

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Urotensin-2 receptor (Homo sapiens (Human)) | BDBM86516 (ETPDCFWKYCV | Human U-II | L-Ala-Gly-L-Thr-L-Ala-L...) | Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 311: 204-12 (2004) Article DOI: 10.1124/jpet.104.068320 BindingDB Entry DOI: 10.7270/Q2H993RW | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM86516 (ETPDCFWKYCV | Human U-II | L-Ala-Gly-L-Thr-L-Ala-L...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 311: 204-12 (2004) Article DOI: 10.1124/jpet.104.068320 BindingDB Entry DOI: 10.7270/Q2H993RW | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urotensin-2 receptor (RAT) | BDBM50302272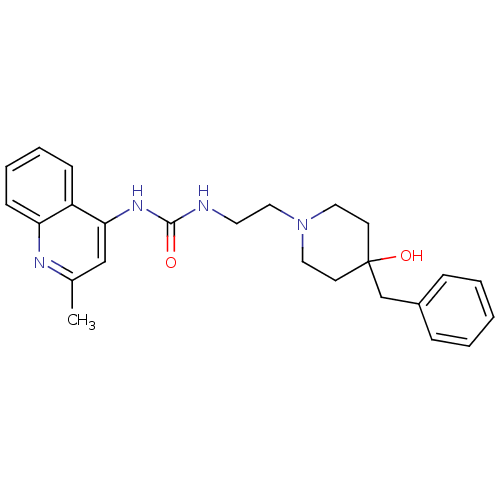 (1-(2-(4-benzyl-4-hydroxypiperidin-1-yl)ethyl)-3-(2...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 311: 204-12 (2004) Article DOI: 10.1124/jpet.104.068320 BindingDB Entry DOI: 10.7270/Q2H993RW | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50330345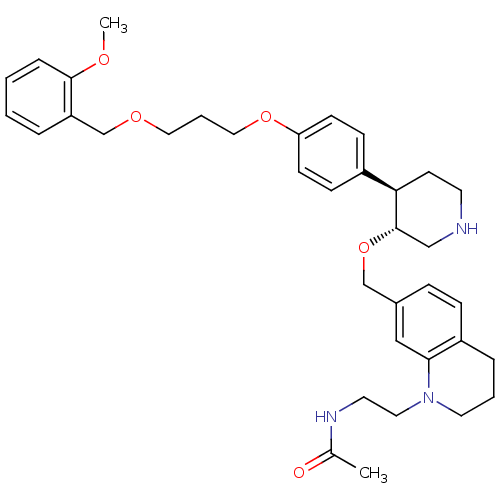 (CHEMBL1276275 | N-(2-(7-(((3R,4R)-4-(4-(3-(2-metho...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0390 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant rennin in buffer assessed as accumulation of angiotensin 1 using human tetradecapeptide by immunoassay | J Med Chem 52: 3689-702 (2009) Article DOI: 10.1021/jm900022f BindingDB Entry DOI: 10.7270/Q2PC3391 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50259452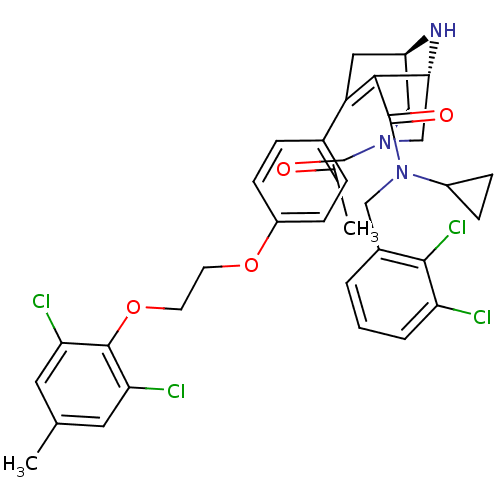 ((1R,5S)-3-Acetyl-7-{4-[2-(2,6-dichloro-4-methyl-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant rennin in buffer assessed as accumulation of angiotensin 1 using human tetradecapeptide by immunoassay | J Med Chem 52: 3689-702 (2009) Article DOI: 10.1021/jm900022f BindingDB Entry DOI: 10.7270/Q2PC3391 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50259464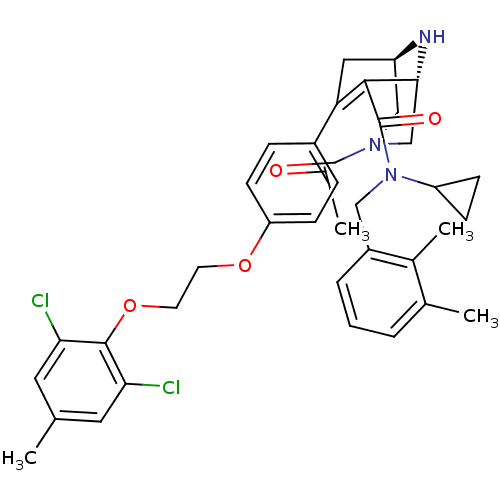 ((1R,5S)-7-{4-[2-(2,6-Dichloro-4-methyl-phenoxy)-et...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant rennin in buffer assessed as accumulation of angiotensin 1 using human tetradecapeptide by immunoassay | J Med Chem 52: 3689-702 (2009) Article DOI: 10.1021/jm900022f BindingDB Entry DOI: 10.7270/Q2PC3391 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50259432 ((1R,5S)-3-Acetyl-7-{4-[3-(2-methoxy-benzyloxy)-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant rennin in buffer assessed as accumulation of angiotensin 1 using human tetradecapeptide by immunoassay | J Med Chem 52: 3689-702 (2009) Article DOI: 10.1021/jm900022f