

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Substance-K receptor (Homo sapiens (Human)) | BDBM50030096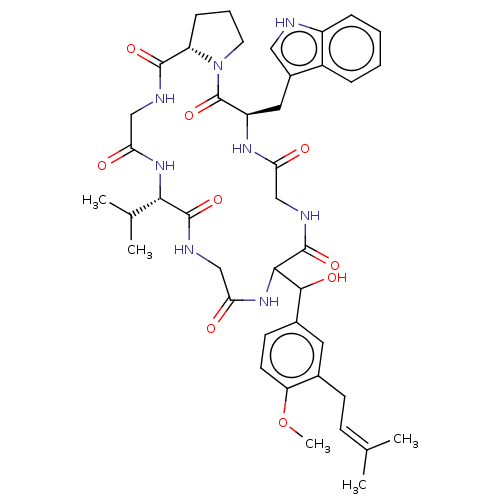 ((R)-11-{Hydroxy-[4-methoxy-3-(3-methyl-but-2-enyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Displacement of [125I]-NKA substance P binding to human urinary bladder membrane protein (NK2) | J Med Chem 37: 356-63 (1994) BindingDB Entry DOI: 10.7270/Q21N805R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50030095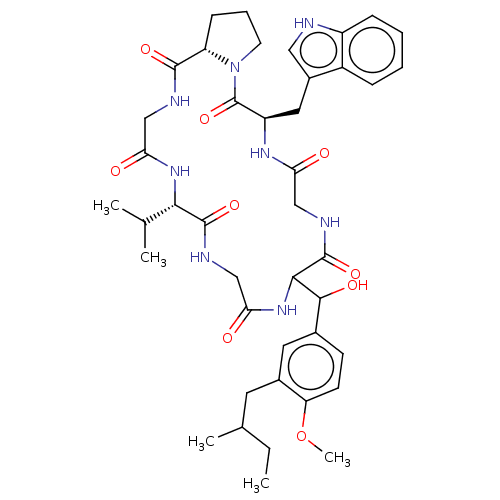 ((R)-11-{Hydroxy-[4-methoxy-3-(2-methyl-butyl)-phen...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Displacement of [125I]-NKA substance P binding to human urinary bladder membrane protein (NK2) | J Med Chem 37: 356-63 (1994) BindingDB Entry DOI: 10.7270/Q21N805R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030095 ((R)-11-{Hydroxy-[4-methoxy-3-(2-methyl-butyl)-phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Displacement of [125I]- substance P binding to human astrocytoma cells (NK1) | J Med Chem 37: 356-63 (1994) BindingDB Entry DOI: 10.7270/Q21N805R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030096 ((R)-11-{Hydroxy-[4-methoxy-3-(3-methyl-but-2-enyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Displacement of [125I]- substance P binding to human astrocytoma cells (NK1) | J Med Chem 37: 356-63 (1994) BindingDB Entry DOI: 