 Found 652 hits with Last Name = 'blaney' and Initial = 'pm'
Found 652 hits with Last Name = 'blaney' and Initial = 'pm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50394918
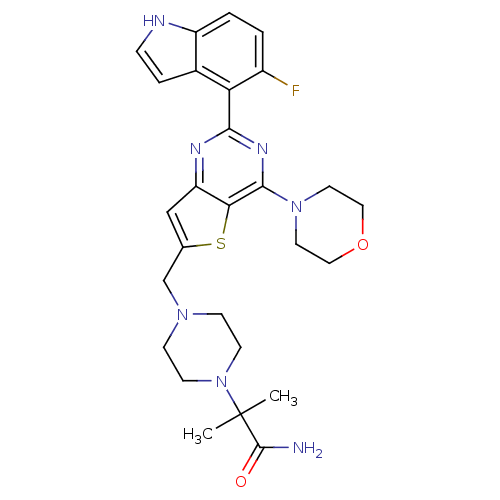
(CHEMBL2165504)Show SMILES CC(C)(N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2c(F)ccc3[nH]ccc23)CC1)C(N)=O Show InChI InChI=1S/C27H32FN7O2S/c1-27(2,26(29)36)35-9-7-33(8-10-35)16-17-15-21-23(38-17)25(34-11-13-37-14-12-34)32-24(31-21)22-18-5-6-30-20(18)4-3-19(22)28/h3-6,15,30H,7-14,16H2,1-2H3,(H2,29,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4 in human liver microsomes preincubated for 30 mins |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50394917
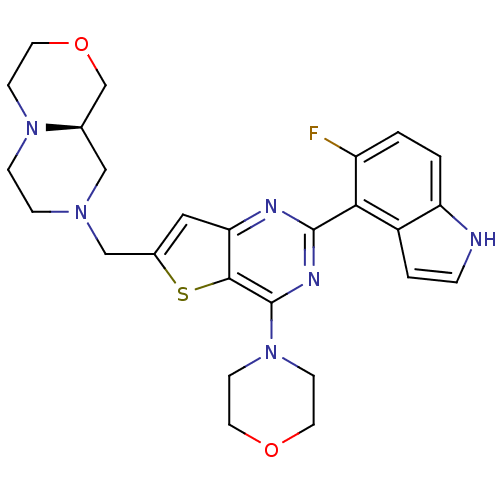
(CHEMBL2165505)Show SMILES Fc1ccc2[nH]ccc2c1-c1nc(N2CCOCC2)c2sc(CN3CCN4CCOC[C@H]4C3)cc2n1 |r| Show InChI InChI=1S/C26H29FN6O2S/c27-20-1-2-21-19(3-4-28-21)23(20)25-29-22-13-18(15-31-5-6-32-7-12-35-16-17(32)14-31)36-24(22)26(30-25)33-8-10-34-11-9-33/h1-4,13,17,28H,5-12,14-16H2/t17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Time dependent inhibition of CYP3A4 in human liver microsomes preincubated for 30 mins |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50394916
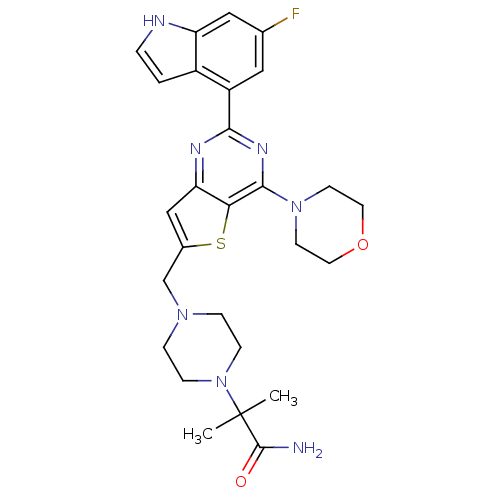
(CHEMBL2165506)Show SMILES CC(C)(N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cc(F)cc3[nH]ccc23)CC1)C(N)=O Show InChI InChI=1S/C27H32FN7O2S/c1-27(2,26(29)36)35-7-5-33(6-8-35)16-18-15-22-23(38-18)25(34-9-11-37-12-10-34)32-24(31-22)20-13-17(28)14-21-19(20)3-4-30-21/h3-4,13-15,30H,5-12,16H2,1-2H3,(H2,29,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assay |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50392144
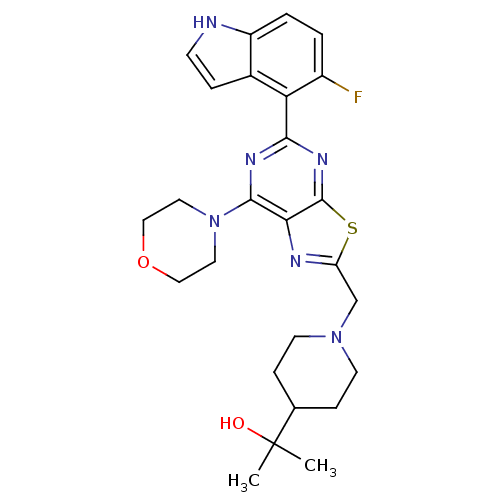
(CHEMBL2152774)Show SMILES CC(C)(O)C1CCN(Cc2nc3c(nc(nc3s2)-c2c(F)ccc3[nH]ccc23)N2CCOCC2)CC1 Show InChI InChI=1S/C26H31FN6O2S/c1-26(2,34)16-6-9-32(10-7-16)15-20-29-22-24(33-11-13-35-14-12-33)30-23(31-25(22)36-20)21-17-5-8-28-19(17)4-3-18(21)27/h3-5,8,16,28,34H,6-7,9-15H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110delta using PIP2 as substrate assessed as PIP3 formation by fluorescence polarization assay |
Bioorg Med Chem Lett 22: 4296-302 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.027
BindingDB Entry DOI: 10.7270/Q2HM59J8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50394910
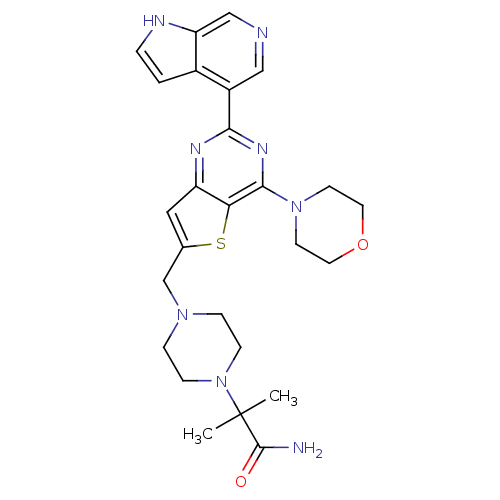
(CHEMBL2165512)Show SMILES CC(C)(N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cncc3[nH]ccc23)CC1)C(N)=O Show InChI InChI=1S/C26H32N8O2S/c1-26(2,25(27)35)34-7-5-32(6-8-34)16-17-13-20-22(37-17)24(33-9-11-36-12-10-33)31-23(30-20)19-14-28-15-21-18(19)3-4-29-21/h3-4,13-15,29H,5-12,16H2,1-2H3,(H2,27,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assay |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50394913

