 Found 542 hits with Last Name = 'bodin' and Initial = 'c'
Found 542 hits with Last Name = 'bodin' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50026603

(Buprenorphine | CHEBI:3216)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@]([H])(C1)[C@](C)(O)C(C)(C)C)ccc3O |r,TLB:25:17:4.5.6:9.15.14,18:17:4.5.6:9.15.14,THB:10:9:17:4.5.6,3:4:17:9.15.14,26:23:16.1:18.19| Show InChI InChI=1S/C29H41NO4/c1-25(2,3)26(4,32)20-15-27-10-11-29(20,33-5)24-28(27)12-13-30(16-17-6-7-17)21(27)14-18-8-9-19(31)23(34-24)22(18)28/h8-9,17,20-21,24,31-32H,6-7,10-16H2,1-5H3/t20-,21-,24-,26+,27-,28+,29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against delta-opiate receptor (human) using [3H]-DPDPE radioligand |
J Med Chem 44: 3378-90 (2001)
BindingDB Entry DOI: 10.7270/Q23X87B8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50105091
(2-[8-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-4-oxo-...)Show SMILES CNC(=O)CN1CN(c2ccccc2)C2(CCN(Cc3cc4OCOc4cc3Cl)CC2)C1=O Show InChI InChI=1S/C24H27ClN4O4/c1-26-22(30)14-28-15-29(18-5-3-2-4-6-18)24(23(28)31)7-9-27(10-8-24)13-17-11-20-21(12-19(17)25)33-16-32-20/h2-6,11-12H,7-10,13-16H2,1H3,(H,26,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu opiate receptor |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50105091

(2-[8-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-4-oxo-...)Show SMILES CNC(=O)CN1CN(c2ccccc2)C2(CCN(Cc3cc4OCOc4cc3Cl)CC2)C1=O Show InChI InChI=1S/C24H27ClN4O4/c1-26-22(30)14-28-15-29(18-5-3-2-4-6-18)24(23(28)31)7-9-27(10-8-24)13-17-11-20-21(12-19(17)25)33-16-32-20/h2-6,11-12H,7-10,13-16H2,1H3,(H,26,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu-opiate receptor (human) using [3H]DAMGO radioligand |
J Med Chem 44: 3378-90 (2001)
BindingDB Entry DOI: 10.7270/Q23X87B8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50105085

(17-cyclobutylmethyl-4,5alpha-epoxymorphinan-3,6alp...)Show SMILES O[C@H]1CC[C@@]2(O)[C@H]3Cc4ccc(O)c5O[C@@H]1[C@]2(CCN3CC1CCC1)c45 |r| Show InChI InChI=1S/C21H27NO4/c23-14-5-4-13-10-16-21(25)7-6-15(24)19-20(21,17(13)18(14)26-19)8-9-22(16)11-12-2-1-3-12/h4-5,12,15-16,19,23-25H,1-3,6-11H2/t15-,16+,19-,20-,21+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu-opiate receptor (human) using [3H]DAMGO radioligand |
J Med Chem 44: 3378-90 (2001)
BindingDB Entry DOI: 10.7270/Q23X87B8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50000092

((-)-(etorphine) | (-)-morphine | (1S,5R,13R,14S)-1...)Show SMILES CN1CC[C@@]23[C@H]4Oc5c2c(C[C@@H]1[C@@H]3C=C[C@@H]4O)ccc5O |r,c:16,TLB:13:12:8.9.10:3.2.1| Show InChI InChI=1S/C17H19NO3/c1-18-7-6-17-10-3-5-13(20)16(17)21-15-12(19)4-2-9(14(15)17)8-11(10)18/h2-5,10-11,13,16,19-20H,6-8H2,1H3/t10-,11+,13-,16-,17-/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu-opiate receptor (human) using [3H]DAMGO radioligand |
J Med Chem 44: 3378-90 (2001)
BindingDB Entry DOI: 10.7270/Q23X87B8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50105094
(1-[1-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-piperi...)Show SMILES Clc1cc2OCOc2cc1CN1CCC(CC1)n1c2ccccc2[nH]c1=O Show InChI InChI=1S/C20H20ClN3O3/c21-15-10-19-18(26-12-27-19)9-13(15)11-23-7-5-14(6-8-23)24-17-4-2-1-3-16(17)22-20(24)25/h1-4,9-10,14H,5-8,11-12H2,(H,22,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu opiate receptor |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50026603

(Buprenorphine | CHEBI:3216)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@]([H])(C1)[C@](C)(O)C(C)(C)C)ccc3O |r,TLB:25:17:4.5.6:9.15.14,18:17:4.5.6:9.15.14,THB:10:9:17:4.5.6,3:4:17:9.15.14,26:23:16.1:18.19| Show InChI InChI=1S/C29H41NO4/c1-25(2,3)26(4,32)20-15-27-10-11-29(20,33-5)24-28(27)12-13-30(16-17-6-7-17)21(27)14-18-8-9-19(31)23(34-24)22(18)28/h8-9,17,20-21,24,31-32H,6-7,10-16H2,1-5H3/t20-,21-,24-,26+,27-,28+,29-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against opioid receptor kappa 1 using [3H]- U-69,593 radioligand |
J Med Chem 44: 3378-90 (2001)
BindingDB Entry DOI: 10.7270/Q23X87B8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50105094

(1-[1-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-piperi...)Show SMILES Clc1cc2OCOc2cc1CN1CCC(CC1)n1c2ccccc2[nH]c1=O Show InChI InChI=1S/C20H20ClN3O3/c21-15-10-19-18(26-12-27-19)9-13(15)11-23-7-5-14(6-8-23)24-17-4-2-1-3-16(17)22-20(24)25/h1-4,9-10,14H,5-8,11-12H2,(H,22,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu-opiate receptor (human) using [3H]DAMGO radioligand |
J Med Chem 44: 3378-90 (2001)
BindingDB Entry DOI: 10.7270/Q23X87B8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50008984

(4-(4-Chloro-benzyl)-2-(1-methyl-azepan-4-yl)-2H-ph...)Show InChI InChI=1S/C22H28N2O/c1-2-22(25)24(20-11-7-4-8-12-20)21-14-17-23(18-15-21)16-13-19-9-5-3-6-10-19/h3-12,21H,2,13-18H2,1H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu-opiate receptor (human) using [3H]DAMGO radioligand |
J Med Chem 44: 3378-90 (2001)
BindingDB Entry DOI: 10.7270/Q23X87B8 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50000788

((morphine)4-methyl-(1S,5R,13R,14S,17R)-12-oxa-4-az...)Show SMILES Oc1ccc2C[C@H]3N(CC=C)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O |r| Show InChI InChI=1S/C19H21NO4/c1-2-8-20-9-7-18-15-11-3-4-12(21)16(15)24-17(18)13(22)5-6-19(18,23)14(20)10-11/h2-4,14,17,21,23H,1,5-10H2/t14-,17+,18+,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against opioid receptor kappa 1 using [3H]- U-69,593 radioligand |
J Med Chem 44: 3378-90 (2001)
BindingDB Entry DOI: 10.7270/Q23X87B8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50105080
(1-[1-(6-Nitro-benzo[1,3]dioxol-5-ylmethyl)-piperid...)Show SMILES [O-][N+](=O)c1cc2OCOc2cc1CN1CCC(CC1)n1[c-]2ccccc2nc1=[OH+] Show InChI InChI=1S/C20H19N4O5/c25-20-21-15-3-1-2-4-16(15)23(20)14-5-7-22(8-6-14)11-13-9-18-19(29-12-28-18)10-17(13)24(26)27/h1-4,9-10,14H,5-8,11-12H2/q-1/p+1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu-opiate receptor (human) using [3H]DAMGO radioligand |
J Med Chem 44: 3378-90 (2001)
BindingDB Entry DOI: 10.7270/Q23X87B8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50017698
(4-(4-(4-chlorophenyl)-4-hydroxypiperidin-1-yl)-N,N...)Show SMILES CN(C)C(=O)C(CCN1CCC(O)(CC1)c1ccc(Cl)cc1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C29H33ClN2O2/c1-31(2)27(33)29(24-9-5-3-6-10-24,25-11-7-4-8-12-25)19-22-32-20-17-28(34,18-21-32)23-13-15-26(30)16-14-23/h3-16,34H,17-22H2,1-2H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu-opiate receptor (human) using [3H]DAMGO radioligand |
J Med Chem 44: 3378-90 (2001)
BindingDB Entry DOI: 10.7270/Q23X87B8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50105080

