 Found 1646 hits with Last Name = 'bloomfield' and Initial = 'gc'
Found 1646 hits with Last Name = 'bloomfield' and Initial = 'gc' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
B2 bradykinin receptor
(RAT) | BDBM50370083
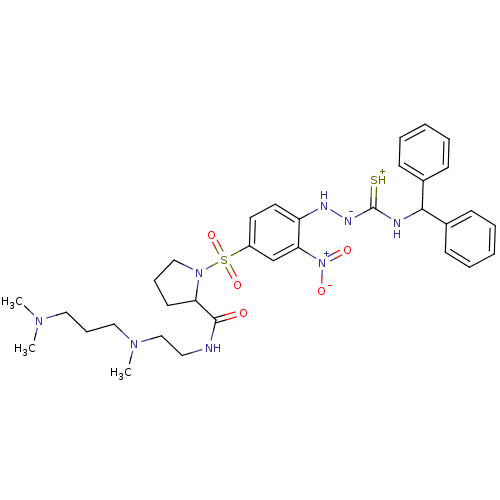
(CHEMBL1907651)Show SMILES CN(C)CCCN(C)CCNC(=O)C1CCCN1S(=O)(=O)c1ccc(N[N-]C(=[SH+])NC(c2ccccc2)c2ccccc2)c(c1)[N+]([O-])=O Show InChI InChI=1S/C33H44N8O5S2/c1-38(2)20-11-21-39(3)23-19-34-32(42)29-16-10-22-40(29)48(45,46)27-17-18-28(30(24-27)41(43)44)36-37-33(47)35-31(25-12-6-4-7-13-25)26-14-8-5-9-15-26/h4-9,12-15,17-18,24,29,31,36H,10-11,16,19-23H2,1-3H3,(H3,34,35,37,42,47) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranes |
J Med Chem 45: 2160-72 (2002)
BindingDB Entry DOI: 10.7270/Q2X067SG |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(RAT) | BDBM50370077
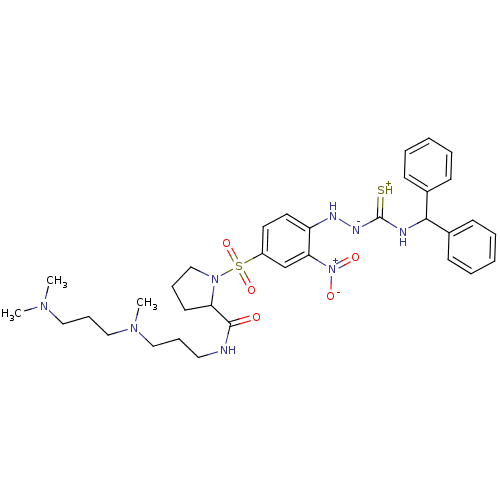
(CHEMBL1907652)Show SMILES CN(C)CCCN(C)CCCNC(=O)C1CCCN1S(=O)(=O)c1ccc(N[N-]C(=[SH+])NC(c2ccccc2)c2ccccc2)c(c1)[N+]([O-])=O Show InChI InChI=1S/C34H46N8O5S2/c1-39(2)21-12-23-40(3)22-11-20-35-33(43)30-17-10-24-41(30)49(46,47)28-18-19-29(31(25-28)42(44)45)37-38-34(48)36-32(26-13-6-4-7-14-26)27-15-8-5-9-16-27/h4-9,13-16,18-19,25,30,32,37H,10-12,17,20-24H2,1-3H3,(H3,35,36,38,43,48) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranes |
J Med Chem 45: 2160-72 (2002)
BindingDB Entry DOI: 10.7270/Q2X067SG |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(RAT) | BDBM50409120
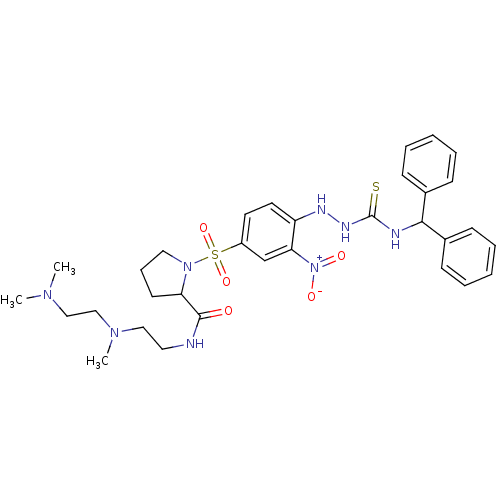
(CHEMBL2112044 | CHEMBL2112937)Show SMILES CN(C)CCN(C)CCNC(=O)C1CCCN1S(=O)(=O)c1ccc(NN\C([S-])=[NH+]\C(c2ccccc2)c2ccccc2)c(c1)[N+]([O-])=O Show InChI InChI=1S/C32H42N8O5S2/c1-37(2)21-22-38(3)20-18-33-31(41)28-15-10-19-39(28)47(44,45)26-16-17-27(29(23-26)40(42)43)35-36-32(46)34-30(24-11-6-4-7-12-24)25-13-8-5-9-14-25/h4-9,11-14,16-17,23,28,30,35H,10,15,18-22H2,1-3H3,(H,33,41)(H2,34,36,46) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-BK to rat bradykinin B2 receptor expressed in NG108-15 neuroblastoma-glioma hybrid cell membranes |
J Med Chem 43: 769-71 (2000)
BindingDB Entry DOI: 10.7270/Q2XD10W5 |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(RAT) | BDBM50113263
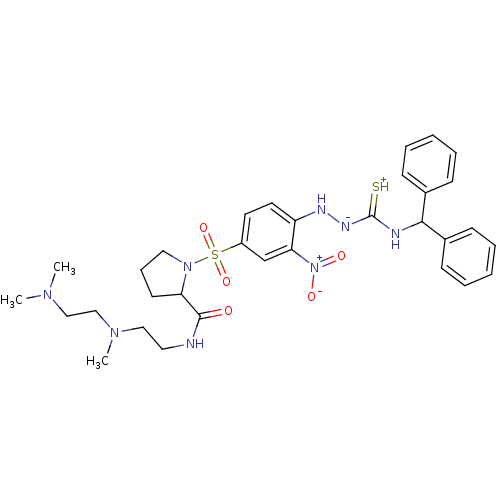
((S)-1-[4-(4-benzhydrylthiosemicarbazido)-3-nitrobe...)Show SMILES CN(C)CCN(C)CCNC(=O)C1CCCN1S(=O)(=O)c1ccc(N[N-]C(=[SH+])NC(c2ccccc2)c2ccccc2)c(c1)[N+]([O-])=O Show InChI InChI=1S/C32H42N8O5S2/c1-37(2)21-22-38(3)20-18-33-31(41)28-15-10-19-39(28)47(44,45)26-16-17-27(29(23-26)40(42)43)35-36-32(46)34-30(24-11-6-4-7-12-24)25-13-8-5-9-14-25/h4-9,11-14,16-17,23,28,30,35H,10,15,18-22H2,1-3H3,(H3,33,34,36,41,46) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranes |
J Med Chem 45: 2160-72 (2002)
BindingDB Entry DOI: 10.7270/Q2X067SG |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(RAT) | BDBM50113263

