

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM8610 (1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of CYP3A4 activity in human liver microsomes using midazolam as a substrate by LC/MS analysis | Drug Metab Dispos 41: 814-26 (2013) Article DOI: 10.1124/dmd.112.048355 BindingDB Entry DOI: 10.7270/Q2NC62XH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM8610 (1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of CYP3A4 activity in human liver microsomes using nifedipine as a substrate by LC/MS analysis | Drug Metab Dispos 41: 814-26 (2013) Article DOI: 10.1124/dmd.112.048355 BindingDB Entry DOI: 10.7270/Q2NC62XH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM8610 (1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of CYP3A4 activity in human liver microsomes using testosterone as a substrate by LC/MS analysis | Drug Metab Dispos 41: 814-26 (2013) Article DOI: 10.1124/dmd.112.048355 BindingDB Entry DOI: 10.7270/Q2NC62XH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50088527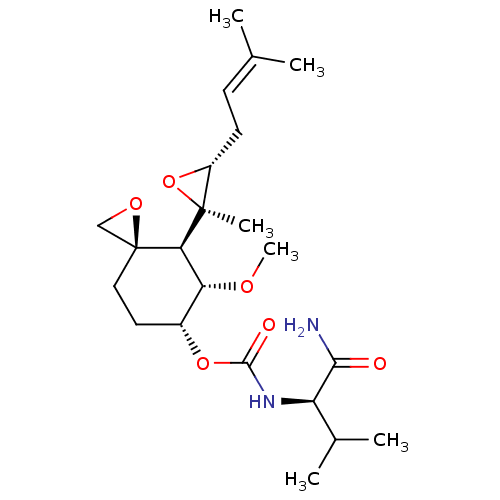 (CHEMBL3527358) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | 5.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of CYP3A4 activity in human liver microsomes using midazolam as a substrate by LC/MS analysis | Drug Metab Dispos 41: 814-26 (2013) Article DOI: 10.1124/dmd.112.048355 BindingDB Entry DOI: 10.7270/Q2NC62XH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50088527 (CHEMBL3527358) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | 2.87E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of CYP3A4 activity in human liver microsomes using testosterone as a substrate by LC/MS analysis | Drug Metab Dispos 41: 814-26 (2013) Article DOI: 10.1124/dmd.112.048355 BindingDB Entry DOI: 10.7270/Q2NC62XH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM50088527 (CHEMBL3527358) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | 4.03E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of CYP3A4 activity in human liver microsomes using nifedipine as a substrate by LC/MS analysis | Drug Metab Dispos 41: 814-26 (2013) Article DOI: 10.1124/dmd.112.048355 BindingDB Entry DOI: 10.7270/Q2NC62XH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50315562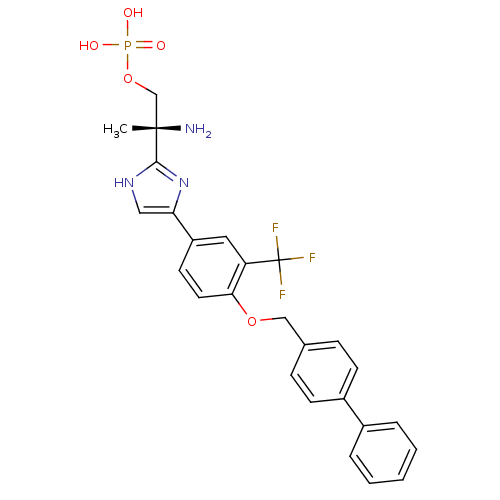 ((R)-2-amino-2-(4-(4-(biphenyl-4-ylmethoxy)-3-(trif...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor expressed in HEK293T cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 2520-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.098 BindingDB Entry DOI: 10.7270/Q2XS5VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50315559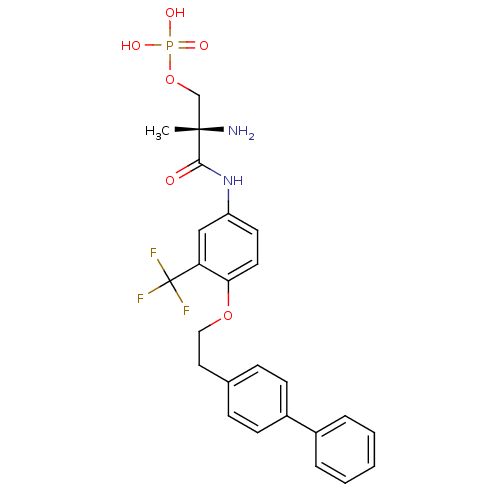 ((S)-2-amino-3-(4-(2-(biphenyl-4-yl)ethoxy)-3-(trif...