(1-[1-(6-Nitro-benzo[1,3]dioxol-5-ylmethyl)-piperid...)Show SMILES [O-][N+](=O)c1cc2OCOc2cc1CN1CCC(CC1)n1[c-]2ccccc2nc1=[OH+] Show InChI InChI=1S/C20H19N4O5/c25-20-21-15-3-1-2-4-16(15)23(20)14-5-7-22(8-6-14)11-13-9-18-19(29-12-28-18)10-17(13)24(26)27/h1-4,9-10,14H,5-8,11-12H2/q-1/p+1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu opiate receptor |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50400147
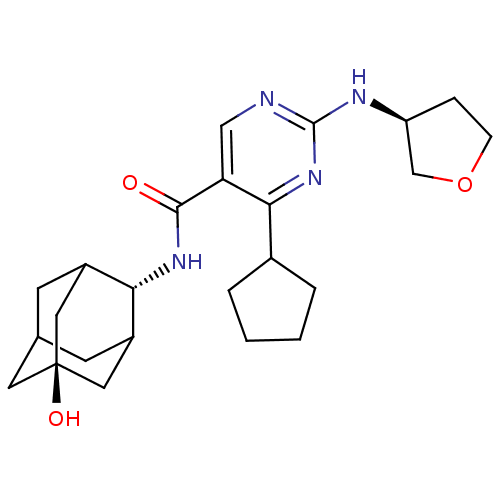
(CHEMBL2179014)Show SMILES O[C@@]12CC3CC(C1)[C@H](NC(=O)c1cnc(N[C@H]4CCOC4)nc1C1CCCC1)C(C3)C2 |r,wU:7.8,16.16,wD:1.0,TLB:0:1:7:29.3.4,8:7:6.30.1:29.3.4,8:7:4:6.1.2,THB:0:1:7.28.29:4,2:1:7:29.3.4,2:3:7:6.30.1,30:28:4:6.1.2,30:1:7.28.29:4,(33.95,-38.8,;32.42,-38.79,;31.38,-37.67,;29.96,-38.21,;29.31,-39.49,;30.34,-40.56,;31.64,-40.01,;30.35,-42.31,;29.02,-43.08,;27.68,-42.32,;27.68,-40.78,;26.35,-43.09,;25.02,-42.33,;23.69,-43.1,;23.69,-44.64,;22.35,-45.41,;21.02,-44.64,;19.61,-45.27,;18.58,-44.13,;19.35,-42.79,;20.86,-43.11,;25.02,-45.41,;26.36,-44.64,;27.69,-45.41,;27.86,-46.94,;29.37,-47.26,;30.13,-45.92,;29.1,-44.78,;31.15,-40.98,;29.98,-39.66,;32.56,-40.42,)| Show InChI InChI=1S/C24H34N4O3/c29-22(27-20-16-7-14-8-17(20)11-24(30,9-14)10-16)19-12-25-23(26-18-5-6-31-13-18)28-21(19)15-3-1-2-4-15/h12,14-18,20,30H,1-11,13H2,(H,27,29)(H,25,26,28)/t14?,16?,17?,18-,20-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 by HPLC assay |
J Med Chem 55: 10652-61 (2012)
Article DOI: 10.1021/jm3013163
BindingDB Entry DOI: 10.7270/Q2BG2Q4S |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50026603

(Buprenorphine | CHEBI:3216)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@]([H])(C1)[C@](C)(O)C(C)(C)C)ccc3O |r,TLB:25:17:4.5.6:9.15.14,18:17:4.5.6:9.15.14,THB:10:9:17:4.5.6,3:4:17:9.15.14,26:23:16.1:18.19| Show InChI InChI=1S/C29H41NO4/c1-25(2,3)26(4,32)20-15-27-10-11-29(20,33-5)24-28(27)12-13-30(16-17-6-7-17)21(27)14-18-8-9-19(31)23(34-24)22(18)28/h8-9,17,20-21,24,31-32H,6-7,10-16H2,1-5H3/t20-,21-,24-,26+,27-,28+,29-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu-opiate receptor (human) using [3H]DAMGO radioligand |
J Med Chem 44: 3378-90 (2001)
BindingDB Entry DOI: 10.7270/Q23X87B8 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50105094

(1-[1-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-piperi...)Show SMILES Clc1cc2OCOc2cc1CN1CCC(CC1)n1c2ccccc2[nH]c1=O Show InChI InChI=1S/C20H20ClN3O3/c21-15-10-19-18(26-12-27-19)9-13(15)11-23-7-5-14(6-8-23)24-17-4-2-1-3-16(17)22-20(24)25/h1-4,9-10,14H,5-8,11-12H2,(H,22,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against opioid receptor kappa 1 using [3H]- U-69,593 radioligand |
J Med Chem 44: 3378-90 (2001)
BindingDB Entry DOI: 10.7270/Q23X87B8 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50105094

(1-[1-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-piperi...)Show SMILES Clc1cc2OCOc2cc1CN1CCC(CC1)n1c2ccccc2[nH]c1=O Show InChI InChI=1S/C20H20ClN3O3/c21-15-10-19-18(26-12-27-19)9-13(15)11-23-7-5-14(6-8-23)24-17-4-2-1-3-16(17)22-20(24)25/h1-4,9-10,14H,5-8,11-12H2,(H,22,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against opioid receptor kappa 1 by using [3H]U-69593 as radioligand |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50000788

((morphine)4-methyl-(1S,5R,13R,14S,17R)-12-oxa-4-az...)Show SMILES Oc1ccc2C[C@H]3N(CC=C)CC[C@@]45[C@@H](Oc1c24)C(=O)CC[C@@]35O |r| Show InChI InChI=1S/C19H21NO4/c1-2-8-20-9-7-18-15-11-3-4-12(21)16(15)24-17(18)13(22)5-6-19(18,23)14(20)10-11/h2-4,14,17,21,23H,1,5-10H2/t14-,17+,18+,19-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu-opiate receptor (human) using [3H]DAMGO radioligand |
J Med Chem 44: 3378-90 (2001)
BindingDB Entry DOI: 10.7270/Q23X87B8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50105072
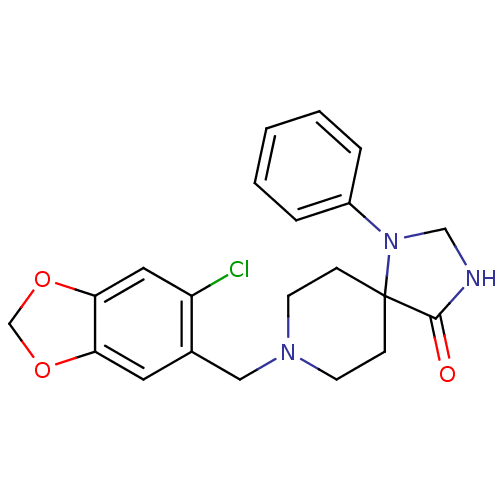
(8-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-1-phenyl-...)Show SMILES Clc1cc2OCOc2cc1CN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C21H22ClN3O3/c22-17-11-19-18(27-14-28-19)10-15(17)12-24-8-6-21(7-9-24)20(26)23-13-25(21)16-4-2-1-3-5-16/h1-5,10-11H,6-9,12-14H2,(H,23,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu opiate receptor |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50105072

(8-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-1-phenyl-...)Show SMILES Clc1cc2OCOc2cc1CN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C21H22ClN3O3/c22-17-11-19-18(27-14-28-19)10-15(17)12-24-8-6-21(7-9-24)20(26)23-13-25(21)16-4-2-1-3-5-16/h1-5,10-11H,6-9,12-14H2,(H,23,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu-opiate receptor (human) using [3H]DAMGO radioligand |
J Med Chem 44: 3378-90 (2001)
BindingDB Entry DOI: 10.7270/Q23X87B8 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50105091