((S)-1-[4-(4-benzhydrylthiosemicarbazido)-3-nitrobe...)Show SMILES CN(C)CCN(C)CCNC(=O)C1CCCN1S(=O)(=O)c1ccc(N[N-]C(=[SH+])NC(c2ccccc2)c2ccccc2)c(c1)[N+]([O-])=O Show InChI InChI=1S/C32H42N8O5S2/c1-37(2)21-22-38(3)20-18-33-31(41)28-15-10-19-39(28)47(44,45)26-16-17-27(29(23-26)40(42)43)35-36-32(46)34-30(24-11-6-4-7-12-24)25-13-8-5-9-14-25/h4-9,11-14,16-17,23,28,30,35H,10,15,18-22H2,1-3H3,(H3,33,34,36,41,46) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranes |
J Med Chem 45: 2160-72 (2002)
BindingDB Entry DOI: 10.7270/Q2X067SG |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(RAT) | BDBM50409120

(CHEMBL2112044 | CHEMBL2112937)Show SMILES CN(C)CCN(C)CCNC(=O)C1CCCN1S(=O)(=O)c1ccc(NN\C([S-])=[NH+]\C(c2ccccc2)c2ccccc2)c(c1)[N+]([O-])=O Show InChI InChI=1S/C32H42N8O5S2/c1-37(2)21-22-38(3)20-18-33-31(41)28-15-10-19-39(28)47(44,45)26-16-17-27(29(23-26)40(42)43)35-36-32(46)34-30(24-11-6-4-7-12-24)25-13-8-5-9-14-25/h4-9,11-14,16-17,23,28,30,35H,10,15,18-22H2,1-3H3,(H,33,41)(H2,34,36,46) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-BK to rat bradykinin B2 receptor expressed in NG108-15 neuroblastoma-glioma hybrid cell membranes |
J Med Chem 43: 769-71 (2000)
BindingDB Entry DOI: 10.7270/Q2XD10W5 |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(Homo sapiens (Human)) | BDBM50409527
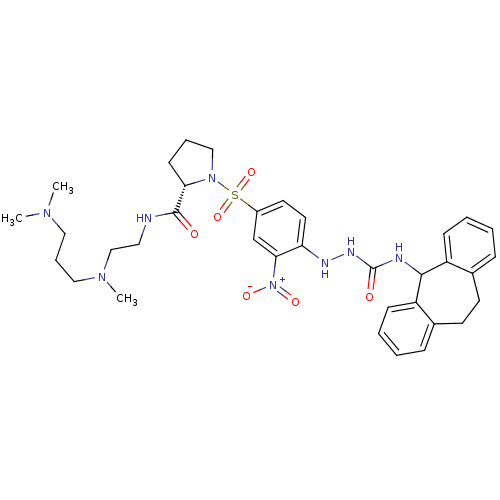
(CHEMBL2112283)Show SMILES CN(C)CCCN(C)CCNC(=O)[C@@H]1CCCN1S(=O)(=O)c1ccc(NNC(=O)NC2c3ccccc3CCc3ccccc23)c(c1)[N+]([O-])=O |r| Show InChI InChI=1S/C35H46N8O6S/c1-40(2)20-9-21-41(3)23-19-36-34(44)31-14-8-22-42(31)50(48,49)27-17-18-30(32(24-27)43(46)47)38-39-35(45)37-33-28-12-6-4-10-25(28)15-16-26-11-5-7-13-29(26)33/h4-7,10-13,17-18,24,31,33,38H,8-9,14-16,19-23H2,1-3H3,(H,36,44)(H2,37,39,45)/t31-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]BK (1 nM) from human Bradykinin receptor B2 expressed in Cos-7 cells |
J Med Chem 45: 2160-72 (2002)
BindingDB Entry DOI: 10.7270/Q2X067SG |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50151885

(1-[4-(2,2-Diphenyl-ethylamino)-3-(morpholine-4-car...)Show SMILES CN(C)CCCN(CCCN(C)C)C(=O)C1CCN(CC1)S(=O)(=O)c1ccc(NCC(c2ccccc2)c2ccccc2)c(c1)C(=O)N1CCOCC1 Show InChI InChI=1S/C41H58N6O5S/c1-43(2)21-11-23-45(24-12-22-44(3)4)40(48)35-19-25-47(26-20-35)53(50,51)36-17-18-39(37(31-36)41(49)46-27-29-52-30-28-46)42-32-38(33-13-7-5-8-14-33)34-15-9-6-10-16-34/h5-10,13-18,31,35,38,42H,11-12,19-30,32H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Bradykinin receptor B1 expressed in HEK293 cells |
J Med Chem 47: 4642-4 (2004)
Article DOI: 10.1021/jm049747g
BindingDB Entry DOI: 10.7270/Q2J965V7 |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(Homo sapiens (Human)) | BDBM50409529
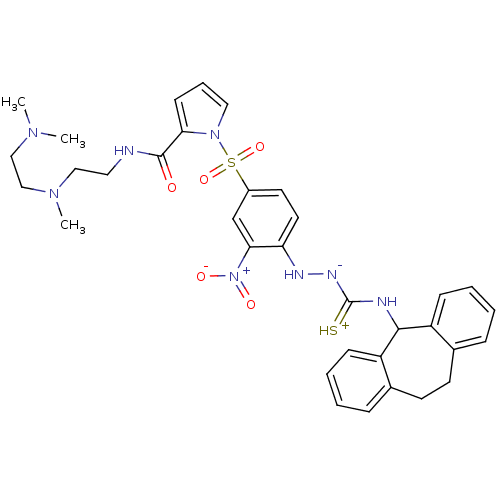
(CHEMBL2112221)Show SMILES CN(C)CCN(C)CCNC(=O)c1cccn1S(=O)(=O)c1ccc(N[N-]C(=[SH+])NC2c3ccccc3CCc3ccccc23)c(c1)[N+]([O-])=O Show InChI InChI=1S/C34H40N8O5S2/c1-39(2)21-22-40(3)20-18-35-33(43)30-13-8-19-41(30)49(46,47)26-16-17-29(31(23-26)42(44)45)37-38-34(48)36-32-27-11-6-4-9-24(27)14-15-25-10-5-7-12-28(25)32/h4-13,16-17,19,23,32,37H,14-15,18,20-22H2,1-3H3,(H3,35,36,38,43,48) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]BK (1 nM) from human Bradykinin receptor B2 expressed in Cos-7 cells |
J Med Chem 45: 2160-72 (2002)
BindingDB Entry DOI: 10.7270/Q2X067SG |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(RAT) | BDBM50085685

