

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50042149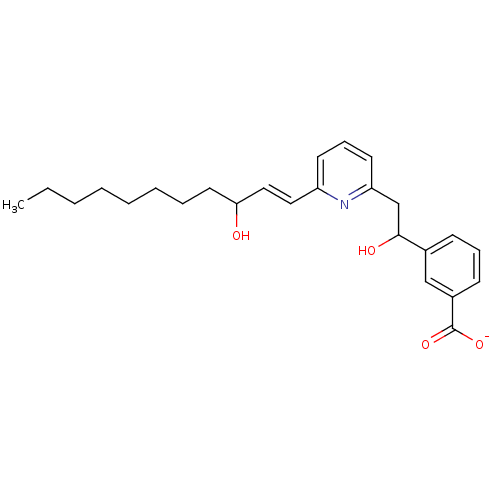 (CHEMBL112520 | Lithium; 3-{1-hydroxy-2-[6-(3-hydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3H]- LTB4 binding on human whole cells | J Med Chem 36: 3308-20 (1993) BindingDB Entry DOI: 10.7270/Q21R6R4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Relaxin receptor 1 (Homo sapiens (Human)) | BDBM50563230 (CHEMBL4754949) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of Eu3+-labelled H2 relaxin from human RXFP1 expressed in human HEK-293T cells in presence of 10 % FCS by competition binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01533 BindingDB Entry DOI: 10.7270/Q2XW4PHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50042153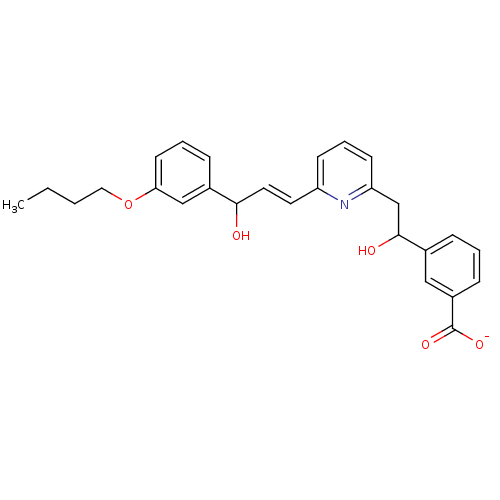 (CHEMBL113288 | Lithium; 3-(2-{7-[3-(3-butoxy-pheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3H]- LTB4 binding on human whole cells | J Med Chem 36: 3308-20 (1993) BindingDB Entry DOI: 10.7270/Q21R6R4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50042150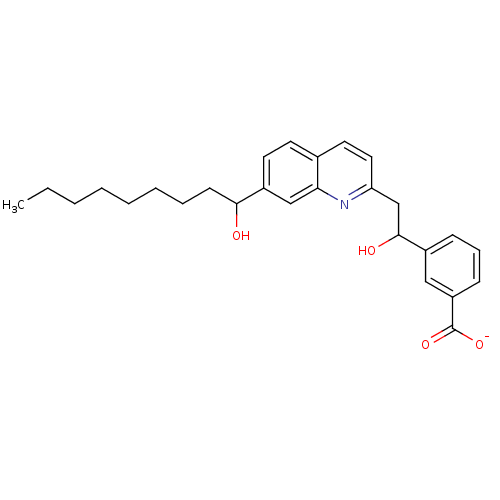 (CHEMBL326397 | Lithium; 3-{1-hydroxy-2-[7-(1-hydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3H]- LTB4 binding on human whole cells | J Med Chem 36: 3308-20 (1993) BindingDB Entry DOI: 10.7270/Q21R6R4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50368705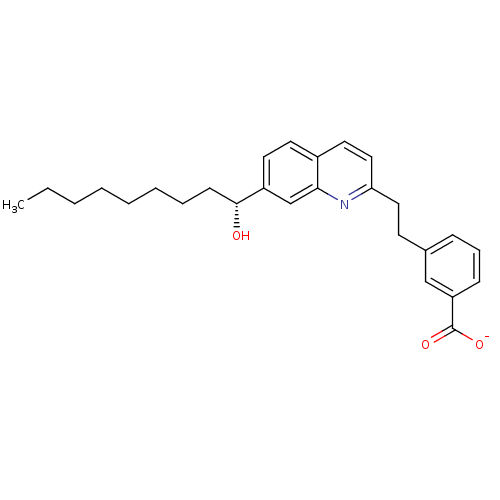 (CHEMBL1788228) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3H]- LTB4 binding on human whole cells | J Med Chem 36: 3308-20 (1993) BindingDB Entry DOI: 10.7270/Q21R6R4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50042148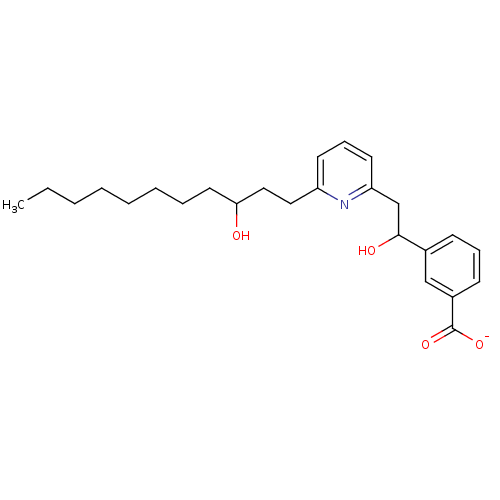 (CHEMBL111811 | Lithium; 3-{1-hydroxy-2-[6-(3-hydro...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3H]- LTB4 binding on human whole cells | J Med Chem 36: 3308-20 (1993) BindingDB Entry DOI: 10.7270/Q21R6R4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50042142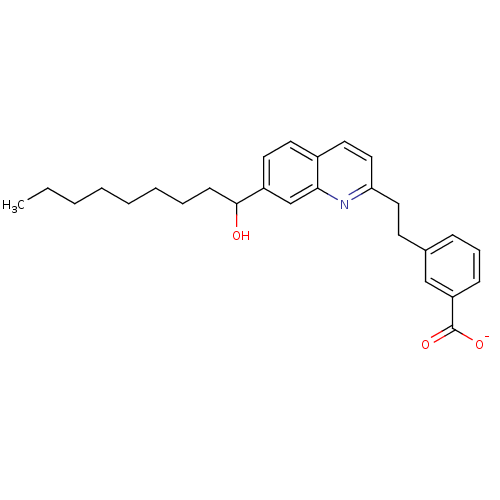 ((R)-Lithium; 3-{2-[7-(1-hydroxy-nonyl)-quinolin-2-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3H]- LTB4 binding on human whole cells | J Med Chem 36: 3308-20 (1993) BindingDB Entry DOI: 10.7270/Q21R6R4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50042143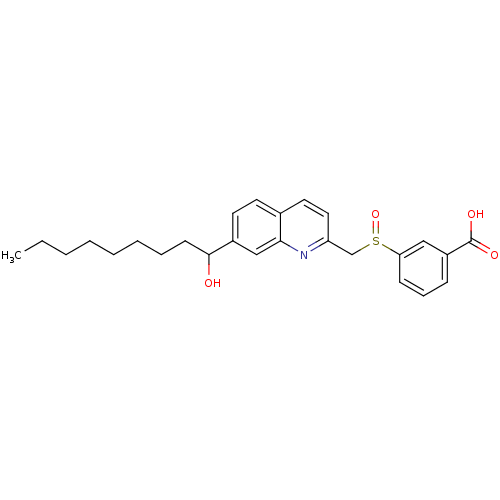 (3-[7-(1-Hydroxy-nonyl)-quinolin-2-ylmethanesulfiny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3H]- LTB4 binding on human whole cells | J Med Chem 36: 3308-20 (1993) BindingDB Entry DOI: 10.7270/Q21R6R4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001610 (7-[3-(4-Acetyl-3-methoxy-2-propyl-phenoxy)-propoxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smithkline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against Leukotriene B4 receptor on intact differentiated U-937 cells in competitive binding assay with [3H]-LTB4 | J Med Chem 36: 3333-40 (1993) BindingDB Entry DOI: 10.7270/Q2BG2N1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50001611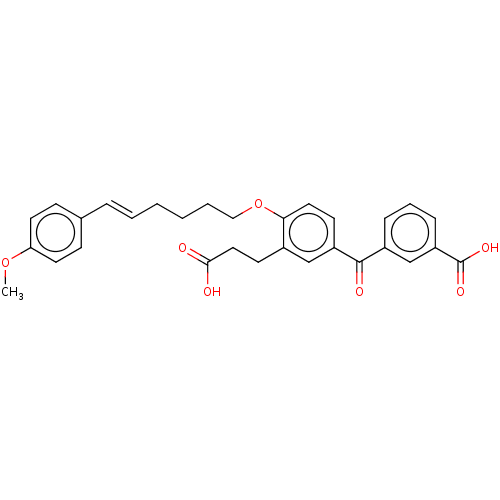 ((E)-3-{3-(2-Carboxy-ethyl)-4-[6-(4-methoxy-phenyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | PubMed | 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smithkline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against Leukotriene B4 receptor on intact differentiated U-937 cells in competitive binding assay with [3H]-LTB4 | J Med Chem 36: 3333-40 (1993) BindingDB Entry DOI: 10.7270/Q2BG2N1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50042151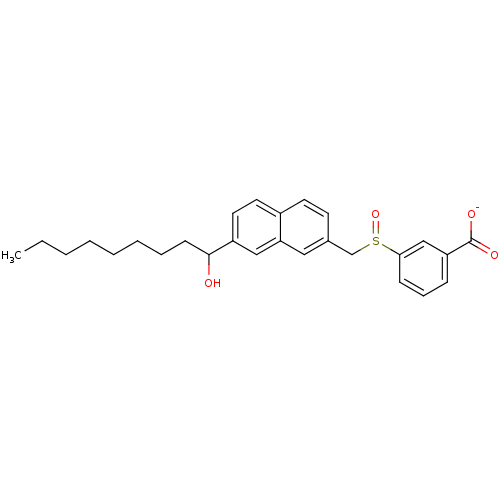 (CHEMBL115826 | Lithium; 3-[7-(1-hydroxy-nonyl)-nap...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3H]- LTB4 binding on human whole cells | J Med Chem 36: 3308-20 (1993) BindingDB Entry DOI: 10.7270/Q21R6R4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50042146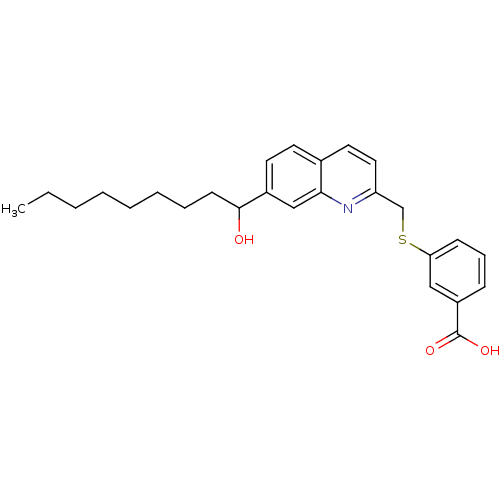 (3-[7-(1-Hydroxy-nonyl)-quinolin-2-ylmethylsulfanyl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3H]- LTB4 binding on human whole cells | J Med Chem 36: 3308-20 (1993) BindingDB Entry DOI: 10.7270/Q21R6R4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM79408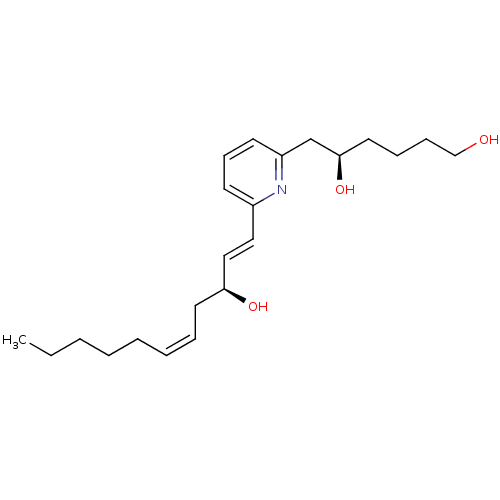 ((5R)-6-[6-[(1E,3S,5Z)-3-hydroxyundeca-1,5-dienyl]-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3H]- LTB4 binding on human whole cells | J Med Chem 36: 3308-20 (1993) BindingDB Entry DOI: 10.7270/Q21R6R4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50042145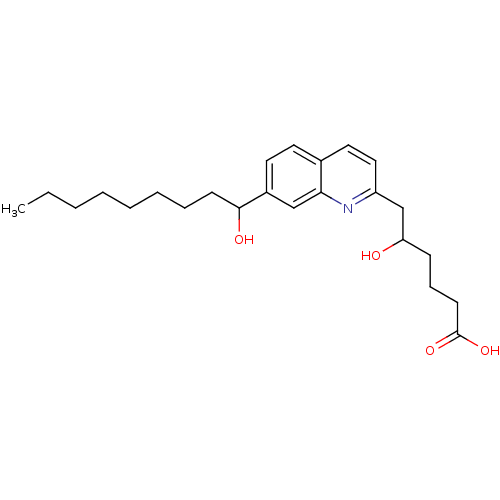 (5-Hydroxy-6-[7-(1-hydroxy-nonyl)-quinolin-2-yl]-he...