

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prothrombin (Homo sapiens (Human)) | BDBM50410910 (CHEMBL377651) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 16: 2882-5 (2006) Article DOI: 10.1016/j.bmcl.2006.03.012 BindingDB Entry DOI: 10.7270/Q2H41SN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM50410912 (CHEMBL379174) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics Curated by ChEMBL | Assay Description Inhibition of beta tryptase | Bioorg Med Chem Lett 16: 2882-5 (2006) Article DOI: 10.1016/j.bmcl.2006.03.012 BindingDB Entry DOI: 10.7270/Q2H41SN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50410910 (CHEMBL377651) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics Curated by ChEMBL | Assay Description Inhibition of plasmin | Bioorg Med Chem Lett 16: 2882-5 (2006) Article DOI: 10.1016/j.bmcl.2006.03.012 BindingDB Entry DOI: 10.7270/Q2H41SN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50410912 (CHEMBL379174) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics Curated by ChEMBL | Assay Description Inhibition of plasmin | Bioorg Med Chem Lett 16: 2882-5 (2006) Article DOI: 10.1016/j.bmcl.2006.03.012 BindingDB Entry DOI: 10.7270/Q2H41SN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM50410910 (CHEMBL377651) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics Curated by ChEMBL | Assay Description Inhibition of beta tryptase | Bioorg Med Chem Lett 16: 2882-5 (2006) Article DOI: 10.1016/j.bmcl.2006.03.012 BindingDB Entry DOI: 10.7270/Q2H41SN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50410911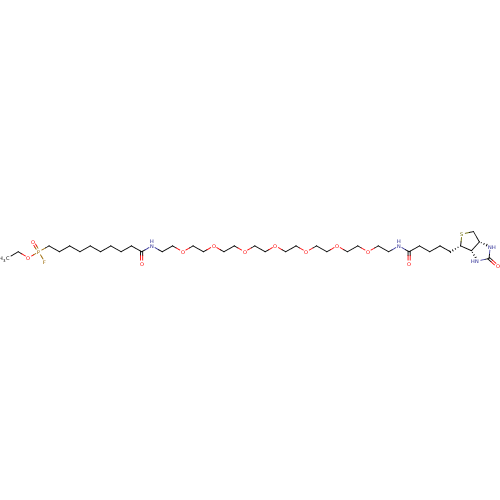 (CHEMBL377737) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 16: 2882-5 (2006) Article DOI: 10.1016/j.bmcl.2006.03.012 BindingDB Entry DOI: 10.7270/Q2H41SN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tryptase beta-2 (Homo sapiens (Human)) | BDBM50410911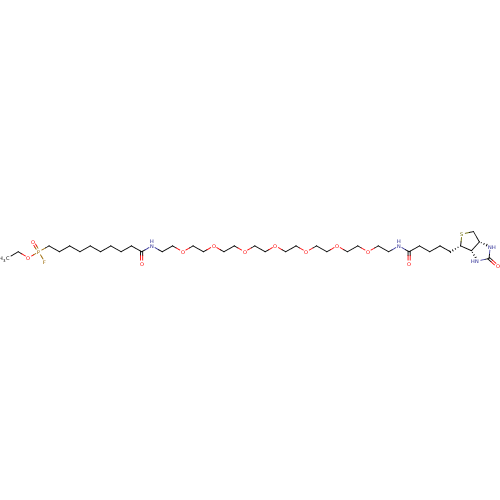 (CHEMBL377737) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics Curated by ChEMBL | Assay Description Inhibition of beta tryptase | Bioorg Med Chem Lett 16: 2882-5 (2006) Article DOI: 10.1016/j.bmcl.2006.03.012 BindingDB Entry DOI: 10.7270/Q2H41SN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50410911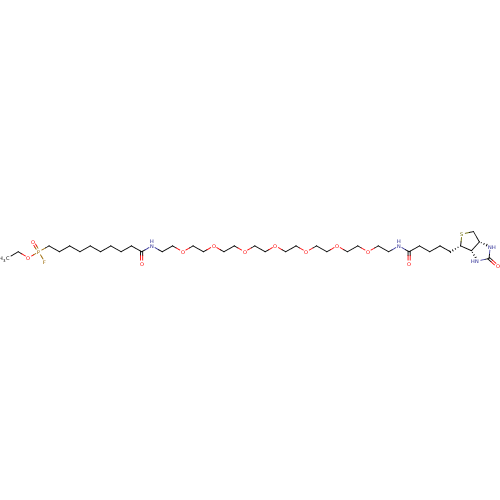 (CHEMBL377737) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics Curated by ChEMBL | Assay Description Inhibition of plasmin | Bioorg Med Chem Lett 16: 2882-5 (2006) Article DOI: 10.1016/j.bmcl.2006.03.012 BindingDB Entry DOI: 10.7270/Q2H41SN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50410912 (CHEMBL379174) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera Genomics Curated by ChEMBL | Assay Description Inhibition of thrombin | Bioorg Med Chem Lett 16: 2882-5 (2006) Article DOI: 10.1016/j.bmcl.2006.03.012 BindingDB Entry DOI: 10.7270/Q2H41SN2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Legumain (Mus musculus) | BDBM50364367 (CHEMBL1950283) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University School of Medicine Curated by ChEMBL | Assay Description Inhibition of mouse recombinant legumain using Cbz-Ala-Ala-Asn-AMC as substrate measured every 30 secs for 2.5 hrs by fluorimetry | Bioorg Med Chem Lett 22: 1340-3 (2012) Article DOI: 10.1016/j.bmcl.2011.12.079 BindingDB Entry DOI: 10.7270/Q2H132GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Legumain (Mus musculus) | BDBM50364353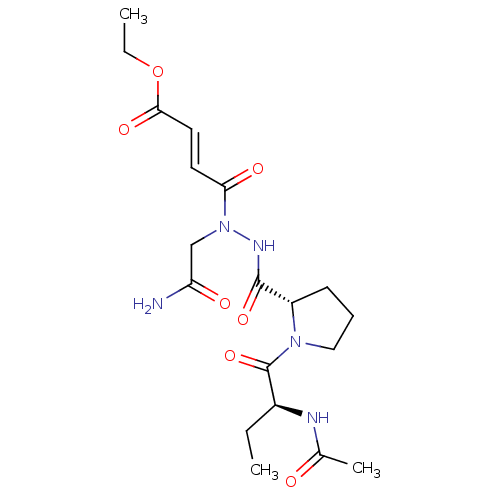 (CHEMBL1950109) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University School of Medicine Curated by ChEMBL | Assay Description Inhibition of mouse recombinant legumain using Cbz-Ala-Ala-Asn-AMC as substrate measured every 30 secs for 2.5 hrs by fluorimetry | Bioorg Med Chem Lett 22: 1340-3 (2012) Article DOI: 10.1016/j.bmcl.2011.12.079 BindingDB Entry DOI: 10.7270/Q2H132GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Rattus norvegicus) | BDBM36331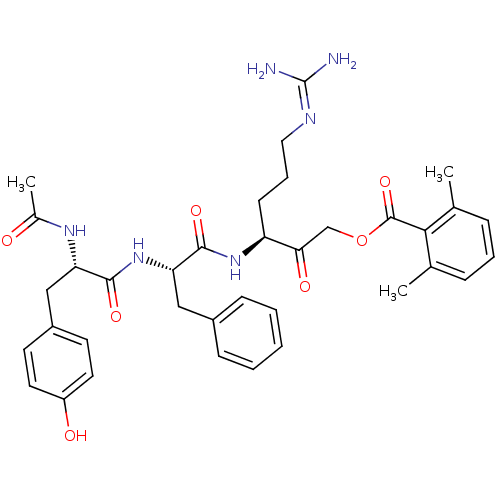 (Ac-YFR-AMOK 10b) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Stanford University | Assay Description Protease enzyme inhibition assay targeting diverse member of the cysteine protease families:cathepsin B, Z, and H | Nat Chem Biol 1: 33-8 (2005) Article DOI: 10.1038/nchembio707 BindingDB Entry DOI: 10.7270/Q22V2DGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Legumain (Mus musculus) | BDBM50364366 (CHEMBL1950282) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University School of Medicine Curated by ChEMBL | Assay Description Inhibition of mouse recombinant