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor expressed in HEK293T cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 2520-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.098 BindingDB Entry DOI: 10.7270/Q2XS5VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50315556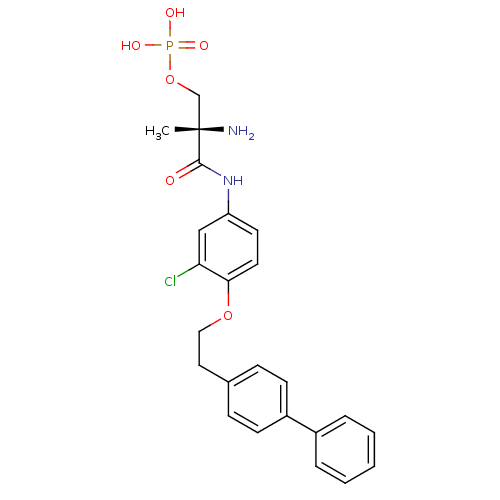 ((S)-2-amino-3-(4-(2-(biphenyl-4-yl)ethoxy)-3-chlor...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor expressed in HEK293T cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 2520-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.098 BindingDB Entry DOI: 10.7270/Q2XS5VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50249266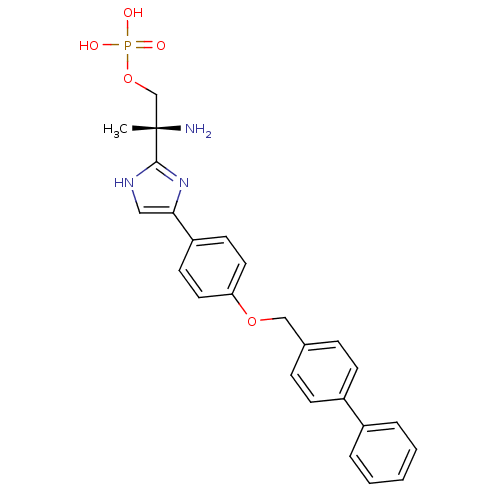 ((R)-2-amino-2-(4-(4-(biphenyl-4-ylmethoxy)phenyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.03 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor expressed in HEK293T cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 2520-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.098 BindingDB Entry DOI: 10.7270/Q2XS5VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50249240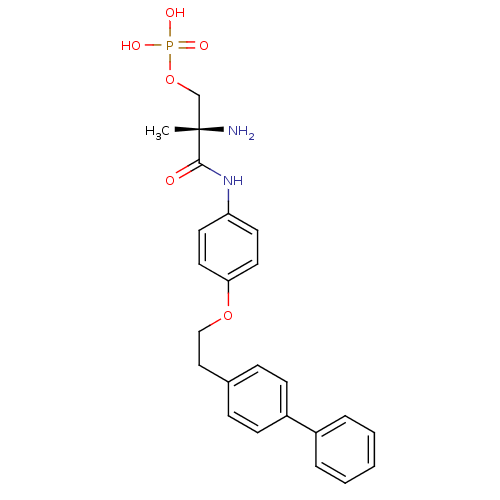 ((S)-2-amino-3-(4-(2-(biphenyl-4-yl)ethoxy)phenylam...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.05 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor expressed in HEK293T cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 2520-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.098 BindingDB Entry DOI: 10.7270/Q2XS5VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50315557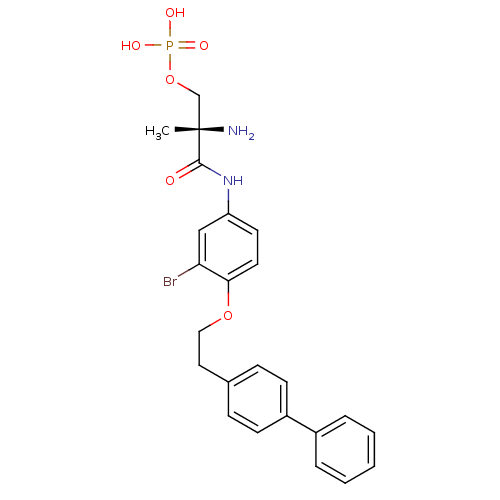 ((S)-2-amino-3-(4-(2-(biphenyl-4-yl)ethoxy)-3-bromo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor expressed in HEK293T cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 2520-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.098 BindingDB Entry DOI: 10.7270/Q2XS5VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 4 (Homo sapiens (Human)) | BDBM50315559 ((S)-2-amino-3-(4-(2-(biphenyl-4-yl)ethoxy)-3-(trif...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P4 receptor expressed in HEK293T cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 2520-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.098 BindingDB Entry DOI: 10.7270/Q2XS5VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50315555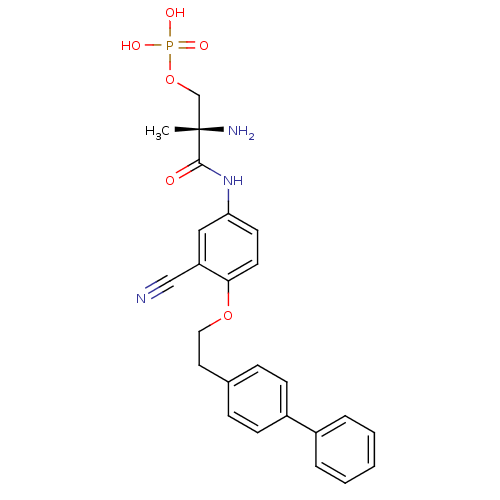 ((S)-2-amino-3-(4-(2-(biphenyl-4-yl)ethoxy)-3-cyano...