(4-benzhydrylamino(thioxo)methylhydrazine-3-nitrobe...)Show SMILES NC(=O)c1ccc(N[N-]C(=[SH+])NC(c2ccccc2)c2ccccc2)c(c1)[N+]([O-])=O Show InChI InChI=1S/C21H19N5O3S/c22-20(27)16-11-12-17(18(13-16)26(28)29)24-25-21(30)23-19(14-7-3-1-4-8-14)15-9-5-2-6-10-15/h1-13,19H,(H5,22,23,24,25,27,30) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-BK to rat bradykinin B2 receptor expressed in NG108-15 neuroblastoma-glioma hybrid cell membranes |
J Med Chem 43: 769-71 (2000)
BindingDB Entry DOI: 10.7270/Q2XD10W5 |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(Homo sapiens (Human)) | BDBM50409528
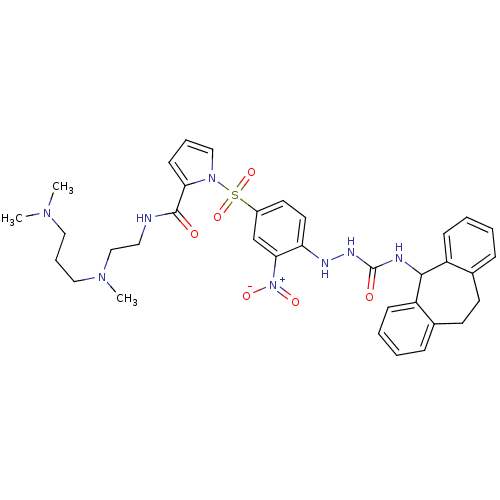
(CHEMBL2112220)Show SMILES CN(C)CCCN(C)CCNC(=O)c1cccn1S(=O)(=O)c1ccc(NNC(=O)NC2c3ccccc3CCc3ccccc23)c(c1)[N+]([O-])=O Show InChI InChI=1S/C35H42N8O6S/c1-40(2)20-9-21-41(3)23-19-36-34(44)31-14-8-22-42(31)50(48,49)27-17-18-30(32(24-27)43(46)47)38-39-35(45)37-33-28-12-6-4-10-25(28)15-16-26-11-5-7-13-29(26)33/h4-8,10-14,17-18,22,24,33,38H,9,15-16,19-21,23H2,1-3H3,(H,36,44)(H2,37,39,45) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]BK (1 nM) from human Bradykinin receptor B2 expressed in Cos-7 cells |
J Med Chem 45: 2160-72 (2002)
BindingDB Entry DOI: 10.7270/Q2X067SG |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50390424
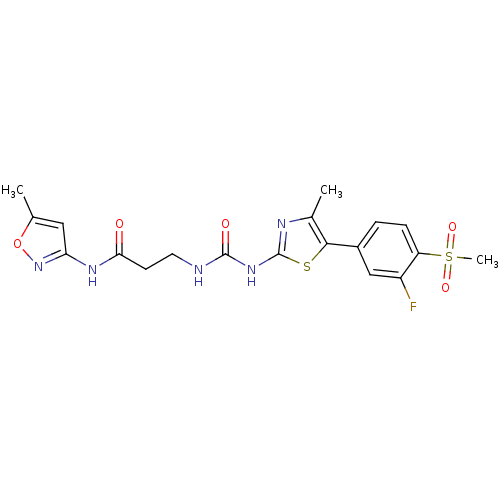
(CHEMBL2071340)Show SMILES Cc1cc(NC(=O)CCNC(=O)Nc2nc(C)c(s2)-c2ccc(c(F)c2)S(C)(=O)=O)no1 Show InChI InChI=1S/C19H20FN5O5S2/c1-10-8-15(25-30-10)23-16(26)6-7-21-18(27)24-19-22-11(2)17(31-19)12-4-5-14(13(20)9-12)32(3,28)29/h4-5,8-9H,6-7H2,1-3H3,(H,23,25,26)(H2,21,22,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110delta using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(RAT) | BDBM50085684
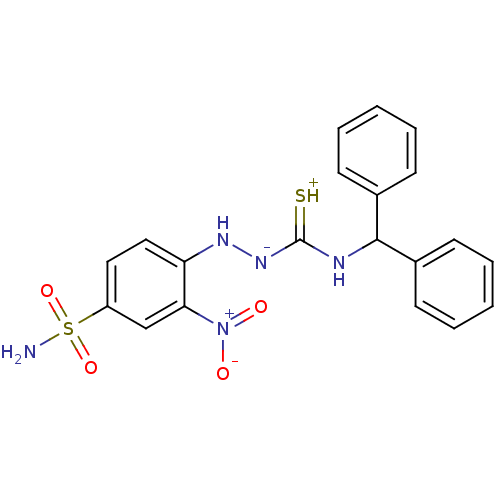
(4-benzhydrylamino(thioxo)methylhydrazine-3-nitro-1...)Show SMILES NS(=O)(=O)c1ccc(N[N-]C(=[SH+])NC(c2ccccc2)c2ccccc2)c(c1)[N+]([O-])=O Show InChI InChI=1S/C20H19N5O4S2/c21-31(28,29)16-11-12-17(18(13-16)25(26)27)23-24-20(30)22-19(14-7-3-1-4-8-14)15-9-5-2-6-10-15/h1-13,19,23H,(H4,21,22,24,28,29,30) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-BK to rat bradykinin B2 receptor expressed in NG108-15 neuroblastoma-glioma hybrid cell membranes |
J Med Chem 43: 769-71 (2000)
BindingDB Entry DOI: 10.7270/Q2XD10W5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50390409
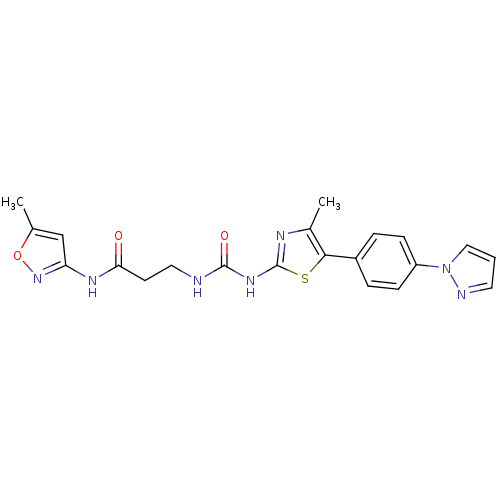
(CHEMBL1986603)Show SMILES Cc1cc(NC(=O)CCNC(=O)Nc2nc(C)c(s2)-c2ccc(cc2)-n2cccn2)no1 Show InChI InChI=1S/C21H21N7O3S/c1-13-12-17(27-31-13)25-18(29)8-10-22-20(30)26-21-24-14(2)19(32-21)15-4-6-16(7-5-15)28-11-3-9-23-28/h3-7,9,11-12H,8,10H2,1-2H3,(H,25,27,29)(H2,22,24,26,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110delta using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(Homo sapiens (Human)) | BDBM50370080
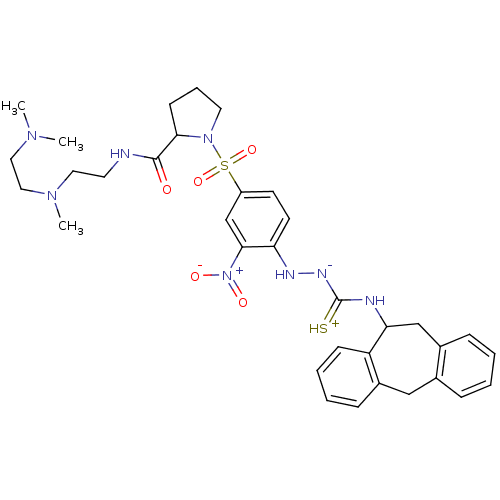
(CHEMBL1907656)Show SMILES CN(C)CCN(C)CCNC(=O)C1CCCN1S(=O)(=O)c1ccc(N[N-]C(=[SH+])NC2Cc3ccccc3Cc3ccccc23)c(c1)[N+]([O-])=O Show InChI InChI=1S/C34H44N8O5S2/c1-39(2)19-20-40(3)18-16-35-33(43)31-13-8-17-41(31)49(46,47)27-14-15-29(32(23-27)42(44)45)37-38-34(48)36-30-22-25-10-5-4-9-24(25)21-26-11-6-7-12-28(26)30/h4-7,9-12,14-15,23,30-31,37H,8,13,16-22H2,1-3H3,(H3,35,36,38,43,48) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]BK (1 nM) from human Bradykinin receptor B2 expressed in Cos-7 cells |
J Med Chem 45: 2160-72 (2002)
BindingDB Entry DOI: 10.7270/Q2X067SG |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50390408
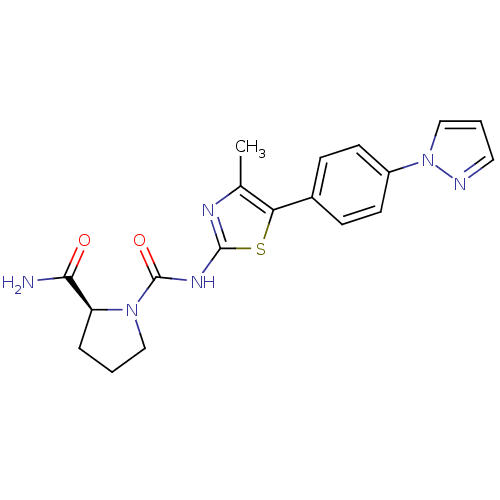
(CHEMBL2071338)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccc(cc1)-n1cccn1 |r| Show InChI InChI=1S/C19H20N6O2S/c1-12-16(13-5-7-14(8-6-13)25-11-3-9-21-25)28-18(22-12)23-19(27)24-10-2-4-15(24)17(20)26/h3,5-9,11,15H,2,4,10H2,1H3,(H2,20,26)(H,22,23,27)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110alpha by KinaseGlo assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(RAT) | BDBM50370081
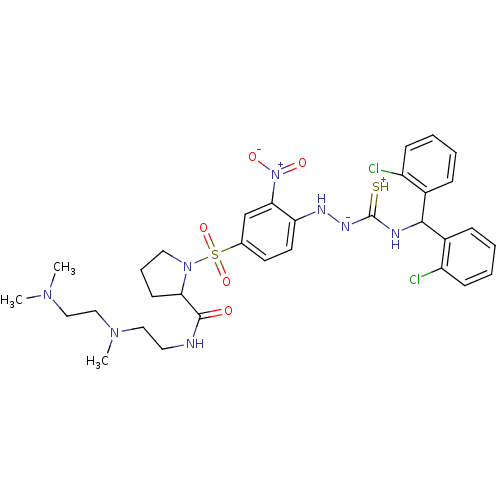