legumain using Cbz-Ala-Ala-Asn-AMC as substrate measured every 30 secs for 2.5 hrs by fluorimetry | Bioorg Med Chem Lett 22: 1340-3 (2012) Article DOI: 10.1016/j.bmcl.2011.12.079 BindingDB Entry DOI: 10.7270/Q2H132GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Legumain (Mus musculus) | BDBM228618 (BDBM50364365 | US9345789, LI-2) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University School of Medicine Curated by ChEMBL | Assay Description Inhibition of mouse recombinant legumain using Cbz-Ala-Ala-Asn-AMC as substrate measured every 30 secs for 2.5 hrs by fluorimetry | Bioorg Med Chem Lett 22: 1340-3 (2012) Article DOI: 10.1016/j.bmcl.2011.12.079 BindingDB Entry DOI: 10.7270/Q2H132GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Legumain (Mus musculus) | BDBM50364368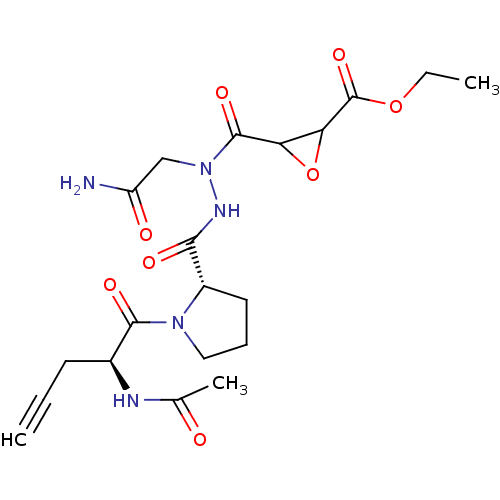 (CHEMBL1950284) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University School of Medicine Curated by ChEMBL | Assay Description Inhibition of mouse recombinant legumain using Cbz-Ala-Ala-Asn-AMC as substrate measured every 30 secs for 2.5 hrs by fluorimetry | Bioorg Med Chem Lett 22: 1340-3 (2012) Article DOI: 10.1016/j.bmcl.2011.12.079 BindingDB Entry DOI: 10.7270/Q2H132GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Legumain (Mus musculus) | BDBM36332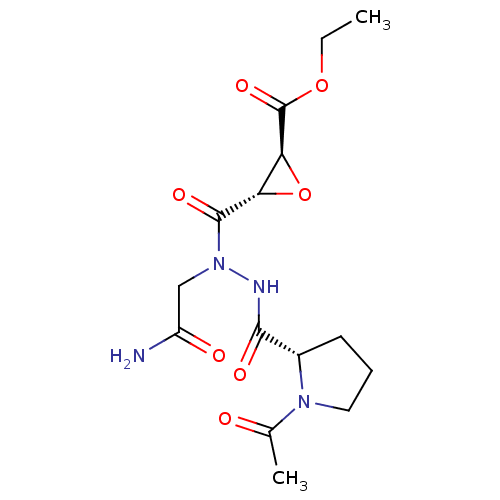 (LI-1 | Legumain Inhibitor -1) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University School of Medicine Curated by ChEMBL | Assay Description Inhibition of mouse recombinant legumain using Cbz-Ala-Ala-Asn-AMC as substrate measured every 30 secs for 2.5 hrs by fluorimetry | Bioorg Med Chem Lett 22: 1340-3 (2012) Article DOI: 10.1016/j.bmcl.2011.12.079 BindingDB Entry DOI: 10.7270/Q2H132GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Legumain (Mus musculus) | BDBM50364356 (CHEMBL1950112) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University School of Medicine Curated by ChEMBL | Assay Description Inhibition of mouse recombinant legumain using Cbz-Ala-Ala-Asn-AMC as substrate measured every 30 secs for 2.5 hrs by fluorimetry | Bioorg Med Chem Lett 22: 1340-3 (2012) Article DOI: 10.1016/j.bmcl.2011.12.079 BindingDB Entry DOI: 10.7270/Q2H132GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Legumain (Homo sapiens (Human)) | BDBM228617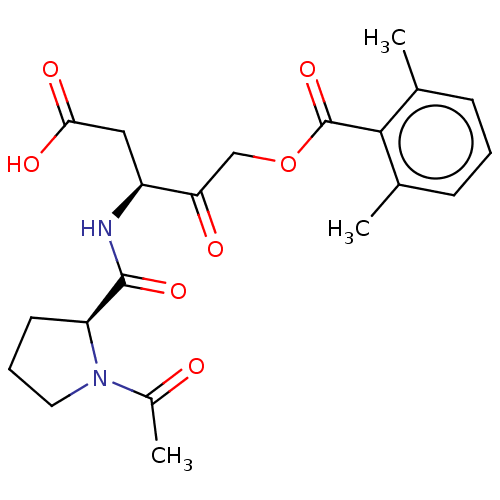 (US9345789, LI-0) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11.5 | n/a | n/a | n/a | n/a | 5.8 | n/a |