(CHEMBL2165509)Show SMILES Cc1cc2c(cccc2[nH]1)-c1nc(N2CCOCC2)c2sc(CN3CCN(CC3)C(C)(C)C(N)=O)cc2n1 Show InChI InChI=1S/C28H35N7O2S/c1-18-15-21-20(5-4-6-22(21)30-18)25-31-23-16-19(38-24(23)26(32-25)34-11-13-37-14-12-34)17-33-7-9-35(10-8-33)28(2,3)27(29)36/h4-6,15-16,30H,7-14,17H2,1-3H3,(H2,29,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assay |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50394920
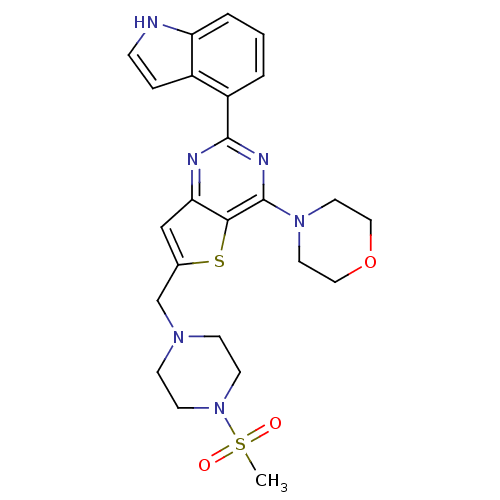
(CHEMBL2165672)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ccc23)CC1 Show InChI InChI=1S/C24H28N6O3S2/c1-35(31,32)30-9-7-28(8-10-30)16-17-15-21-22(34-17)24(29-11-13-33-14-12-29)27-23(26-21)19-3-2-4-20-18(19)5-6-25-20/h2-6,15,25H,7-14,16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assay |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50394921
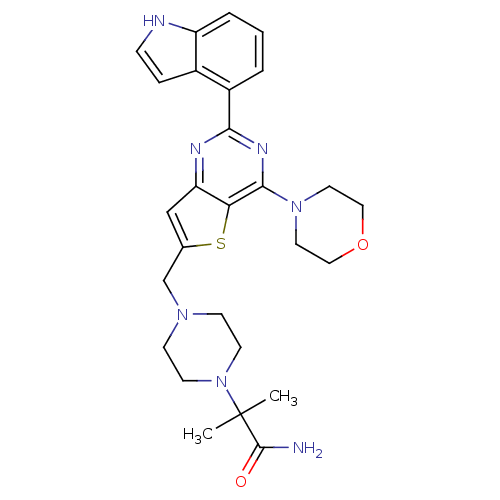
(CHEMBL2165671)Show SMILES CC(C)(N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ccc23)CC1)C(N)=O Show InChI InChI=1S/C27H33N7O2S/c1-27(2,26(28)35)34-10-8-32(9-11-34)17-18-16-22-23(37-18)25(33-12-14-36-15-13-33)31-24(30-22)20-4-3-5-21-19(20)6-7-29-21/h3-7,16,29H,8-15,17H2,1-2H3,(H2,28,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assay |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50394908
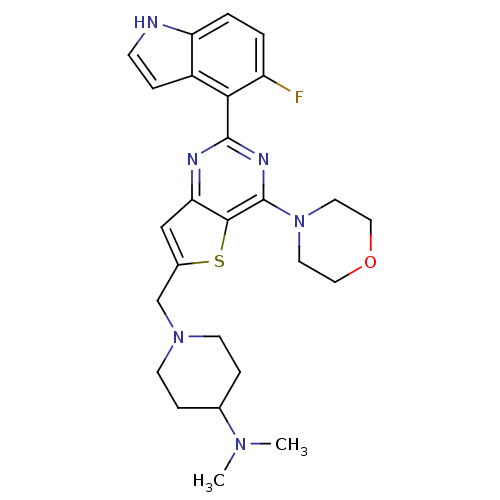
(CHEMBL2165666)Show SMILES CN(C)C1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2c(F)ccc3[nH]ccc23)CC1 Show InChI InChI=1S/C26H31FN6OS/c1-31(2)17-6-9-32(10-7-17)16-18-15-22-24(35-18)26(33-11-13-34-14-12-33)30-25(29-22)23-19-5-8-28-21(19)4-3-20(23)27/h3-5,8,15,17,28H,6-7,9-14,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assay |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50392145
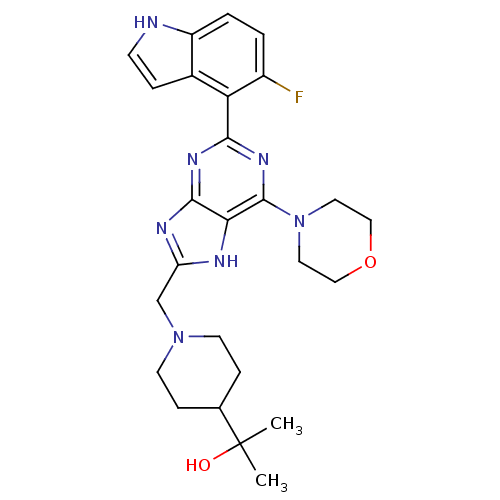
(CHEMBL2152775)Show SMILES CC(C)(O)C1CCN(Cc2nc3nc(nc(N4CCOCC4)c3[nH]2)-c2c(F)ccc3[nH]ccc23)CC1 Show InChI InChI=1S/C26H32FN7O2/c1-26(2,35)16-6-9-33(10-7-16)15-20-29-22-24(30-20)31-23(32-25(22)34-11-13-36-14-12-34)21-17-5-8-28-19(17)4-3-18(21)27/h3-5,8,16,28,35H,6-7,9-15H2,1-2H3,(H,29,30,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110delta using PIP2 as substrate assessed as PIP3 formation by fluorescence polarization assay |
Bioorg Med Chem Lett 22: 4296-302 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.027
BindingDB Entry DOI: 10.7270/Q2HM59J8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50394893
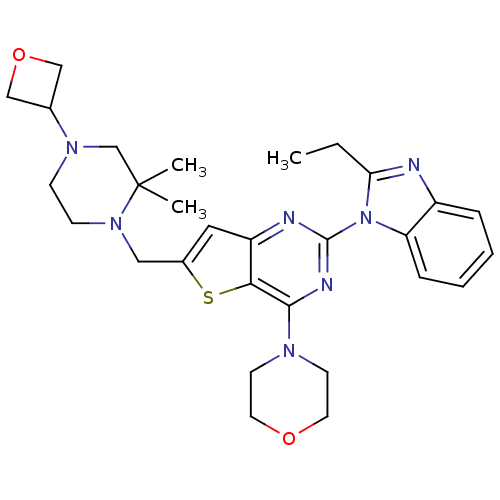
(CHEMBL2165502)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2sc(CN3CCN(CC3(C)C)C3COC3)cc2n1 Show InChI InChI=1S/C29H37N7O2S/c1-4-25-30-22-7-5-6-8-24(22)36(25)28-31-23-15-21(39-26(23)27(32-28)33-11-13-37-14-12-33)16-35-10-9-34(19-29(35,2)3)20-17-38-18-20/h5-8,15,20H,4,9-14,16-19H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assay |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50394909
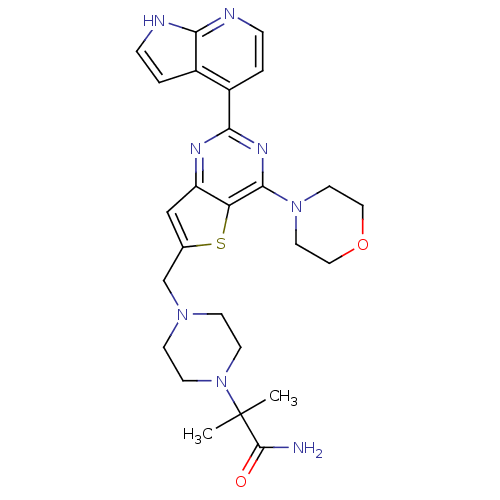
(CHEMBL2165513)Show SMILES CC(C)(N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2ccnc3[nH]ccc23)CC1)C(N)=O Show InChI InChI=1S/C26H32N8O2S/c1-26(2,25(27)35)34-9-7-32(8-10-34)16-17-15-20-21(37-17)24(33-11-13-36-14-12-33)31-23(30-20)19-4-6-29-22-18(19)3-5-28-22/h3-6,15H,7-14,16H2,1-2H3,(H2,27,35)(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assay |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50394903
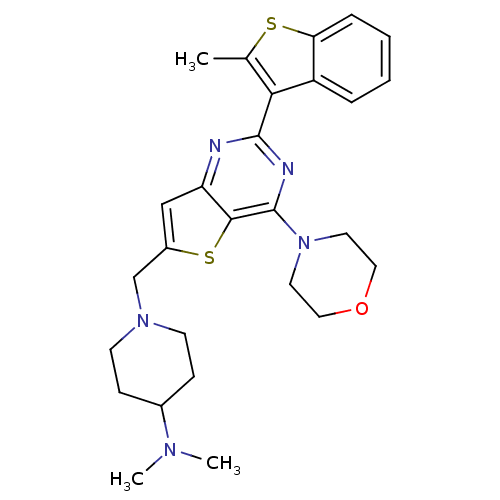
(CHEMBL2165492)Show SMILES CN(C)C1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2c(C)sc3ccccc23)CC1 Show InChI InChI=1S/C27H33N5OS2/c1-18-24(21-6-4-5-7-23(21)34-18)26-28-22-16-20(17-31-10-8-19(9-11-31)30(2)3)35-25(22)27(29-26)32-12-14-33-15-13-32/h4-7,16,19H,8-15,17H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assay |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50392141

