

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50414304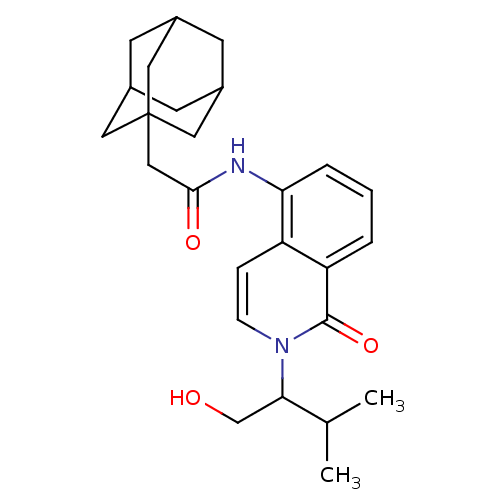 (CHEMBL550030) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0501 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at P2X7 receptor in human THP1 cells assessed as inhibition of BzATP-induced ethidium uptake | J Med Chem 52: 3123-41 (2009) Article DOI: 10.1021/jm801528x BindingDB Entry DOI: 10.7270/Q2251KDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50414307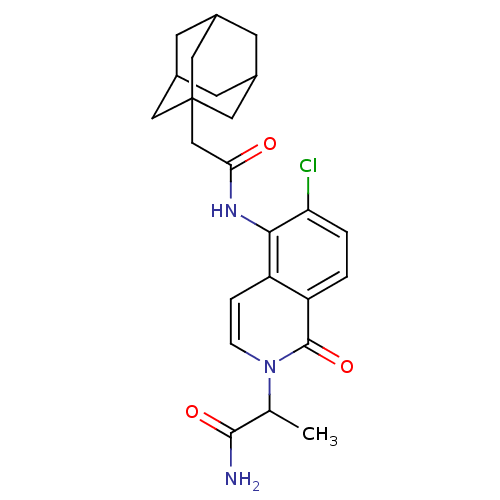 (CHEMBL560506) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0794 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at P2X7 receptor in human THP1 cells assessed as inhibition of BzATP-induced ethidium uptake | J Med Chem 52: 3123-41 (2009) Article DOI: 10.1021/jm801528x BindingDB Entry DOI: 10.7270/Q2251KDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50414305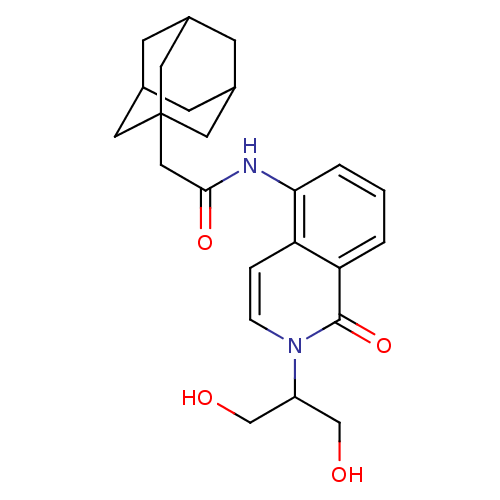 (CHEMBL551360) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at P2X7 receptor in human THP1 cells assessed as inhibition of BzATP-induced ethidium uptake | J Med Chem 52: 3123-41 (2009) Article DOI: 10.1021/jm801528x BindingDB Entry DOI: 10.7270/Q2251KDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50414314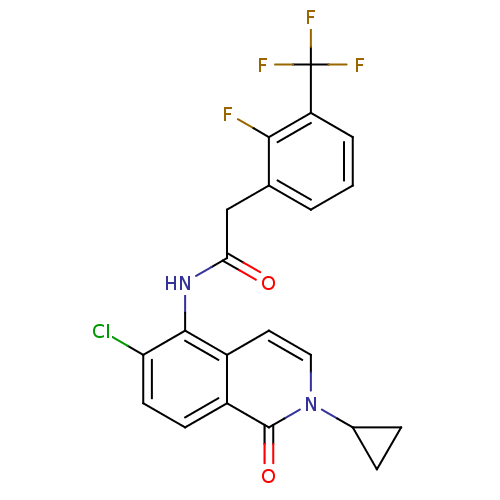 (CHEMBL564882) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.316 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at P2X7 receptor in human THP1 cells assessed as inhibition of BzATP-induced ethidium uptake | J Med Chem 52: 3123-41 (2009) Article DOI: 10.1021/jm801528x BindingDB Entry DOI: 10.7270/Q2251KDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50423388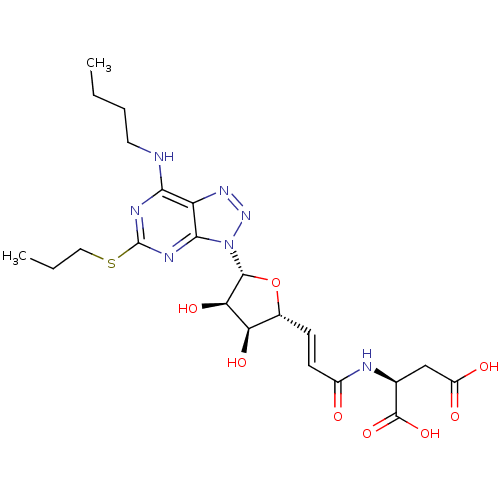 (CHEMBL251024) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human P2Y12 receptor assessed as ADP-induced human platelet aggregation | Bioorg Med Chem Lett 17: 6013-8 (2007) Article DOI: 10.1016/j.bmcl.2007.07.057 BindingDB Entry DOI: 10.7270/Q2JD4Z2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50414309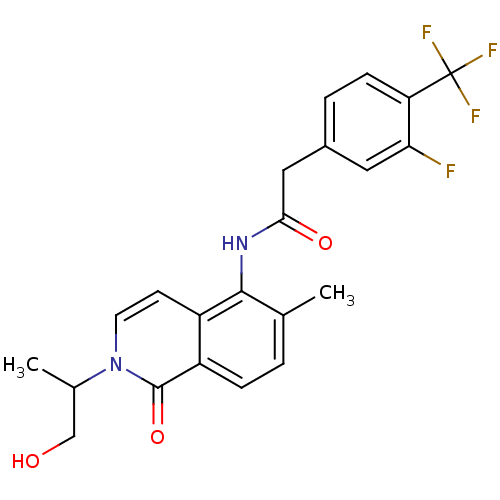 (CHEMBL551362) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at P2X7 receptor in human THP1 cells assessed as inhibition of BzATP-induced ethidium uptake | J Med Chem 52: 3123-41 (2009) Article DOI: 10.1021/jm801528x BindingDB Entry DOI: 10.7270/Q2251KDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2Y purinoceptor 12 (Homo sapiens (Human)) | BDBM50423387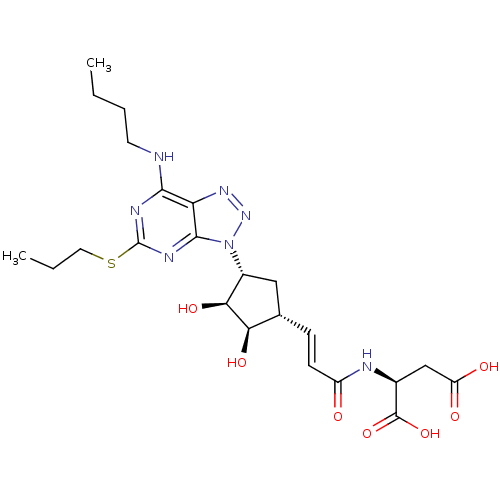 (CHEMBL437204) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human P2Y12 receptor assessed as ADP-induced human platelet aggregation | Bioorg Med Chem Lett 17: 6013-8 (2007) Article DOI: 10.1016/j.bmcl.2007.07.057 BindingDB Entry DOI: 10.7270/Q2JD4Z2Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50414306 (CHEMBL556680) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at P2X7 receptor in human THP1 cells assessed as inhibition of BzATP-induced ethidium uptake | J Med Chem 52: 3123-41 (2009) Article DOI: 10.1021/jm801528x BindingDB Entry DOI: 10.7270/Q2251KDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50126727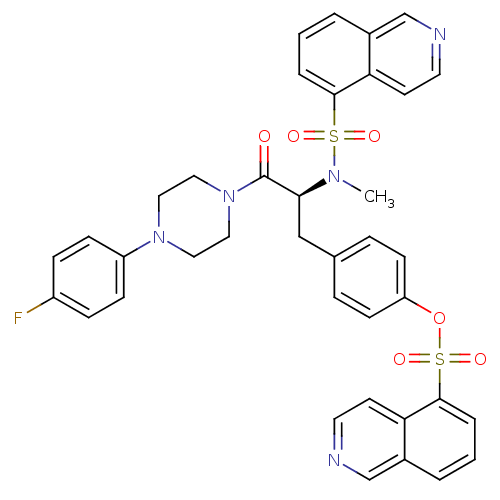 ((S)-4-(3-(4-(4-fluorophenyl)piperazin-1-yl)-2-(N-m...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.26 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor expressed in HEK293 cells assessed as inhibition of BzATP-induced calcium influx | J Med Chem 52: 3123-41 (2009) Article DOI: 10.1021/jm801528x BindingDB Entry DOI: 10.7270/Q2251KDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50414350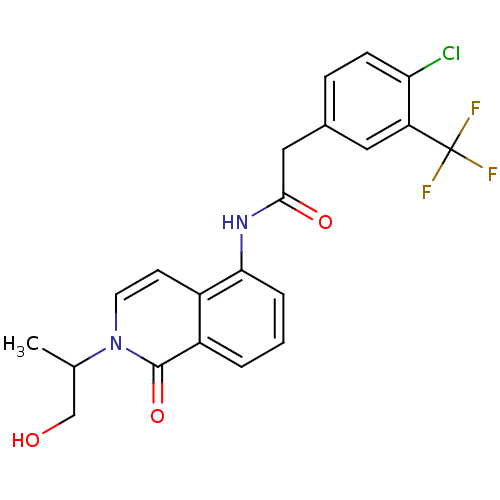 (CHEMBL550436) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.26 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at P2X7 receptor in human THP1 cells assessed as inhibition of BzATP-induced ethidium uptake | J Med Chem 52: 3123-41 (2009) Article DOI: 10.1021/jm801528x BindingDB Entry DOI: 10.7270/Q2251KDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50414313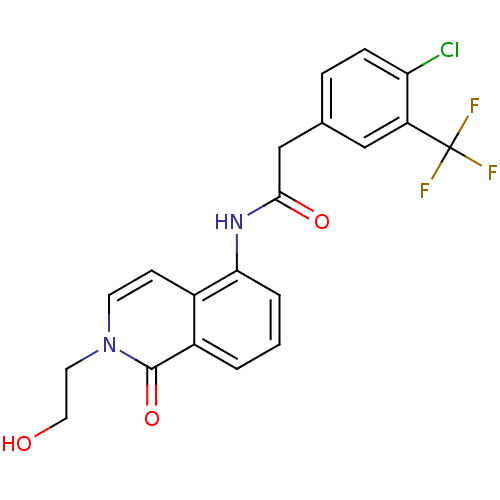 (CHEMBL560219) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at P2X7 receptor in human THP1 cells assessed as inhibition of BzATP-induced ethidium uptake | J Med Chem 52: 3123-41 (2009) Article DOI: 10.1021/jm801528x BindingDB Entry DOI: 10.7270/Q2251KDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50188311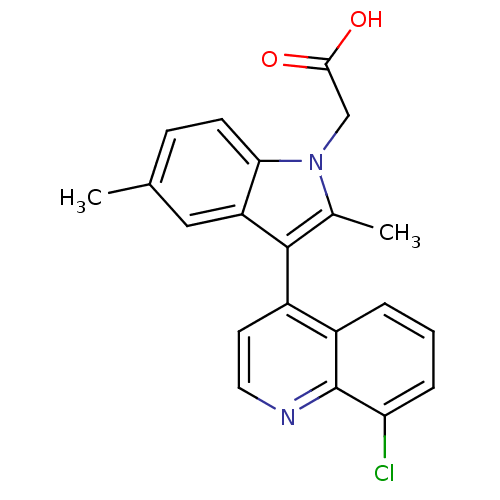 (2-(3-(8-chloroquinolin-4-yl)-2,5-dimethyl-1H-indol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]PGD2 from human CRTh2 expressed in HEK293 cells | Bioorg Med Chem Lett 16: 4287-90 (2006) Article DOI: 10.1016/j.bmcl.2006.05.062 BindingDB Entry DOI: 10.7270/Q2G44PX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50414312 (CHEMBL552513) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.51 