The Board of Trustees of the Leland Stanford Junior University US Patent | Assay Description Assay buffers consist of 20 mM citric acid, 60 mM disodium hydrogen orthophosphate, 1 mM EDTA, 0.1% CHAPS, 4 mM DTT, pH 5.8 for legumain, 50 mM dihyd... | US Patent US9345789 (2016) BindingDB Entry DOI: 10.7270/Q2610Z63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Legumain (Mus musculus) | BDBM36332 (LI-1 | Legumain Inhibitor -1) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11.5 | n/a | n/a | n/a | n/a | 5.8 | 25 |
Stanford University | Assay Description IC50 measurements and enzyme kinetics assays were performed on a Spectramax M5 fluorescent plate reader (Molecular Devices). | ACS Chem Biol 5: 233-43 (2010) Article DOI: 10.1021/cb900232a BindingDB Entry DOI: 10.7270/Q2Z31X0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Rattus norvegicus) | BDBM36328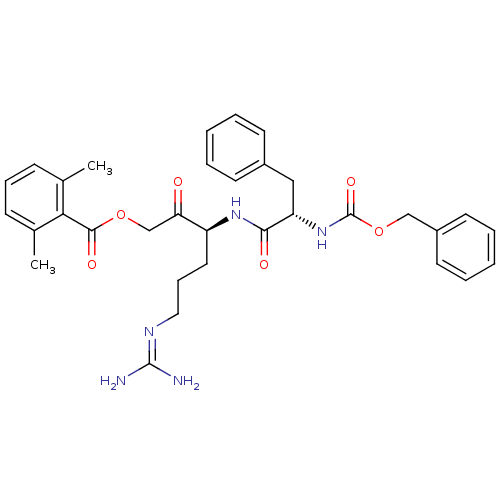 (Z-FR-AMOK 9b) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Stanford University | Assay Description Protease enzyme inhibition assay targeting diverse member of the cysteine protease families:cathepsin B, Z, and H | Nat Chem Biol 1: 33-8 (2005) Article DOI: 10.1038/nchembio707 BindingDB Entry DOI: 10.7270/Q22V2DGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Legumain (Mus musculus) | BDBM50364358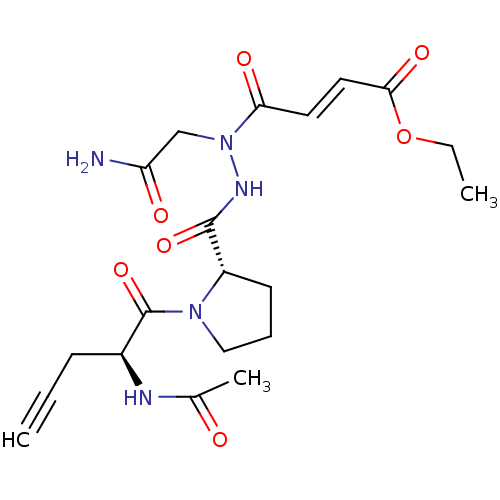 (CHEMBL1950273) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University School of Medicine Curated by ChEMBL | Assay Description Inhibition of mouse recombinant legumain using Cbz-Ala-Ala-Asn-AMC as substrate measured every 30 secs for 2.5 hrs by fluorimetry | Bioorg Med Chem Lett 22: 1340-3 (2012) Article DOI: 10.1016/j.bmcl.2011.12.079 BindingDB Entry DOI: 10.7270/Q2H132GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 1 (Mus musculus) | BDBM81407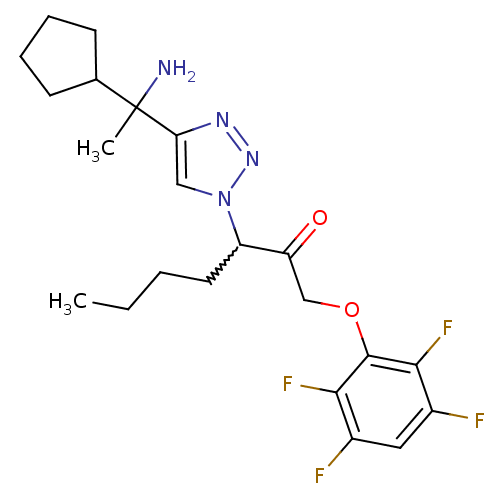 (ML4118 | ML4118R | ML4118S) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford School of Medicine | Assay Description DPAP1 inhibtion was measured using DPAP1-specific fluorogenic assay. IC50 