(2-[8-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-4-oxo-...)Show SMILES CNC(=O)CN1CN(c2ccccc2)C2(CCN(Cc3cc4OCOc4cc3Cl)CC2)C1=O Show InChI InChI=1S/C24H27ClN4O4/c1-26-22(30)14-28-15-29(18-5-3-2-4-6-18)24(23(28)31)7-9-27(10-8-24)13-17-11-20-21(12-19(17)25)33-16-32-20/h2-6,11-12H,7-10,13-16H2,1H3,(H,26,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against opioid receptor kappa 1 by using [3H]U-69593 as radioligand |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50105091

(2-[8-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-4-oxo-...)Show SMILES CNC(=O)CN1CN(c2ccccc2)C2(CCN(Cc3cc4OCOc4cc3Cl)CC2)C1=O Show InChI InChI=1S/C24H27ClN4O4/c1-26-22(30)14-28-15-29(18-5-3-2-4-6-18)24(23(28)31)7-9-27(10-8-24)13-17-11-20-21(12-19(17)25)33-16-32-20/h2-6,11-12H,7-10,13-16H2,1H3,(H,26,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against opioid receptor kappa 1 using [3H]- U-69,593 radioligand |
J Med Chem 44: 3378-90 (2001)
BindingDB Entry DOI: 10.7270/Q23X87B8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50105066

(1-[1-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-piperi...)Show SMILES Cc1ccc2[nH]c(=O)n(C3CCN(Cc4cc5OCOc5cc4Cl)CC3)c2c1 Show InChI InChI=1S/C21H22ClN3O3/c1-13-2-3-17-18(8-13)25(21(26)23-17)15-4-6-24(7-5-15)11-14-9-19-20(10-16(14)22)28-12-27-19/h2-3,8-10,15H,4-7,11-12H2,1H3,(H,23,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu-opiate receptor (human) using [3H]DAMGO radioligand |
J Med Chem 44: 3378-90 (2001)
BindingDB Entry DOI: 10.7270/Q23X87B8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50105066

(1-[1-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-piperi...)Show SMILES Cc1ccc2[nH]c(=O)n(C3CCN(Cc4cc5OCOc5cc4Cl)CC3)c2c1 Show InChI InChI=1S/C21H22ClN3O3/c1-13-2-3-17-18(8-13)25(21(26)23-17)15-4-6-24(7-5-15)11-14-9-19-20(10-16(14)22)28-12-27-19/h2-3,8-10,15H,4-7,11-12H2,1H3,(H,23,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu opiate receptor |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50105066

(1-[1-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-piperi...)Show SMILES Cc1ccc2[nH]c(=O)n(C3CCN(Cc4cc5OCOc5cc4Cl)CC3)c2c1 Show InChI InChI=1S/C21H22ClN3O3/c1-13-2-3-17-18(8-13)25(21(26)23-17)15-4-6-24(7-5-15)11-14-9-19-20(10-16(14)22)28-12-27-19/h2-3,8-10,15H,4-7,11-12H2,1H3,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against opioid receptor kappa 1 by using [3H]U-69593 as radioligand |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50105066

(1-[1-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-piperi...)Show SMILES Cc1ccc2[nH]c(=O)n(C3CCN(Cc4cc5OCOc5cc4Cl)CC3)c2c1 Show InChI InChI=1S/C21H22ClN3O3/c1-13-2-3-17-18(8-13)25(21(26)23-17)15-4-6-24(7-5-15)11-14-9-19-20(10-16(14)22)28-12-27-19/h2-3,8-10,15H,4-7,11-12H2,1H3,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against opioid receptor kappa 1 using [3H]- U-69,593 radioligand |
J Med Chem 44: 3378-90 (2001)
BindingDB Entry DOI: 10.7270/Q23X87B8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM82507
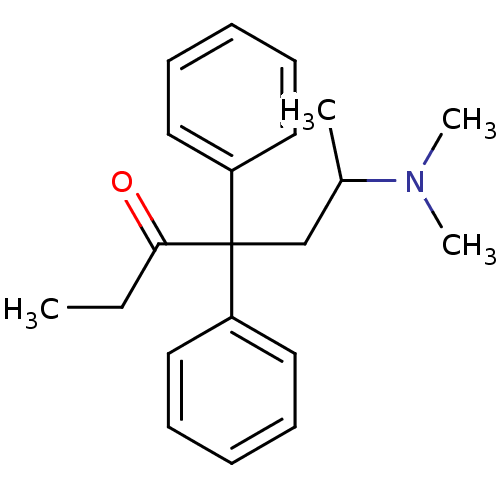
((+/-)-Methadone | CAS_5967-73-7 | METHADONE | Meth...)Show InChI InChI=1S/C21H27NO/c1-5-20(23)21(16-17(2)22(3)4,18-12-8-6-9-13-18)19-14-10-7-11-15-19/h6-15,17H,5,16H2,1-4H3 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
UniChem
Similars
| DrugBank
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu-opiate receptor (human) using [3H]DAMGO radioligand |
J Med Chem 44: 3378-90 (2001)
BindingDB Entry DOI: 10.7270/Q23X87B8 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50105072

(8-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-1-phenyl-...)Show SMILES Clc1cc2OCOc2cc1CN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C21H22ClN3O3/c22-17-11-19-18(27-14-28-19)10-15(17)12-24-8-6-21(7-9-24)20(26)23-13-25(21)16-4-2-1-3-5-16/h1-5,10-11H,6-9,12-14H2,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against opioid receptor kappa 1 by using [3H]U-69593 as radioligand |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50105072