BindingDB Entry DOI: 10.7270/Q2PC3391 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin receptor type B (Homo sapiens (Human)) | BDBM50395673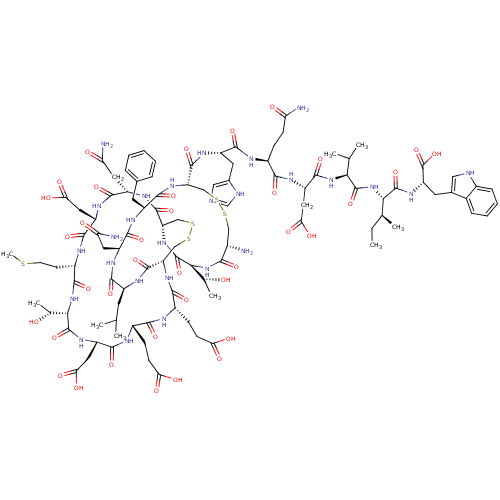 (CHEMBL2165327) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.208 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Displacement of [I125]ET1 from recombinant ETB receptor expressed in CHO cells after 2 hrs by TopCount analysis | J Med Chem 55: 7849-61 (2012) Article DOI: 10.1021/jm3009103 BindingDB Entry DOI: 10.7270/Q21C1XZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50259431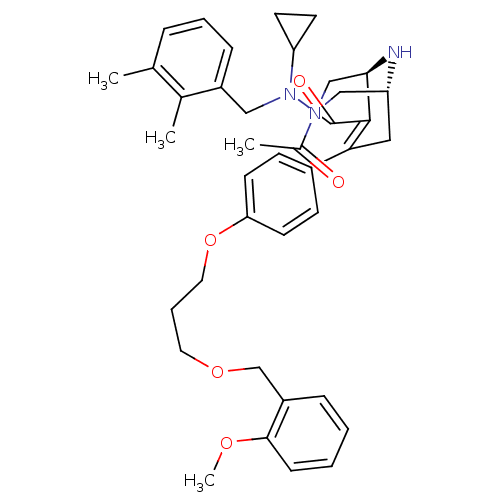 ((1R,5S)-3-Acetyl-7-{4-[3-(2-methoxy-benzyloxy)-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant rennin in buffer assessed as accumulation of angiotensin 1 using human tetradecapeptide by immunoassay | J Med Chem 52: 3689-702 (2009) Article DOI: 10.1021/jm900022f BindingDB Entry DOI: 10.7270/Q2PC3391 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50259444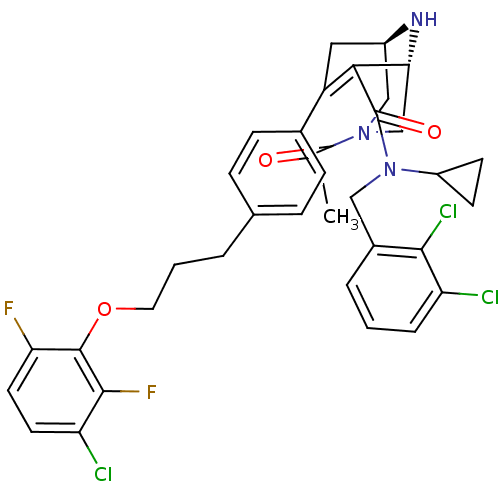 ((1R,5S)-3-Acetyl-7-{4-[3-(3-chloro-2,6-difluoro-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant rennin in buffer assessed as accumulation of angiotensin 1 using human tetradecapeptide by immunoassay | J Med Chem 52: 3689-702 (2009) Article DOI: 10.1021/jm900022f BindingDB Entry DOI: 10.7270/Q2PC3391 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50259445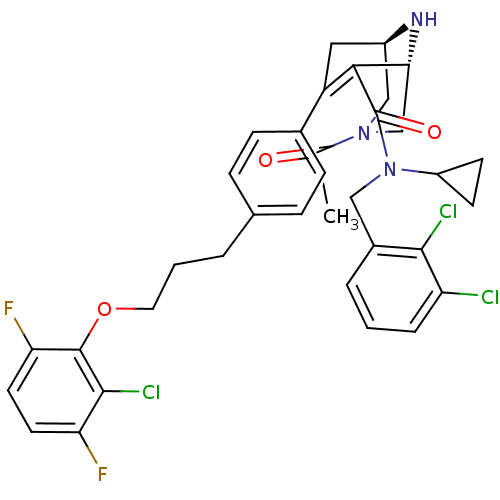 ((1R,5S)-3-Acetyl-7-{4-[3-(2-chloro-3,6-difluoro-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant rennin in buffer assessed as accumulation of angiotensin 1 using human tetradecapeptide by immunoassay | J Med Chem 52: 3689-702 (2009) Article DOI: 10.1021/jm900022f BindingDB Entry DOI: 10.7270/Q2PC3391 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50259457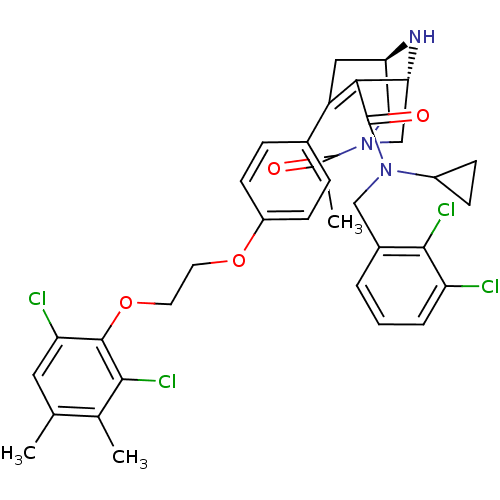 ((1R,5S)-3-Acetyl-7-{4-[2-(2,6-dichloro-3,4-dimethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant rennin in buffer assessed as accumulation of angiotensin 1 using human tetradecapeptide by immunoassay | J Med Chem 52: 3689-702 (2009) Article DOI: 10.1021/jm900022f BindingDB Entry DOI: 10.7270/Q2PC3391 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50395625 (CHEMBL2165339) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Displacement of [I125]ET1 from recombinant ETA receptor expressed in CHO cells after 2 hrs by TopCount analysis | J Med Chem 55: 7849-61 (2012) Article DOI: 10.1021/jm3009103 BindingDB Entry DOI: 10.7270/Q21C1XZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50259456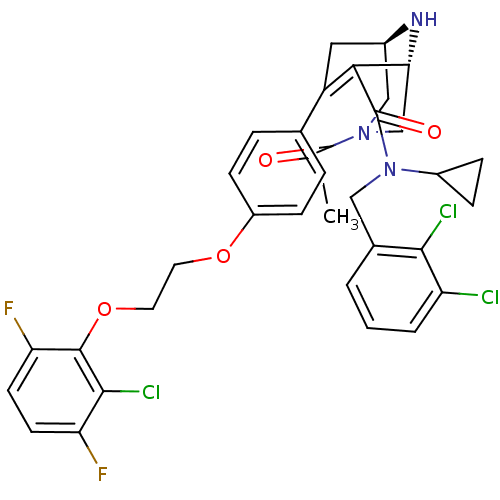 ((1R,5S)-3-Acetyl-7-{4-[2-(2-chloro-3,6-difluoro-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant rennin in buffer assessed as accumulation of angiotensin 1 using human tetradecapeptide by immunoassay | J Med Chem 52: 3689-702 (2009) Article DOI: 10.1021/jm900022f BindingDB Entry DOI: 10.7270/Q2PC3391 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50259453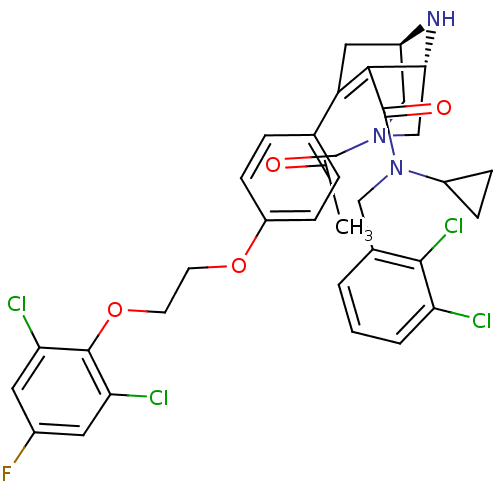 ((1R,5S)-3-Acetyl-7-{4-[2-(2,6-dichloro-4-fluoro-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant rennin in buffer assessed as accumulation of angiotensin 1 using human tetradecapeptide by immunoassay | J Med Chem 52: 3689-702 (2009) Article DOI: 10.1021/jm900022f BindingDB Entry DOI: 10.7270/Q2PC3391 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50259465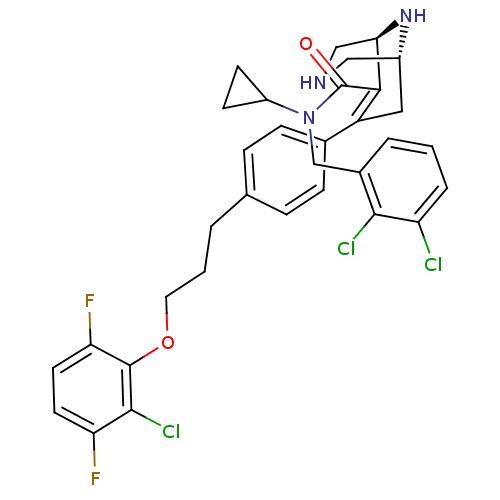 ((1R,5S)-7-{4-[3-(2-Chloro-3,6-difluoro-phenoxy)-pr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant rennin in buffer assessed as accumulation of angiotensin 1 using human tetradecapeptide by immunoassay | J Med Chem 52: 3689-702 (2009) Article DOI: 10.1021/jm900022f BindingDB Entry DOI: 10.7270/Q2PC3391 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50105051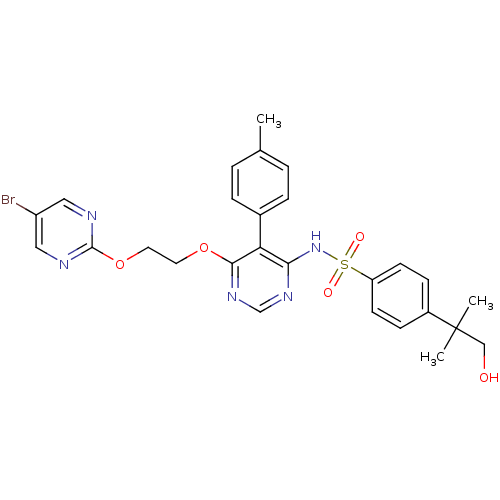 (CHEMBL112624 | N-{6-[2-(5-Bromo-pyrimidin-2-yloxy)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Displacement of [I125]ET1 from recombinant ETA receptor expressed in CHO cells after 2 hrs by TopCount analysis | J Med Chem 55: 7849-61 (2012) Article DOI: 10.1021/jm3009103 BindingDB Entry DOI: 10.7270/Q21C1XZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50395619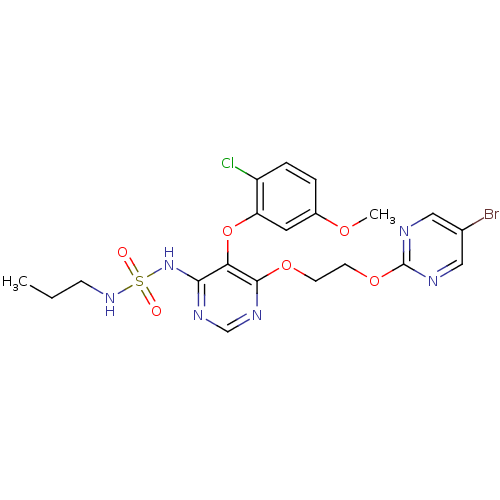 (CHEMBL2163692) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Displacement of [I125]ET1 from recombinant ETA receptor expressed in CHO cells after 2 hrs by TopCount analysis | J Med Chem 55: 7849-61 (2012) Article DOI: 10.1021/jm3009103 BindingDB Entry DOI: 10.7270/Q21C1XZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50311803 ((1S,5S)-7-(4-(3-(2-chloro-3,6-difluorophenoxy)prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Inhibition of human rennin in buffer assessed as accumulation of angiotensin 1 using human tetradecapeptide by immunoassay | Bioorg Med Chem Lett 19: 6762-5 (2009) Article DOI: 10.1016/j.bmcl.2009.09.104 BindingDB Entry DOI: 10.7270/Q23F4PRP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50259443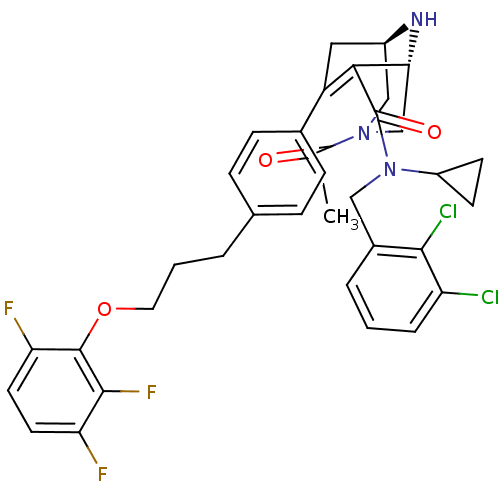 ((1R,5S)-3-Acetyl-7-{4-[3-(2,3,6-trifluoro-phenoxy)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant rennin in buffer assessed as accumulation of angiotensin 1 using human tetradecapeptide by immunoassay | J Med Chem 52: 3689-702 (2009) Article DOI: 10.1021/jm900022f BindingDB Entry DOI: 10.7270/Q2PC3391 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50311806 ((1S,5S)-7-(4-(3-(2-chloro-3,6-difluorophenoxy)prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Inhibition of human rennin in buffer assessed as accumulation of angiotensin 1 using human tetradecapeptide by immunoassay | Bioorg Med Chem Lett 19: 6762-5 (2009) Article DOI: 10.1016/j.bmcl.2009.09.104 BindingDB Entry DOI: 10.7270/Q23F4PRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50395626 (ACT-064992 | MACITENTAN) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Displacement of [I125]ET1 from recombinant ETA receptor expressed in CHO cells after 2 hrs by TopCount analysis | J Med Chem 55: 7849-61 (2012) Article DOI: 10.1021/jm3009103 BindingDB Entry DOI: 10.7270/Q21C1XZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50259459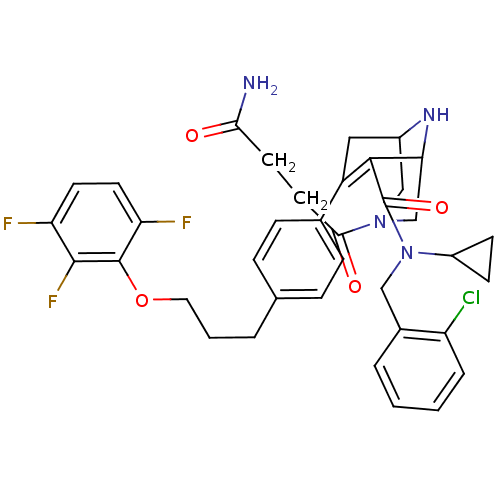 ((rac)-(1RS,5SR)-3-(3-Carbamoyl-propionyl)-7-{4-[3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant rennin in buffer assessed as accumulation of angiotensin 1 using human tetradecapeptide by immunoassay | J Med Chem 52: 3689-702 (2009) Article DOI: 10.1021/jm900022f BindingDB Entry DOI: 10.7270/Q2PC3391 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50259458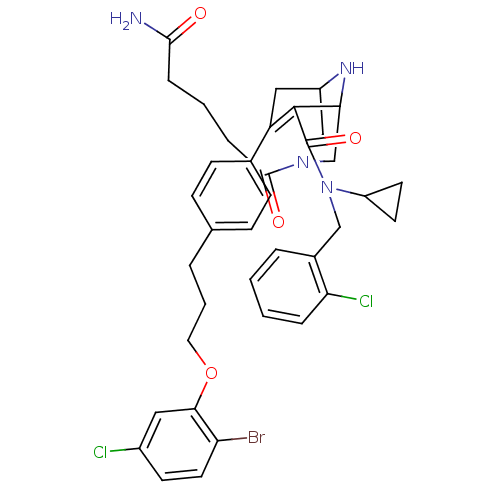 ((rac)-(1RS,5SR)-7-{4-[3-(2-Bromo-5-fluoro-phenoxy)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant rennin in buffer assessed as accumulation of angiotensin 1 using human tetradecapeptide by immunoassay | J Med Chem 52: 3689-702 (2009) Article DOI: 10.1021/jm900022f BindingDB Entry DOI: 10.7270/Q2PC3391 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50311799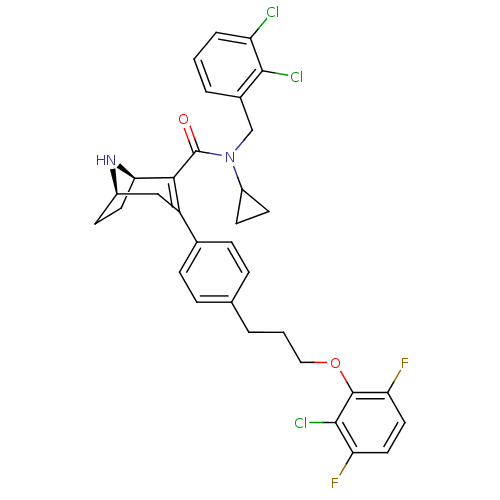 ((1R,5R)-3-(4-(3-(2-chloro-3,6-difluorophenoxy)prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Inhibition of human rennin in buffer assessed as accumulation of angiotensin 1 using human tetradecapeptide by immunoassay | Bioorg Med Chem Lett 19: 6762-5 (2009) Article DOI: 10.1016/j.bmcl.2009.09.104 BindingDB Entry DOI: 10.7270/Q23F4PRP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM17950 ((2S,4S,5S,7S)-5-amino-N-(2-carbamoyl-2,2-dimethyle...