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3H]- LTB4 binding on human whole cells | J Med Chem 36: 3308-20 (1993) BindingDB Entry DOI: 10.7270/Q21R6R4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50042152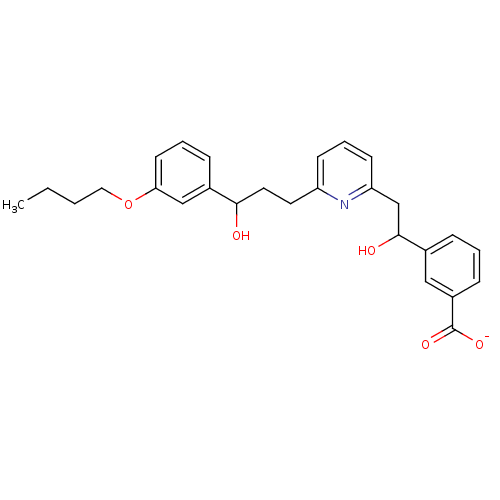 (CHEMBL114454 | Lithium; 3-(2-{6-[3-(3-butoxy-pheny...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3H]- LTB4 binding on human whole cells | J Med Chem 36: 3308-20 (1993) BindingDB Entry DOI: 10.7270/Q21R6R4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50042141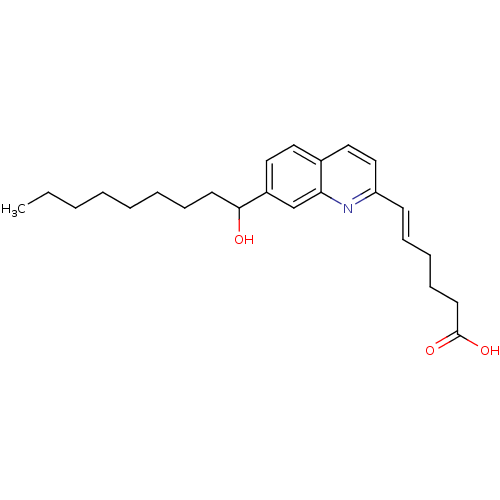 (6-[7-(1-Hydroxy-nonyl)-quinolin-2-yl]-hex-5-enoic ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3H]- LTB4 binding on human whole cells | J Med Chem 36: 3308-20 (1993) BindingDB Entry DOI: 10.7270/Q21R6R4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50042187 (4-[4-(2-Amino-5-benzyl-1-methyl-1H-imidazol-4-ylme...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smithkline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against Leukotriene B4 receptor on intact differentiated U-937 cells in competitive binding assay with [3H]-LTB4 | J Med Chem 36: 3333-40 (1993) BindingDB Entry DOI: 10.7270/Q2BG2N1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50042154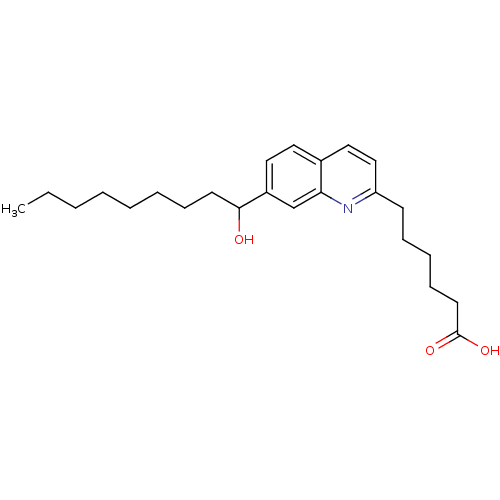 (6-[7-(1-Hydroxy-nonyl)-quinolin-2-yl]-hexanoic aci...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3H]- LTB4 binding on human whole cells | J Med Chem 36: 3308-20 (1993) BindingDB Entry DOI: 10.7270/Q21R6R4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Relaxin receptor 1 (Homo sapiens (Human)) | BDBM50563231 (CHEMBL4780098) | PDB Reactome pathway KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Displacement of Eu3+-labelled H2 relaxin from human RXFP1 expressed in human HEK-293T cells in presence of 10 % FCS by competition binding assay | Citation and Details Article DOI: 10.1021/acs.jmedchem.0c01533 BindingDB Entry DOI: 10.7270/Q2XW4PHG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50042185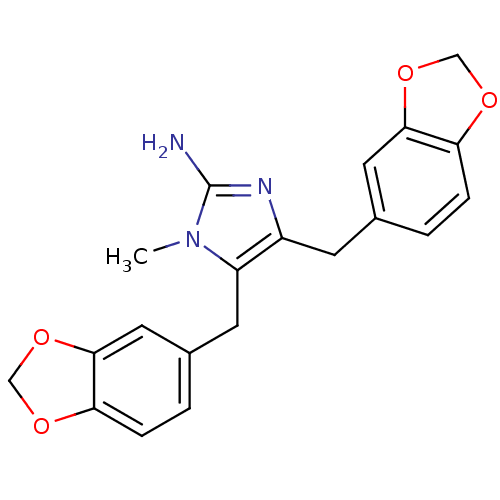 (4,5-Bis-benzo[1,3]dioxol-5-ylmethyl-1-methyl-1H-im...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smithkline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against Leukotriene B4 receptor on intact differentiated U-937 cells in competitive binding assay with [3H]-LTB4 | J Med Chem 36: 3333-40 (1993) BindingDB Entry DOI: 10.7270/Q2BG2N1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50042147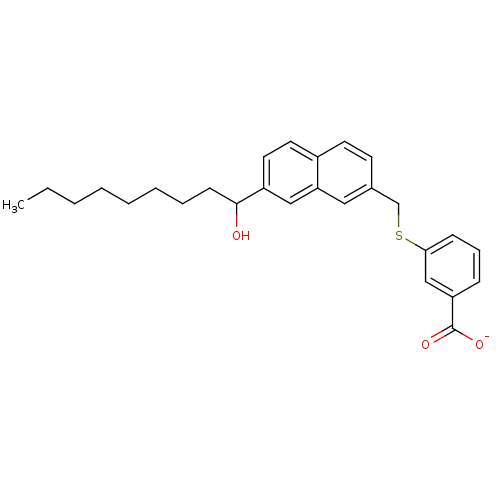 (CHEMBL113401 | Lithium; 3-[7-(1-hydroxy-nonyl)-nap...