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor expressed in HEK293T cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 2520-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.098 BindingDB Entry DOI: 10.7270/Q2XS5VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50315550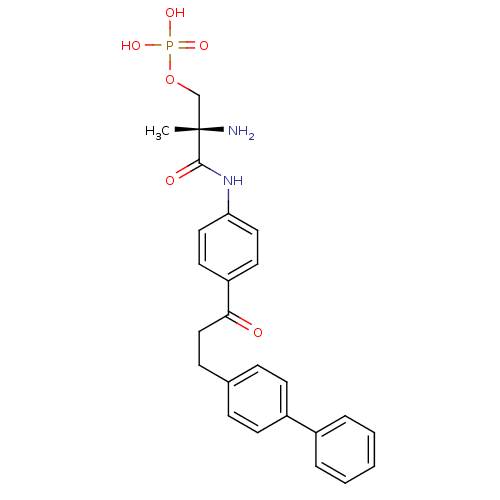 ((S)-2-amino-3-(4-(3-(biphenyl-4-yl)propanoyl)pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.14 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor expressed in HEK293T cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 2520-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.098 BindingDB Entry DOI: 10.7270/Q2XS5VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 4 (Homo sapiens (Human)) | BDBM50315562 ((R)-2-amino-2-(4-(4-(biphenyl-4-ylmethoxy)-3-(trif...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P4 receptor expressed in HEK293T cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 2520-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.098 BindingDB Entry DOI: 10.7270/Q2XS5VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 4 (Homo sapiens (Human)) | BDBM50249266 ((R)-2-amino-2-(4-(4-(biphenyl-4-ylmethoxy)phenyl)-...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.87 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P4 receptor expressed in HEK293T cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 2520-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.098 BindingDB Entry DOI: 10.7270/Q2XS5VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50315558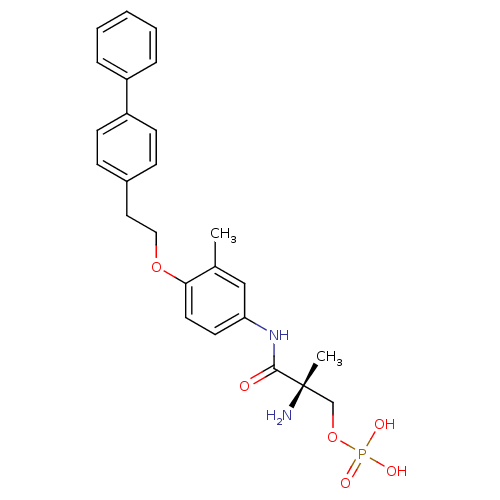 ((S)-2-amino-3-(4-(2-(biphenyl-4-yl)ethoxy)-3-methy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor expressed in HEK293T cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 2520-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.098 BindingDB Entry DOI: 10.7270/Q2XS5VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50315560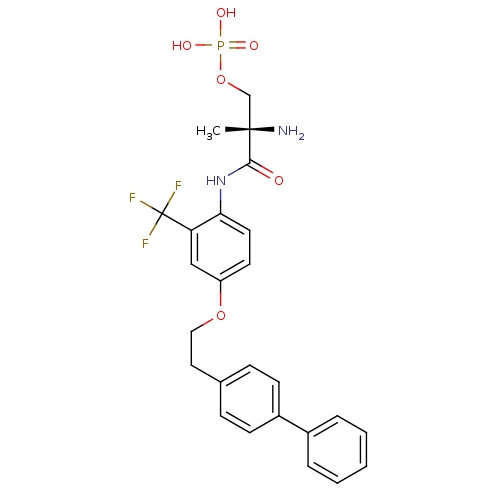 ((S)-2-amino-3-(4-(2-(biphenyl-4-yl)ethoxy)-2-(trif...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor expressed in HEK293T cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 2520-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.098 BindingDB Entry DOI: 10.7270/Q2XS5VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 4 (Homo sapiens (Human)) | BDBM50315561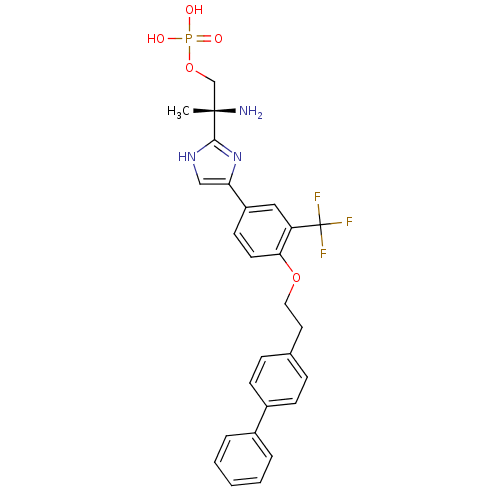 ((R)-2-amino-2-(4-(4-(2-(biphenyl-4-yl)ethoxy)-3-(t...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P4 receptor expressed in HEK293T cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 2520-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.098 BindingDB Entry DOI: 10.7270/Q2XS5VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50315561 ((R)-2-amino-2-(4-(4-(2-(biphenyl-4-yl)ethoxy)-3-(t...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor expressed in HEK293T cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 2520-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.098 BindingDB Entry DOI: 10.7270/Q2XS5VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 5 (Homo sapiens (Human)) | BDBM50315562 ((R)-2-amino-2-(4-(4-(biphenyl-4-ylmethoxy)-3-(trif...