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant rennin in buffer assessed as accumulation of angiotensin 1 using human tetradecapeptide by immunoassay | J Med Chem 52: 3689-702 (2009) Article DOI: 10.1021/jm900022f BindingDB Entry DOI: 10.7270/Q2PC3391 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50259433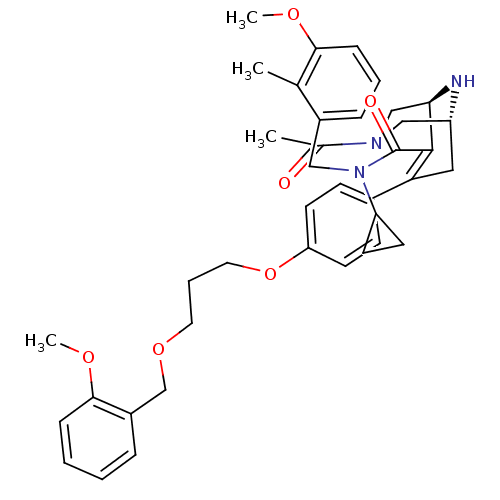 ((1R,5S)-3-Acetyl-7-{4-[3-(2-methoxy-benzyloxy)-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant rennin in buffer assessed as accumulation of angiotensin 1 using human tetradecapeptide by immunoassay | J Med Chem 52: 3689-702 (2009) Article DOI: 10.1021/jm900022f BindingDB Entry DOI: 10.7270/Q2PC3391 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50259461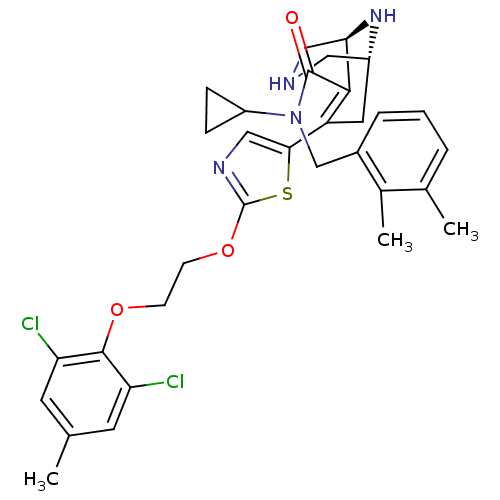 ((1R,5S)-7-{2-[2-(2,6-Dichloro-4-methyl-phenoxy)-et...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant rennin in buffer assessed as accumulation of angiotensin 1 using human tetradecapeptide by immunoassay | J Med Chem 52: 3689-702 (2009) Article DOI: 10.1021/jm900022f BindingDB Entry DOI: 10.7270/Q2PC3391 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50311801 ((1S,5S)-7-(4-(3-(2-chloro-3,6-difluorophenoxy)prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Inhibition of human rennin in buffer assessed as accumulation of angiotensin 1 using human tetradecapeptide by immunoassay | Bioorg Med Chem Lett 19: 6762-5 (2009) Article DOI: 10.1016/j.bmcl.2009.09.104 BindingDB Entry DOI: 10.7270/Q23F4PRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50395633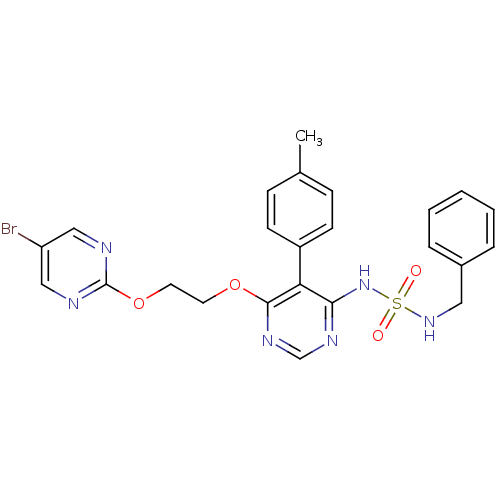 (CHEMBL2165332) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Displacement of [I125]ET1 from recombinant ETA receptor expressed in CHO cells after 2 hrs by TopCount analysis | J Med Chem 55: 7849-61 (2012) Article DOI: 10.1021/jm3009103 BindingDB Entry DOI: 10.7270/Q21C1XZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50311809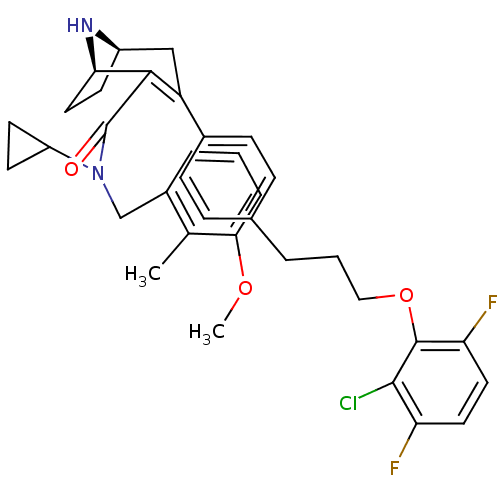 (4-(4-(3-(2-chloro-3,6-difluorophenoxy)propyl)pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Inhibition of human rennin in buffer assessed as accumulation of angiotensin 1 using human tetradecapeptide by immunoassay | Bioorg Med Chem Lett 19: 6762-5 (2009) Article DOI: 10.1016/j.bmcl.2009.09.104 BindingDB Entry DOI: 10.7270/Q23F4PRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50259428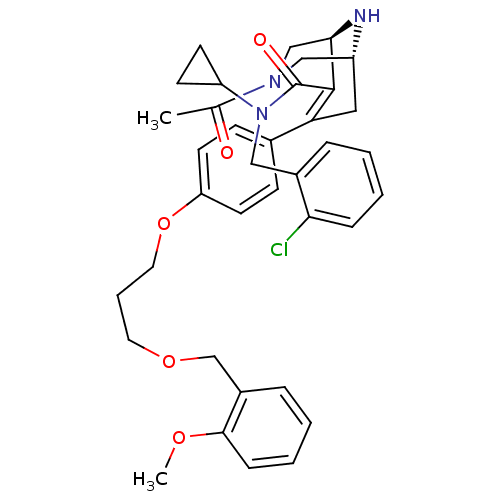 ((1R,5S)-3-Acetyl-7-{4-[3-(2-methoxy-benzyloxy)-pro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant rennin in buffer assessed as accumulation of angiotensin 1 using human tetradecapeptide by immunoassay | J Med Chem 52: 3689-702 (2009) Article DOI: 10.1021/jm900022f BindingDB Entry DOI: 10.7270/Q2PC3391 