DPAP1 were determined after 30 min incubation of parasite lysates with 5 n... | Chem Biol 17: 808-19 (2010) Article DOI: 10.1016/j.chembiol.2010.06.007 BindingDB Entry DOI: 10.7270/Q2R20ZTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM36812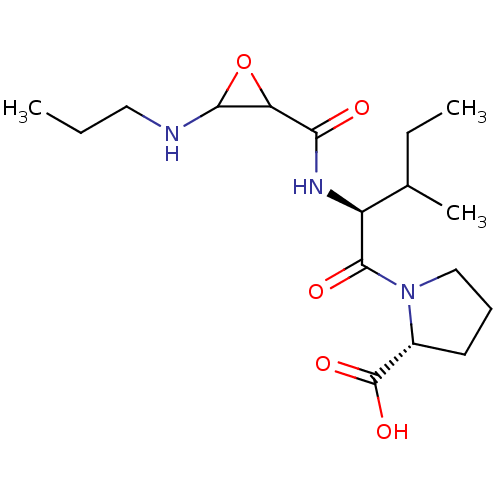 (CA-074) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | 5.5 | 25 |
University of California, San Francisco | Assay Description Inhibition assay using pre-treatment of recombinant cathepsin-L-like cysteine protease cruzain lacking the carboxy-terminal domain or cathepsin B fr... | Chem Biol 7: 27-38 (2000) Article DOI: 10.1016/s1074-5521(00)00061-2 BindingDB Entry DOI: 10.7270/Q24B2ZN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM36813 (CA-074b) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | 5.5 | 25 |
University of California, San Francisco | Assay Description Inhibition assay using pre-treatment of recombinant cathepsin-L-like cysteine protease cruzain lacking the carboxy-terminal domain or cathepsin B fr... | Chem Biol 7: 27-38 (2000) Article DOI: 10.1016/s1074-5521(00)00061-2 BindingDB Entry DOI: 10.7270/Q24B2ZN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Rattus norvegicus) | BDBM36327 (Z-FG-AOMK 9a) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Stanford University | Assay Description Protease enzyme inhibition assay targeting diverse member of the cysteine protease families:cathepsin B, Z, and H | Nat Chem Biol 1: 33-8 (2005) Article DOI: 10.1038/nchembio707 BindingDB Entry DOI: 10.7270/Q22V2DGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM228619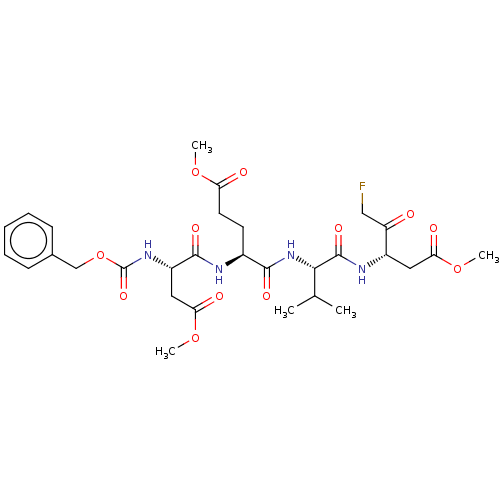 (US9345789, Z-DEVD-FMK) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 130 | n/a | n/a | n/a | n/a | 5.8 | n/a |
The Board of Trustees of the Leland Stanford Junior University US Patent | Assay Description Assay buffers consist of 20 mM citric acid, 60 mM disodium hydrogen orthophosphate, 1 mM EDTA, 0.1% CHAPS, 4 mM DTT, pH 5.8 for legumain, 50 mM dihyd... | US Patent US9345789 (2016) BindingDB Entry DOI: 10.7270/Q2610Z63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Mus musculus) | BDBM228619 (US9345789, Z-DEVD-FMK) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | 7.4 | 25 |
