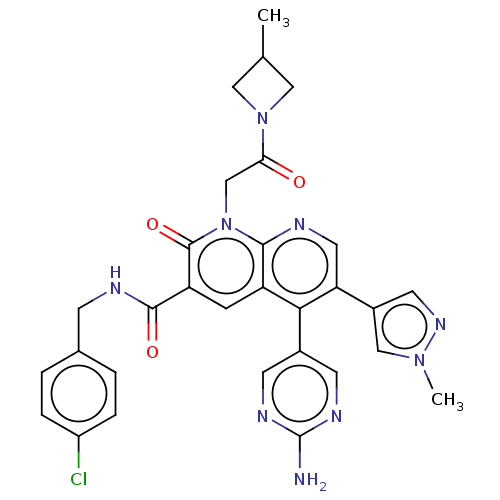 (US9315499, 6005) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 120 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The assay conditions are the following: 10 mM HEPES pH 7.5, 25 mM KCl, 7.5 mM NaCl, 5 mM MgCl2, 0.2 mg BSA/mL, 1 mM TCEP, 1.5% glycerol, 5% DMSO, 235... | US Patent US9315499 (2016) BindingDB Entry DOI: 10.7270/Q2028QCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase catalytic subunit (Human cytomegalovirus (HCMV strain AD169) ) | BDBM222919 (US9315499, 5015) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 120 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The assay conditions are the following: 10 mM HEPES pH 7.5, 25 mM KCl, 7.5 mM NaCl, 5 mM MgCl2, 0.2 mg BSA/mL, 1 mM TCEP, 1.5% glycerol, 5% DMSO, 235... | US Patent US9315499 (2016) BindingDB Entry DOI: 10.7270/Q2028QCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase catalytic subunit (Human cytomegalovirus (HCMV strain AD169) ) | BDBM222913 (US9315499, 4040) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 130 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The assay conditions are the following: 10 mM HEPES pH 7.5, 25 mM KCl, 7.5 mM NaCl, 5 mM MgCl2, 0.2 mg BSA/mL, 1 mM TCEP, 1.5% glycerol, 5% DMSO, 235... | US Patent US9315499 (2016) BindingDB Entry DOI: 10.7270/Q2028QCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase catalytic subunit (Human cytomegalovirus (HCMV strain AD169) ) | BDBM222921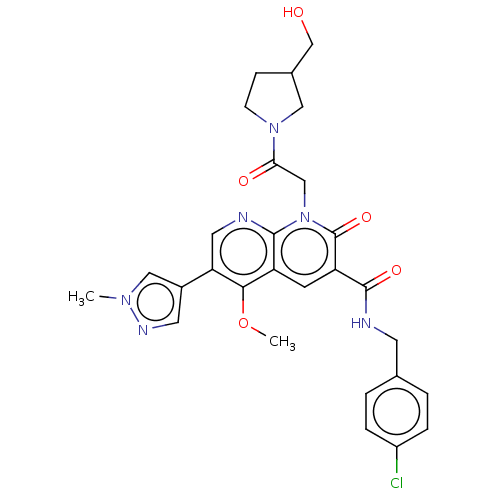 (US9315499, 6003) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 140 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The assay conditions are the following: 10 mM HEPES pH 7.5, 25 mM KCl, 7.5 mM NaCl, 5 mM MgCl2, 0.2 mg BSA/mL, 1 mM TCEP, 1.5% glycerol, 5% DMSO, 235... | US Patent US9315499 (2016) BindingDB Entry DOI: 10.7270/Q2028QCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50175302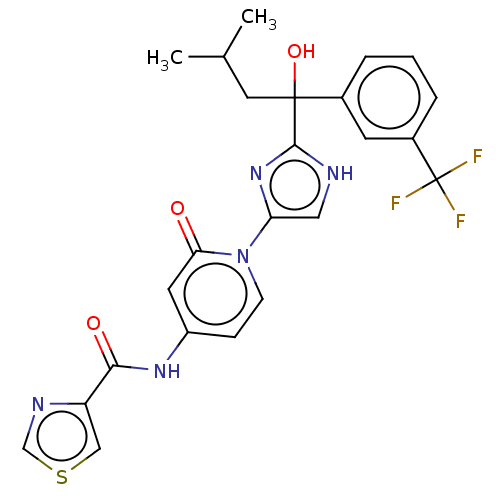 (CHEMBL3808401) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | ACS Med Chem Lett 7: 525-30 (2016) Article DOI: 10.1021/acsmedchemlett.6b00064 BindingDB Entry DOI: 10.7270/Q24B337T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase catalytic subunit (Human cytomegalovirus (HCMV strain AD169) ) | BDBM222927 (US9315499, 6018) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 160 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The assay conditions are the following: 10 mM HEPES pH 7.5, 25 mM KCl, 7.5 mM NaCl, 5 mM MgCl2, 0.2 mg BSA/mL, 1 mM TCEP, 1.5% glycerol, 5% DMSO, 235... | US Patent US9315499 (2016) BindingDB Entry DOI: 10.7270/Q2028QCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase catalytic subunit (Human cytomegalovirus (HCMV strain AD169) ) | BDBM222914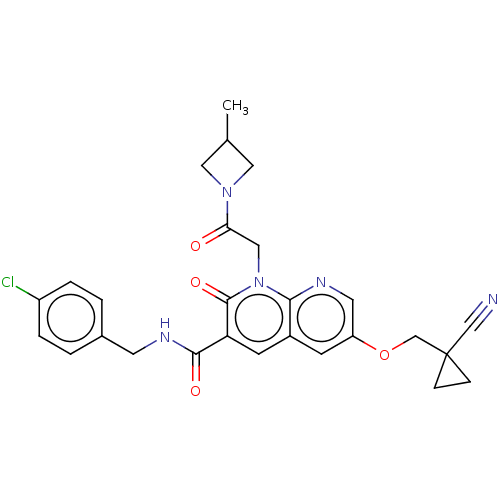 (US9315499, 4056) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 170 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The assay conditions are the following: 10 mM HEPES pH 7.5, 25 mM KCl, 7.5 mM NaCl, 5 mM MgCl2, 0.2 mg BSA/mL, 1 mM TCEP, 1.5% glycerol, 5% DMSO, 235... | US Patent US9315499 (2016) BindingDB Entry DOI: 10.7270/Q2028QCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase catalytic subunit (Human cytomegalovirus (HCMV strain AD169) ) | BDBM222907 (US9315499, 4017) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 180 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The assay conditions are the following: 10 mM HEPES pH 7.5, 25 mM KCl, 7.5 mM NaCl, 5 mM MgCl2, 0.2 mg BSA/mL, 1 mM TCEP, 1.5% glycerol, 5% DMSO, 235... | US Patent US9315499 (2016) BindingDB Entry DOI: 10.7270/Q2028QCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase catalytic subunit (Human cytomegalovirus (HCMV strain AD169) ) | BDBM222923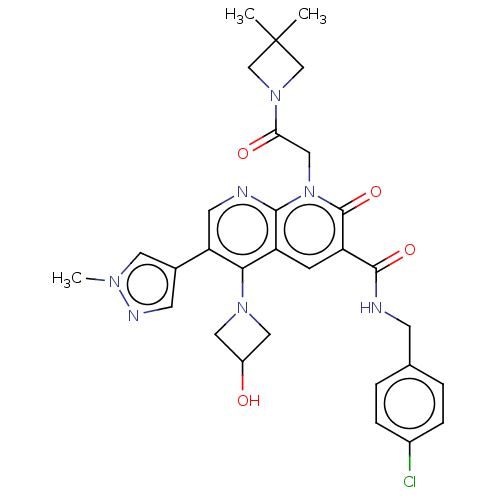 (US9315499, 6008) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 180 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The assay conditions are the following: 10 mM HEPES pH 7.5, 25 