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50259454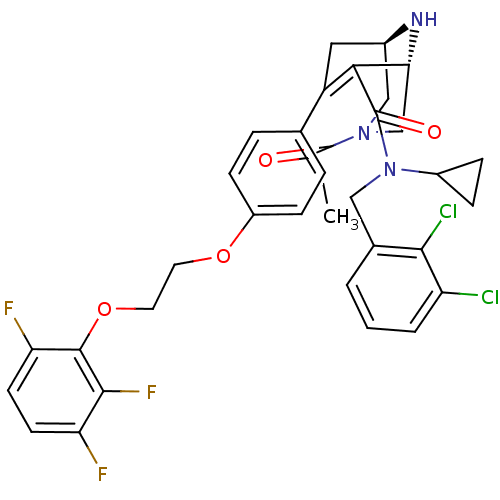 ((1R,5S)-3-Acetyl-7-{4-[2-(2,3,6-trifluoro-phenoxy)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant rennin in buffer assessed as accumulation of angiotensin 1 using human tetradecapeptide by immunoassay | J Med Chem 52: 3689-702 (2009) Article DOI: 10.1021/jm900022f BindingDB Entry DOI: 10.7270/Q2PC3391 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50259466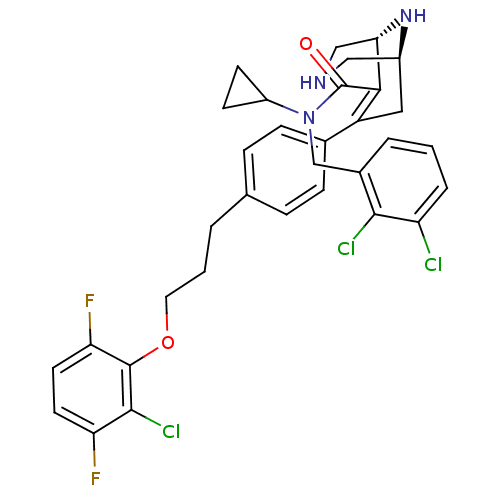 ((1S,5R)-7-{4-[3-(2-Chloro-3,6-difluoro-phenoxy)-pr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant rennin in buffer assessed as accumulation of angiotensin 1 using human tetradecapeptide by immunoassay | J Med Chem 52: 3689-702 (2009) Article DOI: 10.1021/jm900022f BindingDB Entry DOI: 10.7270/Q2PC3391 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50395627 (CHEMBL2165338) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Displacement of [I125]ET1 from recombinant ETA receptor expressed in CHO cells after 2 hrs by TopCount analysis | J Med Chem 55: 7849-61 (2012) Article DOI: 10.1021/jm3009103 BindingDB Entry DOI: 10.7270/Q21C1XZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50259441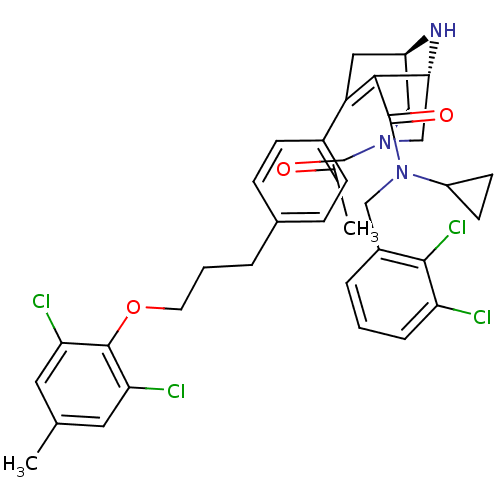 ((1R,5S)-3-Acetyl-7-{4-[3-(2,6-dichloro-4-methyl-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.830 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant rennin in buffer assessed as accumulation of angiotensin 1 using human tetradecapeptide by immunoassay | J Med Chem 52: 3689-702 (2009) Article DOI: 10.1021/jm900022f BindingDB Entry DOI: 10.7270/Q2PC3391 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50311807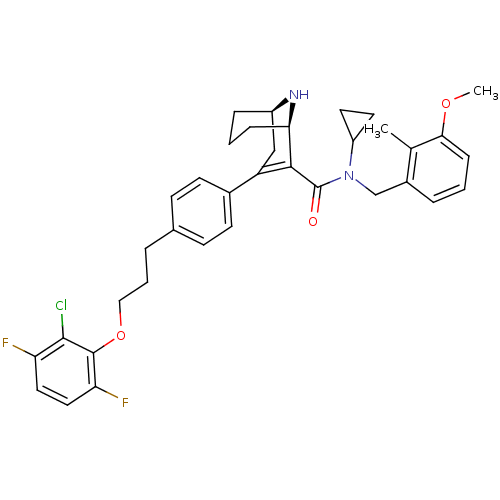 ((1R,5R)-3-(4-(3-(2-chloro-3,6-difluorophenoxy)prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Inhibition of human rennin in buffer assessed as accumulation of angiotensin 1 using human tetradecapeptide by immunoassay | Bioorg Med Chem Lett 19: 6762-5 (2009) Article DOI: 10.1016/j.bmcl.2009.09.104 BindingDB Entry DOI: 10.7270/Q23F4PRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50395636 (CHEMBL2165329) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Displacement of [I125]ET1 from recombinant ETA receptor expressed in CHO cells after 2 hrs by TopCount analysis | J Med Chem 55: 7849-61 (2012) Article DOI: 10.1021/jm3009103 BindingDB Entry DOI: 10.7270/Q21C1XZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50259442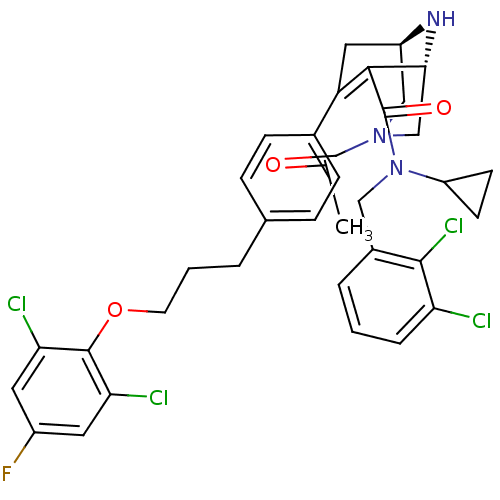 ((1R,5S)-3-Acetyl-7-{4-[3-(2,6-dichloro-4-fluoro-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant rennin in buffer assessed as accumulation of angiotensin 1 using human tetradecapeptide by immunoassay | J Med Chem 52: 3689-702 (2009) Article DOI: 10.1021/jm900022f BindingDB Entry DOI: 10.7270/Q2PC3391 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50259418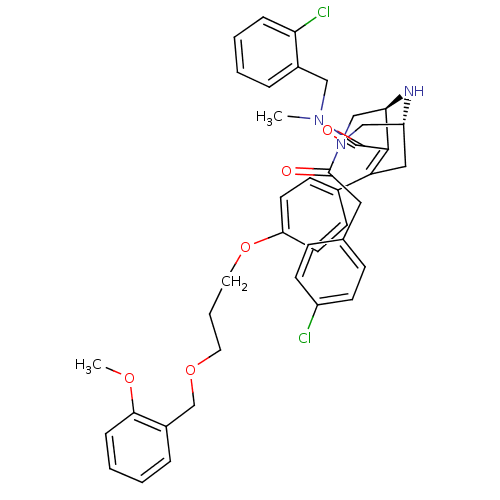 ((1R,5S)-3-[2-(4-Chloro-phenyl)-acetyl]-7{4-[3-(2-m...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant rennin in buffer assessed as accumulation of angiotensin 1 using human tetradecapeptide by immunoassay | J Med Chem 52: 3689-702 (2009) Article DOI: 10.1021/jm900022f BindingDB Entry DOI: 10.7270/Q2PC3391 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50395617 (CHEMBL2163694) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Displacement of [I125]ET1 from recombinant ETA receptor expressed in CHO cells after 2 hrs by TopCount analysis | J Med Chem 55: 7849-61 (2012) Article DOI: 10.1021/jm3009103 BindingDB Entry DOI: 10.7270/Q21C1XZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50395662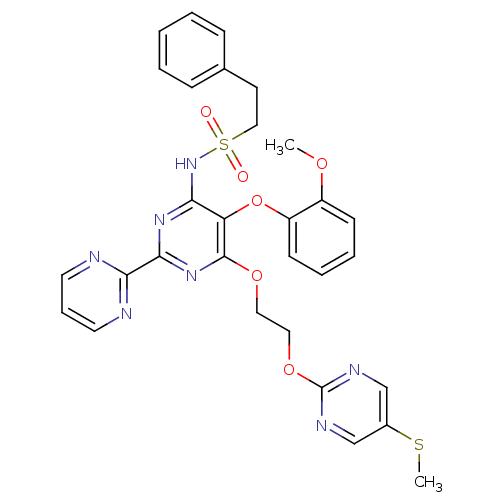 (CHEMBL2163704) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Displacement of [I125]ET1 from recombinant ETA receptor expressed in CHO cells after 2 hrs by TopCount analysis | J Med Chem 55: 7849-61 (2012) Article DOI: 10.1021/jm3009103 BindingDB Entry DOI: 10.7270/Q21C1XZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50046798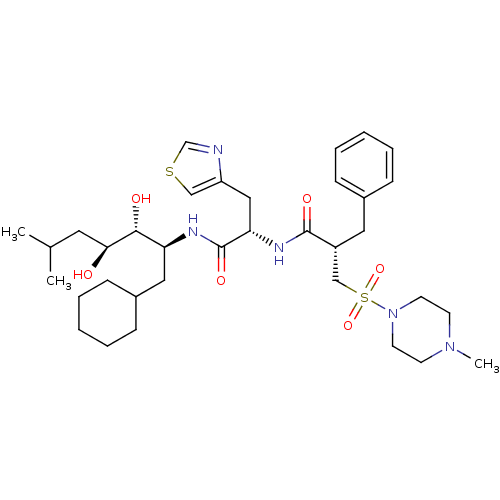 (2-Benzyl-N-[1-(1-cyclohexylmethyl-2,3-dihydroxy-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant rennin in human plasma assessed as accumulation of angiotensin 1 using human tetradecapeptide by immunoassay | J Med Chem 52: 3689-702 (2009) Article DOI: 10.1021/jm900022f BindingDB Entry DOI: 10.7270/Q2PC3391 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50146652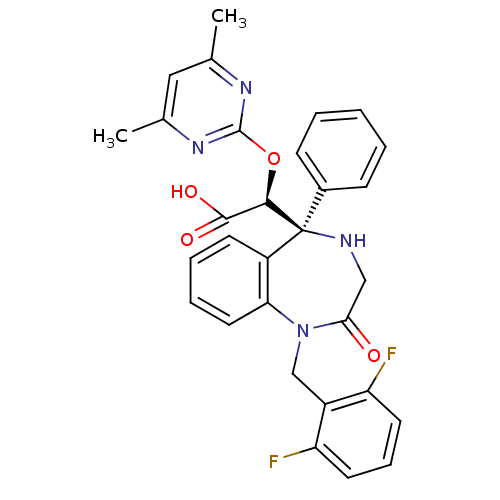 ((S)-[(S)-1-(2,6-Difluoro-benzyl)-2-oxo-5-phenyl-2,...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Antagonist activity towards human recombinant Endothelin A receptor expressed in chinese hamster ovary (CHO) cells determined using [125I]-ET-1 as ra... | J Med Chem 47: 2776-95 (2004) Article DOI: 10.1021/jm031115r BindingDB Entry DOI: 10.7270/Q2Z89BVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50311800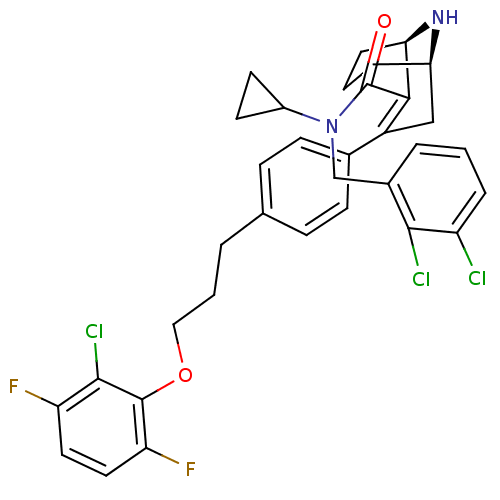 ((1R,5R)-3-(4-(3-(2-chloro-3,6-difluorophenoxy)prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Inhibition of human rennin in buffer assessed as