(CHEMBL1907654)Show SMILES CN(C)CCN(C)CCNC(=O)C1CCCN1S(=O)(=O)c1ccc(N[N-]C(=[SH+])NC(c2ccccc2Cl)c2ccccc2Cl)c(c1)[N+]([O-])=O Show InChI InChI=1S/C32H40Cl2N8O5S2/c1-39(2)19-20-40(3)18-16-35-31(43)28-13-8-17-41(28)49(46,47)22-14-15-27(29(21-22)42(44)45)37-38-32(48)36-30(23-9-4-6-11-25(23)33)24-10-5-7-12-26(24)34/h4-7,9-12,14-15,21,28,30,37H,8,13,16-20H2,1-3H3,(H3,35,36,38,43,48) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5.46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranes |
J Med Chem 45: 2160-72 (2002)
BindingDB Entry DOI: 10.7270/Q2X067SG |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(RAT) | BDBM50370078
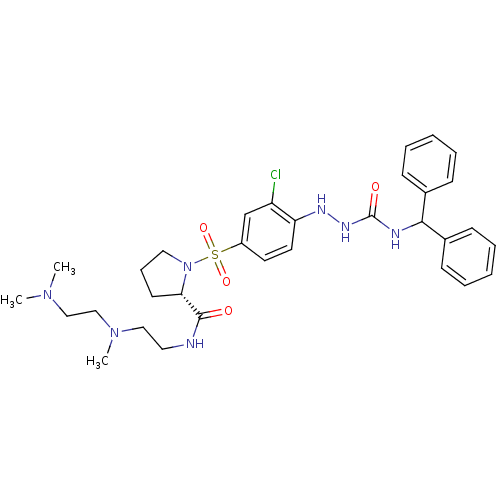
(CHEMBL1907653)Show SMILES CN(C)CCN(C)CCNC(=O)[C@@H]1CCCN1S(=O)(=O)c1ccc(NNC(=O)NC(c2ccccc2)c2ccccc2)c(Cl)c1 |r| Show InChI InChI=1S/C32H42ClN7O4S/c1-38(2)21-22-39(3)20-18-34-31(41)29-15-10-19-40(29)45(43,44)26-16-17-28(27(33)23-26)36-37-32(42)35-30(24-11-6-4-7-12-24)25-13-8-5-9-14-25/h4-9,11-14,16-17,23,29-30,36H,10,15,18-22H2,1-3H3,(H,34,41)(H2,35,37,42)/t29-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranes |
J Med Chem 45: 2160-72 (2002)
BindingDB Entry DOI: 10.7270/Q2X067SG |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50151894
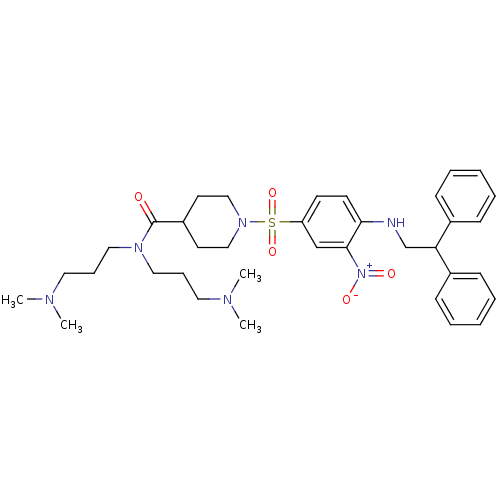
(1-[4-(2,2-Diphenyl-ethylamino)-3-nitro-benzenesulf...)Show SMILES CN(C)CCCN(CCCN(C)C)C(=O)C1CCN(CC1)S(=O)(=O)c1ccc(NCC(c2ccccc2)c2ccccc2)c(c1)[N+]([O-])=O Show InChI InChI=1S/C36H50N6O5S/c1-38(2)21-11-23-40(24-12-22-39(3)4)36(43)31-19-25-41(26-20-31)48(46,47)32-17-18-34(35(27-32)42(44)45)37-28-33(29-13-7-5-8-14-29)30-15-9-6-10-16-30/h5-10,13-18,27,31,33,37H,11-12,19-26,28H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Bradykinin receptor B1 expressed in HEK293 cells |
J Med Chem 47: 4642-4 (2004)
Article DOI: 10.1021/jm049747g
BindingDB Entry DOI: 10.7270/Q2J965V7 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50151884
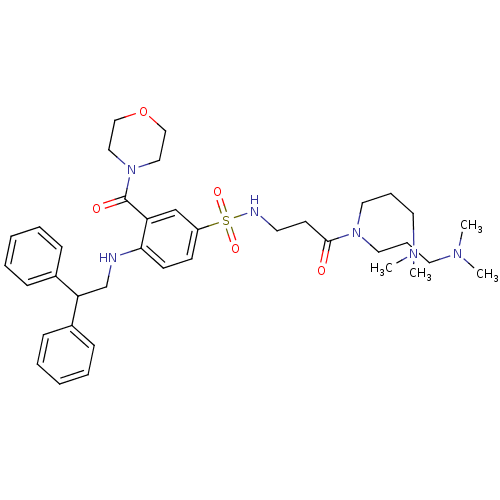
(CHEMBL185551 | N,N-Bis-(3-dimethylamino-propyl)-3-...)Show SMILES CN(C)CCCN(CCCN(C)C)C(=O)CCNS(=O)(=O)c1ccc(NCC(c2ccccc2)c2ccccc2)c(c1)C(=O)N1CCOCC1 Show InChI InChI=1S/C38H54N6O5S/c1-41(2)21-11-23-43(24-12-22-42(3)4)37(45)19-20-40-50(47,48)33-17-18-36(34(29-33)38(46)44-25-27-49-28-26-44)39-30-35(31-13-7-5-8-14-31)32-15-9-6-10-16-32/h5-10,13-18,29,35,39-40H,11-12,19-28,30H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Bradykinin receptor B1 expressed in HEK293 cells |
J Med Chem 47: 4642-4 (2004)
Article DOI: 10.1021/jm049747g
BindingDB Entry DOI: 10.7270/Q2J965V7 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50151886
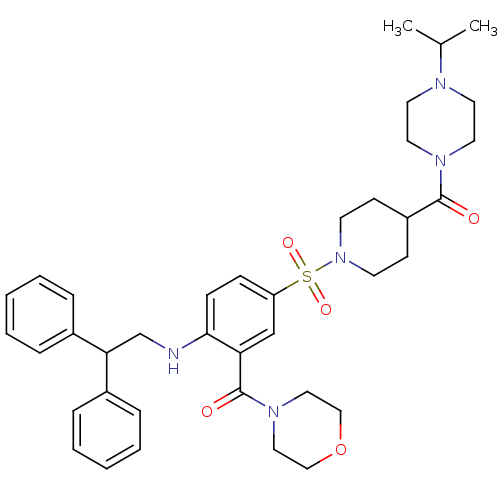
(CHEMBL181695 | {2-(2,2-Diphenyl-ethylamino)-5-[4-(...)Show SMILES CC(C)N1CCN(CC1)C(=O)C1CCN(CC1)S(=O)(=O)c1ccc(NCC(c2ccccc2)c2ccccc2)c(c1)C(=O)N1CCOCC1 Show InChI InChI=1S/C38H49N5O5S/c1-29(2)40-19-21-41(22-20-40)37(44)32-15-17-43(18-16-32)49(46,47)33-13-14-36(34(27-33)38(45)42-23-25-48-26-24-42)39-28-35(30-9-5-3-6-10-30)31-11-7-4-8-12-31/h3-14,27,29,32,35,39H,15-26,28H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Bradykinin receptor B1 expressed in HEK293 cells |
J Med Chem 47: 4642-4 (2004)
Article DOI: 10.1021/jm049747g
BindingDB Entry DOI: 10.7270/Q2J965V7 |
More data for this
Ligand-Target Pair | |
B1 bradykinin receptor
(Homo sapiens (Human)) | BDBM50151891
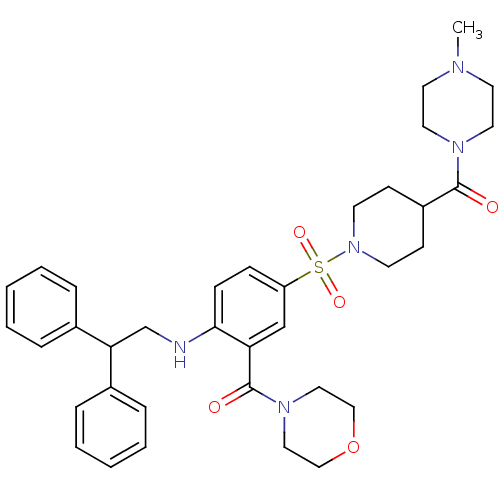
(CHEMBL273869 | {2-(2,2-Diphenyl-ethylamino)-5-[4-(...)Show SMILES CN1CCN(CC1)C(=O)C1CCN(CC1)S(=O)(=O)c1ccc(NCC(c2ccccc2)c2ccccc2)c(c1)C(=O)N1CCOCC1 Show InChI InChI=1S/C36H45N5O5S/c1-38-18-20-39(21-19-38)35(42)30-14-16-41(17-15-30)47(44,45)31-12-13-34(32(26-31)36(43)40-22-24-46-25-23-40)37-27-33(28-8-4-2-5-9-28)29-10-6-3-7-11-29/h2-13,26,30,33,37H,14-25,27H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity for Bradykinin receptor B1 expressed in HEK293 cells |
J Med Chem 47: 4642-4 (2004)
Article DOI: 10.1021/jm049747g
BindingDB Entry DOI: 10.7270/Q2J965V7 |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(RAT) | BDBM50409527