10.7270/Q21N805R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030099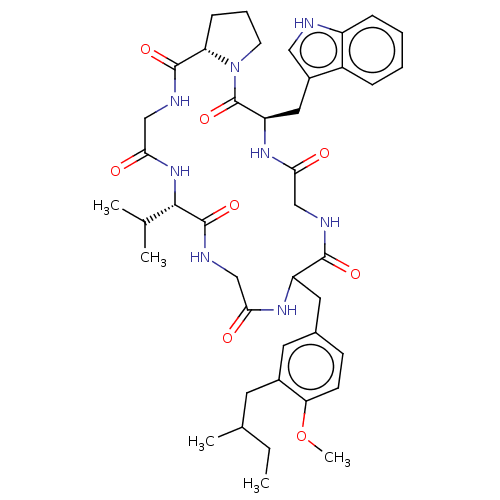 ((R)-5-(1H-Indol-3-ylmethyl)-17-isopropyl-11-[4-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Displacement of [125I]- substance P binding to human astrocytoma cells (NK1) | J Med Chem 37: 356-63 (1994) BindingDB Entry DOI: 10.7270/Q21N805R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50030092 ((R)-11-{Hydroxy-[4-hydroxy-3-(3-methyl-but-2-enyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Displacement of [125I]-NKA substance P binding to human urinary bladder membrane protein (NK2) | J Med Chem 37: 356-63 (1994) BindingDB Entry DOI: 10.7270/Q21N805R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030092 ((R)-11-{Hydroxy-[4-hydroxy-3-(3-methyl-but-2-enyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Displacement of [125I]- substance P binding to human astrocytoma cells (NK1) | J Med Chem 37: 356-63 (1994) BindingDB Entry DOI: 10.7270/Q21N805R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50030094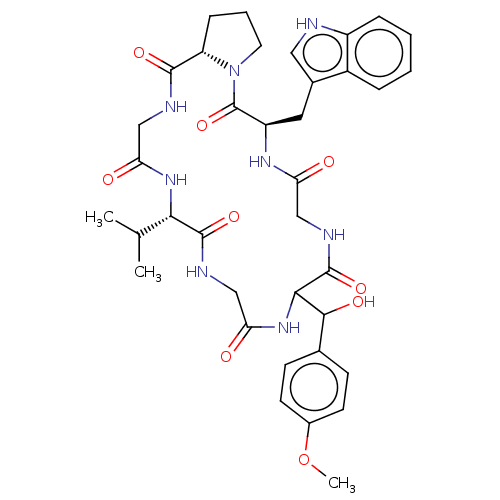 ((R)-11-[Hydroxy-(4-methoxy-phenyl)-methyl]-5-(1H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Displacement of [125I]-NKA substance P binding to human urinary bladder membrane protein (NK2) | J Med Chem 37: 356-63 (1994) BindingDB Entry DOI: 10.7270/Q21N805R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030094 ((R)-11-[Hydroxy-(4-methoxy-phenyl)-methyl]-5-(1H-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Displacement of [125I]- substance P binding to human astrocytoma cells (NK1) | J Med Chem 37: 356-63 (1994) BindingDB Entry DOI: 10.7270/Q21N805R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50407271 (CHEMBL2112592) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Displacement of [125I]-NKA substance P binding to human urinary bladder membrane protein (NK2) | J Med Chem 37: 356-63 (1994) BindingDB Entry DOI: 10.7270/Q21N805R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50407272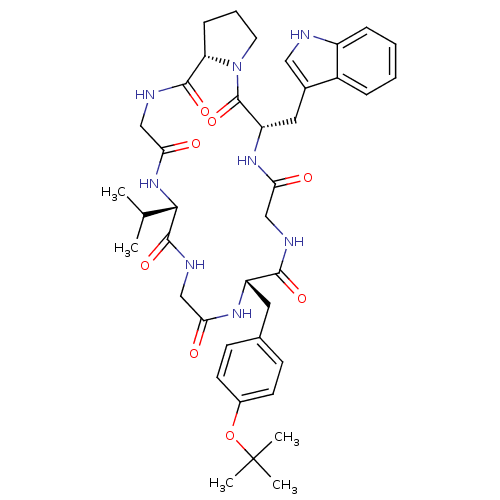 (CHEMBL2111789) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Displacement of [125I]-NKA substance P binding to human urinary bladder membrane protein (NK2) | J Med Chem 37: 356-63 (1994) BindingDB Entry DOI: 10.7270/Q21N805R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50407271 (CHEMBL2112592) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Displacement of [125I]- substance P binding to human astrocytoma cells (NK1) | J Med Chem 37: 356-63 (1994) BindingDB Entry DOI: 10.7270/Q21N805R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030091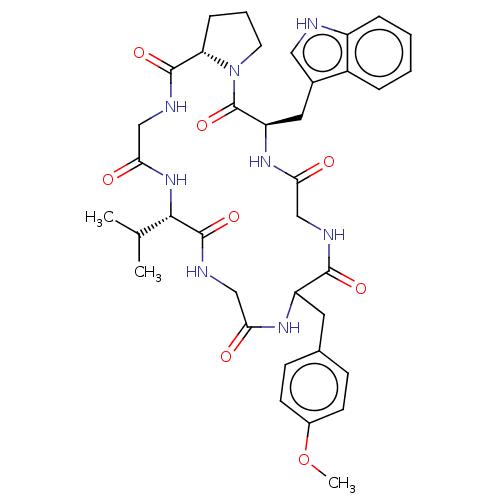 ((R)-5-(1H-Indol-3-ylmethyl)-17-isopropyl-11-(4-met...