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at P2X7 receptor in human THP1 cells assessed as inhibition of BzATP-induced ethidium uptake | J Med Chem 52: 3123-41 (2009) Article DOI: 10.1021/jm801528x BindingDB Entry DOI: 10.7270/Q2251KDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50188301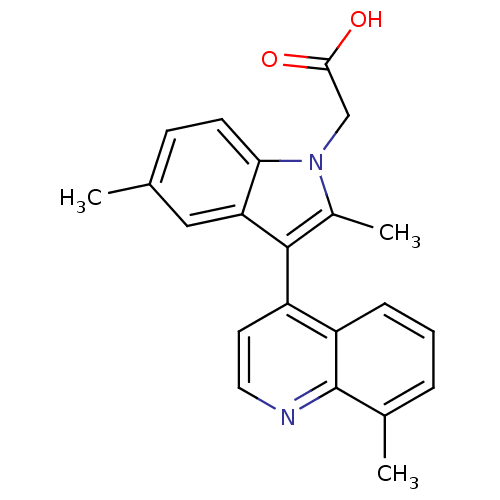 (2-(2,5-dimethyl-3-(8-methylquinolin-4-yl)-1H-indol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]PGD2 from human CRTh2 expressed in HEK293 cells | Bioorg Med Chem Lett 16: 4287-90 (2006) Article DOI: 10.1016/j.bmcl.2006.05.062 BindingDB Entry DOI: 10.7270/Q2G44PX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50414303 (CHEMBL560563) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at P2X7 receptor in human THP1 cells assessed as effect on BzATP-induced Yo-Pro uptake | J Med Chem 52: 3123-41 (2009) Article DOI: 10.1021/jm801528x BindingDB Entry DOI: 10.7270/Q2251KDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50414311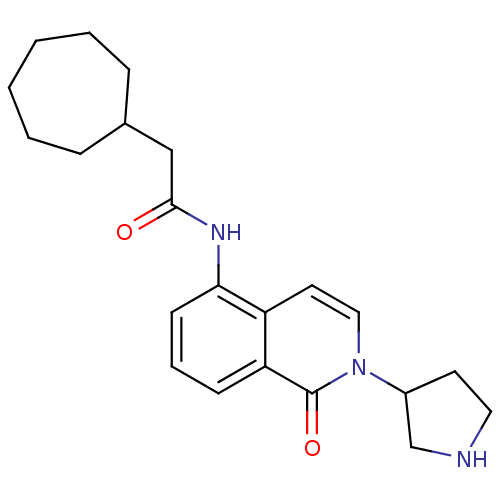 (CHEMBL552512) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.16 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at P2X7 receptor in human THP1 cells assessed as inhibition of BzATP-induced ethidium uptake | J Med Chem 52: 3123-41 (2009) Article DOI: 10.1021/jm801528x BindingDB Entry DOI: 10.7270/Q2251KDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50414349 (CHEMBL564535) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.98 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at P2X7 receptor in human THP1 cells assessed as inhibition of BzATP-induced ethidium uptake | J Med Chem 52: 3123-41 (2009) Article DOI: 10.1021/jm801528x BindingDB Entry DOI: 10.7270/Q2251KDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50339997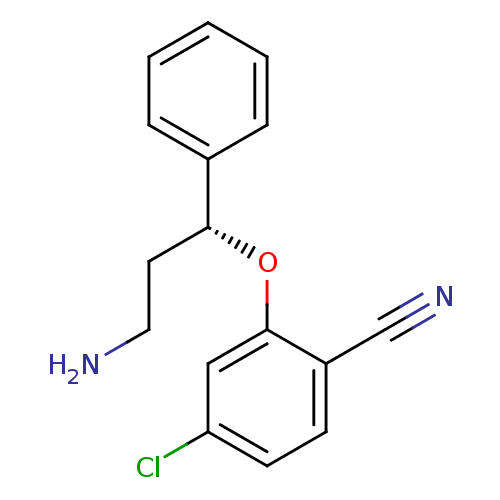 ((R)-2-(3-amino-1-phenylpropoxy)-4-chlorobenzonitri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood Curated by ChEMBL | Assay Description Inhibition of human recombinant iNOS | Bioorg Med Chem Lett 21: 2468-71 (2011) Article DOI: 10.1016/j.bmcl.2011.02.061 BindingDB Entry DOI: 10.7270/Q29G5N4S | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50339998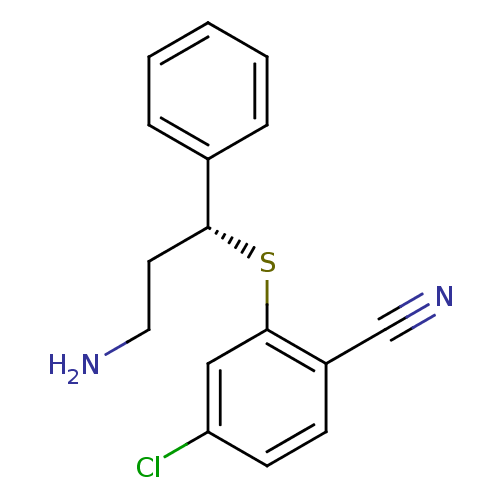 ((R)-2-(3-amino-1-phenylpropylthio)-4-chlorobenzoni...