(CHEMBL2112283)Show SMILES CN(C)CCCN(C)CCNC(=O)[C@@H]1CCCN1S(=O)(=O)c1ccc(NNC(=O)NC2c3ccccc3CCc3ccccc23)c(c1)[N+]([O-])=O |r| Show InChI InChI=1S/C35H46N8O6S/c1-40(2)20-9-21-41(3)23-19-36-34(44)31-14-8-22-42(31)50(48,49)27-17-18-30(32(24-27)43(46)47)38-39-35(45)37-33-28-12-6-4-10-25(28)15-16-26-11-5-7-13-29(26)33/h4-7,10-13,17-18,24,31,33,38H,8-9,14-16,19-23H2,1-3H3,(H,36,44)(H2,37,39,45)/t31-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 10.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranes |
J Med Chem 45: 2160-72 (2002)
BindingDB Entry DOI: 10.7270/Q2X067SG |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50390426
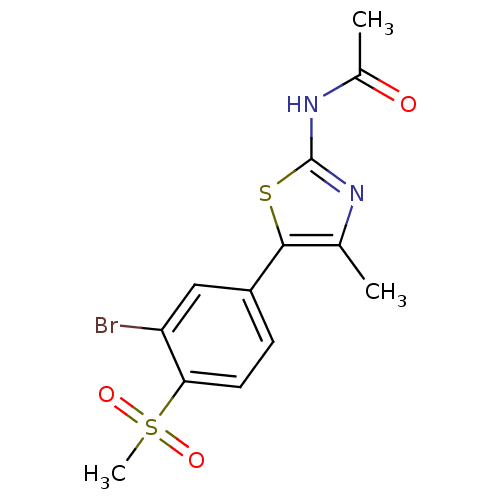
(CHEMBL2071329)Show SMILES CC(=O)Nc1nc(C)c(s1)-c1ccc(c(Br)c1)S(C)(=O)=O Show InChI InChI=1S/C13H13BrN2O3S2/c1-7-12(20-13(15-7)16-8(2)17)9-4-5-11(10(14)6-9)21(3,18)19/h4-6H,1-3H3,(H,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110alpha by KinaseGlo assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(RAT) | BDBM50370080