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Displacement of [125I]- substance P binding to human astrocytoma cells (NK1) | J Med Chem 37: 356-63 (1994) BindingDB Entry DOI: 10.7270/Q21N805R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50030091 ((R)-5-(1H-Indol-3-ylmethyl)-17-isopropyl-11-(4-met...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 7.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Displacement of [125I]-NKA substance P binding to human urinary bladder membrane protein (NK2) | J Med Chem 37: 356-63 (1994) BindingDB Entry DOI: 10.7270/Q21N805R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50407272 (CHEMBL2111789) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Displacement of [125I]- substance P binding to human astrocytoma cells (NK1) | J Med Chem 37: 356-63 (1994) BindingDB Entry DOI: 10.7270/Q21N805R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030097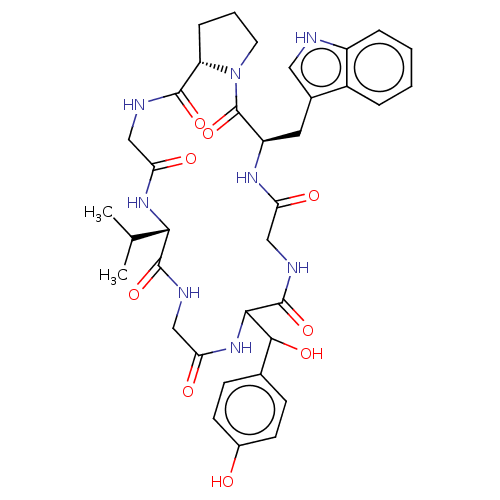 ((R)-11-[Hydroxy-(4-hydroxy-phenyl)-methyl]-5-(1H-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Displacement of [125I]- substance P binding to human astrocytoma cells (NK1) | J Med Chem 37: 356-63 (1994) BindingDB Entry DOI: 10.7270/Q21N805R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50030097 ((R)-11-[Hydroxy-(4-hydroxy-phenyl)-methyl]-5-(1H-i...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Displacement of [125I]-NKA substance P binding to human urinary bladder membrane protein (NK2) | J Med Chem 37: 356-63 (1994) BindingDB Entry DOI: 