(CHEMBL2152771)Show SMILES CC(C)(O)C1CCN(Cc2ccc3nc(nc(N4CCOCC4)c3n2)-c2cnc(N)nc2)CC1 Show InChI InChI=1S/C24H32N8O2/c1-24(2,33)17-5-7-31(8-6-17)15-18-3-4-19-20(28-18)22(32-9-11-34-12-10-32)30-21(29-19)16-13-26-23(25)27-14-16/h3-4,13-14,17,33H,5-12,15H2,1-2H3,(H2,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110delta using PIP2 as substrate assessed as PIP3 formation by fluorescence polarization assay |
Bioorg Med Chem Lett 22: 4296-302 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.027
BindingDB Entry DOI: 10.7270/Q2HM59J8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50394897
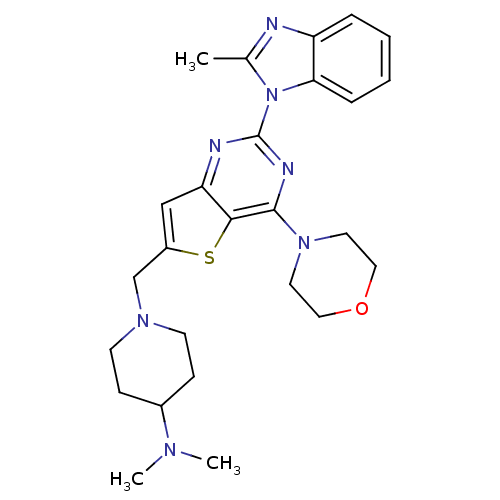
(CHEMBL2165498)Show SMILES CN(C)C1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-n2c(C)nc3ccccc23)CC1 Show InChI InChI=1S/C26H33N7OS/c1-18-27-21-6-4-5-7-23(21)33(18)26-28-22-16-20(17-31-10-8-19(9-11-31)30(2)3)35-24(22)25(29-26)32-12-14-34-15-13-32/h4-7,16,19H,8-15,17H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assay |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50392139
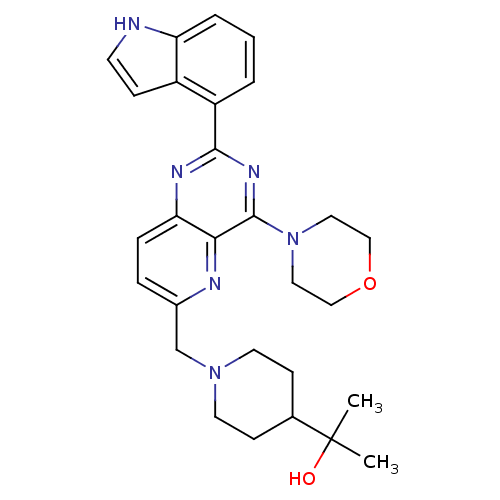
(CHEMBL2152769)Show SMILES CC(C)(O)C1CCN(Cc2ccc3nc(nc(N4CCOCC4)c3n2)-c2cccc3[nH]ccc23)CC1 Show InChI InChI=1S/C28H34N6O2/c1-28(2,35)19-9-12-33(13-10-19)18-20-6-7-24-25(30-20)27(34-14-16-36-17-15-34)32-26(31-24)22-4-3-5-23-21(22)8-11-29-23/h3-8,11,19,29,35H,9-10,12-18H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110delta using PIP2 as substrate assessed as PIP3 formation by fluorescence polarization assay |
Bioorg Med Chem Lett 22: 4296-302 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.027
BindingDB Entry DOI: 10.7270/Q2HM59J8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50394918

(CHEMBL2165504)Show SMILES CC(C)(N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2c(F)ccc3[nH]ccc23)CC1)C(N)=O Show InChI InChI=1S/C27H32FN7O2S/c1-27(2,26(29)36)35-9-7-33(8-10-35)16-17-15-21-23(38-17)25(34-11-13-37-14-12-34)32-24(31-21)22-18-5-6-30-20(18)4-3-19(22)28/h3-6,15,30H,7-14,16H2,1-2H3,(H2,29,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assay |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM25028

(4-[2-(1H-indazol-4-yl)-6-[(4-methanesulfonylpipera...)Show SMILES CS(=O)(=O)N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ncc23)CC1 Show InChI InChI=1S/C23H27N7O3S2/c1-35(31,32)30-7-5-28(6-8-30)15-16-13-20-21(34-16)23(29-9-11-33-12-10-29)26-22(25-20)17-3-2-4-19-18(17)14-24-27-19/h2-4,13-14H,5-12,15H2,1H3,(H,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assay |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50394919
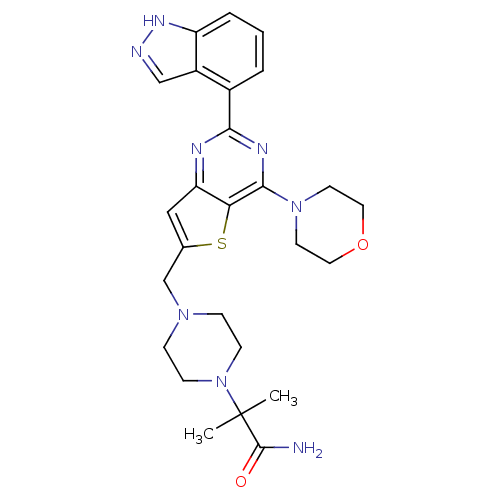
(CHEMBL2165503)Show SMILES CC(C)(N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cccc3[nH]ncc23)CC1)C(N)=O Show InChI InChI=1S/C26H32N8O2S/c1-26(2,25(27)35)34-8-6-32(7-9-34)16-17-14-21-22(37-17)24(33-10-12-36-13-11-33)30-23(29-21)18-4-3-5-20-19(18)15-28-31-20/h3-5,14-15H,6-13,16H2,1-2H3,(H2,27,35)(H,28,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assay |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50394915