(8-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-1-phenyl-...)Show SMILES Clc1cc2OCOc2cc1CN1CCC2(CC1)N(CNC2=O)c1ccccc1 Show InChI InChI=1S/C21H22ClN3O3/c22-17-11-19-18(27-14-28-19)10-15(17)12-24-8-6-21(7-9-24)20(26)23-13-25(21)16-4-2-1-3-5-16/h1-5,10-11H,6-9,12-14H2,(H,23,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against opioid receptor kappa 1 using [3H]- U-69,593 radioligand |
J Med Chem 44: 3378-90 (2001)
BindingDB Entry DOI: 10.7270/Q23X87B8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50105111
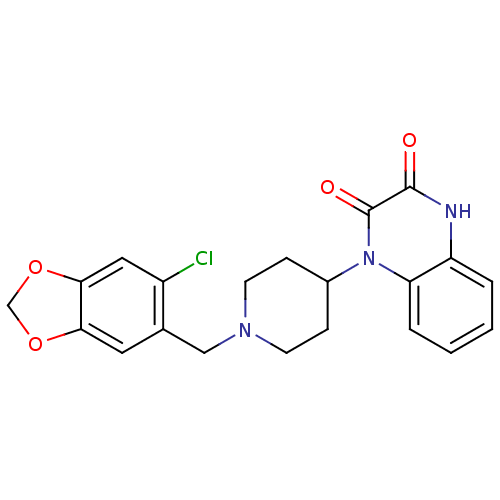
(1-[1-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-piperi...)Show SMILES Clc1cc2OCOc2cc1CN1CCC(CC1)n1c2ccccc2[nH]c(=O)c1=O Show InChI InChI=1S/C21H20ClN3O4/c22-15-10-19-18(28-12-29-19)9-13(15)11-24-7-5-14(6-8-24)25-17-4-2-1-3-16(17)23-20(26)21(25)27/h1-4,9-10,14H,5-8,11-12H2,(H,23,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu opiate receptor |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50105060
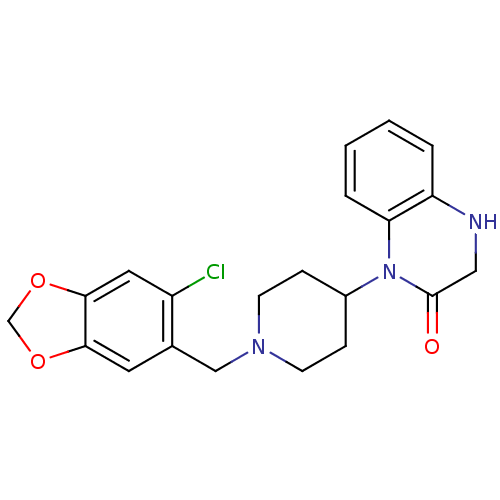
(1-[1-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-piperi...)Show SMILES Clc1cc2OCOc2cc1CN1CCC(CC1)N1C(=O)CNc2ccccc12 Show InChI InChI=1S/C21H22ClN3O3/c22-16-10-20-19(27-13-28-20)9-14(16)12-24-7-5-15(6-8-24)25-18-4-2-1-3-17(18)23-11-21(25)26/h1-4,9-10,15,23H,5-8,11-13H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu-opiate receptor (human) using [3H]DAMGO radioligand |
J Med Chem 44: 3378-90 (2001)
BindingDB Entry DOI: 10.7270/Q23X87B8 |
More data for this
Ligand-Target Pair | |
Group 10 secretory phospholipase A2
(Homo sapiens (Human)) | BDBM50262998
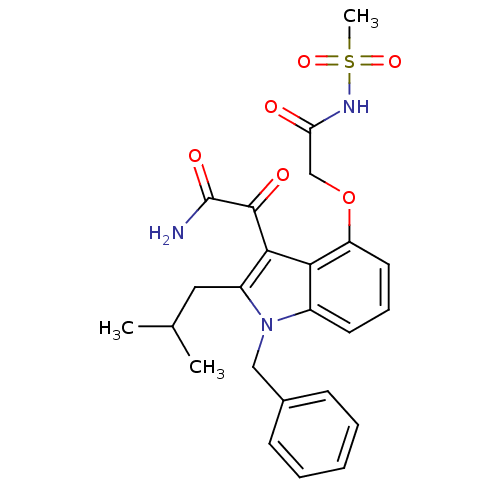
(CHEMBL477548 | mesyl-2-(3-(2-amino-2-oxoacetyl)-1-...)Show SMILES CC(C)Cc1c(C(=O)C(N)=O)c2c(OCC(=O)NS(C)(=O)=O)cccc2n1Cc1ccccc1 Show InChI InChI=1S/C24H27N3O6S/c1-15(2)12-18-22(23(29)24(25)30)21-17(27(18)13-16-8-5-4-6-9-16)10-7-11-19(21)33-14-20(28)26-34(3,31)32/h4-11,15H,12-14H2,1-3H3,(H2,25,30)(H,26,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Binding affinity to sPLA2X (unknown origin) |
Bioorg Med Chem Lett 24: 5251-5 (2014)
Article DOI: 10.1016/j.bmcl.2014.09.058
BindingDB Entry DOI: 10.7270/Q2668FS8 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50400149
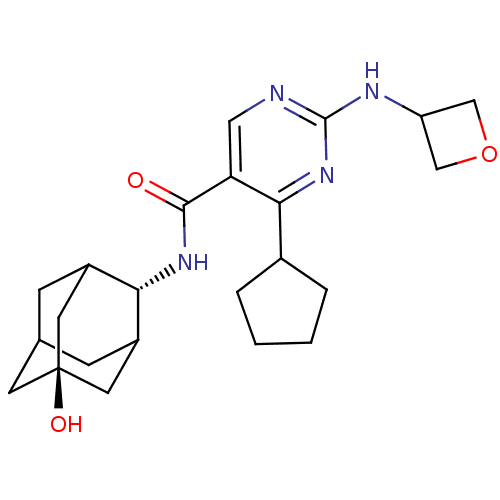
(CHEMBL2179456)Show SMILES O[C@@]12CC3CC(C1)[C@H](NC(=O)c1cnc(NC4COC4)nc1C1CCCC1)C(C3)C2 |r,wU:7.8,wD:1.0,TLB:0:1:7:28.3.4,8:7:6.29.1:28.3.4,8:7:4:6.1.2,THB:0:1:7.27.28:4,2:1:7:28.3.4,2:3:7:6.29.1,29:27:4:6.1.2,29:1:7.27.28:4,(75.52,-27,;73.99,-26.98,;72.94,-25.86,;71.53,-26.41,;70.88,-27.68,;71.91,-28.76,;73.21,-28.2,;71.92,-30.5,;70.59,-31.27,;69.25,-30.51,;69.24,-28.97,;67.92,-31.28,;66.58,-30.52,;65.26,-31.29,;65.25,-32.84,;63.92,-33.6,;62.59,-32.83,;62.19,-31.35,;60.71,-31.74,;61.1,-33.23,;66.59,-33.61,;67.93,-32.83,;69.26,-33.6,;69.42,-35.13,;70.93,-35.45,;71.7,-34.11,;70.67,-32.97,;72.72,-29.17,;71.55,-27.85,;74.13,-28.61,)| Show InChI InChI=1S/C23H32N4O3/c28-21(26-19-15-5-13-6-16(19)9-23(29,7-13)8-15)18-10-24-22(25-17-11-30-12-17)27-20(18)14-3-1-2-4-14/h10,13-17,19,29H,1-9,11-12H2,(H,26,28)(H,24,25,27)/t13?,15?,16?,19-,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 by HTRF assay |
J Med Chem 55: 10652-61 (2012)
Article DOI: 10.1021/jm3013163
BindingDB Entry DOI: 10.7270/Q2BG2Q4S |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50105091

(2-[8-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-4-oxo-...)Show SMILES CNC(=O)CN1CN(c2ccccc2)C2(CCN(Cc3cc4OCOc4cc3Cl)CC2)C1=O Show InChI InChI=1S/C24H27ClN4O4/c1-26-22(30)14-28-15-29(18-5-3-2-4-6-18)24(23(28)31)7-9-27(10-8-24)13-17-11-20-21(12-19(17)25)33-16-32-20/h2-6,11-12H,7-10,13-16H2,1H3,(H,26,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Inhibition of ligand binding to human delta opioid receptor. |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50400160
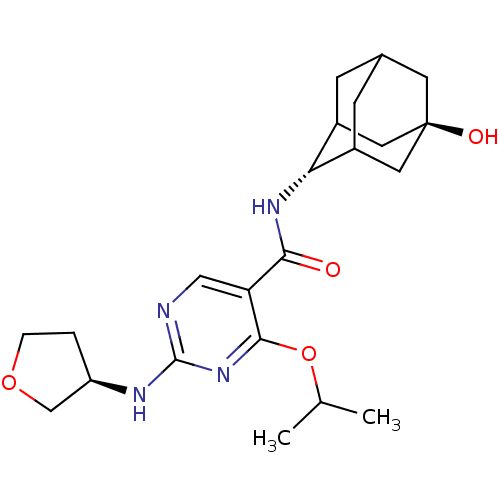
(CHEMBL2179442)Show SMILES CC(C)Oc1nc(N[C@@H]2CCOC2)ncc1C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2 |r,wU:19.20,wD:26.29,8.7,TLB:27:26:19:23.22.21,18:19:29.25.26:23.22.21,18:19:21:29.26.28,THB:27:26:19.24.23:21,28:26:19:23.22.21,28:22:19:29.25.26,25:24:21:29.26.28,25:26:19.24.23:21,(11.53,-55.4,;10.19,-54.63,;8.86,-55.4,;10.19,-53.09,;8.86,-52.32,;7.52,-53.09,;6.19,-52.32,;4.85,-53.09,;3.52,-52.32,;2.11,-52.94,;1.08,-51.8,;1.85,-50.46,;3.36,-50.79,;6.19,-50.78,;7.52,-50.01,;8.85,-50.77,;10.18,-50,;10.18,-48.46,;11.52,-50.76,;12.85,-49.99,;12.84,-48.24,;11.81,-47.17,;12.46,-45.9,;12.48,-47.34,;13.65,-48.66,;15.06,-48.1,;14.92,-46.47,;16.45,-46.48,;13.88,-45.35,;14.14,-47.69,)| Show InChI InChI=1S/C22H32N4O4/c1-12(2)30-20-17(10-23-21(26-20)24-16-3-4-29-11-16)19(27)25-18-14-5-13-6-15(18)9-22(28,7-13)8-14/h10,12-16,18,28H,3-9,11H2,1-2H3,(H,25,27)(H,23,24,26)/t13?,14?,15?,16-,18-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 by HTRF assay |
J Med Chem 55: 10652-61 (2012)
Article DOI: 10.1021/jm3013163
BindingDB Entry DOI: 10.7270/Q2BG2Q4S |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50105091