10.7270/Q21N805R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50030098 ((R)-11-(4-Hydroxy-benzyl)-5-(1H-indol-3-ylmethyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Displacement of [125I]-NKA substance P binding to human urinary bladder membrane protein (NK2) | J Med Chem 37: 356-63 (1994) BindingDB Entry DOI: 10.7270/Q21N805R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030090 ((R)-11-(1H-Indol-5-ylmethyl)-5-(1H-indol-3-ylmethy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Displacement of [125I]- substance P binding to human astrocytoma cells (NK1) | J Med Chem 37: 356-63 (1994) BindingDB Entry DOI: 10.7270/Q21N805R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-K receptor (Homo sapiens (Human)) | BDBM50030090 ((R)-11-(1H-Indol-5-ylmethyl)-5-(1H-indol-3-ylmethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Displacement of [125I]-NKA substance P binding to human urinary bladder membrane protein (NK2) | J Med Chem 37: 356-63 (1994) BindingDB Entry DOI: 10.7270/Q21N805R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM50030098 ((R)-11-(4-Hydroxy-benzyl)-5-(1H-indol-3-ylmethyl)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sterling Winthrop Pharmaceuticals Research Division Curated by ChEMBL | Assay Description Displacement of [125I]- substance P binding to human astrocytoma cells (NK1) | J Med Chem 37: 356-63 (1994) BindingDB Entry DOI: 