(CHEMBL2165507)Show SMILES CC(C)(N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2cc(cc3[nH]ccc23)C#N)CC1)C(N)=O Show InChI InChI=1S/C28H32N8O2S/c1-28(2,27(30)37)36-7-5-34(6-8-36)17-19-15-23-24(39-19)26(35-9-11-38-12-10-35)33-25(32-23)21-13-18(16-29)14-22-20(21)3-4-31-22/h3-4,13-15,31H,5-12,17H2,1-2H3,(H2,30,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assay |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50394911
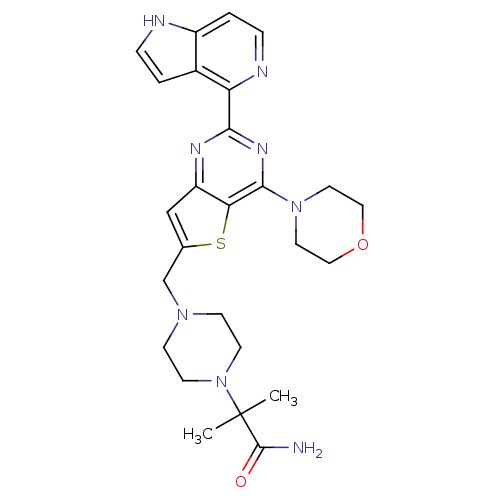
(CHEMBL2165511)Show SMILES CC(C)(N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2nccc3[nH]ccc23)CC1)C(N)=O Show InChI InChI=1S/C26H32N8O2S/c1-26(2,25(27)35)34-9-7-32(8-10-34)16-17-15-20-22(37-17)24(33-11-13-36-14-12-33)31-23(30-20)21-18-3-5-28-19(18)4-6-29-21/h3-6,15,28H,7-14,16H2,1-2H3,(H2,27,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assay |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50392138
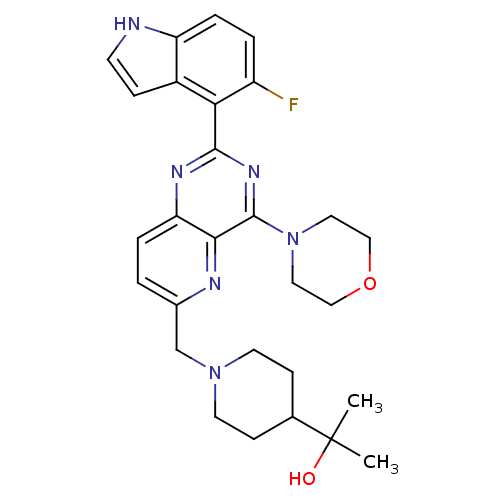
(CHEMBL2152768)Show SMILES CC(C)(O)C1CCN(Cc2ccc3nc(nc(N4CCOCC4)c3n2)-c2c(F)ccc3[nH]ccc23)CC1 Show InChI InChI=1S/C28H33FN6O2/c1-28(2,36)18-8-11-34(12-9-18)17-19-3-5-23-25(31-19)27(35-13-15-37-16-14-35)33-26(32-23)24-20-7-10-30-22(20)6-4-21(24)29/h3-7,10,18,30,36H,8-9,11-17H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110delta using PIP2 as substrate assessed as PIP3 formation by fluorescence polarization assay |
Bioorg Med Chem Lett 22: 4296-302 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.027
BindingDB Entry DOI: 10.7270/Q2HM59J8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50392146

(CHEMBL2152776)Show SMILES CC(C)(O)C1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2c(F)ccc3[nH]ccc23)CC1 Show InChI InChI=1S/C27H32FN5O2S/c1-27(2,34)17-6-9-32(10-7-17)16-18-15-22-24(36-18)26(33-11-13-35-14-12-33)31-25(30-22)23-19-5-8-29-21(19)4-3-20(23)28/h3-5,8,15,17,29,34H,6-7,9-14,16H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110delta using PIP2 as substrate assessed as PIP3 formation by fluorescence polarization assay |
Bioorg Med Chem Lett 22: 4296-302 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.027
BindingDB Entry DOI: 10.7270/Q2HM59J8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50394895

(CHEMBL2165500)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2sc(CN3CCN(CC3)S(C)(=O)=O)cc2n1 Show InChI InChI=1S/C25H31N7O3S2/c1-3-22-26-19-6-4-5-7-21(19)32(22)25-27-20-16-18(17-29-8-10-31(11-9-29)37(2,33)34)36-23(20)24(28-25)30-12-14-35-15-13-30/h4-7,16H,3,8-15,17H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assay |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50394896
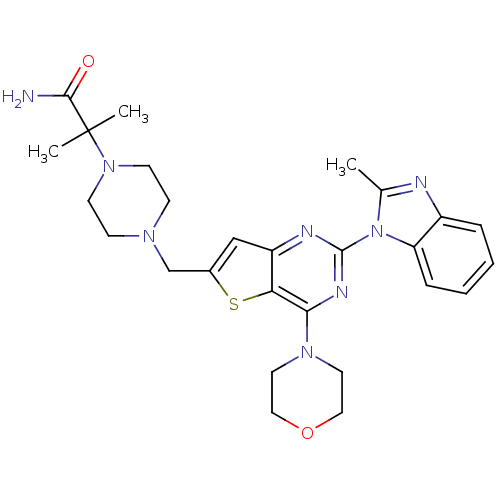
(CHEMBL2165499)Show SMILES Cc1nc2ccccc2n1-c1nc(N2CCOCC2)c2sc(CN3CCN(CC3)C(C)(C)C(N)=O)cc2n1 Show InChI InChI=1S/C27H34N8O2S/c1-18-29-20-6-4-5-7-22(20)35(18)26-30-21-16-19(38-23(21)24(31-26)33-12-14-37-15-13-33)17-32-8-10-34(11-9-32)27(2,3)25(28)36/h4-7,16H,8-15,17H2,1-3H3,(H2,28,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assay |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Isocitrate dehydrogenase [NADP] cytoplasmic [R132H]
(Homo sapiens (Human)) | BDBM390498
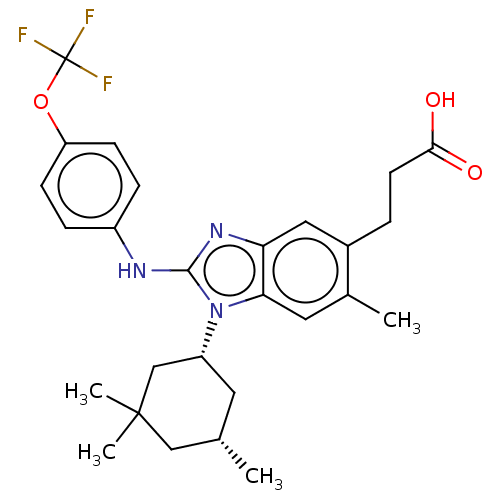
((�) 3-(6-methyl-2-{[4-(trifluoromethoxy)phe...)Show SMILES C[C@H]1C[C@H](CC(C)(C)C1)n1c(Nc2ccc(OC(F)(F)F)cc2)nc2cc(CCC(O)=O)c(C)cc12 |r| Show InChI InChI=1S/C27H32F3N3O3/c1-16-11-20(15-26(3,4)14-16)33-23-12-17(2)18(5-10-24(34)35)13-22(23)32-25(33)31-19-6-8-21(9-7-19)36-27(28,29)30/h6-9,12-13,16,20H,5,10-11,14-15H2,1-4H3,(H,31,32)(H,34,35)/t16-,20+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co. Ltd
| Assay Description
mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by... |
Bioorg Med Chem 14: 1378-90 (2006)
BindingDB Entry DOI: 10.7270/Q2ZS2ZW7 |
More data for this
Ligand-Target Pair | |
Isocitrate dehydrogenase [NADP] cytoplasmic [R132H]
(Homo sapiens (Human)) | BDBM390499
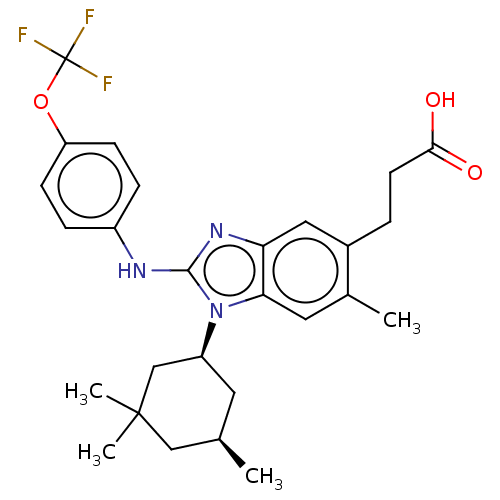
(US10442772, Example 2-162-2 | US9957235, 2-162-1 |...)Show SMILES C[C@@H]1C[C@@H](CC(C)(C)C1)n1c(Nc2ccc(OC(F)(F)F)cc2)nc2cc(CCC(O)=O)c(C)cc12 Show InChI InChI=1S/C27H32F3N3O3/c1-16-11-20(15-26(3,4)14-16)33-23-12-17(2)18(5-10-24(34)35)13-22(23)32-25(33)31-19-6-8-21(9-7-19)36-27(28,29)30/h6-9,12-13,16,20H,5,10-11,14-15H2,1-4H3,(H,31,32)(H,34,35)/t16-,20+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT
US Patent
| Assay Description
The biochemical reactions were performed at 32° C. in 384-well plates using a reaction volume of 41 μL and the following assay buffer conditions... |
US Patent US10442772 (2019)
BindingDB Entry DOI: 10.7270/Q21R6SVH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50394894
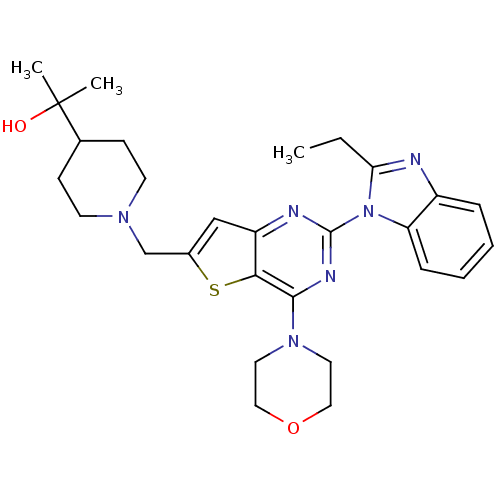
(CHEMBL2165501)Show SMILES CCc1nc2ccccc2n1-c1nc(N2CCOCC2)c2sc(CN3CCC(CC3)C(C)(C)O)cc2n1 Show InChI InChI=1S/C28H36N6O2S/c1-4-24-29-21-7-5-6-8-23(21)34(24)27-30-22-17-20(18-32-11-9-19(10-12-32)28(2,3)35)37-25(22)26(31-27)33-13-15-36-16-14-33/h5-8,17,19,35H,4,9-16,18H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assay |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50394900