Stanford University | Assay Description IC50 measurements and enzyme kinetics assays were performed on a Spectramax M5 fluorescent plate reader (Molecular Devices). | ACS Chem Biol 5: 233-43 (2010) Article DOI: 10.1021/cb900232a BindingDB Entry DOI: 10.7270/Q2Z31X0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Legumain (Mus musculus) | BDBM50364354 (CHEMBL1950110) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 141 | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University School of Medicine Curated by ChEMBL | Assay Description Inhibition of mouse recombinant legumain using Cbz-Ala-Ala-Asn-AMC as substrate measured every 30 secs for 2.5 hrs by fluorimetry | Bioorg Med Chem Lett 22: 1340-3 (2012) Article DOI: 10.1016/j.bmcl.2011.12.079 BindingDB Entry DOI: 10.7270/Q2H132GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Legumain (Homo sapiens (Human)) | BDBM228617 (US9345789, LI-0) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 145 | n/a | n/a | n/a | n/a | 5.8 | n/a |
The Board of Trustees of the Leland Stanford Junior University US Patent | Assay Description Assay buffers consist of 20 mM citric acid, 60 mM disodium hydrogen orthophosphate, 1 mM EDTA, 0.1% CHAPS, 4 mM DTT, pH 5.8 for legumain, 50 mM dihyd... | US Patent US9345789 (2016) BindingDB Entry DOI: 10.7270/Q2610Z63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Legumain (Mus musculus) | BDBM50364355 (CHEMBL1950111) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 148 | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University School of Medicine Curated by ChEMBL | Assay Description Inhibition of mouse recombinant legumain using Cbz-Ala-Ala-Asn-AMC as substrate measured every 30 secs for 2.5 hrs by fluorimetry | Bioorg Med Chem Lett 22: 1340-3 (2012) Article DOI: 10.1016/j.bmcl.2011.12.079 BindingDB Entry DOI: 10.7270/Q2H132GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Legumain (Mus musculus) | BDBM50364360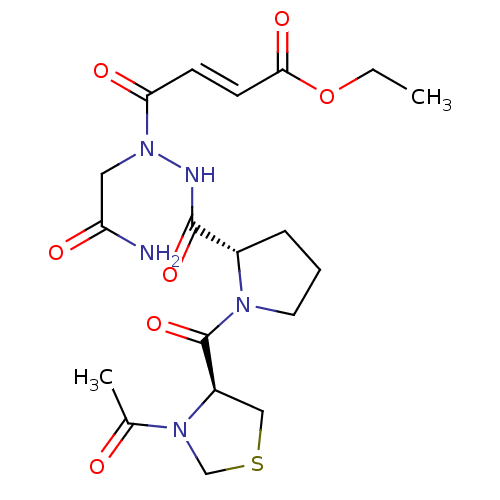 (CHEMBL1950275) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 167 | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University School of Medicine Curated by ChEMBL | Assay Description Inhibition of mouse recombinant legumain using Cbz-Ala-Ala-Asn-AMC as substrate measured every 30 secs for 2.5 hrs by fluorimetry | Bioorg Med Chem Lett 22: 1340-3 (2012) Article DOI: 10.1016/j.bmcl.2011.12.079 BindingDB Entry DOI: 10.7270/Q2H132GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 1 (Mus musculus) | BDBM81407 (ML4118 | ML4118R | ML4118S) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 186 | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford School of Medicine | Assay Description DPAP1 inhibtion was measured using DPAP1-specific fluorogenic assay. IC50 DPAP1 were determined after 30 min incubation of parasite lysates with 5 n... | Chem Biol 17: 808-19 (2010) Article DOI: 10.1016/j.chembiol.2010.06.007 BindingDB Entry DOI: 10.7270/Q2R20ZTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Legumain (Mus musculus) | BDBM50364357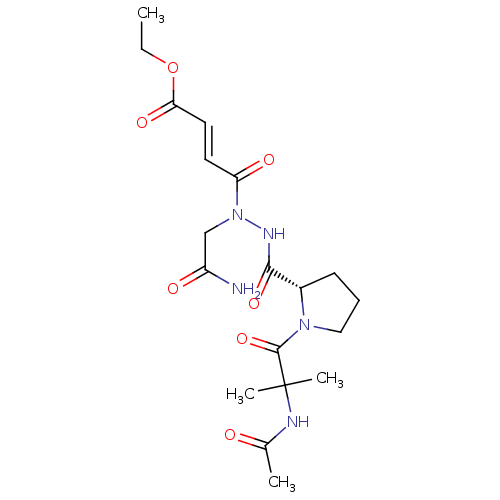 (CHEMBL1950272) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 198 | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University School of Medicine Curated by ChEMBL | Assay Description Inhibition of mouse recombinant legumain using Cbz-Ala-Ala-Asn-AMC as substrate measured every 30 secs for 2.5 hrs by fluorimetry | Bioorg Med Chem Lett 22: 1340-3 (2012) Article DOI: 10.1016/j.bmcl.2011.12.079 BindingDB Entry DOI: 10.7270/Q2H132GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Rattus norvegicus) | BDBM36330 (AC-YFG-AMOK 10a) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | 5.5 | 37 |
Stanford University | Assay Description Protease enzyme inhibition assay targeting diverse member of the cysteine protease families:cathepsin B, Z, and H | Nat Chem Biol 1: 33-8 (2005) Article DOI: 10.1038/nchembio707 BindingDB Entry DOI: 10.7270/Q22V2DGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM81515 (VEA-499) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 250 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Stanford University | Assay Description The fluorogenic substrate library screen was performed with hSENP1 in low salt tris buffer at final concentration of 2uM. Fluorophore release (AFC) ... | Chem Biol 18: 722-32 (2011) Article DOI: 10.1016/j.chembiol.2011.05.008 BindingDB Entry DOI: 10.7270/Q2542M2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Legumain (Mus musculus) | BDBM50364363 (CHEMBL1950278) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 