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood Curated by ChEMBL | Assay Description Inhibition of human recombinant iNOS | Bioorg Med Chem Lett 21: 2468-71 (2011) Article DOI: 10.1016/j.bmcl.2011.02.061 BindingDB Entry DOI: 10.7270/Q29G5N4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50340003 ((R)-2-(3-amino-1-phenylpropylthio)-6-methylnicotin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood Curated by ChEMBL | Assay Description Inhibition of human recombinant iNOS | Bioorg Med Chem Lett 21: 2468-71 (2011) Article DOI: 10.1016/j.bmcl.2011.02.061 BindingDB Entry DOI: 10.7270/Q29G5N4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50188300 (2-(2,5-dimethyl-3-(7-(trifluoromethyl)quinolin-4-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]PGD2 from human CRTh2 expressed in HEK293 cells | Bioorg Med Chem Lett 16: 4287-90 (2006) Article DOI: 10.1016/j.bmcl.2006.05.062 BindingDB Entry DOI: 10.7270/Q2G44PX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50340004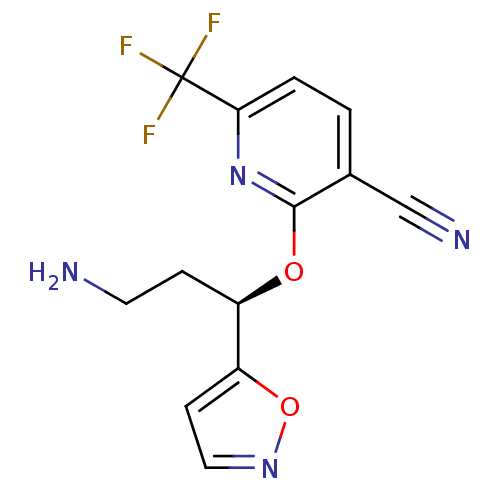 ((R)-2-(3-amino-1-(isoxazol-5-yl)propoxy)-6-(triflu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood Curated by ChEMBL | Assay Description Inhibition of human recombinant iNOS | Bioorg Med Chem Lett 21: 2468-71 (2011) Article DOI: 10.1016/j.bmcl.2011.02.061 BindingDB Entry DOI: 10.7270/Q29G5N4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50340002 (2-[[(1R)-3-Amino-1-phenylpropyl]oxy]-4-chloro-5-fl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood Curated by ChEMBL | Assay Description Inhibition of human recombinant iNOS | Bioorg Med Chem Lett 21: 2468-71 (2011) Article DOI: 10.1016/j.bmcl.2011.02.061 BindingDB Entry DOI: 10.7270/Q29G5N4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50188305 (2-(2,5-dimethyl-3-(8-(trifluoromethyl)quinolin-4-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]PGD2 from human CRTh2 expressed in HEK293 cells | Bioorg Med Chem Lett 16: 4287-90 (2006) Article DOI: 10.1016/j.bmcl.2006.05.062 BindingDB Entry DOI: 10.7270/Q2G44PX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50126705 ((S)-4-(3-(4-(4-fluorophenyl)piperazin-1-yl)-2-(iso...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.31 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor expressed in HEK293 cells assessed as inhibition of BzATP-induced calcium influx | J Med Chem 52: 3123-41 (2009) Article DOI: 10.1021/jm801528x BindingDB Entry DOI: 10.7270/Q2251KDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50414310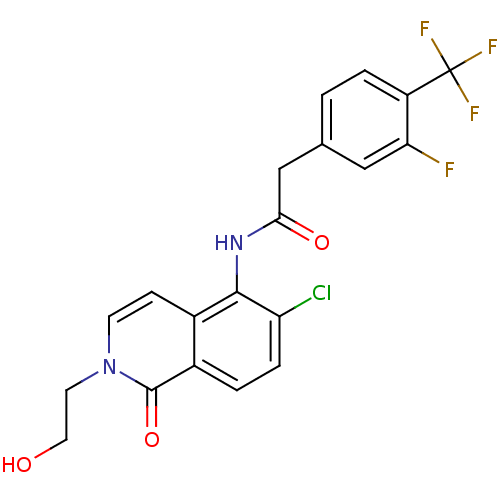 (CHEMBL563255) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.31 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at P2X7 receptor in human THP1 cells assessed as inhibition of BzATP-induced ethidium uptake | J Med Chem 52: 3123-41 (2009) Article DOI: 10.1021/jm801528x BindingDB Entry DOI: 10.7270/Q2251KDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50414330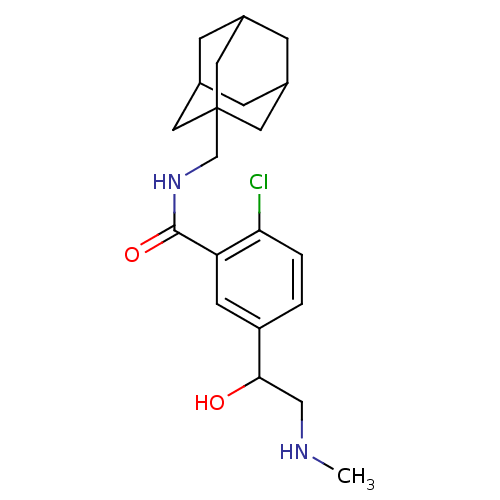 (CHEMBL563379) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.31 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at P2X7 receptor in human THP1 cells assessed as inhibition of BzATP-induced ethidium uptake | J Med Chem 52: 3123-41 (2009) Article DOI: 10.1021/jm801528x BindingDB Entry DOI: 10.7270/Q2251KDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50188309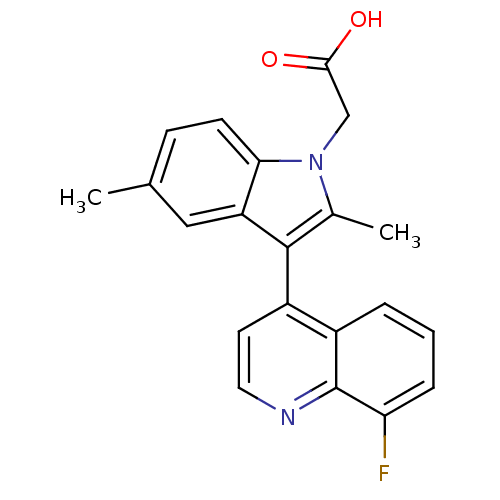 (2-(3-(8-fluoroquinolin-4-yl)-2,5-dimethyl-1H-indol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]PGD2 from human CRTh2 expressed in HEK293 cells | Bioorg Med Chem Lett 16: 4287-90 (2006) Article DOI: 10.1016/j.bmcl.2006.05.062 BindingDB Entry DOI: 10.7270/Q2G44PX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50414302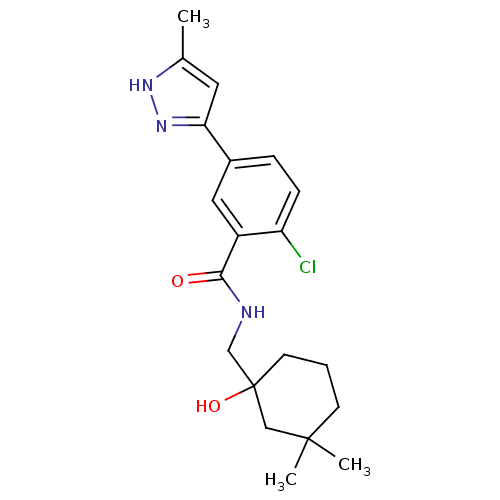 (CHEMBL560616) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.94 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at P2X7 receptor in human THP1 cells assessed as effect on BzATP-induced Yo-Pro uptake | J Med Chem 52: 3123-41 (2009) Article DOI: 10.1021/jm801528x BindingDB Entry DOI: 10.7270/Q2251KDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50414282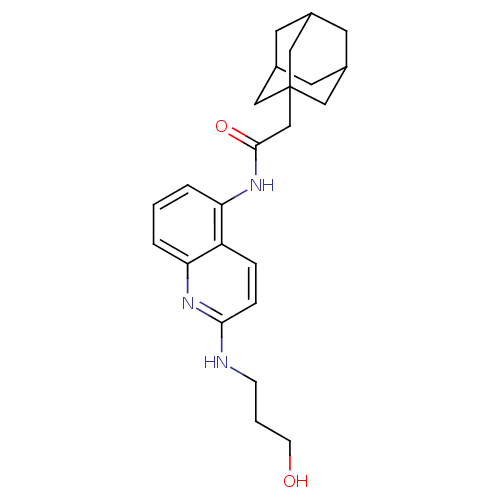 (CHEMBL551301) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.94 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at P2X7 receptor in human THP1 cells assessed as inhibition of BzATP-induced ethidium uptake | J Med Chem 52: 3123-41 (2009) Article DOI: 10.1021/jm801528x BindingDB Entry DOI: 10.7270/Q2251KDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50188307 (2-(3-(7-chloroquinolin-4-yl)-2,5-dimethyl-1H-indol...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]PGD2 from human CRTh2 expressed in HEK293 cells | Bioorg Med Chem Lett 16: 4287-90 (2006) Article DOI: 10.1016/j.bmcl.2006.05.062 BindingDB Entry DOI: 10.7270/Q2G44PX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Homo sapiens (Human)) | BDBM50339996 ((R)-4-chloro-2-(3-(methylamino)-1-phenylpropoxy)be...