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P5 receptor expressed in HEK293T cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 2520-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.098 BindingDB Entry DOI: 10.7270/Q2XS5VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 5 (Homo sapiens (Human)) | BDBM50249266 ((R)-2-amino-2-(4-(4-(biphenyl-4-ylmethoxy)phenyl)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P5 receptor expressed in HEK293T cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 2520-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.098 BindingDB Entry DOI: 10.7270/Q2XS5VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 3A4 (Homo sapiens (Human)) | BDBM8610 (1-[4-(4-{[(2R,4S)-2-(2,4-dichlorophenyl)-2-(1H-imi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of CYP3A4 activity in human liver microsomes assessed as dibenzo fluuorescene oxidation up to 40 uM | Drug Metab Dispos 41: 814-26 (2013) Article DOI: 10.1124/dmd.112.048355 BindingDB Entry DOI: 10.7270/Q2NC62XH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 4 (Homo sapiens (Human)) | BDBM50249240 ((S)-2-amino-3-(4-(2-(biphenyl-4-yl)ethoxy)phenylam...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P4 receptor expressed in HEK293T cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 2520-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.098 BindingDB Entry DOI: 10.7270/Q2XS5VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 4 (Homo sapiens (Human)) | BDBM50315550 ((S)-2-amino-3-(4-(3-(biphenyl-4-yl)propanoyl)pheny...) | NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P4 receptor expressed in HEK293T cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 2520-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.098 BindingDB Entry DOI: 10.7270/Q2XS5VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50249266 ((R)-2-amino-2-(4-(4-(biphenyl-4-ylmethoxy)phenyl)-...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P3 receptor expressed in HEK293T cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 2520-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.098 BindingDB Entry DOI: 10.7270/Q2XS5VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50315551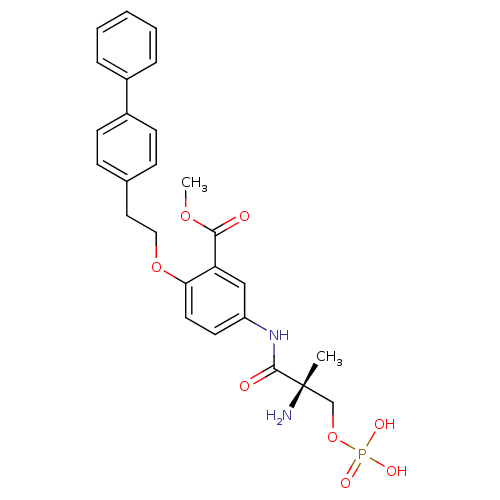 ((S)-methyl 5-(2-amino-2-methyl-3-(phosphonooxy)pro...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor expressed in HEK293T cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 2520-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.098 BindingDB Entry DOI: 10.7270/Q2XS5VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 5 (Homo sapiens (Human)) | BDBM50249240 ((S)-2-amino-3-(4-(2-(biphenyl-4-yl)ethoxy)phenylam...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 43.