(CHEMBL1907656)Show SMILES CN(C)CCN(C)CCNC(=O)C1CCCN1S(=O)(=O)c1ccc(N[N-]C(=[SH+])NC2Cc3ccccc3Cc3ccccc23)c(c1)[N+]([O-])=O Show InChI InChI=1S/C34H44N8O5S2/c1-39(2)19-20-40(3)18-16-35-33(43)31-13-8-17-41(31)49(46,47)27-14-15-29(32(23-27)42(44)45)37-38-34(48)36-30-22-25-10-5-4-9-24(25)21-26-11-6-7-12-28(26)30/h4-7,9-12,14-15,23,30-31,37H,8,13,16-22H2,1-3H3,(H3,35,36,38,43,48) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 14.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranes |
J Med Chem 45: 2160-72 (2002)
BindingDB Entry DOI: 10.7270/Q2X067SG |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50390419
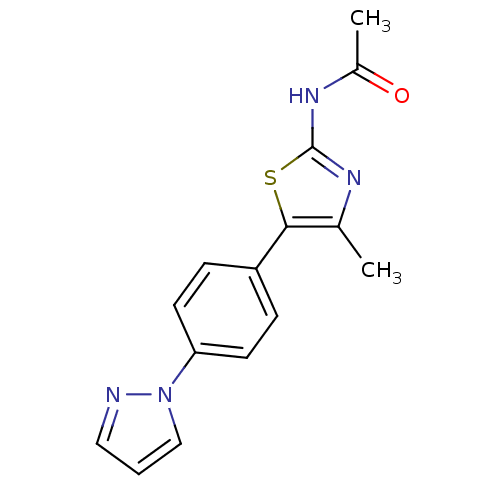
(CHEMBL2071333)Show InChI InChI=1S/C15H14N4OS/c1-10-14(21-15(17-10)18-11(2)20)12-4-6-13(7-5-12)19-9-3-8-16-19/h3-9H,1-2H3,(H,17,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110delta using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50390425
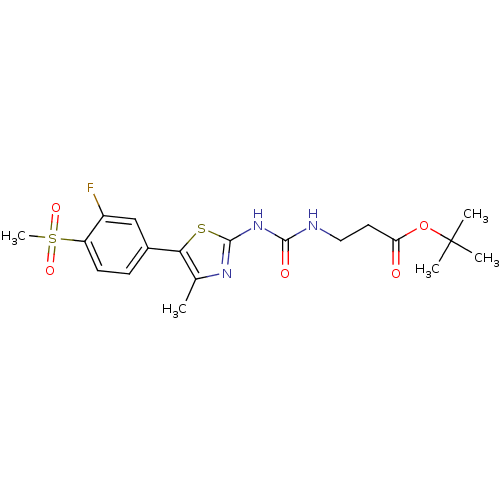
(CHEMBL2071341)Show SMILES Cc1nc(NC(=O)NCCC(=O)OC(C)(C)C)sc1-c1ccc(c(F)c1)S(C)(=O)=O Show InChI InChI=1S/C19H24FN3O5S2/c1-11-16(12-6-7-14(13(20)10-12)30(5,26)27)29-18(22-11)23-17(25)21-9-8-15(24)28-19(2,3)4/h6-7,10H,8-9H2,1-5H3,(H2,21,22,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110gamma using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50390423
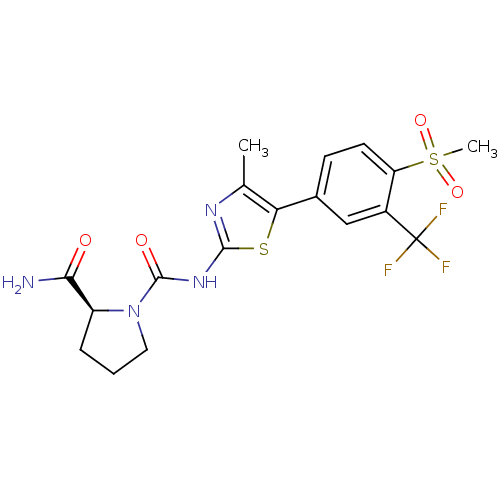
(CHEMBL2071337)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccc(c(c1)C(F)(F)F)S(C)(=O)=O |r| Show InChI InChI=1S/C18H19F3N4O4S2/c1-9-14(10-5-6-13(31(2,28)29)11(8-10)18(19,20)21)30-16(23-9)24-17(27)25-7-3-4-12(25)15(22)26/h5-6,8,12H,3-4,7H2,1-2H3,(H2,22,26)(H,23,24,27)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110alpha by KinaseGlo assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50390418
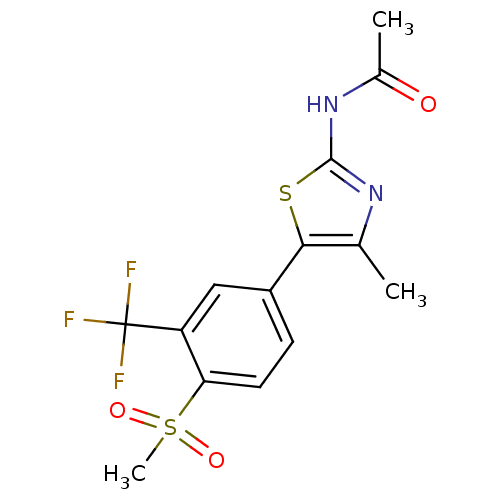
(CHEMBL2071332)Show SMILES CC(=O)Nc1nc(C)c(s1)-c1ccc(c(c1)C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C14H13F3N2O3S2/c1-7-12(23-13(18-7)19-8(2)20)9-4-5-11(24(3,21)22)10(6-9)14(15,16)17/h4-6H,1-3H3,(H,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110alpha by KinaseGlo assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50390419