10.7270/Q21N805R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383996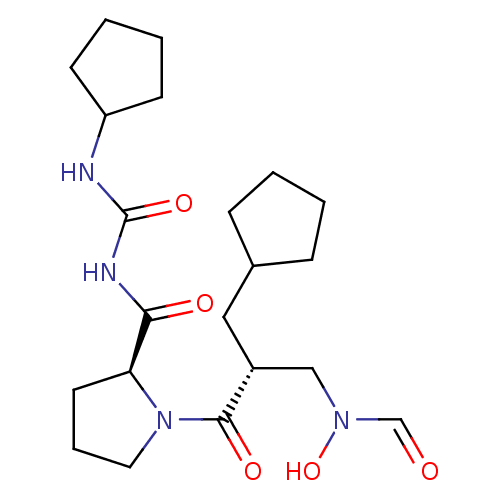 (CHEMBL2032134) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383999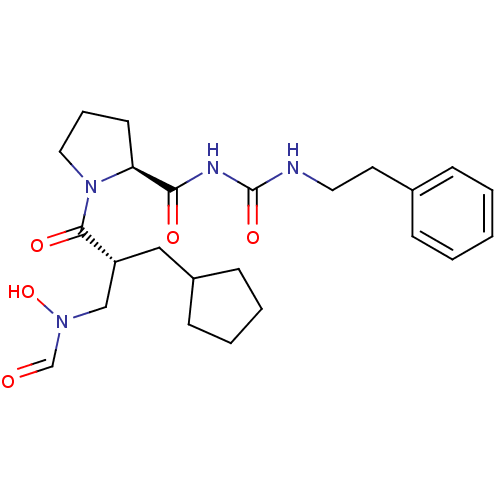 (CHEMBL2032137) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383966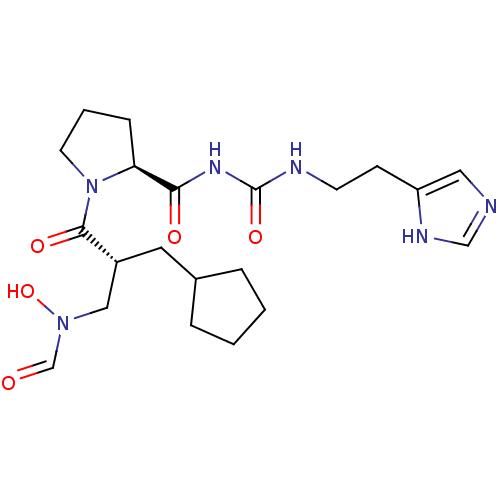 (CHEMBL2032148) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383998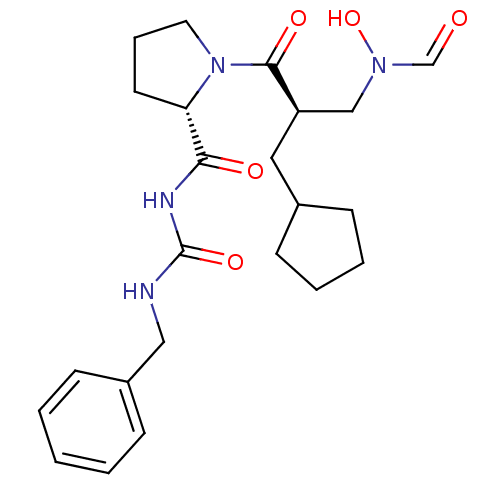 (CHEMBL2032136) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50384000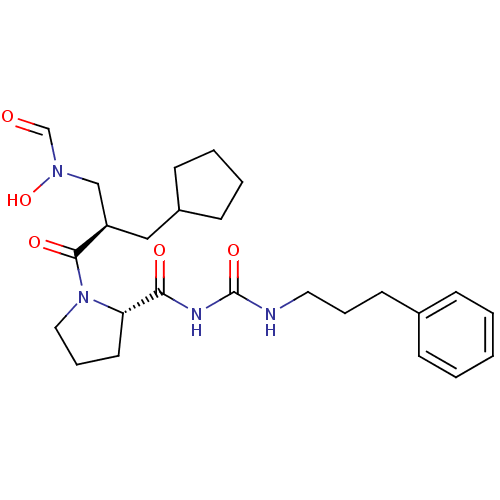 (CHEMBL2032138) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383997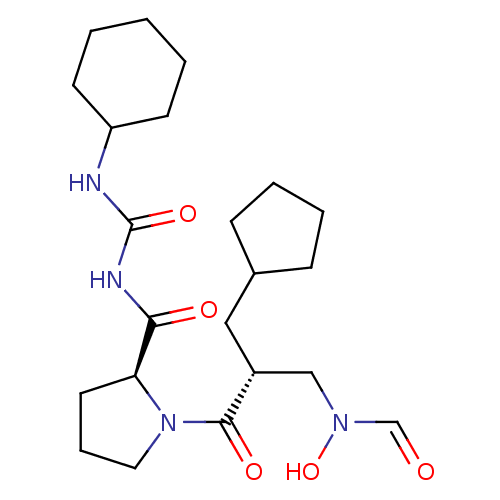 (CHEMBL2032135) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383994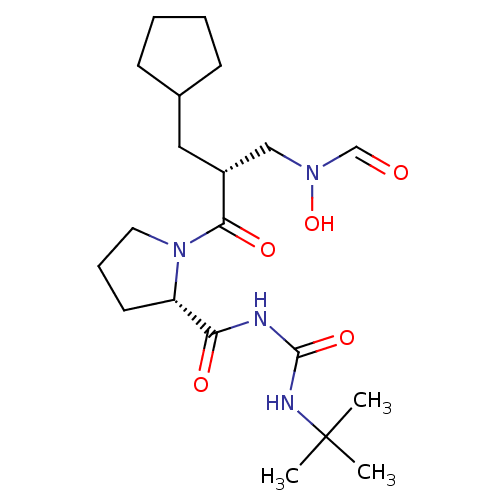 (CHEMBL2032132) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383987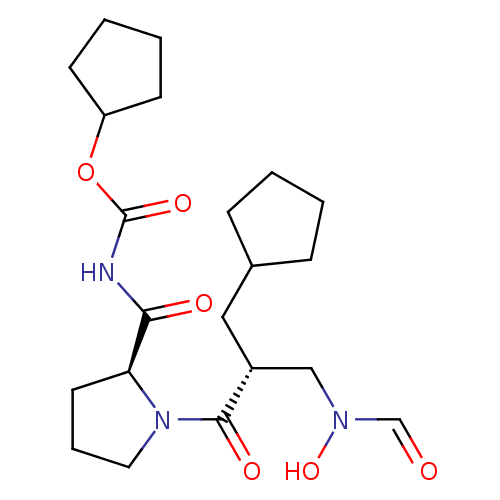 (CHEMBL2032125) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383982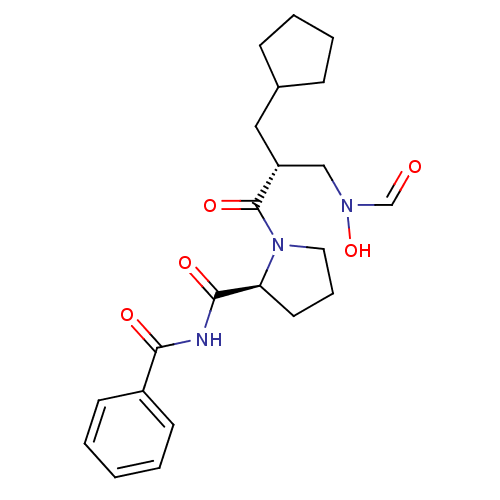 (CHEMBL2032119) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383995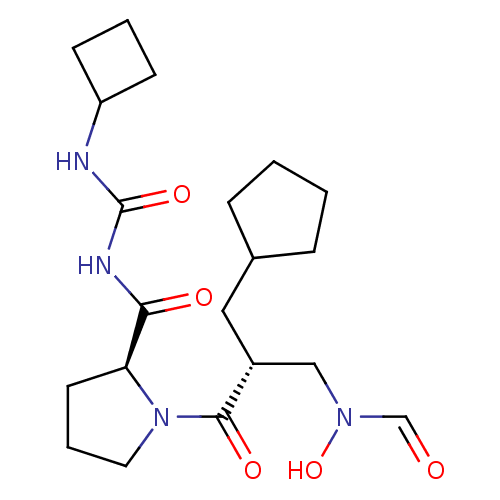 (CHEMBL2032133) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383988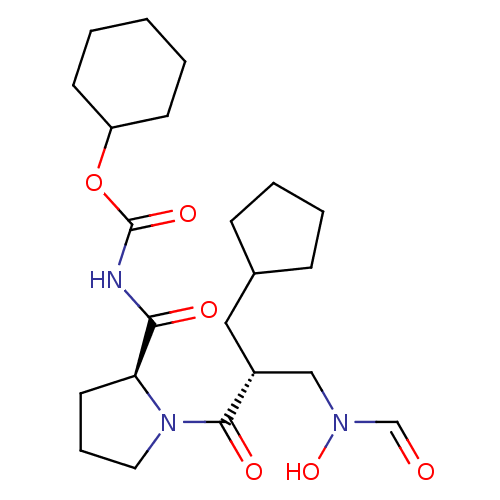 (CHEMBL2032126) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383993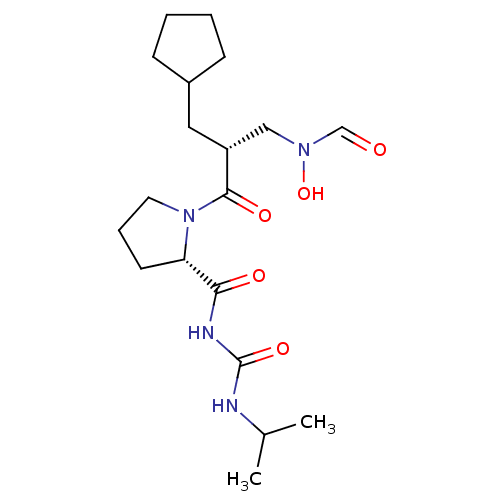 (CHEMBL2032131) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50384003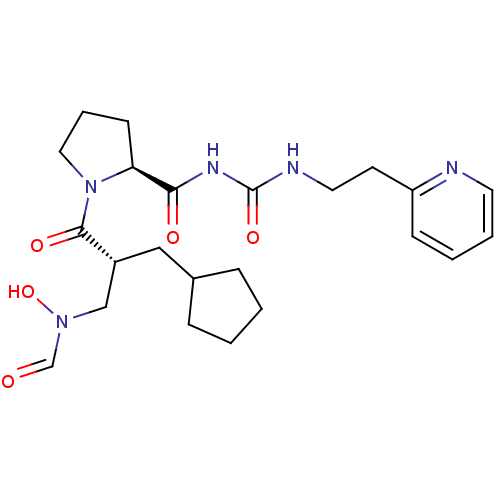 (CHEMBL2032142) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383968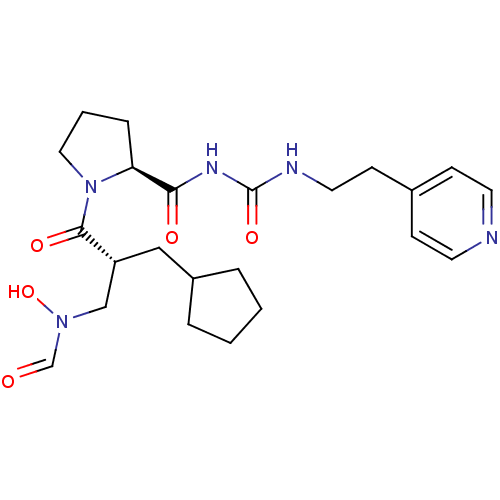 (CHEMBL2032140) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50384002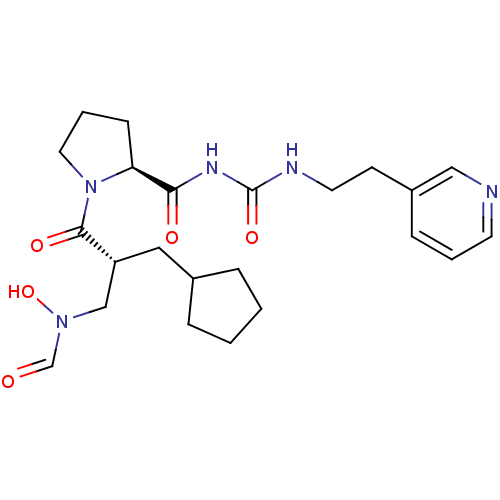 (CHEMBL2032141) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383972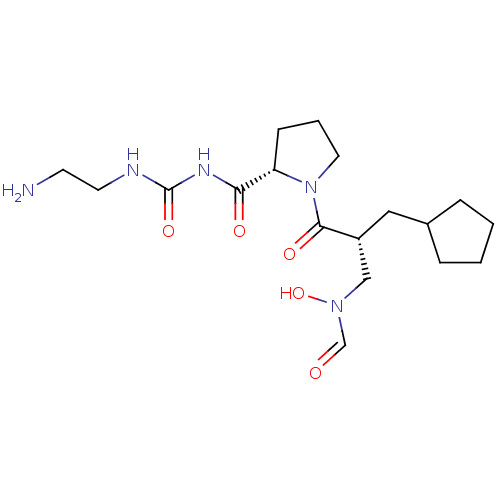 (CHEMBL2032146) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383969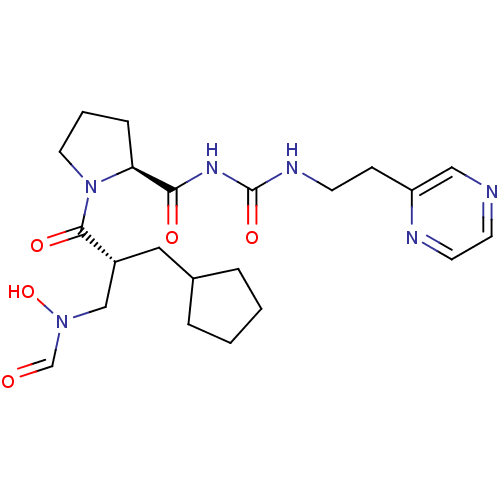 (CHEMBL2032143) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383980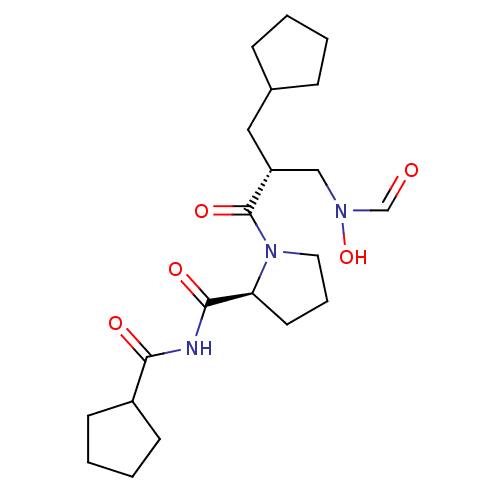 (CHEMBL2032117) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383992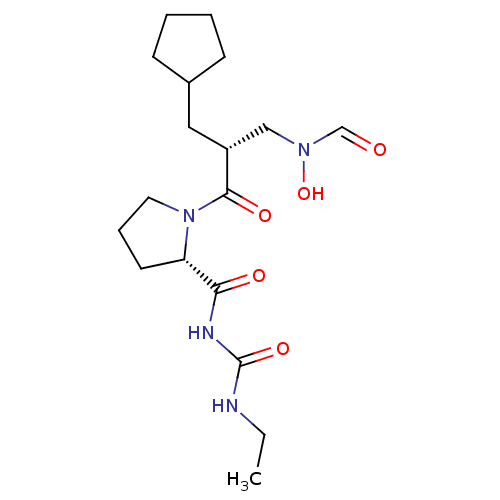 (CHEMBL2032130) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383965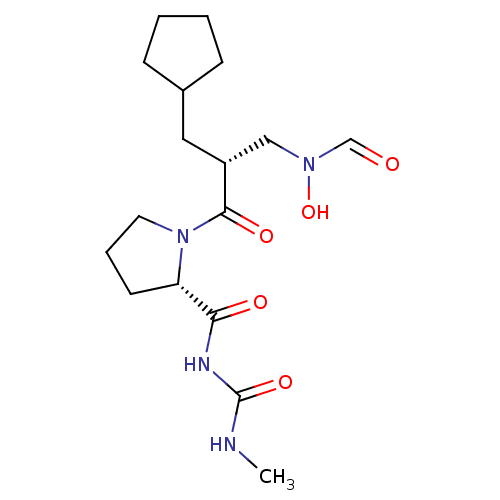 (CHEMBL2032129) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383967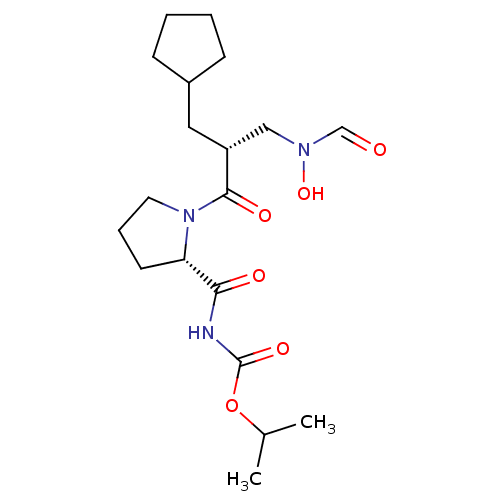 (CHEMBL2032122) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383981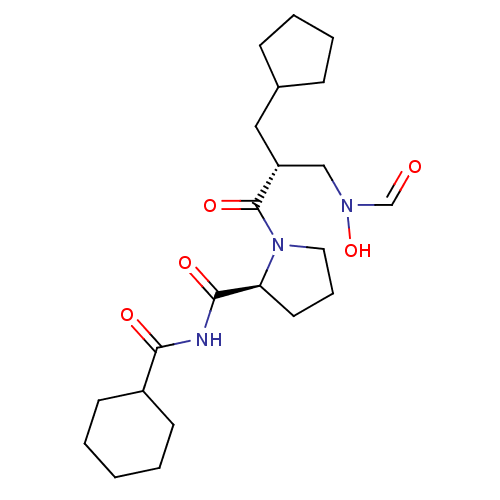 (CHEMBL2032118) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383986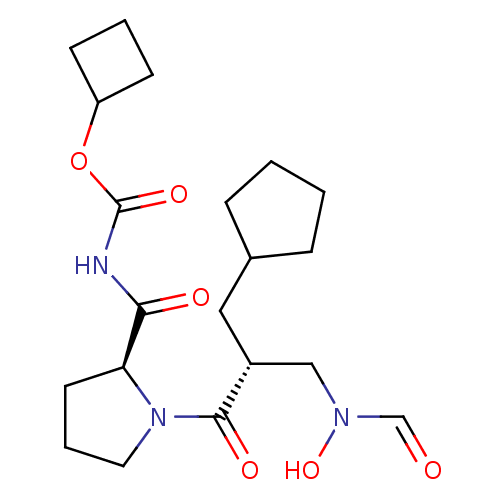 (CHEMBL2032124) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383985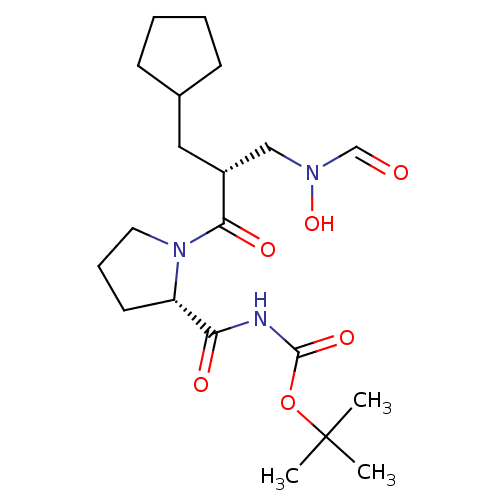 (CHEMBL2032123) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Staphylococcus