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P5 receptor expressed in HEK293T cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 2520-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.098 BindingDB Entry DOI: 10.7270/Q2XS5VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 5 (Homo sapiens (Human)) | BDBM50315559 ((S)-2-amino-3-(4-(2-(biphenyl-4-yl)ethoxy)-3-(trif...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 87.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P5 receptor expressed in HEK293T cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 2520-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.098 BindingDB Entry DOI: 10.7270/Q2XS5VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50315553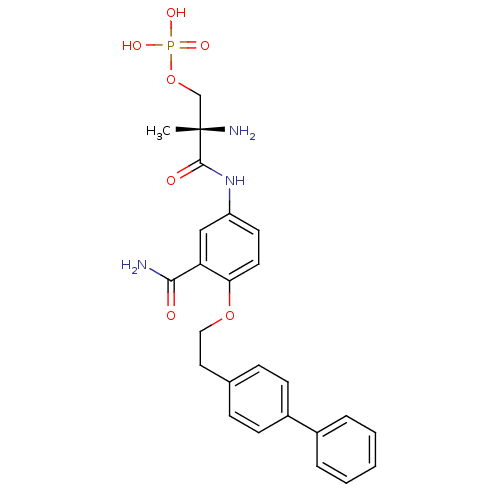 ((S)-2-amino-3-(4-(2-(biphenyl-4-yl)ethoxy)-3-carba...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor expressed in HEK293T cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 2520-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.098 BindingDB Entry DOI: 10.7270/Q2XS5VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 5 (Homo sapiens (Human)) | BDBM50315561 ((R)-2-amino-2-(4-(4-(2-(biphenyl-4-yl)ethoxy)-3-(t...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 184 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P5 receptor expressed in HEK293T cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 2520-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.098 BindingDB Entry DOI: 10.7270/Q2XS5VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50249240 ((S)-2-amino-3-(4-(2-(biphenyl-4-yl)ethoxy)phenylam...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P3 receptor expressed in HEK293T cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 2520-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.098 BindingDB Entry DOI: 10.7270/Q2XS5VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50315556 ((S)-2-amino-3-(4-(2-(biphenyl-4-yl)ethoxy)-3-chlor...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 285 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P3 receptor expressed in HEK293T cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 2520-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.098 BindingDB Entry DOI: 10.7270/Q2XS5VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50315554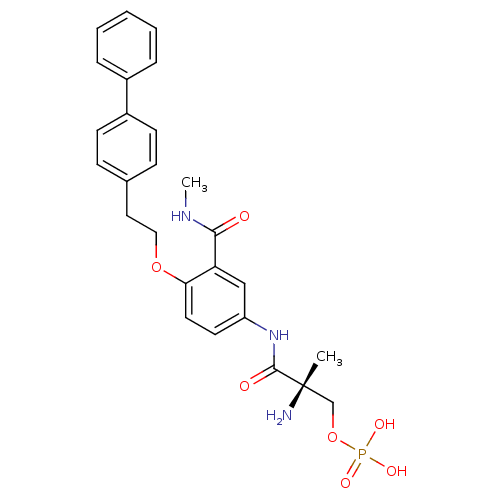 ((S)-2-amino-3-(4-(2-(biphenyl-4-yl)ethoxy)-3-(meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor expressed in HEK293T cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 2520-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.098 BindingDB Entry DOI: 10.7270/Q2XS5VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50315560 ((S)-2-amino-3-(4-(2-(biphenyl-4-yl)ethoxy)-2-(trif...