(CHEMBL2071333)Show InChI InChI=1S/C15H14N4OS/c1-10-14(21-15(17-10)18-11(2)20)12-4-6-13(7-5-12)19-9-3-8-16-19/h3-9H,1-2H3,(H,17,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110alpha by KinaseGlo assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50390426

(CHEMBL2071329)Show SMILES CC(=O)Nc1nc(C)c(s1)-c1ccc(c(Br)c1)S(C)(=O)=O Show InChI InChI=1S/C13H13BrN2O3S2/c1-7-12(20-13(15-7)16-8(2)17)9-4-5-11(10(14)6-9)21(3,18)19/h4-6H,1-3H3,(H,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110gamma using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(Homo sapiens (Human)) | BDBM50370081

(CHEMBL1907654)Show SMILES CN(C)CCN(C)CCNC(=O)C1CCCN1S(=O)(=O)c1ccc(N[N-]C(=[SH+])NC(c2ccccc2Cl)c2ccccc2Cl)c(c1)[N+]([O-])=O Show InChI InChI=1S/C32H40Cl2N8O5S2/c1-39(2)19-20-40(3)18-16-35-31(43)28-13-8-17-41(28)49(46,47)22-14-15-27(29(21-22)42(44)45)37-38-32(48)36-30(23-9-4-6-11-25(23)33)24-10-5-7-12-26(24)34/h4-7,9-12,14-15,21,28,30,37H,8,13,16-20H2,1-3H3,(H3,35,36,38,43,48) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 34.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]BK (1 nM) from human Bradykinin receptor B2 expressed in Cos-7 cells |
J Med Chem 45: 2160-72 (2002)
BindingDB Entry DOI: 10.7270/Q2X067SG |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50390415
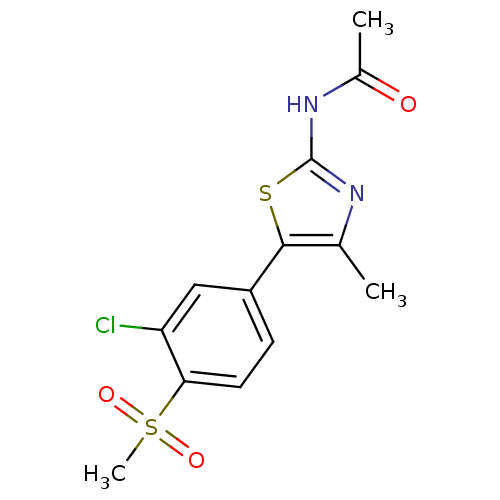
(CHEMBL2071328)Show SMILES CC(=O)Nc1nc(C)c(s1)-c1ccc(c(Cl)c1)S(C)(=O)=O Show InChI InChI=1S/C13H13ClN2O3S2/c1-7-12(20-13(15-7)16-8(2)17)9-4-5-11(10(14)6-9)21(3,18)19/h4-6H,1-3H3,(H,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110alpha by KinaseGlo assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50390411
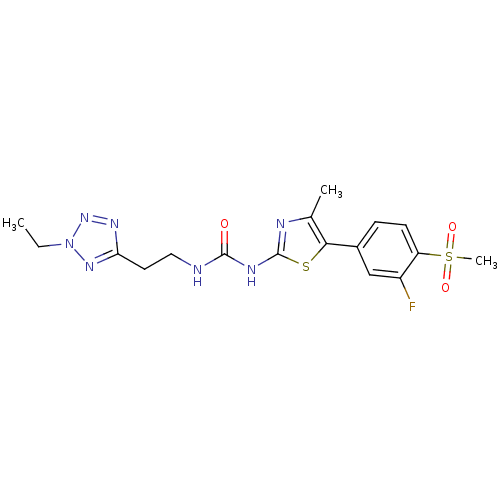
(CHEMBL2071342)Show SMILES CCn1nnc(CCNC(=O)Nc2nc(C)c(s2)-c2ccc(c(F)c2)S(C)(=O)=O)n1 Show InChI InChI=1S/C17H20FN7O3S2/c1-4-25-23-14(22-24-25)7-8-19-16(26)21-17-20-10(2)15(29-17)11-5-6-13(12(18)9-11)30(3,27)28/h5-6,9H,4,7-8H2,1-3H3,(H2,19,20,21,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110gamma using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(RAT) | BDBM50085698
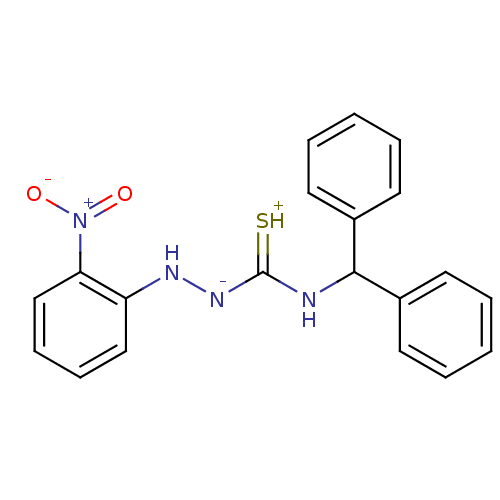
(CHEMBL164636 | benzhydrylamino-2-nitrobenzylhydraz...)Show SMILES [O-][N+](=O)c1ccccc1N[N-]C(=[SH+])NC(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C20H18N4O2S/c25-24(26)18-14-8-7-13-17(18)22-23-20(27)21-19(15-9-3-1-4-10-15)16-11-5-2-6-12-16/h1-14,19,22H,(H2,21,23,27) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of binding of [3H]-BK to rat bradykinin B2 receptor expressed in NG108-15 neuroblastoma-glioma hybrid cell membranes |
J Med Chem 43: 769-71 (2000)
BindingDB Entry DOI: 10.7270/Q2XD10W5 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50390417
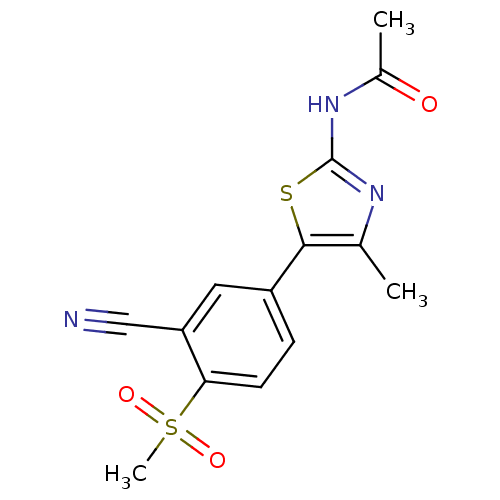
(CHEMBL2071331)Show SMILES CC(=O)Nc1nc(C)c(s1)-c1ccc(c(c1)C#N)S(C)(=O)=O Show InChI InChI=1S/C14H13N3O3S2/c1-8-13(21-14(16-8)17-9(2)18)10-4-5-12(22(3,19)20)11(6-10)7-15/h4-6H,1-3H3,(H,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110alpha by KinaseGlo assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50390422
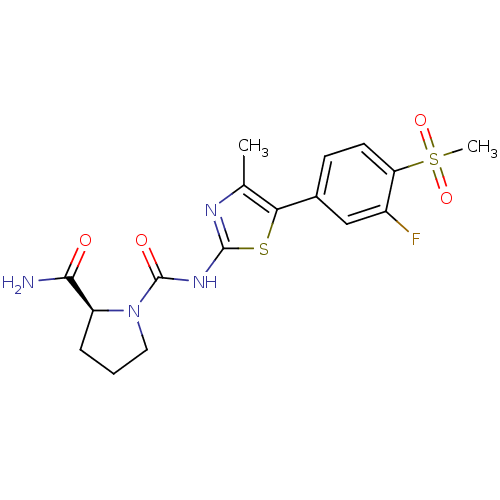
(CHEMBL2071336)Show SMILES Cc1nc(NC(=O)N2CCC[C@H]2C(N)=O)sc1-c1ccc(c(F)c1)S(C)(=O)=O |r| Show InChI InChI=1S/C17H19FN4O4S2/c1-9-14(10-5-6-13(11(18)8-10)28(2,25)26)27-16(20-9)21-17(24)22-7-3-4-12(22)15(19)23/h5-6,8,12H,3-4,7H2,1-2H3,(H2,19,23)(H,20,21,24)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110alpha by KinaseGlo assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50390415

(CHEMBL2071328)Show SMILES CC(=O)Nc1nc(C)c(s1)-c1ccc(c(Cl)c1)S(C)(=O)=O Show InChI InChI=1S/C13H13ClN2O3S2/c1-7-12(20-13(15-7)16-8(2)17)9-4-5-11(10(14)6-9)21(3,18)19/h4-6H,1-3H3,(H,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110gamma using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50390418