aureus) | BDBM50383996 (CHEMBL2032134) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Staphylococcus aureus PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled assay | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383984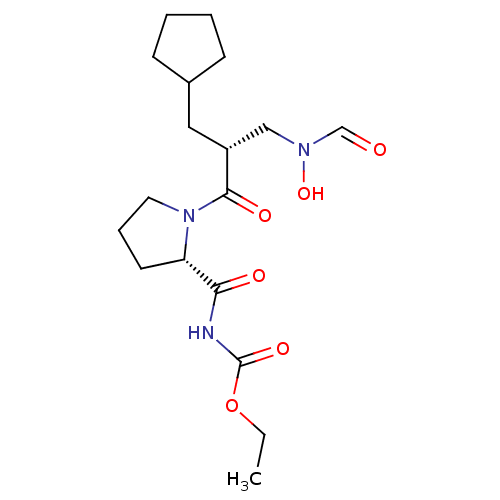 (CHEMBL2032121) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 3-phosphoinositide-dependent protein kinase 1 (Homo sapiens (Human)) | BDBM50341250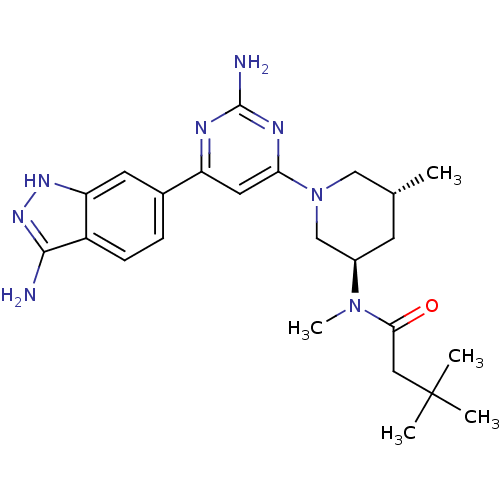 (CHEMBL1765751 | N-{(3R,5R)-1-[2-Amino-6-(3-amino-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of PDK1 | J Med Chem 54: 1871-95 (2011) Article DOI: 10.1021/jm101527u BindingDB Entry DOI: 10.7270/Q2HQ406Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383989 (CHEMBL2032127) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.660 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptide deformylase (Streptococcus pneumoniae serotype 4 (strain ATCC B...) | BDBM50383978 (CHEMBL2032115) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Curated by ChEMBL | Assay Description Inhibition of Streptococcus pneumoniae PDF assessed as formate release from fMAS peptide substrate after 20 mins by formate dehydrogenase coupled ass... | Bioorg Med Chem Lett 22: 4028-32 (2012) Article DOI: 10.1016/j.bmcl.2012.04.086 BindingDB Entry DOI: 10.7270/Q2K64K3V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 482 total ) | Next | Last >> |