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P3 receptor expressed in HEK293T cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 2520-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.098 BindingDB Entry DOI: 10.7270/Q2XS5VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50315557 ((S)-2-amino-3-(4-(2-(biphenyl-4-yl)ethoxy)-3-bromo...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P3 receptor expressed in HEK293T cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 2520-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.098 BindingDB Entry DOI: 10.7270/Q2XS5VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50315553 ((S)-2-amino-3-(4-(2-(biphenyl-4-yl)ethoxy)-3-carba...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P3 receptor expressed in HEK293T cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 2520-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.098 BindingDB Entry DOI: 10.7270/Q2XS5VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50315554 ((S)-2-amino-3-(4-(2-(biphenyl-4-yl)ethoxy)-3-(meth...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P3 receptor expressed in HEK293T cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 2520-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.098 BindingDB Entry DOI: 10.7270/Q2XS5VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 5 (Homo sapiens (Human)) | BDBM50315550 ((S)-2-amino-3-(4-(3-(biphenyl-4-yl)propanoyl)pheny...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P5 receptor expressed in HEK293T cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 2520-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.098 BindingDB Entry DOI: 10.7270/Q2XS5VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50315555 ((S)-2-amino-3-(4-(2-(biphenyl-4-yl)ethoxy)-3-cyano...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P3 receptor expressed in HEK293T cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 2520-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.098 BindingDB Entry DOI: 10.7270/Q2XS5VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50315562 ((R)-2-amino-2-(4-(4-(biphenyl-4-ylmethoxy)-3-(trif...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P3 receptor expressed in HEK293T cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 2520-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.098 BindingDB Entry DOI: 10.7270/Q2XS5VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50315558 ((S)-2-amino-3-(4-(2-(biphenyl-4-yl)ethoxy)-3-methy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P3 receptor expressed in HEK293T cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 2520-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.098 BindingDB Entry DOI: 10.7270/Q2XS5VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50315550 ((S)-2-amino-3-(4-(3-(biphenyl-4-yl)propanoyl)pheny...