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Charnwood Curated by ChEMBL | Assay Description Inhibition of human recombinant iNOS | Bioorg Med Chem Lett 21: 2468-71 (2011) Article DOI: 10.1016/j.bmcl.2011.02.061 BindingDB Entry DOI: 10.7270/Q29G5N4S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50414283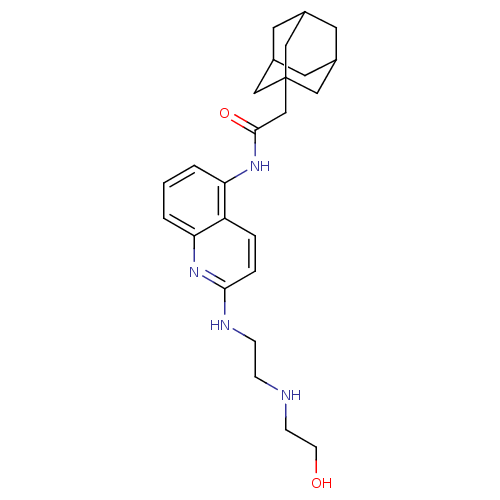 (CHEMBL562308) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at P2X7 receptor in human THP1 cells assessed as inhibition of BzATP-induced ethidium uptake | J Med Chem 52: 3123-41 (2009) Article DOI: 10.1021/jm801528x BindingDB Entry DOI: 10.7270/Q2251KDR | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50414331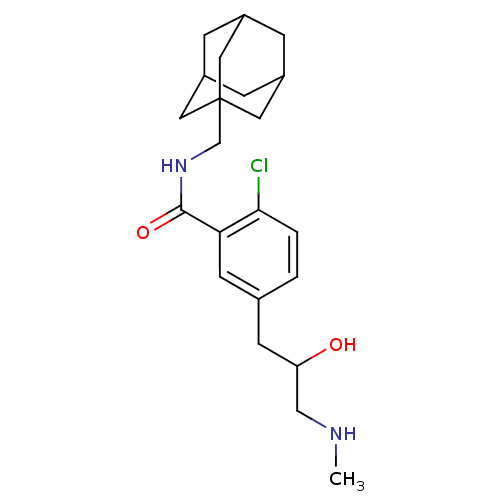 (CHEMBL551377) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at P2X7 receptor in human THP1 cells assessed as inhibition of BzATP-induced ethidium uptake | J Med Chem 52: 3123-41 (2009) Article DOI: 10.1021/jm801528x BindingDB Entry DOI: 10.7270/Q2251KDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50414339 (CHEMBL180397) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor expressed in human U373 cells assessed as inhibition of BzATP-induced Yo-Pro uptake | J Med Chem 52: 3123-41 (2009) Article DOI: 10.1021/jm801528x BindingDB Entry DOI: 10.7270/Q2251KDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50352058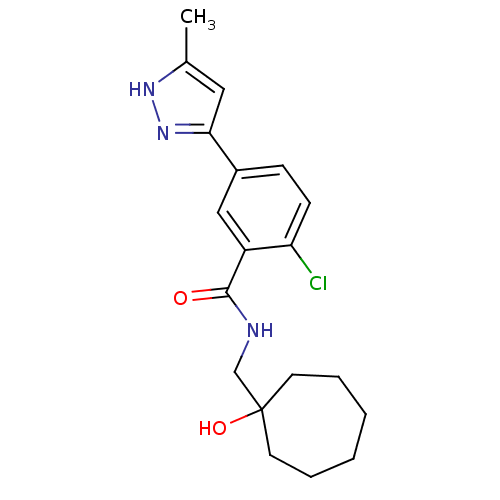 (CHEMBL560241) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at P2X7 receptor in human THP1 cells assessed as effect on BzATP-induced Yo-Pro uptake | J Med Chem 52: 3123-41 (2009) Article DOI: 10.1021/jm801528x BindingDB Entry DOI: 10.7270/Q2251KDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50414301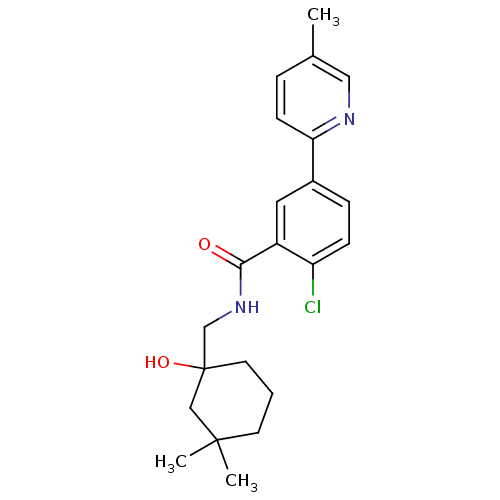 (CHEMBL563152) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at P2X7 receptor in human THP1 cells assessed as effect on BzATP-induced Yo-Pro uptake | J Med Chem 52: 3123-41 (2009) Article DOI: 10.1021/jm801528x BindingDB Entry DOI: 10.7270/Q2251KDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50412149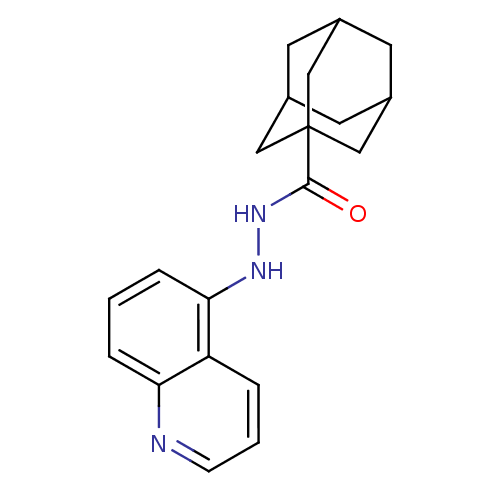 (CHEMBL497967) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at P2X7 receptor in human THP1 cells assessed as inhibition of BzATP-induced ethidium uptake | J Med Chem 52: 3123-41 (2009) Article DOI: 10.1021/jm801528x BindingDB Entry DOI: 10.7270/Q2251KDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor 2 (Homo sapiens (Human)) | BDBM50188299 (2-(3-(8-methoxyquinolin-4-yl)-2,5-dimethyl-1H-indo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca R&D Charnwood Curated by ChEMBL | Assay Description Displacement of [3H]PGD2 from human CRTh2 expressed in HEK293 cells | Bioorg Med Chem Lett 16: 4287-90 (2006) Article DOI: 10.1016/j.bmcl.2006.05.062 BindingDB Entry DOI: 10.7270/Q2G44PX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50410956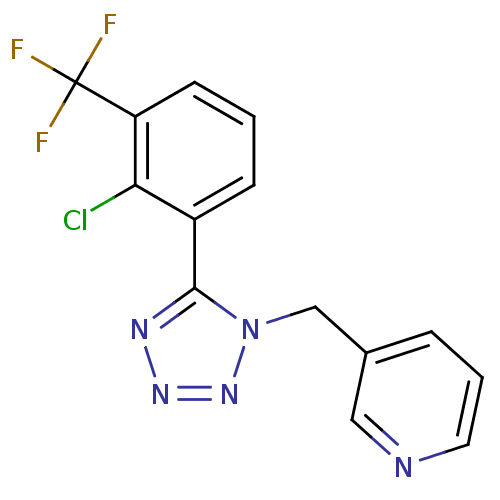 (CHEMBL437703) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at P2X7 receptor in human THP1 cells assessed as inhibition of BzATP-induced ethidium uptake | J Med Chem 52: 3123-41 (2009) Article DOI: 10.1021/jm801528x BindingDB Entry DOI: 10.7270/Q2251KDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50410949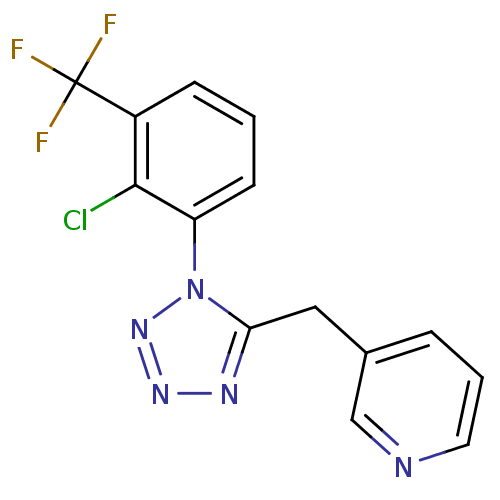 (CHEMBL210835) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at P2X7 receptor in human THP1 cells assessed as inhibition of BzATP-induced ethidium uptake | J Med Chem 52: 3123-41 (2009) Article DOI: 10.1021/jm801528x BindingDB Entry DOI: 10.7270/Q2251KDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50087267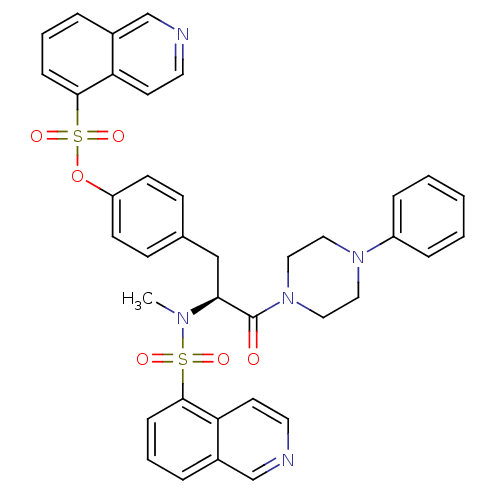 ((1-(N,O-bis(1,5-isoquinolinesulfonyl)-N-methyl-L-t...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at P2X7 receptor in human lymphocytes assessed as inhibition of ATP-induced Ba2+ influx | J Med Chem 52: 3123-41 (2009) Article DOI: 10.1021/jm801528x BindingDB Entry DOI: 10.7270/Q2251KDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50126723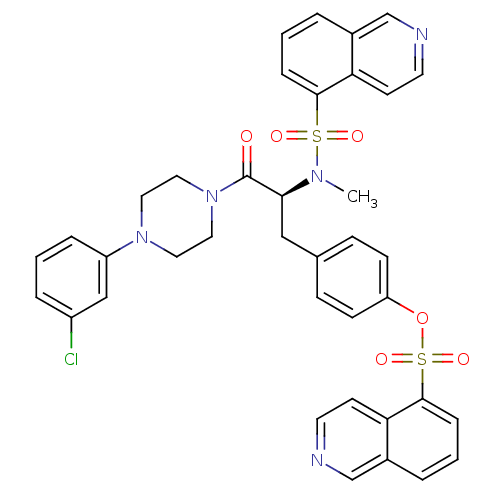 ((S)-4-(3-(4-(3-chlorophenyl)piperazin-1-yl)-2-(N-m...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor expressed in HEK293 cells assessed as inhibition of BzATP-induced calcium influx | J Med Chem 52: 3123-41 (2009) Article DOI: 10.1021/jm801528x BindingDB Entry DOI: 10.7270/Q2251KDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50216395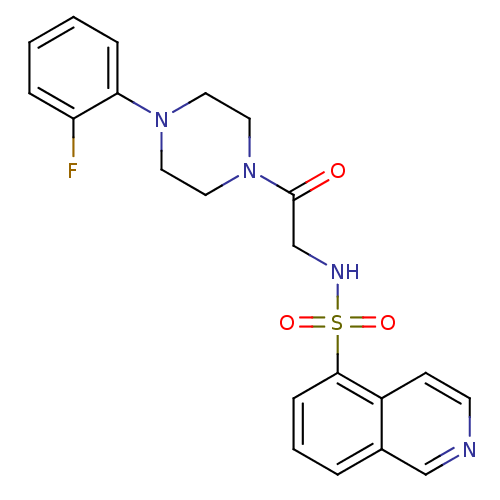 (CHEMBL231032 | N-(2-(4-(2-fluorophenyl)piperazin-1...