accumulation of angiotensin 1 using human tetradecapeptide by immunoassay | Bioorg Med Chem Lett 19: 6762-5 (2009) Article DOI: 10.1016/j.bmcl.2009.09.104 BindingDB Entry DOI: 10.7270/Q23F4PRP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50395621 (CHEMBL2163690) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Displacement of [I125]ET1 from recombinant ETA receptor expressed in CHO cells after 2 hrs by TopCount analysis | J Med Chem 55: 7849-61 (2012) Article DOI: 10.1021/jm3009103 BindingDB Entry DOI: 10.7270/Q21C1XZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50311798 (4-(4-(3-(2-chloro-3,6-difluorophenoxy)propyl)pheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Inhibition of human rennin in buffer assessed as accumulation of angiotensin 1 using human tetradecapeptide by immunoassay | Bioorg Med Chem Lett 19: 6762-5 (2009) Article DOI: 10.1016/j.bmcl.2009.09.104 BindingDB Entry DOI: 10.7270/Q23F4PRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Renin (Homo sapiens (Human)) | BDBM50311805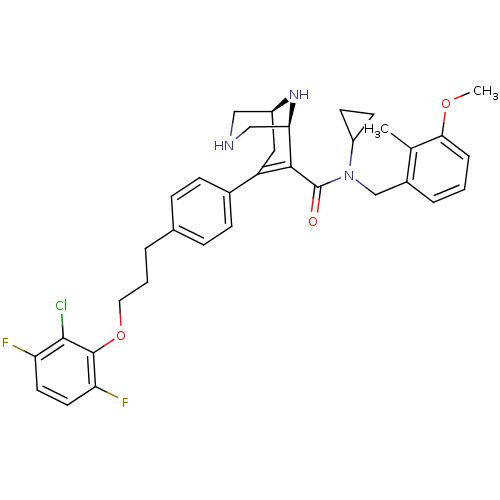 ((1S,5S)-7-(4-(3-(2-chloro-3,6-difluorophenoxy)prop...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.37 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd Curated by ChEMBL | Assay Description Inhibition of human rennin in buffer assessed as accumulation of angiotensin 1 using human tetradecapeptide by immunoassay | Bioorg Med Chem Lett 19: 6762-5 (2009) Article DOI: 10.1016/j.bmcl.2009.09.104 BindingDB Entry DOI: 10.7270/Q23F4PRP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50395635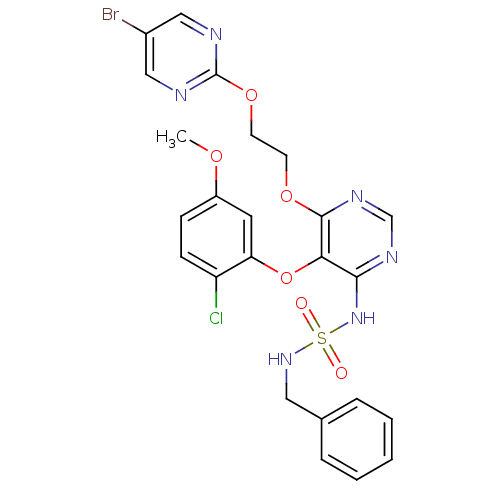 (CHEMBL2165330) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Displacement of [I125]ET1 from recombinant ETA receptor expressed in CHO cells after 2 hrs by TopCount analysis | J Med Chem 55: 7849-61 (2012) Article DOI: 10.1021/jm3009103 BindingDB Entry DOI: 10.7270/Q21C1XZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Endothelin-1 receptor (Homo sapiens (Human)) | BDBM50146717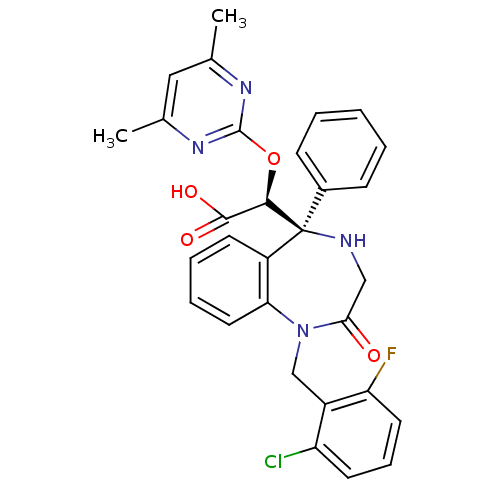 ((S)-[(S)-1-(2-Chloro-6-fluoro-benzyl)-2-oxo-5-phen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Actelion Pharmaceuticals Ltd. Curated by ChEMBL | Assay Description Antagonist activity towards human recombinant Endothelin A receptor expressed in chinese hamster ovary (CHO) cells determined using [125I]-ET-1 as ra... | J Med Chem 47: 2776-95 (2004) Article DOI: 10.1021/jm031115r BindingDB Entry DOI: 10.7270/Q2Z89BVT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 694 total ) | Next | Last >> |