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P3 receptor expressed in HEK293T cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 2520-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.098 BindingDB Entry DOI: 10.7270/Q2XS5VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50315559 ((S)-2-amino-3-(4-(2-(biphenyl-4-yl)ethoxy)-3-(trif...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P3 receptor expressed in HEK293T cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 2520-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.098 BindingDB Entry DOI: 10.7270/Q2XS5VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50315552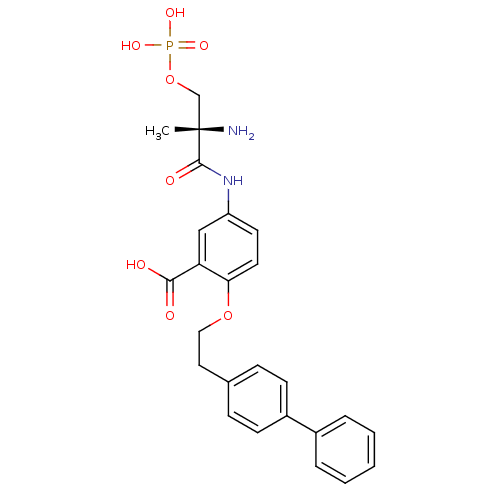 ((S)-5-(2-amino-2-methyl-3-(phosphonooxy)propanamid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P1 receptor expressed in HEK293T cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 2520-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.098 BindingDB Entry DOI: 10.7270/Q2XS5VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50315551 ((S)-methyl 5-(2-amino-2-methyl-3-(phosphonooxy)pro...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P3 receptor expressed in HEK293T cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 2520-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.098 BindingDB Entry DOI: 10.7270/Q2XS5VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50315552 ((S)-5-(2-amino-2-methyl-3-(phosphonooxy)propanamid...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P3 receptor expressed in HEK293T cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 2520-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.098 BindingDB Entry DOI: 10.7270/Q2XS5VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 3 (Homo sapiens (Human)) | BDBM50315561 ((R)-2-amino-2-(4-(4-(2-(biphenyl-4-yl)ethoxy)-3-(t...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Praecis Pharmaceuticals Inc Curated by ChEMBL | Assay Description Displacement of [33P]sphingosine-1-phosphate from human S1P3 receptor expressed in HEK293T cells after 60 mins by scintillation counting | Bioorg Med Chem Lett 20: 2520-4 (2010) Article DOI: 10.1016/j.bmcl.2010.02.098 BindingDB Entry DOI: 10.7270/Q2XS5VJB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Microtubule-associated protein 2 (Homo sapiens (Human)) | BDBM50088521 (CHEMBL3527219) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of MetAP2 activity in HUVEC assessed as inhibition of cell proliferation by MTT assay | Drug Metab Dispos 41: 814-26 (2013) Article DOI: 10.1124/dmd.112.048355 BindingDB Entry DOI: 10.7270/Q2NC62XH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 68 total ) | Next | Last >> |