mM KCl, 7.5 mM NaCl, 5 mM MgCl2, 0.2 mg BSA/mL, 1 mM TCEP, 1.5% glycerol, 5% DMSO, 235... | US Patent US9315499 (2016) BindingDB Entry DOI: 10.7270/Q2028QCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50175303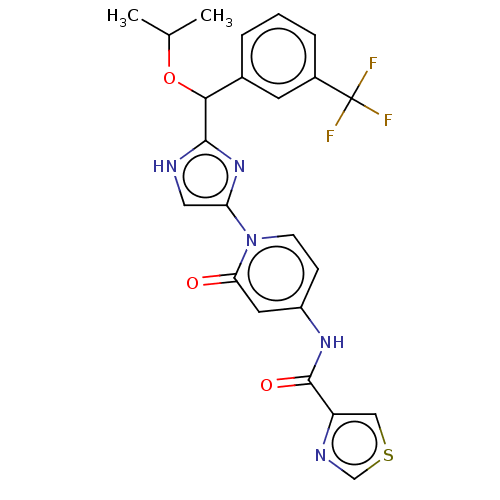 (CHEMBL3810042) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2C8 (unknown origin) | ACS Med Chem Lett 7: 525-30 (2016) Article DOI: 10.1021/acsmedchemlett.6b00064 BindingDB Entry DOI: 10.7270/Q24B337T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase catalytic subunit (Human cytomegalovirus (HCMV strain AD169) ) | BDBM222908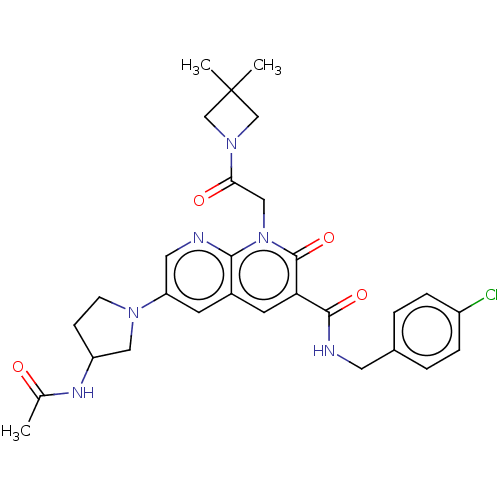 (US9315499, 4018) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 190 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The assay conditions are the following: 10 mM HEPES pH 7.5, 25 mM KCl, 7.5 mM NaCl, 5 mM MgCl2, 0.2 mg BSA/mL, 1 mM TCEP, 1.5% glycerol, 5% DMSO, 235... | US Patent US9315499 (2016) BindingDB Entry DOI: 10.7270/Q2028QCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase catalytic subunit (Human cytomegalovirus (HCMV strain AD169) ) | BDBM222898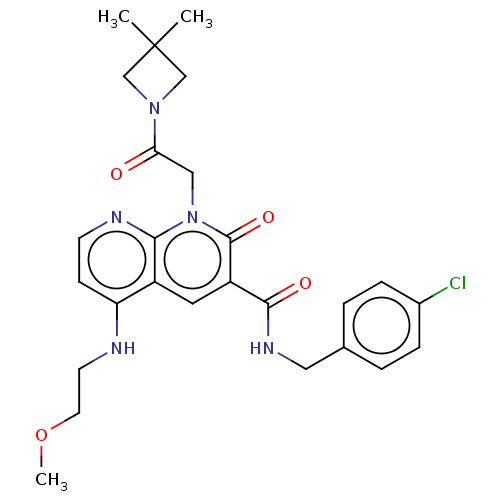 (US9315499, 3008) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 200 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The assay conditions are the following: 10 mM HEPES pH 7.5, 25 mM KCl, 7.5 mM NaCl, 5 mM MgCl2, 0.2 mg BSA/mL, 1 mM TCEP, 1.5% glycerol, 5% DMSO, 235... | US Patent US9315499 (2016) BindingDB Entry DOI: 10.7270/Q2028QCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase catalytic subunit (Human cytomegalovirus (HCMV strain AD169) ) | BDBM222902 (US9315499, 3034) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 200 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The assay conditions are the following: 10 mM HEPES pH 7.5, 25 mM KCl, 7.5 mM NaCl, 5 mM MgCl2, 0.2 mg BSA/mL, 1 mM TCEP, 1.5% glycerol, 5% DMSO, 235... | US Patent US9315499 (2016) BindingDB Entry DOI: 