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SmithKline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of [3H]- LTB4 binding on human whole cells | J Med Chem 36: 3308-20 (1993) BindingDB Entry DOI: 10.7270/Q21R6R4P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50042188 (4,5-Dibenzyl-1H-imidazol-2-ylamine | CHEMBL116278) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smithkline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against Leukotriene B4 receptor on intact differentiated U-937 cells in competitive binding assay with [3H]-LTB4 | J Med Chem 36: 3333-40 (1993) BindingDB Entry DOI: 10.7270/Q2BG2N1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50042190 (4,5-Dibenzyl-1-methyl-1H-imidazol-2-ylamine | CHEM...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smithkline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against Leukotriene B4 receptor on intact differentiated U-937 cells in competitive binding assay with [3H]-LTB4 | J Med Chem 36: 3333-40 (1993) BindingDB Entry DOI: 10.7270/Q2BG2N1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Leukotriene B4 receptor 1 (Homo sapiens (Human)) | BDBM50042186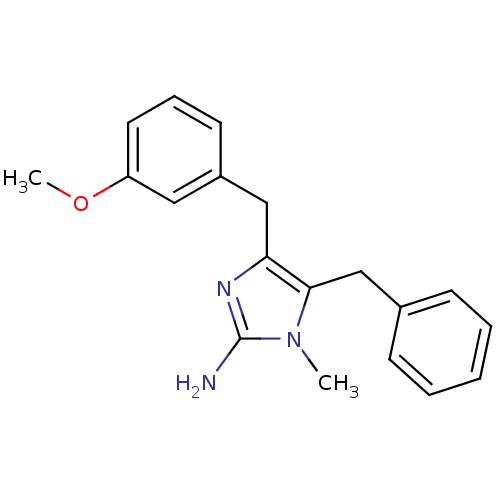 (5-Benzyl-4-(3-methoxy-benzyl)-1-methyl-1H-imidazol...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Smithkline Beecham Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity against Leukotriene B4 receptor on intact differentiated U-937 cells in competitive binding assay with [3H]-LTB4 | J Med Chem 36: 3333-40 (1993) BindingDB Entry DOI: 10.7270/Q2BG2N1M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (RAT) | BDBM50036401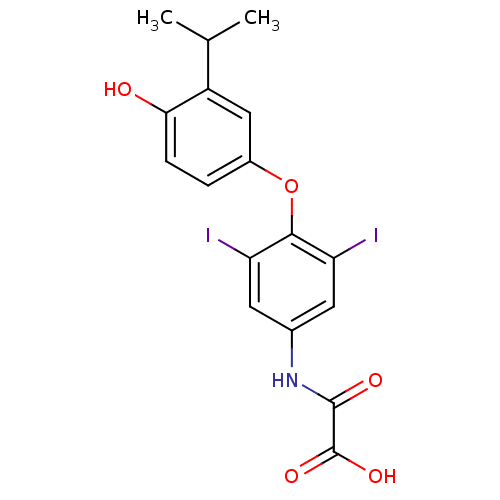 (CHEMBL163221 | N-[4-(4-Hydroxy-3-isopropyl-phenoxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the bound [125I]L-T3 rat liver nuclear L-triiodothyronine receptor | J Med Chem 38: 695-707 (1995) BindingDB Entry DOI: 10.7270/Q2T43TRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (RAT) | BDBM50036427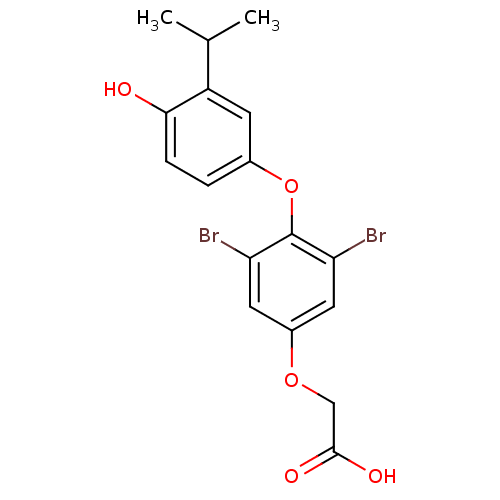 (CHEMBL350265 | [3,5-Dibromo-4-(4-hydroxy-3-isoprop...