(CHEMBL2165495)Show SMILES CN(C)C1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2c[nH]c3ccccc23)CC1 Show InChI InChI=1S/C26H32N6OS/c1-30(2)18-7-9-31(10-8-18)17-19-15-23-24(34-19)26(32-11-13-33-14-12-32)29-25(28-23)21-16-27-22-6-4-3-5-20(21)22/h3-6,15-16,18,27H,7-14,17H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assay |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50394914
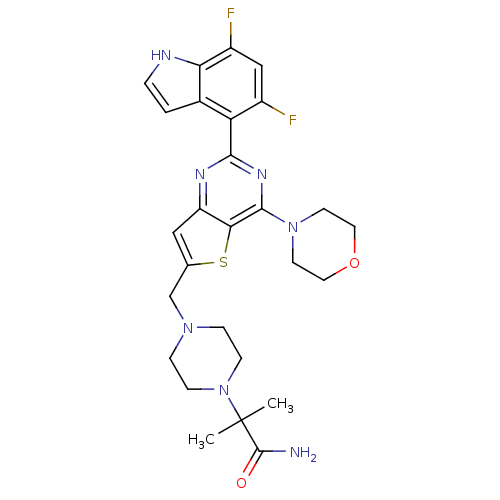
(CHEMBL2165508)Show SMILES CC(C)(N1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2c(F)cc(F)c3[nH]ccc23)CC1)C(N)=O Show InChI InChI=1S/C27H31F2N7O2S/c1-27(2,26(30)37)36-7-5-34(6-8-36)15-16-13-20-23(39-16)25(35-9-11-38-12-10-35)33-24(32-20)21-17-3-4-31-22(17)19(29)14-18(21)28/h3-4,13-14,31H,5-12,15H2,1-2H3,(H2,30,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assay |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50392140
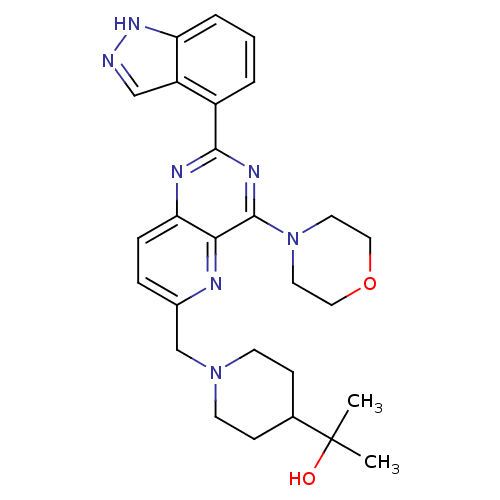
(CHEMBL2152770)Show SMILES CC(C)(O)C1CCN(Cc2ccc3nc(nc(N4CCOCC4)c3n2)-c2cccc3[nH]ncc23)CC1 Show InChI InChI=1S/C27H33N7O2/c1-27(2,35)18-8-10-33(11-9-18)17-19-6-7-23-24(29-19)26(34-12-14-36-15-13-34)31-25(30-23)20-4-3-5-22-21(20)16-28-32-22/h3-7,16,18,35H,8-15,17H2,1-2H3,(H,28,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3K p110delta using PIP2 as substrate assessed as PIP3 formation by fluorescence polarization assay |
Bioorg Med Chem Lett 22: 4296-302 (2012)
Article DOI: 10.1016/j.bmcl.2012.05.027
BindingDB Entry DOI: 10.7270/Q2HM59J8 |
More data for this
Ligand-Target Pair | |
Isocitrate dehydrogenase [NADP] cytoplasmic [R132H]
(Homo sapiens (Human)) | BDBM415800
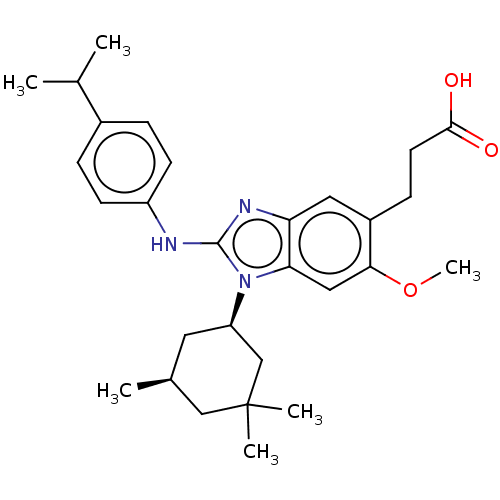
(US10442772, Example 2-170 | US10442772, Example 2-...)Show SMILES COc1cc2n([C@@H]3C[C@H](C)CC(C)(C)C3)c(Nc3ccc(cc3)C(C)C)nc2cc1CCC(O)=O |r| Show InChI InChI=1S/C29H39N3O3/c1-18(2)20-7-10-22(11-8-20)30-28-31-24-14-21(9-12-27(33)34)26(35-6)15-25(24)32(28)23-13-19(3)16-29(4,5)17-23/h7-8,10-11,14-15,18-19,23H,9,12-13,16-17H2,1-6H3,(H,30,31)(H,33,34)/t19-,23+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT
US Patent
| Assay Description
The biochemical reactions were performed at 32° C. in 384-well plates using a reaction volume of 41 μL and the following assay buffer conditions... |
US Patent US10442772 (2019)
BindingDB Entry DOI: 10.7270/Q21R6SVH |
More data for this
Ligand-Target Pair | |
Isocitrate dehydrogenase [NADP] cytoplasmic [R132H]
(Homo sapiens (Human)) | BDBM390662