10.7270/Q2028QCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase catalytic subunit (Human cytomegalovirus (HCMV strain AD169) ) | BDBM222929 (US9315499, 7002) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 210 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The assay conditions are the following: 10 mM HEPES pH 7.5, 25 mM KCl, 7.5 mM NaCl, 5 mM MgCl2, 0.2 mg BSA/mL, 1 mM TCEP, 1.5% glycerol, 5% DMSO, 235... | US Patent US9315499 (2016) BindingDB Entry DOI: 10.7270/Q2028QCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase catalytic subunit (Human cytomegalovirus (HCMV strain AD169) ) | BDBM222890 (US9315499, 1008) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 220 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The assay conditions are the following: 10 mM HEPES pH 7.5, 25 mM KCl, 7.5 mM NaCl, 5 mM MgCl2, 0.2 mg BSA/mL, 1 mM TCEP, 1.5% glycerol, 5% DMSO, 235... | US Patent US9315499 (2016) BindingDB Entry DOI: 10.7270/Q2028QCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50011044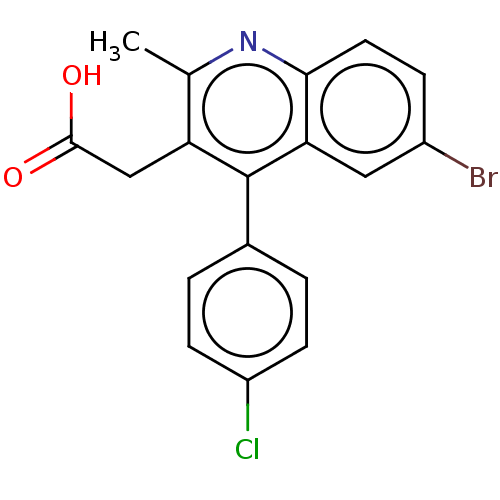 (CHEMBL3259887) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase by LTR cleavage assay | ACS Med Chem Lett 5: 422-7 (2014) Article DOI: 10.1021/ml500002n BindingDB Entry DOI: 10.7270/Q2K64KMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase catalytic subunit (Human cytomegalovirus (HCMV strain AD169) ) | BDBM222900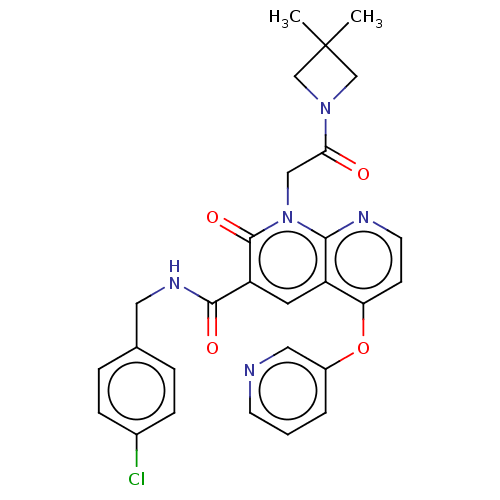 (US9315499, 3025) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 280 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The assay conditions are the following: 10 mM HEPES pH 7.5, 25 mM KCl, 7.5 mM NaCl, 5 mM MgCl2, 0.2 mg BSA/mL, 1 mM TCEP, 1.5% glycerol, 5% DMSO, 235... | US Patent US9315499 (2016) BindingDB Entry DOI: 10.7270/Q2028QCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase catalytic subunit (Human cytomegalovirus (HCMV strain AD169) ) | BDBM222904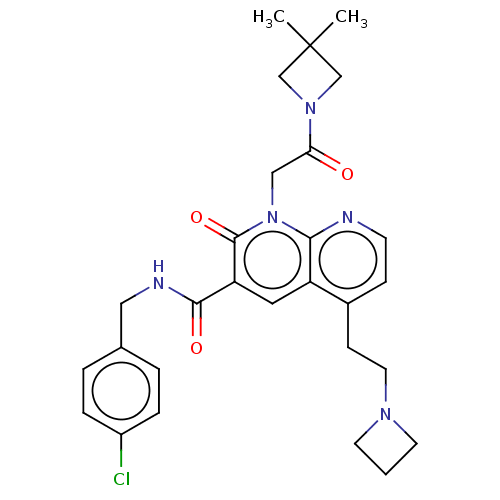 (US9315499, 3043) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 