(CHEMBL2071332)Show SMILES CC(=O)Nc1nc(C)c(s1)-c1ccc(c(c1)C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C14H13F3N2O3S2/c1-7-12(23-13(18-7)19-8(2)20)9-4-5-11(24(3,21)22)10(6-9)14(15,16)17/h4-6H,1-3H3,(H,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110gamma using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50390426

(CHEMBL2071329)Show SMILES CC(=O)Nc1nc(C)c(s1)-c1ccc(c(Br)c1)S(C)(=O)=O Show InChI InChI=1S/C13H13BrN2O3S2/c1-7-12(20-13(15-7)16-8(2)17)9-4-5-11(10(14)6-9)21(3,18)19/h4-6H,1-3H3,(H,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 61 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110delta using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50390417

(CHEMBL2071331)Show SMILES CC(=O)Nc1nc(C)c(s1)-c1ccc(c(c1)C#N)S(C)(=O)=O Show InChI InChI=1S/C14H13N3O3S2/c1-8-13(21-14(16-8)17-9(2)18)10-4-5-12(22(3,19)20)11(6-10)7-15/h4-6H,1-3H3,(H,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110gamma using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(RAT) | BDBM50409529

(CHEMBL2112221)Show SMILES CN(C)CCN(C)CCNC(=O)c1cccn1S(=O)(=O)c1ccc(N[N-]C(=[SH+])NC2c3ccccc3CCc3ccccc23)c(c1)[N+]([O-])=O Show InChI InChI=1S/C34H40N8O5S2/c1-39(2)21-22-40(3)20-18-35-33(43)30-13-8-19-41(30)49(46,47)26-16-17-29(31(23-26)42(44)45)37-38-34(48)36-32-27-11-6-4-9-24(27)14-15-25-10-5-7-12-28(25)32/h4-13,16-17,19,23,32,37H,14-15,18,20-22H2,1-3H3,(H3,35,36,38,43,48) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 64.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of specific binding of [3H]-BK (1 nM) to rat Bradykinin receptor B2 by 50% in NG108-15 cell membranes |
J Med Chem 45: 2160-72 (2002)
BindingDB Entry DOI: 10.7270/Q2X067SG |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50390418

(CHEMBL2071332)Show SMILES CC(=O)Nc1nc(C)c(s1)-c1ccc(c(c1)C(F)(F)F)S(C)(=O)=O Show InChI InChI=1S/C14H13F3N2O3S2/c1-7-12(23-13(18-7)19-8(2)20)9-4-5-11(24(3,21)22)10(6-9)14(15,16)17/h4-6H,1-3H3,(H,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 74 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110delta using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50390415

(CHEMBL2071328)Show SMILES CC(=O)Nc1nc(C)c(s1)-c1ccc(c(Cl)c1)S(C)(=O)=O Show InChI InChI=1S/C13H13ClN2O3S2/c1-7-12(20-13(15-7)16-8(2)17)9-4-5-11(10(14)6-9)21(3,18)19/h4-6H,1-3H3,(H,15,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110delta using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50390417

(CHEMBL2071331)Show SMILES CC(=O)Nc1nc(C)c(s1)-c1ccc(c(c1)C#N)S(C)(=O)=O Show InChI InChI=1S/C14H13N3O3S2/c1-8-13(21-14(16-8)17-9(2)18)10-4-5-12(22(3,19)20)11(6-10)7-15/h4-6H,1-3H3,(H,16,17,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110delta using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50390410
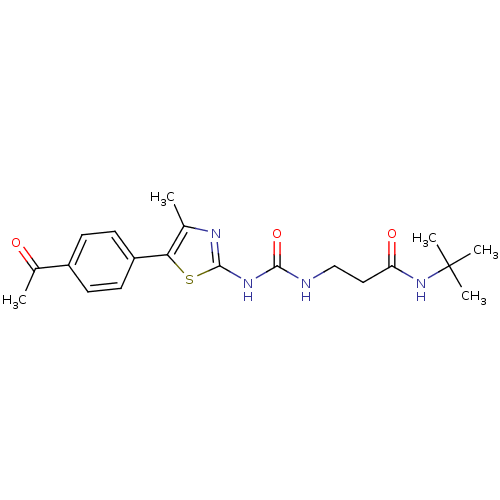
(CHEMBL2069328)Show SMILES CC(=O)c1ccc(cc1)-c1sc(NC(=O)NCCC(=O)NC(C)(C)C)nc1C Show InChI InChI=1S/C20H26N4O3S/c1-12-17(15-8-6-14(7-9-15)13(2)25)28-19(22-12)23-18(27)21-11-10-16(26)24-20(3,4)5/h6-9H,10-11H2,1-5H3,(H,24,26)(H2,21,22,23,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 88 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110gamma using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50390425

(CHEMBL2071341)Show SMILES Cc1nc(NC(=O)NCCC(=O)OC(C)(C)C)sc1-c1ccc(c(F)c1)S(C)(=O)=O Show InChI InChI=1S/C19H24FN3O5S2/c1-11-16(12-6-7-14(13(20)10-12)30(5,26)27)29-18(22-11)23-17(25)21-9-8-15(24)28-19(2,3)4/h6-7,10H,8-9H2,1-5H3,(H2,21,22,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110beta using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
B2 bradykinin receptor
(Homo sapiens (Human)) | BDBM50370077

(CHEMBL1907652)Show SMILES CN(C)CCCN(C)CCCNC(=O)C1CCCN1S(=O)(=O)c1ccc(N[N-]C(=[SH+])NC(c2ccccc2)c2ccccc2)c(c1)[N+]([O-])=O Show InChI InChI=1S/C34H46N8O5S2/c1-39(2)21-12-23-40(3)22-11-20-35-33(43)30-17-10-24-41(30)49(46,47)28-18-19-29(31(25-28)42(44)45)37-38-34(48)36-32(26-13-6-4-7-14-26)27-15-8-5-9-16-27/h4-9,13-16,18-19,25,30,32,37H,10-12,17,20-24H2,1-3H3,(H3,35,36,38,43,48) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 95 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institute for Medical Sciences
Curated by ChEMBL
| Assay Description
Displacement of [3H]BK (1 nM) from human Bradykinin receptor B2 expressed in Cos-7 cells |
J Med Chem 45: 2160-72 (2002)
BindingDB Entry DOI: 10.7270/Q2X067SG |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50390424

(CHEMBL2071340)Show SMILES Cc1cc(NC(=O)CCNC(=O)Nc2nc(C)c(s2)-c2ccc(c(F)c2)S(C)(=O)=O)no1 Show InChI InChI=1S/C19H20FN5O5S2/c1-10-8-15(25-30-10)23-16(26)6-7-21-18(27)24-19-22-11(2)17(31-19)12-4-5-14(13(20)9-12)32(3,28)29/h4-5,8-9H,6-7H2,1-3H3,(H,23,25,26)(H2,21,22,24,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110alpha by KinaseGlo assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50390419

(CHEMBL2071333)Show InChI InChI=1S/C15H14N4OS/c1-10-14(21-15(17-10)18-11(2)20)12-4-6-13(7-5-12)19-9-3-8-16-19/h3-9H,1-2H3,(H,17,18,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant GST-tagged PI3K p110gamma using [32P]ATP after 60 mins by scintillation proximity assay |
Bioorg Med Chem Lett 22: 5445-50 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.042
BindingDB Entry DOI: 10.7270/Q28P61MR |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data