(2-[8-(6-Chloro-benzo[1,3]dioxol-5-ylmethyl)-4-oxo-...)Show SMILES CNC(=O)CN1CN(c2ccccc2)C2(CCN(Cc3cc4OCOc4cc3Cl)CC2)C1=O Show InChI InChI=1S/C24H27ClN4O4/c1-26-22(30)14-28-15-29(18-5-3-2-4-6-18)24(23(28)31)7-9-27(10-8-24)13-17-11-20-21(12-19(17)25)33-16-32-20/h2-6,11-12H,7-10,13-16H2,1H3,(H,26,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against delta-opiate receptor (human) using [3H]-DPDPE radioligand |
J Med Chem 44: 3378-90 (2001)
BindingDB Entry DOI: 10.7270/Q23X87B8 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50400158
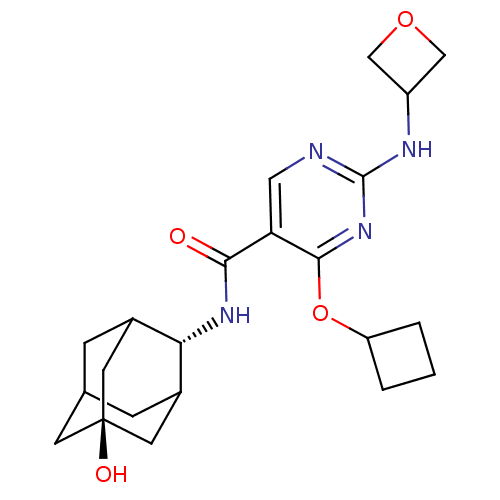
(CHEMBL2179007)Show SMILES O[C@@]12CC3CC(C1)[C@H](NC(=O)c1cnc(NC4COC4)nc1OC1CCC1)C(C3)C2 |r,wU:7.8,wD:1.0,TLB:0:1:7:28.3.4,8:7:6.29.1:28.3.4,8:7:4:6.1.2,THB:0:1:7.27.28:4,2:1:7:28.3.4,2:3:7:6.29.1,29:27:4:6.1.2,29:1:7.27.28:4,(48.03,-46.21,;46.5,-46.19,;45.46,-45.07,;44.04,-45.62,;43.39,-46.89,;44.42,-47.97,;45.72,-47.41,;44.43,-49.71,;43.1,-50.49,;41.76,-49.73,;41.75,-48.18,;40.43,-50.5,;39.09,-49.74,;37.76,-50.51,;37.76,-52.05,;36.42,-52.82,;35.09,-52.05,;34.69,-50.56,;33.2,-50.96,;33.6,-52.45,;39.09,-52.83,;40.43,-52.05,;41.77,-52.82,;41.77,-54.36,;40.68,-55.45,;41.77,-56.54,;42.86,-55.45,;45.23,-48.39,;44.06,-47.06,;46.64,-47.82,)| Show InChI InChI=1S/C22H30N4O4/c27-19(25-18-13-4-12-5-14(18)8-22(28,6-12)7-13)17-9-23-21(24-15-10-29-11-15)26-20(17)30-16-2-1-3-16/h9,12-16,18,28H,1-8,10-11H2,(H,25,27)(H,23,24,26)/t12?,13?,14?,18-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 by HTRF assay |
J Med Chem 55: 10652-61 (2012)
Article DOI: 10.1021/jm3013163
BindingDB Entry DOI: 10.7270/Q2BG2Q4S |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50400168
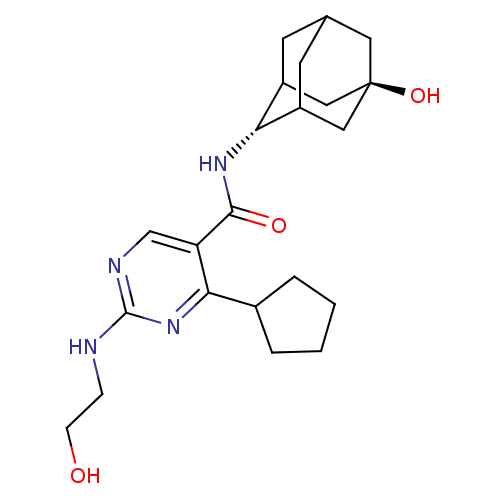
(CHEMBL2179025)Show SMILES OCCNc1ncc(C(=O)N[C@H]2C3CC4CC2C[C@](O)(C4)C3)c(n1)C1CCCC1 |r,wU:11.10,wD:18.19,TLB:19:18:11:15.14.13,10:11:21.17.18:15.14.13,10:11:13:21.18.20,THB:19:18:11.16.15:13,20:18:11:15.14.13,20:14:11:21.17.18,17:16:13:21.18.20,17:18:11.16.15:13,(2.28,-22.72,;3.61,-23.49,;3.61,-25.03,;4.95,-25.8,;6.28,-25.04,;6.28,-23.49,;7.61,-22.72,;8.95,-23.48,;10.28,-22.71,;10.27,-21.17,;11.61,-23.47,;12.95,-22.7,;12.94,-20.95,;11.9,-19.88,;12.56,-18.61,;12.58,-20.05,;13.75,-21.37,;15.16,-20.81,;15.02,-19.18,;16.55,-19.2,;13.97,-18.06,;14.24,-20.4,;8.95,-25.03,;7.61,-25.81,;10.29,-25.8,;10.45,-27.33,;11.96,-27.65,;12.73,-26.31,;11.69,-25.17,)| Show InChI InChI=1S/C22H32N4O3/c27-6-5-23-21-24-12-17(19(26-21)14-3-1-2-4-14)20(28)25-18-15-7-13-8-16(18)11-22(29,9-13)10-15/h12-16,18,27,29H,1-11H2,(H,25,28)(H,23,24,26)/t13?,15?,16?,18-,22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 by HTRF assay |
J Med Chem 55: 10652-61 (2012)
Article DOI: 10.1021/jm3013163
BindingDB Entry DOI: 10.7270/Q2BG2Q4S |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50400157
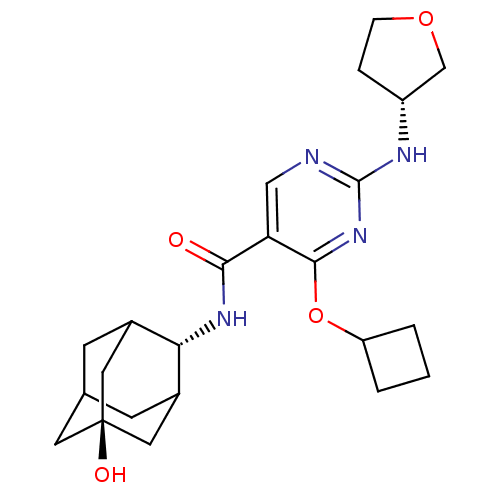
(CHEMBL2179008)Show SMILES O[C@@]12CC3CC(C1)[C@H](NC(=O)c1cnc(N[C@@H]4CCOC4)nc1OC1CCC1)C(C3)C2 |r,wU:7.8,wD:1.0,16.16,TLB:0:1:7:29.3.4,8:7:6.30.1:29.3.4,8:7:4:6.1.2,THB:0:1:7.28.29:4,2:1:7:29.3.4,2:3:7:6.30.1,30:28:4:6.1.2,30:1:7.28.29:4,(61.12,-46.21,;59.58,-46.19,;58.54,-45.07,;57.12,-45.62,;56.47,-46.89,;57.51,-47.97,;58.81,-47.41,;57.51,-49.71,;56.18,-50.49,;54.85,-49.72,;54.84,-48.18,;53.52,-50.5,;52.18,-49.73,;50.85,-50.5,;50.85,-52.05,;49.51,-52.82,;48.18,-52.05,;46.77,-52.67,;45.74,-51.52,;46.51,-50.19,;48.02,-50.51,;52.18,-52.82,;53.52,-52.05,;54.86,-52.82,;54.86,-54.36,;53.77,-55.44,;54.86,-56.53,;55.95,-55.44,;58.31,-48.38,;57.15,-47.06,;59.73,-47.82,)| Show InChI InChI=1S/C23H32N4O4/c28-20(26-19-14-6-13-7-15(19)10-23(29,8-13)9-14)18-11-24-22(25-16-4-5-30-12-16)27-21(18)31-17-2-1-3-17/h11,13-17,19,29H,1-10,12H2,(H,26,28)(H,24,25,27)/t13?,14?,15?,16-,19-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 by HTRF assay |
J Med Chem 55: 10652-61 (2012)
Article DOI: 10.1021/jm3013163
BindingDB Entry DOI: 10.7270/Q2BG2Q4S |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50400161
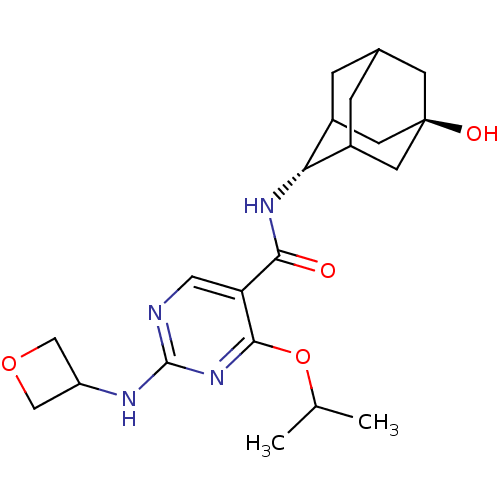
(CHEMBL2179441)Show SMILES CC(C)Oc1nc(NC2COC2)ncc1C(=O)N[C@H]1C2CC3CC1C[C@](O)(C3)C2 |r,wU:18.19,wD:25.28,TLB:26:25:18:22.21.20,17:18:28.24.25:22.21.20,17:18:20:28.25.27,THB:26:25:18.23.22:20,27:25:18:22.21.20,27:21:18:28.24.25,24:23:20:28.25.27,24:25:18.23.22:20,(68.49,-40.84,;67.16,-40.07,;65.82,-40.85,;67.16,-38.53,;65.82,-37.76,;64.48,-38.54,;63.15,-37.77,;61.81,-38.53,;60.47,-37.76,;60.08,-36.27,;58.59,-36.67,;58.99,-38.16,;63.15,-36.22,;64.48,-35.45,;65.81,-36.21,;67.15,-35.44,;67.14,-33.89,;68.48,-36.2,;69.82,-35.42,;69.81,-33.68,;68.77,-32.6,;69.43,-31.33,;69.45,-32.77,;70.62,-34.1,;72.03,-33.53,;71.89,-31.91,;73.42,-31.92,;70.84,-30.78,;71.11,-33.12,)| Show InChI InChI=1S/C21H30N4O4/c1-11(2)29-19-16(8-22-20(25-19)23-15-9-28-10-15)18(26)24-17-13-3-12-4-14(17)7-21(27,5-12)6-13/h8,11-15,17,27H,3-7,9-10H2,1-2H3,(H,24,26)(H,22,23,25)/t12?,13?,14?,17-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 by HTRF assay |