280 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The assay conditions are the following: 10 mM HEPES pH 7.5, 25 mM KCl, 7.5 mM NaCl, 5 mM MgCl2, 0.2 mg BSA/mL, 1 mM TCEP, 1.5% glycerol, 5% DMSO, 235... | US Patent US9315499 (2016) BindingDB Entry DOI: 10.7270/Q2028QCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase catalytic subunit (Human cytomegalovirus (HCMV strain AD169) ) | BDBM222889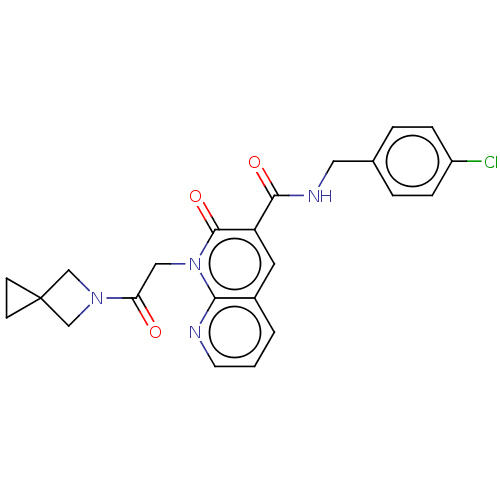 (US9315499, 1006) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 280 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The assay conditions are the following: 10 mM HEPES pH 7.5, 25 mM KCl, 7.5 mM NaCl, 5 mM MgCl2, 0.2 mg BSA/mL, 1 mM TCEP, 1.5% glycerol, 5% DMSO, 235... | US Patent US9315499 (2016) BindingDB Entry DOI: 10.7270/Q2028QCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50011130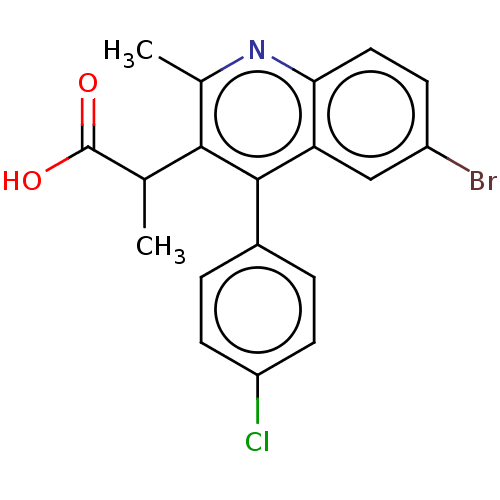 (CHEMBL3259889) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase by LTR cleavage assay | ACS Med Chem Lett 5: 422-7 (2014) Article DOI: 10.1021/ml500002n BindingDB Entry DOI: 10.7270/Q2K64KMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase catalytic subunit (Human cytomegalovirus (HCMV strain AD169) ) | BDBM222912 (US9315499, 4038) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 320 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The assay conditions are the following: 10 mM HEPES pH 7.5, 25 mM KCl, 7.5 mM NaCl, 5 mM MgCl2, 0.2 mg BSA/mL, 1 mM TCEP, 1.5% glycerol, 5% DMSO, 235... | US Patent US9315499 (2016) BindingDB Entry DOI: 10.7270/Q2028QCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50011043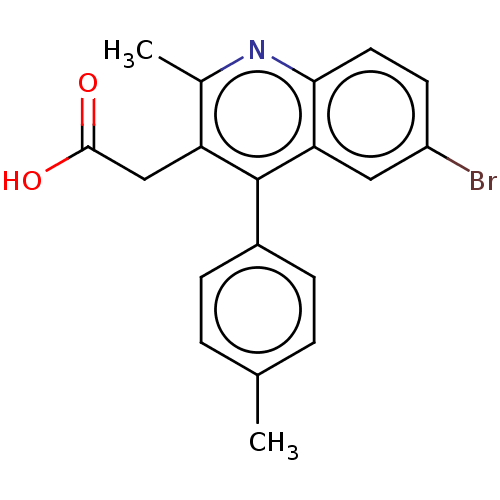 (CHEMBL3259886) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase by LTR cleavage assay | ACS Med Chem Lett 5: 422-7 (2014) Article DOI: 10.1021/ml500002n BindingDB Entry DOI: 10.7270/Q2K64KMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase catalytic subunit (Human cytomegalovirus (HCMV strain