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor expressed in HEK293 cells assessed as inhibition of BzATP-induced calcium influx | J Med Chem 52: 3123-41 (2009) Article DOI: 10.1021/jm801528x BindingDB Entry DOI: 10.7270/Q2251KDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50411885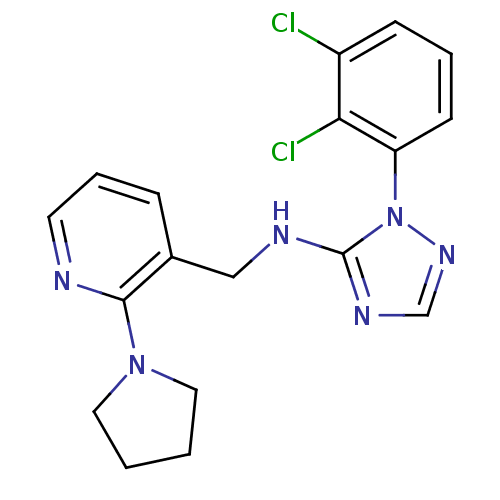 (CHEMBL257045) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at P2X7 receptor in human THP1 cells assessed as inhibition of BzATP-induced ethidium uptake | J Med Chem 52: 3123-41 (2009) Article DOI: 10.1021/jm801528x BindingDB Entry DOI: 10.7270/Q2251KDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Rattus norvegicus (Rat)) | BDBM50411881 (CHEMBL271462) | PDB KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Activity at rat P2X7 receptor expressed in HEK cells assessed as effect on BzATP-induced ethidium uptake | J Med Chem 52: 3123-41 (2009) Article DOI: 10.1021/jm801528x BindingDB Entry DOI: 10.7270/Q2251KDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50126720 ((S)-4-(2-(N-methylisoquinoline-5-sulfonamido)-3-ox...) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15.8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at human P2X7 receptor expressed in HEK293 cells assessed as inhibition of BzATP-induced calcium influx | J Med Chem 52: 3123-41 (2009) Article DOI: 10.1021/jm801528x BindingDB Entry DOI: 10.7270/Q2251KDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50411881 (CHEMBL271462) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15.8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at P2X7 receptor in human THP1 cells assessed as inhibition of BzATP-induced ethidium uptake | J Med Chem 52: 3123-41 (2009) Article DOI: 10.1021/jm801528x BindingDB Entry DOI: 10.7270/Q2251KDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50410957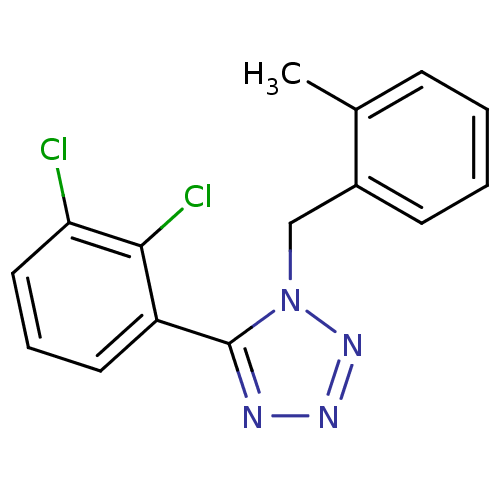 (CHEMBL380239) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15.8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at P2X7 receptor in human THP1 cells assessed as inhibition of BzATP-induced ethidium uptake | J Med Chem 52: 3123-41 (2009) Article DOI: 10.1021/jm801528x BindingDB Entry DOI: 10.7270/Q2251KDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| P2X purinoceptor 7 (Homo sapiens (Human)) | BDBM50414285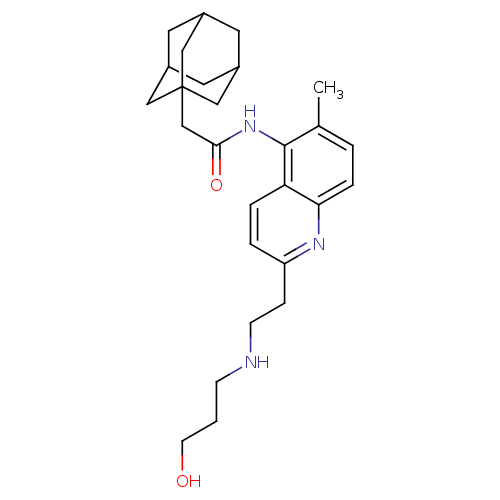 (CHEMBL564366) | PDB KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15.8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Antagonist activity at P2X7 receptor in human THP1 cells assessed as inhibition of BzATP-induced ethidium uptake | J Med Chem 52: 3123-41 (2009) Article DOI: 10.1021/jm801528x BindingDB Entry DOI: 10.7270/Q2251KDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 216 total ) | Next | Last >> |