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba Pharmaceuticals Curated by ChEMBL | Assay Description Ability to inhibit the bound [125I]L-T3 rat liver Nuclear L-triiodothyronine receptor is determined in vitro. | J Med Chem 38: 695-707 (1995) BindingDB Entry DOI: 10.7270/Q2T43TRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (RAT) | BDBM18865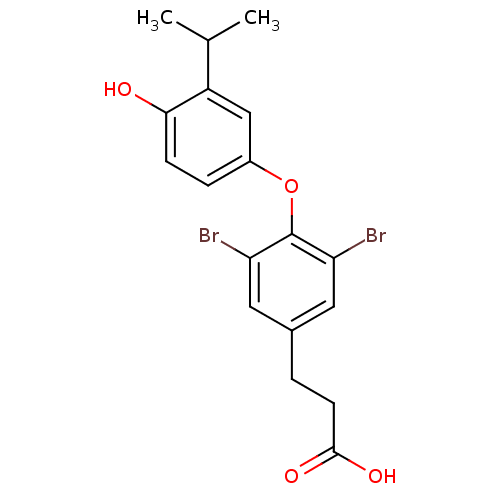 (3-{3,5-dibromo-4-[4-hydroxy-3-(propan-2-yl)phenoxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba Pharmaceuticals Curated by ChEMBL | Assay Description Ability to inhibit the bound [125I]L-T3 rat liver Nuclear L-triiodothyronine receptor is determined in vitro. | J Med Chem 38: 695-707 (1995) BindingDB Entry DOI: 10.7270/Q2T43TRC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thyroid hormone receptor beta (RAT) | BDBM50036399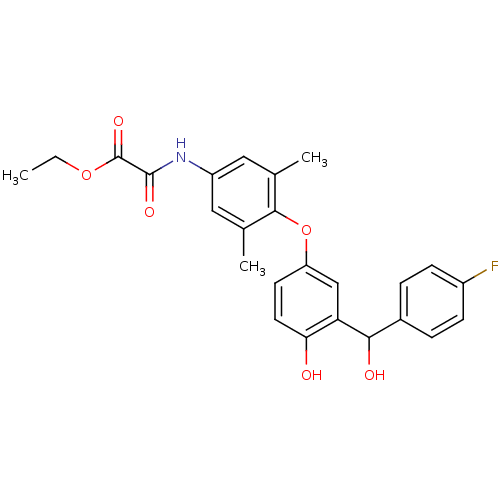 (Axitirome | CHEMBL159682 | N-(4-{3-[(4-Fluoro-phen...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba Pharmaceuticals Curated by ChEMBL | Assay Description Ability to inhibit the bound [125I]L-T3 rat liver Nuclear L-triiodothyronine receptor is determined in vitro. | J Med Chem 38: 695-707 (1995) BindingDB Entry DOI: 10.7270/Q2T43TRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (RAT) | BDBM18862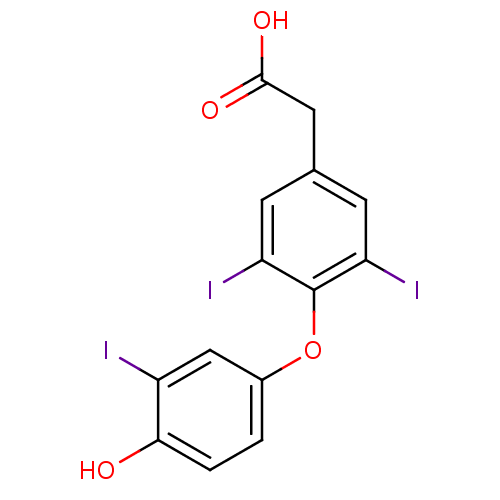 (2-[4-(4-hydroxy-3-iodophenoxy)-3,5-diiodophenyl]ac...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents | PDB PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the bound [125I]L-T3 rat liver nuclear L-triiodothyronine receptor | J Med Chem 38: 695-707 (1995) BindingDB Entry DOI: 10.7270/Q2T43TRC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Thyroid hormone receptor beta (RAT) | BDBM50036402 (CHEMBL46882 | N-[3,5-dimethyl-4-(4'-hydroxy-3'-iso...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the bound [125I]L-T3 rat liver nuclear L-triiodothyronine receptor | J Med Chem 38: 695-707 (1995) BindingDB Entry DOI: 10.7270/Q2T43TRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (RAT) | BDBM50036418 (CHEMBL350049 | N-[4-(4-Hydroxy-3-isopropyl-phenoxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the bound [125I]L-T3 rat liver nuclear L-triiodothyronine receptor | J Med Chem 38: 695-707 (1995) BindingDB Entry DOI: 10.7270/Q2T43TRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (RAT) | BDBM50036423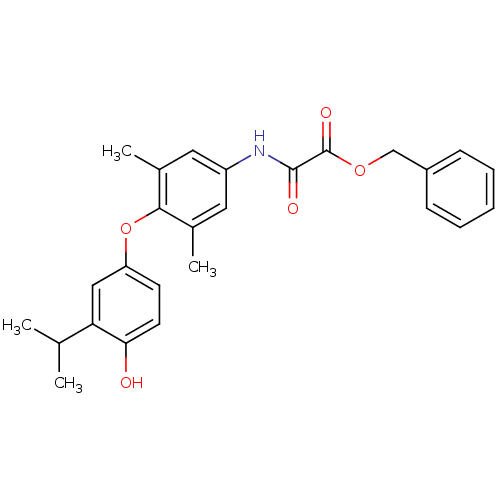 (CHEMBL164421 | N-[4-(4-Hydroxy-3-isopropyl-phenoxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the bound [125I]L-T3 rat liver nuclear L-triiodothyronine receptor | J Med Chem 38: 695-707 (1995) BindingDB Entry DOI: 10.7270/Q2T43TRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (RAT) | BDBM50036411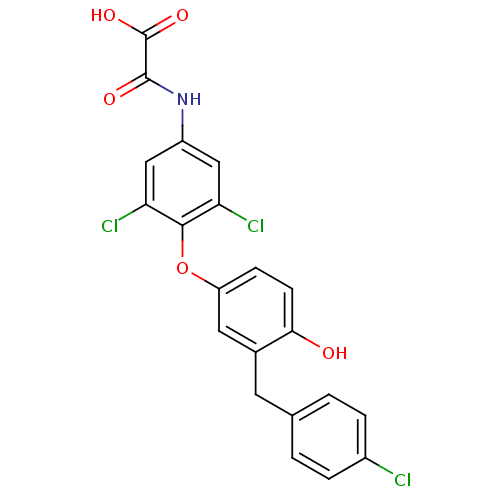 (CHEMBL163324 | N-{3,5-Dichloro-4-[3-(4-chloro-benz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the bound [125I]L-T3 rat liver nuclear L-triiodothyronine receptor | J Med Chem 38: 695-707 (1995) BindingDB Entry DOI: 10.7270/Q2T43TRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (RAT) | BDBM50036410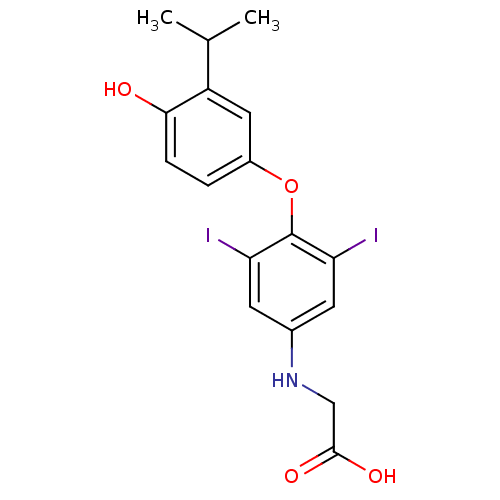 (CHEMBL159976 | [4-(4-Hydroxy-3-isopropyl-phenoxy)-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba Pharmaceuticals Curated by ChEMBL | Assay Description Ability to inhibit the bound [125I]L-T3 rat liver Nuclear L-triiodothyronine receptor is determined in vitro. | J Med Chem 38: 695-707 (1995) BindingDB Entry DOI: 10.7270/Q2T43TRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (RAT) | BDBM50036424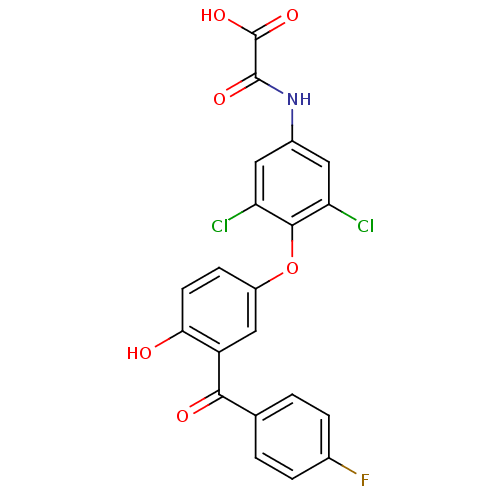 (CHEMBL159334 | N-{3,5-Dichloro-4-[3-(4-fluoro-benz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the bound [125I]L-T3 rat liver nuclear L-triiodothyronine receptor | J Med Chem 38: 695-707 (1995) BindingDB Entry DOI: 10.7270/Q2T43TRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (RAT) | BDBM50036408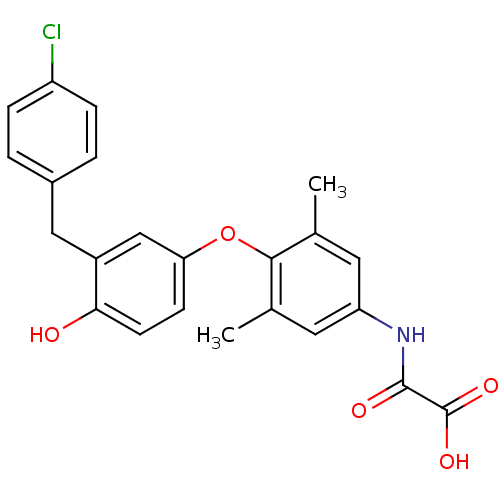 (CHEMBL161828 | N-{4-[3-(4-Chloro-benzyl)-4-hydroxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the bound [125I]L-T3 rat liver nuclear L-triiodothyronine receptor | J Med Chem 38: 695-707 (1995) BindingDB Entry DOI: 10.7270/Q2T43TRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (RAT) | BDBM50036417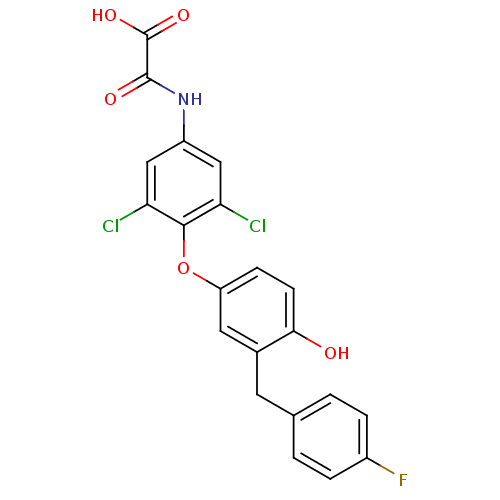 (CHEMBL423977 | N-{3,5-Dichloro-4-[3-(4-fluoro-benz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the bound [125I]L-T3 rat liver nuclear L-triiodothyronine receptor | J Med Chem 38: 695-707 (1995) BindingDB Entry DOI: 10.7270/Q2T43TRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (RAT) | BDBM50036417 (CHEMBL423977 | N-{3,5-Dichloro-4-[3-(4-fluoro-benz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the bound [125I]L-T3 rat liver nuclear L-triiodothyronine receptor | J Med Chem 38: 695-707 (1995) BindingDB Entry DOI: 10.7270/Q2T43TRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (RAT) | BDBM50036404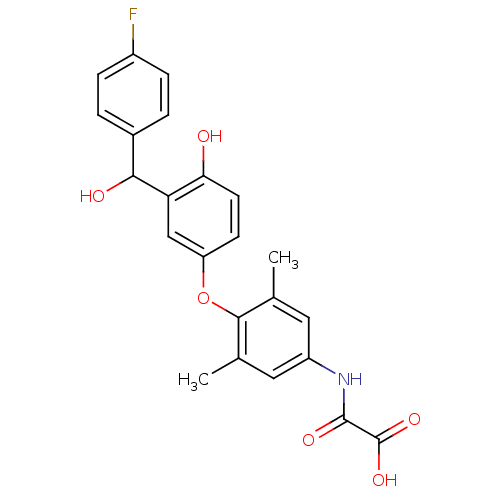 (CHEMBL159595 | N-(4-{3-[(4-Fluoro-phenyl)-hydroxy-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the bound [125I]L-T3 rat liver nuclear L-triiodothyronine receptor | J Med Chem 38: 695-707 (1995) BindingDB Entry DOI: 10.7270/Q2T43TRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (RAT) | BDBM50036395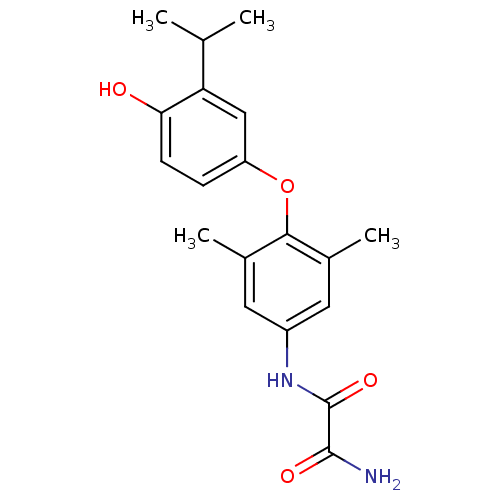 (CHEMBL350199 | N-[4-(4-Hydroxy-3-isopropyl-phenoxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.950 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the bound [125I]L-T3 rat liver nuclear L-triiodothyronine receptor | J Med Chem 38: 695-707 (1995) BindingDB Entry DOI: 10.7270/Q2T43TRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (RAT) | BDBM50036420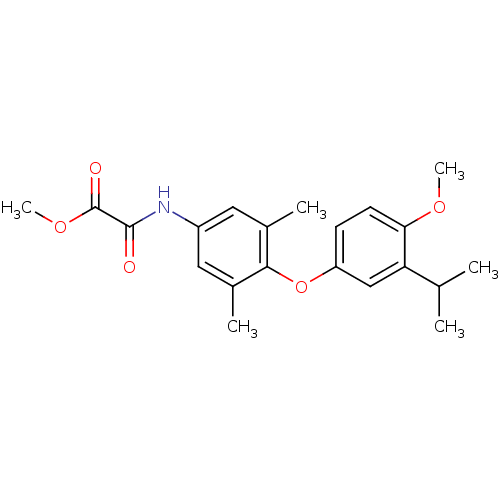 (CHEMBL161535 | N-[4-(3-Isopropyl-4-methoxy-phenoxy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the bound [125I]L-T3 rat liver nuclear L-triiodothyronine receptor | J Med Chem 38: 695-707 (1995) BindingDB Entry DOI: 10.7270/Q2T43TRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (RAT) | BDBM50036406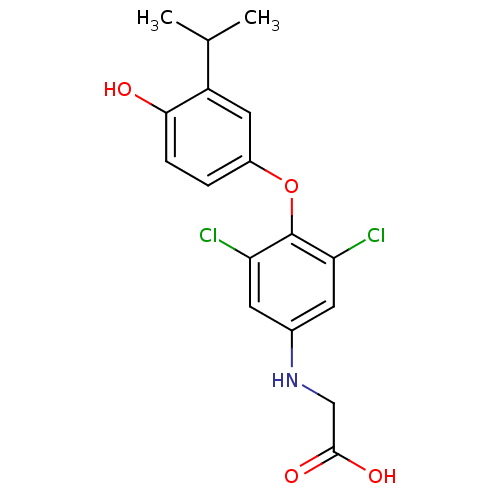 (CHEMBL163380 | [3,5-Dichloro-4-(4-hydroxy-3-isopro...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba Pharmaceuticals Curated by ChEMBL | Assay Description Ability to inhibit the bound [125I]L-T3 rat liver Nuclear L-triiodothyronine receptor is determined in vitro. | J Med Chem 38: 695-707 (1995) BindingDB Entry DOI: 10.7270/Q2T43TRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (RAT) | BDBM50036397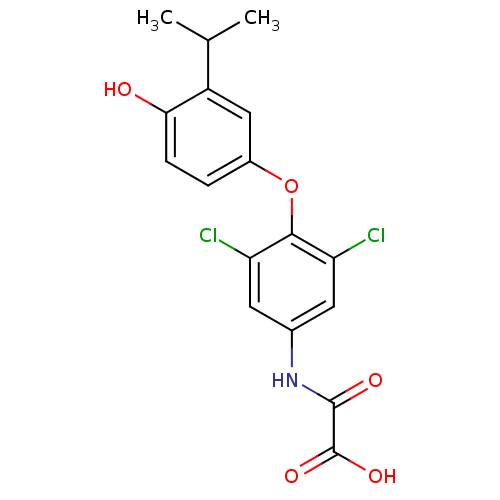 (CHEMBL348983 | N-[3,5-Dichloro-4-(4-hydroxy-3-isop...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the bound [125I]L-T3 rat liver nuclear L-triiodothyronine receptor | J Med Chem 38: 695-707 (1995) BindingDB Entry DOI: 10.7270/Q2T43TRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50105748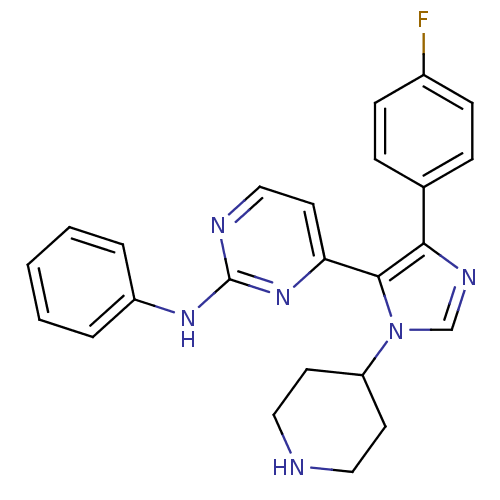 (4-(4-(4-fluorophenyl)-1-(piperidin-4-yl)-1H-imidaz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition against Mitogen-activated protein kinase p38 alpha | Bioorg Med Chem Lett 11: 2867-70 (2001) BindingDB Entry DOI: 10.7270/Q28G8K1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50105762 (4-[4-(4-Fluoro-phenyl)-5-(2-phenylamino-pyrimidin-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition against Mitogen-activated protein kinase p38 alpha | Bioorg Med Chem Lett 11: 2867-70 (2001) BindingDB Entry DOI: 10.7270/Q28G8K1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (RAT) | BDBM50036405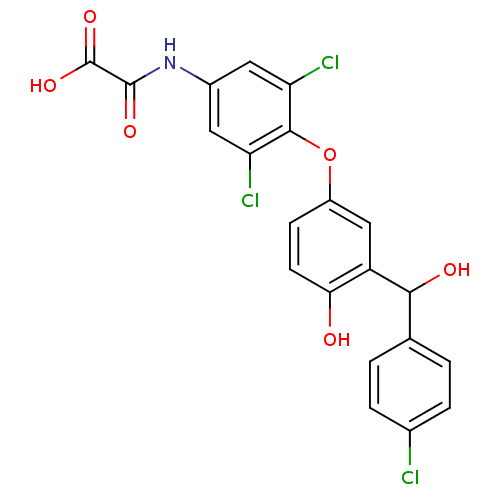 (CHEMBL434476 | N-(3,5-Dichloro-4-{3-[(4-chloro-phe...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the bound [125I]L-T3 rat liver nuclear L-triiodothyronine receptor | J Med Chem 38: 695-707 (1995) BindingDB Entry DOI: 10.7270/Q2T43TRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (RAT) | BDBM50036398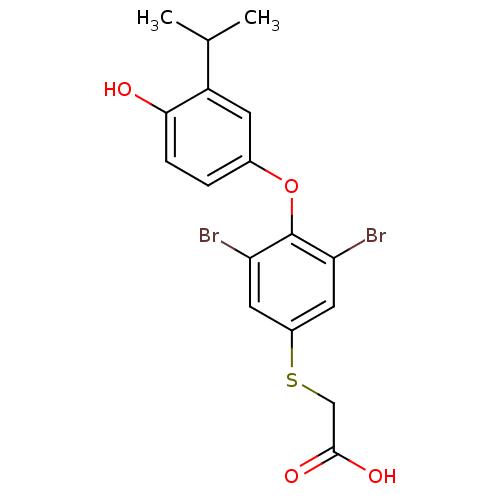 (CHEMBL162692 | [3,5-Dibromo-4-(4-hydroxy-3-isoprop...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba Pharmaceuticals Curated by ChEMBL | Assay Description Ability to inhibit the bound [125I]L-T3 rat liver Nuclear L-triiodothyronine receptor is determined in vitro. | J Med Chem 38: 695-707 (1995) BindingDB Entry DOI: 10.7270/Q2T43TRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (RAT) | BDBM50036426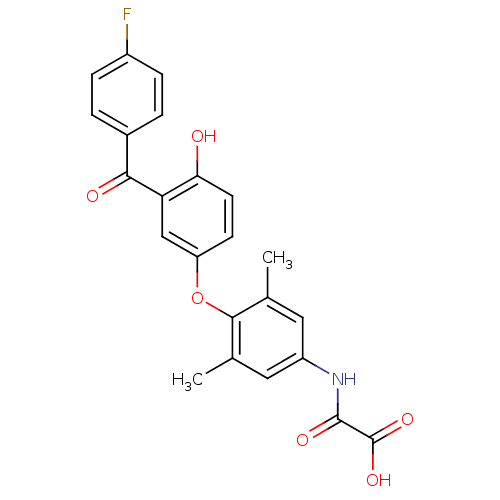 (CHEMBL159610 | N-{4-[3-(4-Fluoro-benzoyl)-4-hydrox...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the bound [125I]L-T3 rat liver nuclear L-triiodothyronine receptor | J Med Chem 38: 695-707 (1995) BindingDB Entry DOI: 10.7270/Q2T43TRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 14 (Homo sapiens (Human)) | BDBM50105746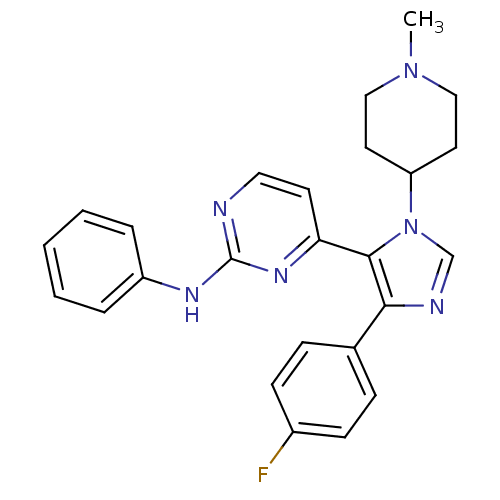 (CHEMBL318892 | {4-[5-(4-Fluoro-phenyl)-3-(1-methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition against Mitogen-activated protein kinase p38 alpha | Bioorg Med Chem Lett 11: 2867-70 (2001) BindingDB Entry DOI: 10.7270/Q28G8K1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (RAT) | BDBM50036415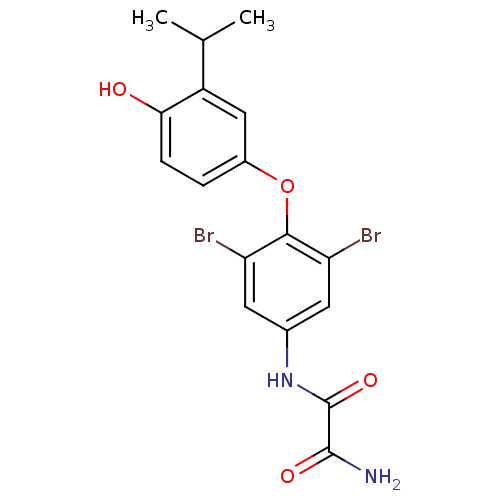 (CHEMBL163268 | N-[3,5-Dibromo-4-(4-hydroxy-3-isopr...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba Pharmaceuticals Curated by ChEMBL | Assay Description In vitro inhibition of the bound [125I]L-T3 rat liver nuclear L-triiodothyronine receptor | J Med Chem 38: 695-707 (1995) BindingDB Entry DOI: 10.7270/Q2T43TRC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 527 total ) | Next | Last >> |