J Med Chem 55: 10652-61 (2012)
Article DOI: 10.1021/jm3013163
BindingDB Entry DOI: 10.7270/Q2BG2Q4S |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50400136
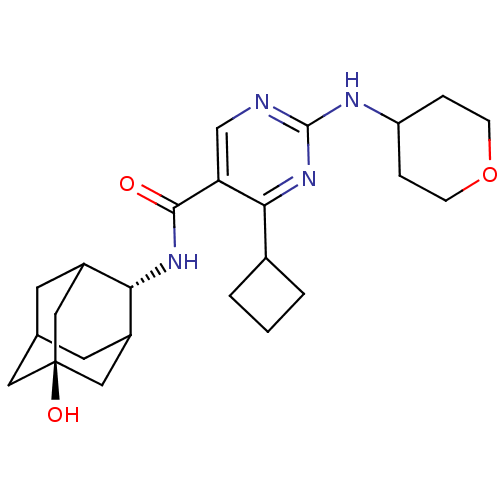
(CHEMBL2179452)Show SMILES O[C@@]12CC3CC(C1)[C@H](NC(=O)c1cnc(NC4CCOCC4)nc1C1CCC1)C(C3)C2 |r,wU:7.8,wD:1.0,TLB:0:1:7:29.3.4,8:7:6.30.1:29.3.4,8:7:4:6.1.2,THB:0:1:7.28.29:4,2:1:7:29.3.4,2:3:7:6.30.1,30:28:4:6.1.2,30:1:7.28.29:4,(73.77,-13.85,;72.24,-13.84,;71.19,-12.72,;69.78,-13.26,;69.13,-14.53,;70.16,-15.61,;71.46,-15.06,;70.17,-17.35,;68.84,-18.14,;67.5,-17.37,;67.5,-15.83,;66.17,-18.15,;64.83,-17.38,;63.5,-18.15,;63.5,-19.7,;62.16,-20.46,;60.83,-19.7,;60.84,-18.16,;59.52,-17.38,;58.17,-18.15,;58.17,-19.69,;59.51,-20.47,;64.83,-20.47,;66.17,-19.7,;67.51,-20.46,;67.9,-21.95,;69.39,-21.55,;68.99,-20.06,;70.97,-16.03,;69.8,-14.7,;72.38,-15.47,)| Show InChI InChI=1S/C24H34N4O3/c29-22(27-20-16-8-14-9-17(20)12-24(30,10-14)11-16)19-13-25-23(26-18-4-6-31-7-5-18)28-21(19)15-2-1-3-15/h13-18,20,30H,1-12H2,(H,27,29)(H,25,26,28)/t14?,16?,17?,20-,24- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 by HPLC assay |
J Med Chem 55: 10652-61 (2012)
Article DOI: 10.1021/jm3013163
BindingDB Entry DOI: 10.7270/Q2BG2Q4S |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50400153
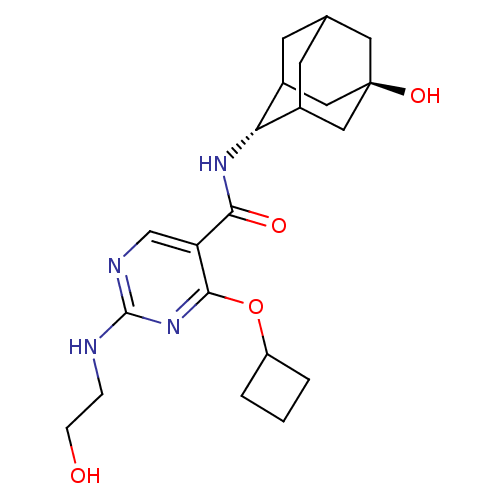
(CHEMBL2179012)Show SMILES OCCNc1ncc(C(=O)N[C@H]2C3CC4CC2C[C@](O)(C4)C3)c(OC2CCC2)n1 |r,wU:11.10,wD:18.19,TLB:19:18:11:15.14.13,10:11:21.17.18:15.14.13,10:11:13:21.18.20,THB:19:18:11.16.15:13,20:18:11:15.14.13,20:14:11:21.17.18,17:16:13:21.18.20,17:18:11.16.15:13,(38.1,-6.82,;39.43,-7.59,;39.43,-9.13,;40.77,-9.9,;42.1,-9.13,;42.1,-7.58,;43.43,-6.81,;44.77,-7.58,;46.1,-6.8,;46.09,-5.26,;47.44,-7.57,;48.77,-6.79,;48.76,-5.05,;47.73,-3.97,;48.38,-2.7,;48.4,-4.14,;49.57,-5.46,;50.98,-4.9,;50.84,-3.27,;52.37,-3.29,;49.79,-2.15,;50.06,-4.49,;44.77,-9.13,;46.11,-9.9,;46.11,-11.44,;45.02,-12.52,;46.11,-13.61,;47.2,-12.52,;43.44,-9.9,)| Show InChI InChI=1S/C21H30N4O4/c26-5-4-22-20-23-11-16(19(25-20)29-15-2-1-3-15)18(27)24-17-13-6-12-7-14(17)10-21(28,8-12)9-13/h11-15,17,26,28H,1-10H2,(H,24,27)(H,22,23,25)/t12?,13?,14?,17-,21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 by HTRF assay |
J Med Chem 55: 10652-61 (2012)
Article DOI: 10.1021/jm3013163
BindingDB Entry DOI: 10.7270/Q2BG2Q4S |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50400167
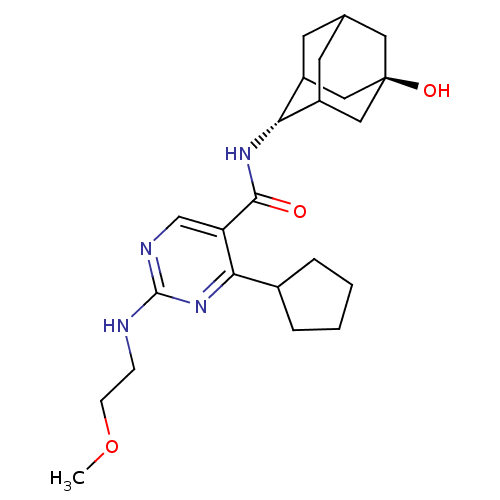
(CHEMBL2179026)Show SMILES COCCNc1ncc(C(=O)N[C@H]2C3CC4CC2C[C@](O)(C4)C3)c(n1)C1CCCC1 |r,wU:12.11,wD:19.20,TLB:20:19:12:16.15.14,11:12:22.18.19:16.15.14,11:12:14:22.19.21,THB:20:19:12.17.16:14,21:19:12:16.15.14,21:15:12:22.18.19,18:17:14:22.19.21,18:19:12.17.16:14,(21.41,-20.78,;21.41,-22.32,;22.74,-23.09,;22.74,-24.63,;24.07,-25.4,;25.41,-24.63,;25.41,-23.09,;26.74,-22.31,;28.07,-23.08,;29.4,-22.3,;29.4,-20.76,;30.74,-23.07,;32.07,-22.29,;32.06,-20.55,;31.03,-19.47,;31.68,-18.2,;31.7,-19.64,;32.87,-20.97,;34.28,-20.4,;34.14,-18.78,;35.67,-18.79,;33.1,-17.66,;33.36,-19.99,;28.08,-24.63,;26.74,-25.4,;29.41,-25.4,;29.58,-26.92,;31.08,-27.24,;31.85,-25.91,;30.82,-24.76,)| Show InChI InChI=1S/C23H34N4O3/c1-30-7-6-24-22-25-13-18(20(27-22)15-4-2-3-5-15)21(28)26-19-16-8-14-9-17(19)12-23(29,10-14)11-16/h13-17,19,29H,2-12H2,1H3,(H,26,28)(H,24,25,27)/t14?,16?,17?,19-,23- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 by HTRF assay |
J Med Chem 55: 10652-61 (2012)
Article DOI: 10.1021/jm3013163
BindingDB Entry DOI: 10.7270/Q2BG2Q4S |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50105069
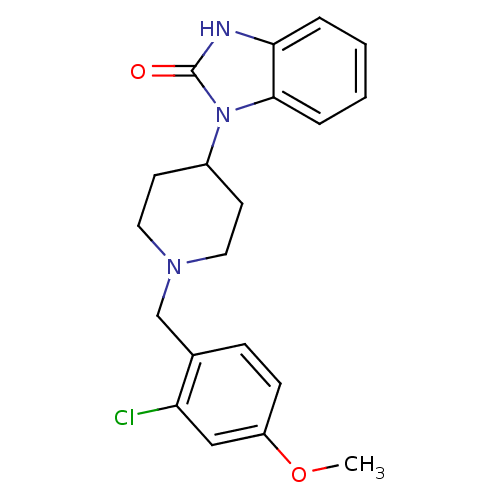
(1-[1-(2-Chloro-4-methoxy-benzyl)-piperidin-4-yl]-1...)Show SMILES COc1ccc(CN2CCC(CC2)n2c3ccccc3[nH]c2=O)c(Cl)c1 Show InChI InChI=1S/C20H22ClN3O2/c1-26-16-7-6-14(17(21)12-16)13-23-10-8-15(9-11-23)24-19-5-3-2-4-18(19)22-20(24)25/h2-7,12,15H,8-11,13H2,1H3,(H,22,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu-opiate receptor (human) using [3H]DAMGO radioligand |
J Med Chem 44: 3378-90 (2001)
BindingDB Entry DOI: 10.7270/Q23X87B8 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50400157