(US10442772, Example 2-247-2 | US9957235, 2-247-1 |...)Show SMILES COc1cc2n(C3CC(C)CC(C)(C)C3)c(Nc3ccc(cc3)C(C)C)nc2cc1CC(O)=O Show InChI InChI=1S/C28H37N3O3/c1-17(2)19-7-9-21(10-8-19)29-27-30-23-12-20(13-26(32)33)25(34-6)14-24(23)31(27)22-11-18(3)15-28(4,5)16-22/h7-10,12,14,17-18,22H,11,13,15-16H2,1-6H3,(H,29,30)(H,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT
US Patent
| Assay Description
The biochemical reactions were performed at 32° C. in 384-well plates using a reaction volume of 41 μL and the following assay buffer conditions... |
US Patent US10442772 (2019)
BindingDB Entry DOI: 10.7270/Q21R6SVH |
More data for this
Ligand-Target Pair | |
Isocitrate dehydrogenase [NADP] cytoplasmic [R132H]
(Homo sapiens (Human)) | BDBM390662

(US10442772, Example 2-247-2 | US9957235, 2-247-1 |...)Show SMILES COc1cc2n(C3CC(C)CC(C)(C)C3)c(Nc3ccc(cc3)C(C)C)nc2cc1CC(O)=O Show InChI InChI=1S/C28H37N3O3/c1-17(2)19-7-9-21(10-8-19)29-27-30-23-12-20(13-26(32)33)25(34-6)14-24(23)31(27)22-11-18(3)15-28(4,5)16-22/h7-10,12,14,17-18,22H,11,13,15-16H2,1-6H3,(H,29,30)(H,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co. Ltd
| Assay Description
mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by... |
Bioorg Med Chem 14: 1378-90 (2006)
BindingDB Entry DOI: 10.7270/Q2ZS2ZW7 |
More data for this
Ligand-Target Pair | |
Isocitrate dehydrogenase [NADP] cytoplasmic [R132H]
(Homo sapiens (Human)) | BDBM390522
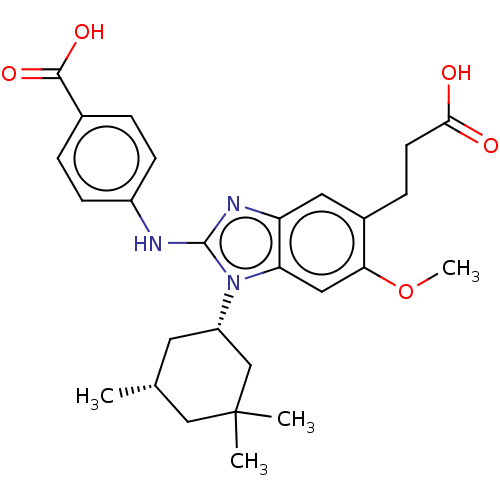
(US9957235, 2-170 | US9957235, 2-170-1 | US9957235,...)Show SMILES COc1cc2n([C@H]3C[C@@H](C)CC(C)(C)C3)c(Nc3ccc(cc3)C(O)=O)nc2cc1CCC(O)=O Show InChI InChI=1S/C27H33N3O5/c1-16-11-20(15-27(2,3)14-16)30-22-13-23(35-4)18(7-10-24(31)32)12-21(22)29-26(30)28-19-8-5-17(6-9-19)25(33)34/h5-6,8-9,12-13,16,20H,7,10-11,14-15H2,1-4H3,(H,28,29)(H,31,32)(H,33,34)/t16-,20+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co. Ltd
| Assay Description
mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by... |
Bioorg Med Chem 14: 1378-90 (2006)
BindingDB Entry DOI: 10.7270/Q2ZS2ZW7 |
More data for this
Ligand-Target Pair | |
Isocitrate dehydrogenase [NADP] cytoplasmic [R132H]
(Homo sapiens (Human)) | BDBM390499

(US10442772, Example 2-162-2 | US9957235, 2-162-1 |...)Show SMILES C[C@@H]1C[C@@H](CC(C)(C)C1)n1c(Nc2ccc(OC(F)(F)F)cc2)nc2cc(CCC(O)=O)c(C)cc12 Show InChI InChI=1S/C27H32F3N3O3/c1-16-11-20(15-26(3,4)14-16)33-23-12-17(2)18(5-10-24(34)35)13-22(23)32-25(33)31-19-6-8-21(9-7-19)36-27(28,29)30/h6-9,12-13,16,20H,5,10-11,14-15H2,1-4H3,(H,31,32)(H,34,35)/t16-,20+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co. Ltd
| Assay Description
mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by... |
Bioorg Med Chem 14: 1378-90 (2006)
BindingDB Entry DOI: 10.7270/Q2ZS2ZW7 |
More data for this
Ligand-Target Pair | |
Isocitrate dehydrogenase [NADP] cytoplasmic [R132H]
(Homo sapiens (Human)) | BDBM390522

(US9957235, 2-170 | US9957235, 2-170-1 | US9957235,...)Show SMILES COc1cc2n([C@H]3C[C@@H](C)CC(C)(C)C3)c(Nc3ccc(cc3)C(O)=O)nc2cc1CCC(O)=O Show InChI InChI=1S/C27H33N3O5/c1-16-11-20(15-27(2,3)14-16)30-22-13-23(35-4)18(7-10-24(31)32)12-21(22)29-26(30)28-19-8-5-17(6-9-19)25(33)34/h5-6,8-9,12-13,16,20H,7,10-11,14-15H2,1-4H3,(H,28,29)(H,31,32)(H,33,34)/t16-,20+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co. Ltd
| Assay Description
mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by... |
Bioorg Med Chem 14: 1378-90 (2006)
BindingDB Entry DOI: 10.7270/Q2ZS2ZW7 |
More data for this
Ligand-Target Pair | |
Isocitrate dehydrogenase [NADP] cytoplasmic [R132H]
(Homo sapiens (Human)) | BDBM390525
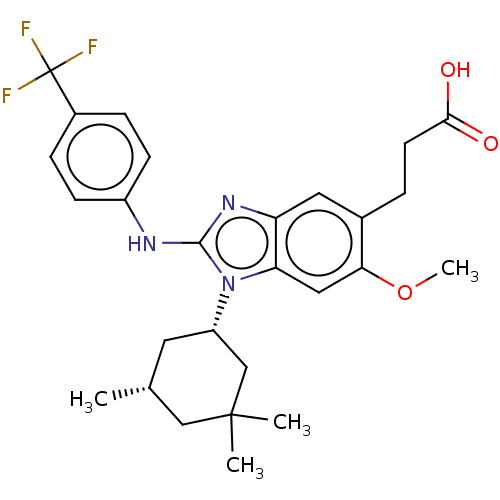
(US10442772, Example 2-171-2 | US9957235, 2-171 | U...)Show SMILES COc1cc2n([C@H]3C[C@@H](C)CC(C)(C)C3)c(Nc3ccc(cc3)C(F)(F)F)nc2cc1CCC(O)=O Show InChI InChI=1S/C27H32F3N3O3/c1-16-11-20(15-26(2,3)14-16)33-22-13-23(36-4)17(5-10-24(34)35)12-21(22)32-25(33)31-19-8-6-18(7-9-19)27(28,29)30/h6-9,12-13,16,20H,5,10-11,14-15H2,1-4H3,(H,31,32)(H,34,35)/t16-,20+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co. Ltd
| Assay Description
mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by... |
Bioorg Med Chem 14: 1378-90 (2006)
BindingDB Entry DOI: 10.7270/Q2ZS2ZW7 |
More data for this
Ligand-Target Pair | |
Isocitrate dehydrogenase [NADP] cytoplasmic [R132H]
(Homo sapiens (Human)) | BDBM390528