AD169) ) | BDBM222916 (US9315499, 4065) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 400 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The assay conditions are the following: 10 mM HEPES pH 7.5, 25 mM KCl, 7.5 mM NaCl, 5 mM MgCl2, 0.2 mg BSA/mL, 1 mM TCEP, 1.5% glycerol, 5% DMSO, 235... | US Patent US9315499 (2016) BindingDB Entry DOI: 10.7270/Q2028QCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase catalytic subunit (Human cytomegalovirus (HCMV strain AD169) ) | BDBM222917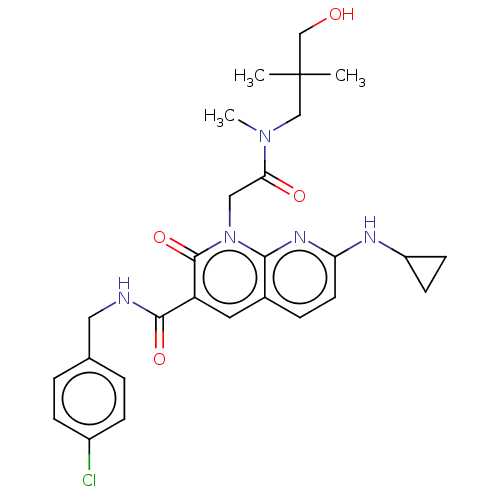 (US9315499, 5004) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 970 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The assay conditions are the following: 10 mM HEPES pH 7.5, 25 mM KCl, 7.5 mM NaCl, 5 mM MgCl2, 0.2 mg BSA/mL, 1 mM TCEP, 1.5% glycerol, 5% DMSO, 235... | US Patent US9315499 (2016) BindingDB Entry DOI: 10.7270/Q2028QCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase catalytic subunit (Human cytomegalovirus (HCMV strain AD169) ) | BDBM222918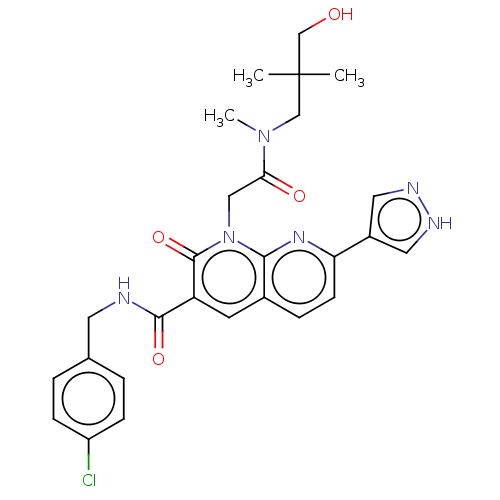 (US9315499, 5011) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The assay conditions are the following: 10 mM HEPES pH 7.5, 25 mM KCl, 7.5 mM NaCl, 5 mM MgCl2, 0.2 mg BSA/mL, 1 mM TCEP, 1.5% glycerol, 5% DMSO, 235... | US Patent US9315499 (2016) BindingDB Entry DOI: 10.7270/Q2028QCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA polymerase catalytic subunit (Human cytomegalovirus (HCMV strain AD169) ) | BDBM222920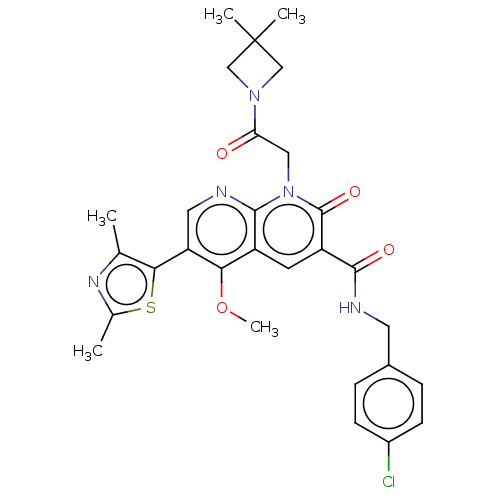 (US9315499, 6001) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Boehringer Ingelheim International GmbH US Patent | Assay Description The assay conditions are the following: 10 mM HEPES pH 7.5, 25 mM KCl, 7.5 mM NaCl, 5 mM MgCl2, 0.2 mg BSA/mL, 1 mM TCEP, 1.5% glycerol, 5% DMSO, 235... | US Patent US9315499 (2016) BindingDB Entry DOI: 10.7270/Q2028QCZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50175303 (CHEMBL3810042) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | ACS