(CHEMBL2179008)Show SMILES O[C@@]12CC3CC(C1)[C@H](NC(=O)c1cnc(N[C@@H]4CCOC4)nc1OC1CCC1)C(C3)C2 |r,wU:7.8,wD:1.0,16.16,TLB:0:1:7:29.3.4,8:7:6.30.1:29.3.4,8:7:4:6.1.2,THB:0:1:7.28.29:4,2:1:7:29.3.4,2:3:7:6.30.1,30:28:4:6.1.2,30:1:7.28.29:4,(61.12,-46.21,;59.58,-46.19,;58.54,-45.07,;57.12,-45.62,;56.47,-46.89,;57.51,-47.97,;58.81,-47.41,;57.51,-49.71,;56.18,-50.49,;54.85,-49.72,;54.84,-48.18,;53.52,-50.5,;52.18,-49.73,;50.85,-50.5,;50.85,-52.05,;49.51,-52.82,;48.18,-52.05,;46.77,-52.67,;45.74,-51.52,;46.51,-50.19,;48.02,-50.51,;52.18,-52.82,;53.52,-52.05,;54.86,-52.82,;54.86,-54.36,;53.77,-55.44,;54.86,-56.53,;55.95,-55.44,;58.31,-48.38,;57.15,-47.06,;59.73,-47.82,)| Show InChI InChI=1S/C23H32N4O4/c28-20(26-19-14-6-13-7-15(19)10-23(29,8-13)9-14)18-11-24-22(25-16-4-5-30-12-16)27-21(18)31-17-2-1-3-17/h11,13-17,19,29H,1-10,12H2,(H,26,28)(H,24,25,27)/t13?,14?,15?,16-,19-,23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 by HTRF assay |
J Med Chem 55: 10652-61 (2012)
Article DOI: 10.1021/jm3013163
BindingDB Entry DOI: 10.7270/Q2BG2Q4S |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50105106
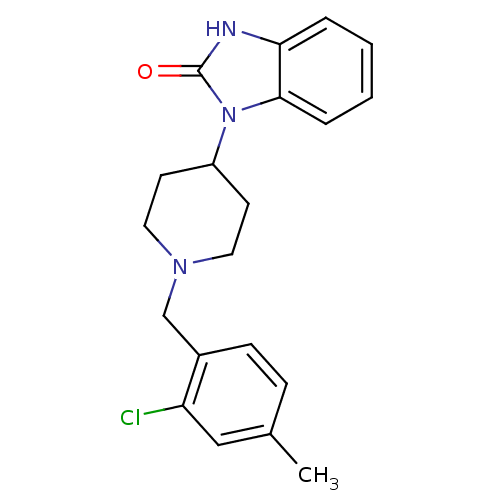
(1-[1-(2-Chloro-4-methyl-benzyl)-piperidin-4-yl]-1,...)Show SMILES Cc1ccc(CN2CCC(CC2)n2c3ccccc3[nH]c2=O)c(Cl)c1 Show InChI InChI=1S/C20H22ClN3O/c1-14-6-7-15(17(21)12-14)13-23-10-8-16(9-11-23)24-19-5-3-2-4-18(19)22-20(24)25/h2-7,12,16H,8-11,13H2,1H3,(H,22,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu opiate receptor |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Homo sapiens (Human)) | BDBM50400173
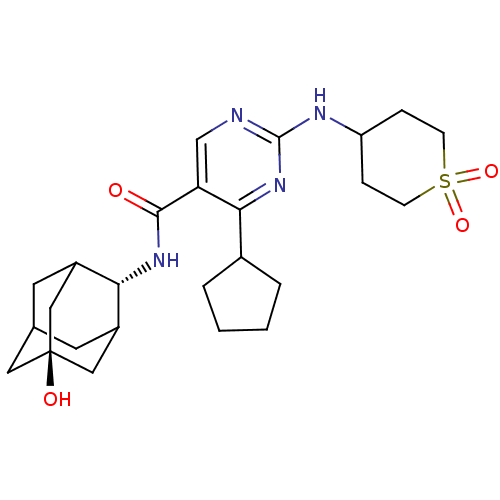
(CHEMBL2179020)Show SMILES O[C@@]12CC3CC(C1)[C@H](NC(=O)c1cnc(NC4CCS(=O)(=O)CC4)nc1C1CCCC1)C(C3)C2 |r,wU:7.8,wD:1.0,TLB:0:1:7:32.3.4,8:7:6.33.1:32.3.4,8:7:4:6.1.2,THB:0:1:7.31.32:4,2:1:7:32.3.4,2:3:7:6.33.1,33:31:4:6.1.2,33:1:7.31.32:4,(74.15,-51.19,;72.62,-51.17,;71.58,-50.05,;70.16,-50.6,;69.51,-51.87,;70.54,-52.95,;71.84,-52.39,;70.55,-54.69,;69.22,-55.47,;67.88,-54.7,;67.88,-53.16,;66.55,-55.48,;65.22,-54.71,;63.89,-55.48,;63.89,-57.03,;62.55,-57.8,;61.22,-57.03,;61.23,-55.49,;59.9,-54.72,;58.56,-55.48,;57.06,-55.87,;57.46,-54.38,;58.56,-57.02,;59.89,-57.8,;65.22,-57.8,;66.56,-57.03,;67.89,-57.8,;68.06,-59.32,;69.57,-59.64,;70.33,-58.3,;69.3,-57.16,;71.35,-53.36,;70.18,-52.04,;72.77,-52.8,)| Show InChI InChI=1S/C25H36N4O4S/c30-23(28-21-17-9-15-10-18(21)13-25(31,11-15)12-17)20-14-26-24(29-22(20)16-3-1-2-4-16)27-19-5-7-34(32,33)8-6-19/h14-19,21,31H,1-13H2,(H,28,30)(H,26,27,29)/t15?,17?,18?,21-,25- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human 11beta-HSD1 by HTRF assay |
J Med Chem 55: 10652-61 (2012)
Article DOI: 10.1021/jm3013163
BindingDB Entry DOI: 10.7270/Q2BG2Q4S |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50105089
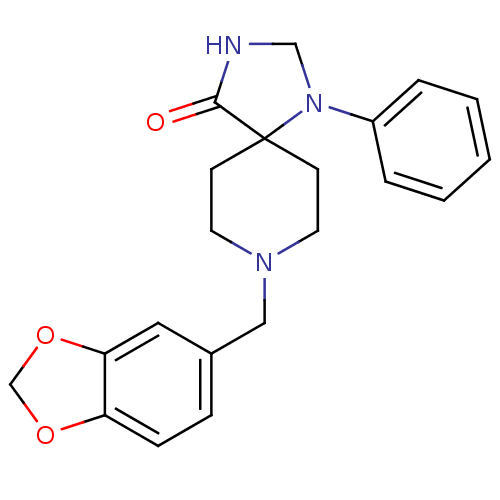
(8-Benzo[1,3]dioxol-5-ylmethyl-1-phenyl-1,3,8-triaz...)Show InChI InChI=1S/C21H23N3O3/c25-20-21(24(14-22-20)17-4-2-1-3-5-17)8-10-23(11-9-21)13-16-6-7-18-19(12-16)27-15-26-18/h1-7,12H,8-11,13-15H2,(H,22,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu-opiate receptor (human) using [3H]DAMGO radioligand |
J Med Chem 44: 3378-90 (2001)
BindingDB Entry DOI: 10.7270/Q23X87B8 |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50105089