(US10442772, Example 2-175-2 | US9957235, 2-172 | U...)Show SMILES CC(C)c1ccc(Nc2nc3cc(CCC(O)=O)ccc3n2[C@H]2C[C@@H](C)CC(C)(C)C2)cc1 Show InChI InChI=1S/C28H37N3O2/c1-18(2)21-8-10-22(11-9-21)29-27-30-24-15-20(7-13-26(32)33)6-12-25(24)31(27)23-14-19(3)16-28(4,5)17-23/h6,8-12,15,18-19,23H,7,13-14,16-17H2,1-5H3,(H,29,30)(H,32,33)/t19-,23+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co. Ltd
| Assay Description
mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by... |
Bioorg Med Chem 14: 1378-90 (2006)
BindingDB Entry DOI: 10.7270/Q2ZS2ZW7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50394917

(CHEMBL2165505)Show SMILES Fc1ccc2[nH]ccc2c1-c1nc(N2CCOCC2)c2sc(CN3CCN4CCOC[C@H]4C3)cc2n1 |r| Show InChI InChI=1S/C26H29FN6O2S/c27-20-1-2-21-19(3-4-28-21)23(20)25-29-22-13-18(15-31-5-6-32-7-12-35-16-17(32)14-31)36-24(22)26(30-25)33-8-10-34-11-9-33/h1-4,13,17,28H,5-12,14-16H2/t17-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assay |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Isocitrate dehydrogenase [NADP] cytoplasmic [R132H]
(Homo sapiens (Human)) | BDBM390499

(US10442772, Example 2-162-2 | US9957235, 2-162-1 |...)Show SMILES C[C@@H]1C[C@@H](CC(C)(C)C1)n1c(Nc2ccc(OC(F)(F)F)cc2)nc2cc(CCC(O)=O)c(C)cc12 Show InChI InChI=1S/C27H32F3N3O3/c1-16-11-20(15-26(3,4)14-16)33-23-12-17(2)18(5-10-24(34)35)13-22(23)32-25(33)31-19-6-8-21(9-7-19)36-27(28,29)30/h6-9,12-13,16,20H,5,10-11,14-15H2,1-4H3,(H,31,32)(H,34,35)/t16-,20+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT
US Patent
| Assay Description
The biochemical reactions were performed at 32° C. in 384-well plates using a reaction volume of 41 μL and the following assay buffer conditions... |
US Patent US10442772 (2019)
BindingDB Entry DOI: 10.7270/Q21R6SVH |
More data for this
Ligand-Target Pair | |
Isocitrate dehydrogenase [NADP] cytoplasmic [R132H]
(Homo sapiens (Human)) | BDBM390543
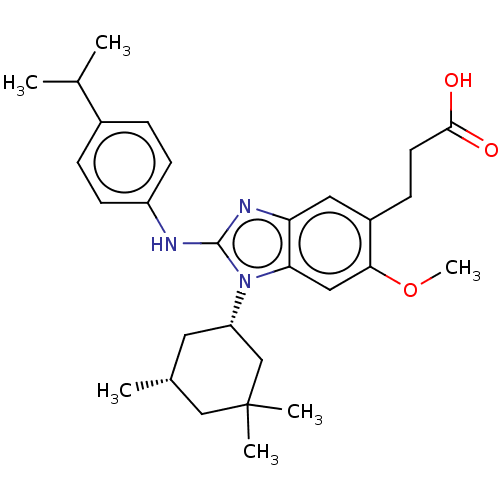
(US10442772, Example 2-170-2 | US9957235, 2-177 | U...)Show SMILES COc1cc2n([C@H]3C[C@@H](C)CC(C)(C)C3)c(Nc3ccc(cc3)C(C)C)nc2cc1CCC(O)=O Show InChI InChI=1S/C29H39N3O3/c1-18(2)20-7-10-22(11-8-20)30-28-31-24-14-21(9-12-27(33)34)26(35-6)15-25(24)32(28)23-13-19(3)16-29(4,5)17-23/h7-8,10-11,14-15,18-19,23H,9,12-13,16-17H2,1-6H3,(H,30,31)(H,33,34)/t19-,23+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT
US Patent
| Assay Description
The biochemical reactions were performed at 32° C. in 384-well plates using a reaction volume of 41 μL and the following assay buffer conditions... |
US Patent US10442772 (2019)
BindingDB Entry DOI: 10.7270/Q21R6SVH |
More data for this
Ligand-Target Pair | |
Isocitrate dehydrogenase [NADP] cytoplasmic [R132H]
(Homo sapiens (Human)) | BDBM390525

(US10442772, Example 2-171-2 | US9957235, 2-171 | U...)Show SMILES COc1cc2n([C@H]3C[C@@H](C)CC(C)(C)C3)c(Nc3ccc(cc3)C(F)(F)F)nc2cc1CCC(O)=O Show InChI InChI=1S/C27H32F3N3O3/c1-16-11-20(15-26(2,3)14-16)33-22-13-23(36-4)17(5-10-24(34)35)12-21(22)32-25(33)31-19-8-6-18(7-9-19)27(28,29)30/h6-9,12-13,16,20H,5,10-11,14-15H2,1-4H3,(H,31,32)(H,34,35)/t16-,20+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT
US Patent
| Assay Description
The biochemical reactions were performed at 32° C. in 384-well plates using a reaction volume of 41 μL and the following assay buffer conditions... |
US Patent US10442772 (2019)
BindingDB Entry DOI: 10.7270/Q21R6SVH |
More data for this
Ligand-Target Pair | |
Isocitrate dehydrogenase [NADP] cytoplasmic [R132H]
(Homo sapiens (Human)) | BDBM390528