Med Chem Lett 7: 525-30 (2016) Article DOI: 10.1021/acsmedchemlett.6b00064 BindingDB Entry DOI: 10.7270/Q24B337T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C8 (Homo sapiens (Human)) | BDBM50175304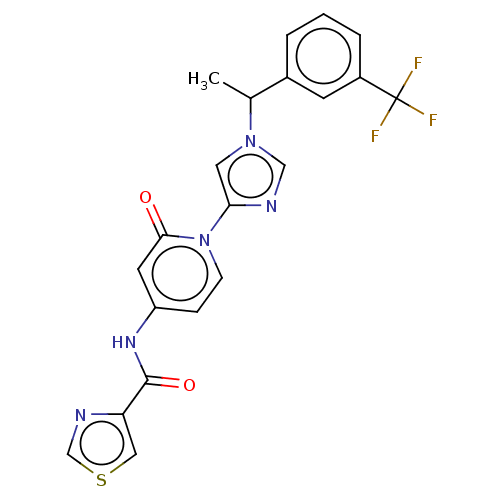 (CHEMBL3808842) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2C8 (unknown origin) | ACS Med Chem Lett 7: 525-30 (2016) Article DOI: 10.1021/acsmedchemlett.6b00064 BindingDB Entry DOI: 10.7270/Q24B337T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50175296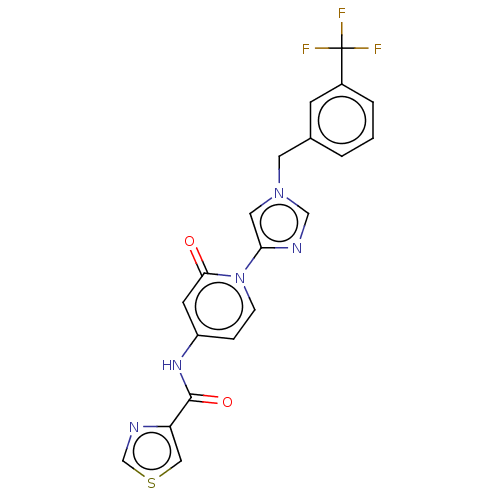 (CHEMBL3809896) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP3A4 (unknown origin) | ACS Med Chem Lett 7: 525-30 (2016) Article DOI: 10.1021/acsmedchemlett.6b00064 BindingDB Entry DOI: 10.7270/Q24B337T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50175304 (CHEMBL3808842) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | ACS Med Chem Lett 7: 525-30 (2016) Article DOI: 10.1021/acsmedchemlett.6b00064 BindingDB Entry DOI: 10.7270/Q24B337T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50175296 (CHEMBL3809896) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | ACS Med Chem Lett 7: 525-30 (2016) Article DOI: 10.1021/acsmedchemlett.6b00064 BindingDB Entry DOI: 10.7270/Q24B337T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 2C9 (Homo sapiens (Human)) | BDBM50175299 (CHEMBL3808805) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of CYP2C9 (unknown origin) | ACS Med Chem Lett 7: 525-30 (2016) Article DOI: 10.1021/acsmedchemlett.6b00064 BindingDB Entry DOI: 10.7270/Q24B337T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50011133 (CHEMBL3259892) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd. Curated by ChEMBL | Assay Description Inhibition of HIV1 integrase by LTR cleavage assay | ACS Med Chem Lett 5: 422-7 (2014) Article DOI: 10.1021/ml500002n BindingDB Entry DOI: 10.7270/Q2K64KMK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 115 total ) | Next | Last >> |