(8-Benzo[1,3]dioxol-5-ylmethyl-1-phenyl-1,3,8-triaz...)Show InChI InChI=1S/C21H23N3O3/c25-20-21(24(14-22-20)17-4-2-1-3-5-17)8-10-23(11-9-21)13-16-6-7-18-19(12-16)27-15-26-18/h1-7,12H,8-11,13-15H2,(H,22,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
F-59000 Lille
Curated by ChEMBL
| Assay Description
Binding affinity against mu opiate receptor |
J Med Chem 44: 3391-401 (2001)
BindingDB Entry DOI: 10.7270/Q2057GN9 |
More data for this
Ligand-Target Pair | |
11-beta-hydroxysteroid dehydrogenase 1
(Mus musculus (mouse)) | BDBM50400155
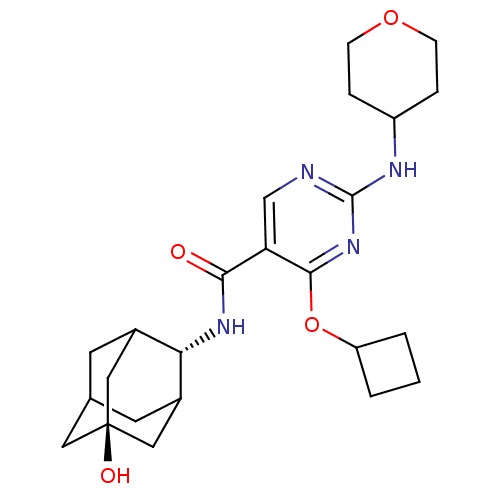
(CHEMBL2179010)Show SMILES O[C@@]12CC3CC(C1)[C@H](NC(=O)c1cnc(NC4CCOCC4)nc1OC1CCC1)C(C3)C2 |r,wU:7.8,wD:1.0,TLB:0:1:7:30.3.4,8:7:6.31.1:30.3.4,8:7:4:6.1.2,THB:0:1:7.29.30:4,2:1:7:30.3.4,2:3:7:6.31.1,31:29:4:6.1.2,31:1:7.29.30:4,(16.09,-4.04,;14.56,-4.02,;13.52,-2.9,;12.1,-3.45,;11.45,-4.72,;12.48,-5.8,;13.78,-5.24,;12.49,-7.54,;11.16,-8.32,;9.82,-7.55,;9.82,-6.01,;8.49,-8.33,;7.15,-7.56,;5.83,-8.33,;5.82,-9.88,;4.49,-10.65,;3.15,-9.88,;3.16,-8.34,;1.84,-7.57,;.5,-8.33,;.49,-9.87,;1.83,-10.65,;7.16,-10.65,;8.5,-9.88,;9.83,-10.65,;9.83,-12.19,;8.75,-13.28,;9.84,-14.37,;10.93,-13.27,;13.29,-6.21,;12.12,-4.89,;14.7,-5.65,)| Show InChI InChI=1S/C24H34N4O4/c29-21(27-20-15-8-14-9-16(20)12-24(30,10-14)11-15)19-13-25-23(26-17-4-6-31-7-5-17)28-22(19)32-18-2-1-3-18/h13-18,20,30H,1-12H2,(H,27,29)(H,25,26,28)/t14?,15?,16?,20-,24- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of mouse 11beta-HSD1 by HTRF assay |
J Med Chem 55: 10652-61 (2012)
Article DOI: 10.1021/jm3013163
BindingDB Entry DOI: 10.7270/Q2BG2Q4S |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data