(US10442772, Example 2-175-2 | US9957235, 2-172 | U...)Show SMILES CC(C)c1ccc(Nc2nc3cc(CCC(O)=O)ccc3n2[C@H]2C[C@@H](C)CC(C)(C)C2)cc1 Show InChI InChI=1S/C28H37N3O2/c1-18(2)21-8-10-22(11-9-21)29-27-30-24-15-20(7-13-26(32)33)6-12-25(24)31(27)23-14-19(3)16-28(4,5)17-23/h6,8-12,15,18-19,23H,7,13-14,16-17H2,1-5H3,(H,29,30)(H,32,33)/t19-,23+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT
US Patent
| Assay Description
The biochemical reactions were performed at 32° C. in 384-well plates using a reaction volume of 41 μL and the following assay buffer conditions... |
US Patent US10442772 (2019)
BindingDB Entry DOI: 10.7270/Q21R6SVH |
More data for this
Ligand-Target Pair | |
Isocitrate dehydrogenase [NADP] cytoplasmic [R132H]
(Homo sapiens (Human)) | BDBM390647
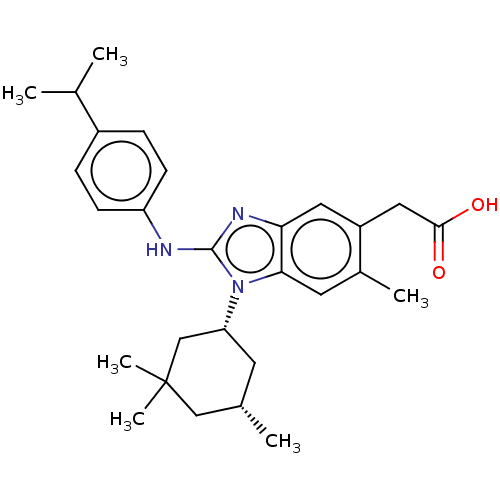
(US10442772, Example 2-237-1 | US9957235, 2-237 | U...)Show SMILES CC(C)c1ccc(Nc2nc3cc(CC(O)=O)c(C)cc3n2[C@@H]2C[C@H](C)CC(C)(C)C2)cc1 |r| Show InChI InChI=1S/C28H37N3O2/c1-17(2)20-7-9-22(10-8-20)29-27-30-24-13-21(14-26(32)33)19(4)12-25(24)31(27)23-11-18(3)15-28(5,6)16-23/h7-10,12-13,17-18,23H,11,14-16H2,1-6H3,(H,29,30)(H,32,33)/t18-,23+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co. Ltd
| Assay Description
mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by... |
Bioorg Med Chem 14: 1378-90 (2006)
BindingDB Entry DOI: 10.7270/Q2ZS2ZW7 |
More data for this
Ligand-Target Pair | |
Isocitrate dehydrogenase [NADP] cytoplasmic [R132H]
(Homo sapiens (Human)) | BDBM415929
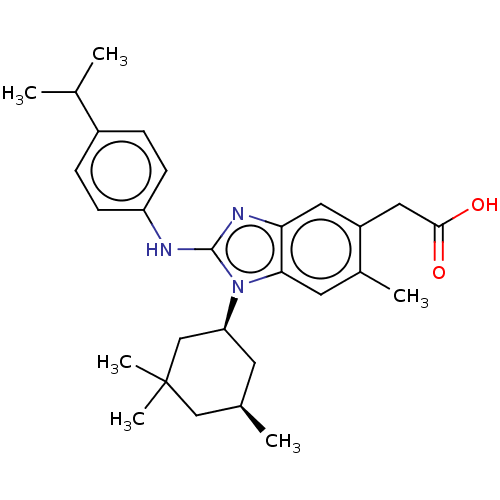
(US10442772, Example 2-237-2)Show SMILES CC(C)c1ccc(Nc2nc3cc(CC(O)=O)c(C)cc3n2[C@H]2C[C@@H](C)CC(C)(C)C2)cc1 |r| Show InChI InChI=1S/C28H37N3O2/c1-17(2)20-7-9-22(10-8-20)29-27-30-24-13-21(14-26(32)33)19(4)12-25(24)31(27)23-11-18(3)15-28(5,6)16-23/h7-10,12-13,17-18,23H,11,14-16H2,1-6H3,(H,29,30)(H,32,33)/t18-,23+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT
US Patent
| Assay Description
The biochemical reactions were performed at 32° C. in 384-well plates using a reaction volume of 41 μL and the following assay buffer conditions... |
US Patent US10442772 (2019)
BindingDB Entry DOI: 10.7270/Q21R6SVH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50394902

(CHEMBL2165493)Show SMILES CN(C)C1CCN(Cc2cc3nc(nc(N4CCOCC4)c3s2)-c2n[nH]c3ccccc23)CC1 Show InChI InChI=1S/C25H31N7OS/c1-30(2)17-7-9-31(10-8-17)16-18-15-21-23(34-18)25(32-11-13-33-14-12-32)27-24(26-21)22-19-5-3-4-6-20(19)28-29-22/h3-6,15,17H,7-14,16H2,1-2H3,(H,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Binding affinity to PI3Kdelta after 30 mins by competitive fluorescence polarization assay |
J Med Chem 55: 5887-900 (2012)
Article DOI: 10.1021/jm3003747
BindingDB Entry DOI: 10.7270/Q2V125XN |
More data for this
Ligand-Target Pair | |
Isocitrate dehydrogenase [NADP] cytoplasmic [R132H]
(Homo sapiens (Human)) | BDBM390660

(US10442772, Example 2-246-2 | US9957235, 2-246-1 |...)Show SMILES COc1cc2n(C3CC(C)CC(C)(C)C3)c(Nc3ccc(OC(F)(F)F)cc3)nc2cc1CC(O)=O Show InChI InChI=1S/C26H30F3N3O4/c1-15-9-18(14-25(2,3)13-15)32-21-12-22(35-4)16(11-23(33)34)10-20(21)31-24(32)30-17-5-7-19(8-6-17)36-26(27,28)29/h5-8,10,12,15,18H,9,11,13-14H2,1-4H3,(H,30,31)(H,33,34) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT
US Patent
| Assay Description
The biochemical reactions were performed at 32° C. in 384-well plates using a reaction volume of 41 μL and the following assay buffer conditions... |
US Patent US10442772 (2019)
BindingDB Entry DOI: 10.7270/Q21R6SVH |
More data for this
Ligand-Target Pair | |
Isocitrate dehydrogenase [NADP] cytoplasmic [R132H]
(Homo sapiens (Human)) | BDBM415926
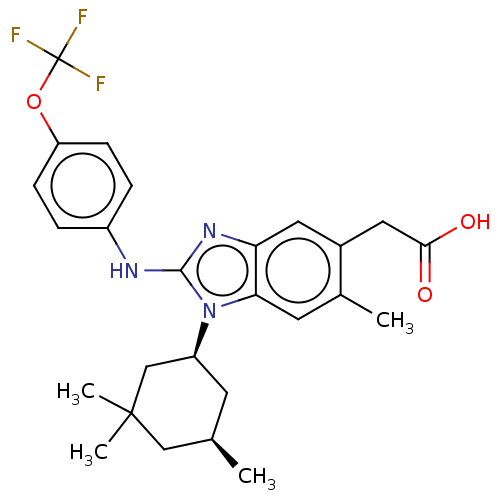
(US10442772, Example 2-236-2)Show SMILES C[C@@H]1C[C@@H](CC(C)(C)C1)n1c(Nc2ccc(OC(F)(F)F)cc2)nc2cc(CC(O)=O)c(C)cc12 |r| Show InChI InChI=1S/C26H30F3N3O3/c1-15-9-19(14-25(3,4)13-15)32-22-10-16(2)17(12-23(33)34)11-21(22)31-24(32)30-18-5-7-20(8-6-18)35-26(27,28)29/h5-8,10-11,15,19H,9,12-14H2,1-4H3,(H,30,31)(H,33,34)/t15-,19+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
BAYER PHARMA AKTIENGESELLSCHAFT
US Patent
| Assay Description
The biochemical reactions were performed at 32° C. in 384-well plates using a reaction volume of 41 μL and the following assay buffer conditions... |
US Patent US10442772 (2019)
BindingDB Entry DOI: 10.7270/Q21R6SVH |
More data for this
Ligand-Target Pair | |
Isocitrate dehydrogenase [NADP] cytoplasmic [R132H]
(Homo sapiens (Human)) | BDBM390460

(US10442772, Example 2-123-2 | US9957235, 2-123 | U...)Show SMILES C[C@@H]1C[C@@H](CC(C)(C)C1)n1c(Nc2ccc(OC(F)(F)F)cc2)nc2cc(OCC(O)=O)ccc12 Show InChI InChI=1S/C25H28F3N3O4/c1-15-10-17(13-24(2,3)12-15)31-21-9-8-19(34-14-22(32)33)11-20(21)30-23(31)29-16-4-6-18(7-5-16)35-25(26,27)28/h4-9,11,15,17H,10,12-14H2,1-3H3,(H,29,30)(H,32,33)/t15-,17+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co. Ltd
| Assay Description
mIDH1 catalyzes the NADPH-dependent reduction of alpha-ketoglutarate (α-KG) to (2R)-2-hydroxyglutarate (2-HG). NADPH consumption was measured by... |
Bioorg Med Chem 14: 1378-90 (2006)
BindingDB Entry DOI: 10.7270/Q2ZS2ZW7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data