267 | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University School of Medicine Curated by ChEMBL | Assay Description Inhibition of mouse recombinant legumain using Cbz-Ala-Ala-Asn-AMC as substrate measured every 30 secs for 2.5 hrs by fluorimetry | Bioorg Med Chem Lett 22: 1340-3 (2012) Article DOI: 10.1016/j.bmcl.2011.12.079 BindingDB Entry DOI: 10.7270/Q2H132GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM36814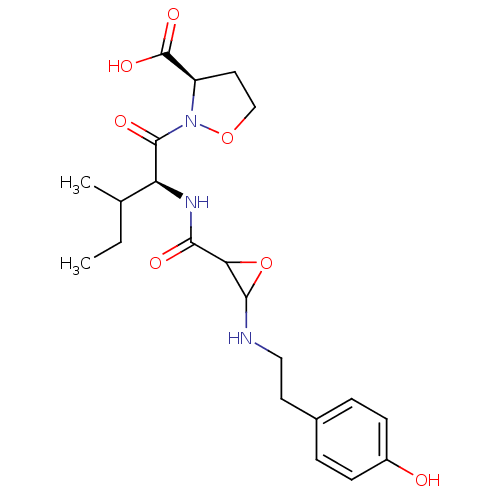 (MB-074) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | 5.5 | 25 |
University of California, San Francisco | Assay Description Inhibition assay using pre-treatment of recombinant cathepsin-L-like cysteine protease cruzain lacking the carboxy-terminal domain or cathepsin B fr... | Chem Biol 7: 27-38 (2000) Article DOI: 10.1016/s1074-5521(00)00061-2 BindingDB Entry DOI: 10.7270/Q24B2ZN5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 1 (Mus musculus) | BDBM81405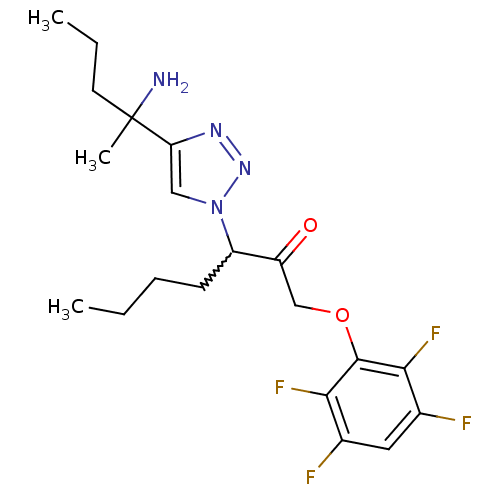 (ML4123) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford School of Medicine | Assay Description DPAP1 inhibtion was measured using DPAP1-specific fluorogenic assay. IC50 DPAP1 were determined after 30 min incubation of parasite lysates with 5 n... | Chem Biol 17: 808-19 (2010) Article DOI: 10.1016/j.chembiol.2010.06.007 BindingDB Entry DOI: 10.7270/Q2R20ZTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 1 (Mus musculus) | BDBM81404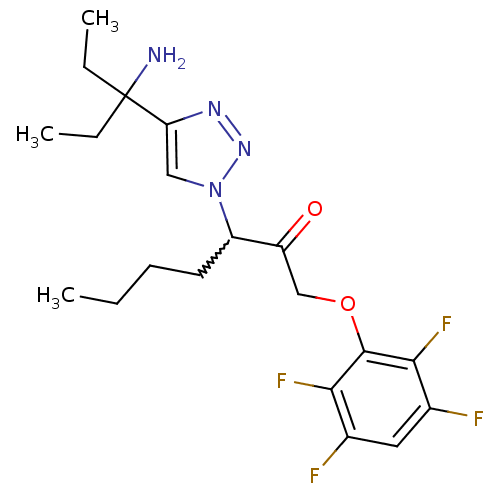 (ML4161) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford School of Medicine | Assay Description DPAP1 inhibtion was measured using DPAP1-specific fluorogenic assay. IC50 DPAP1 were determined after 30 min incubation of parasite lysates with 5 n... | Chem Biol 17: 808-19 (2010) Article DOI: 10.1016/j.chembiol.2010.06.007 BindingDB Entry DOI: 10.7270/Q2R20ZTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 1 (Mus musculus) | BDBM81414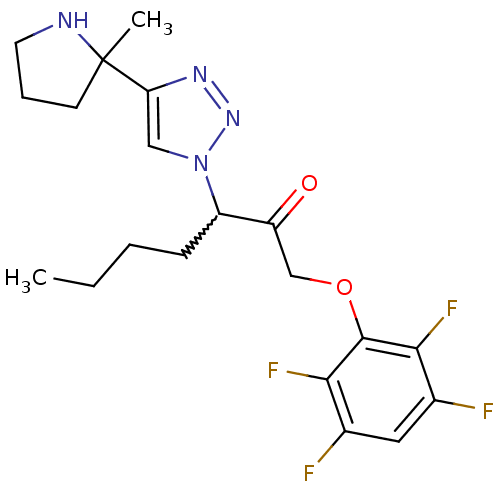 (ML6076) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford School of Medicine | Assay Description DPAP1 inhibtion was measured using DPAP1-specific fluorogenic assay. IC50 DPAP1 were determined after 30 min incubation of parasite lysates with 5 n... | Chem Biol 17: 808-19 (2010) Article DOI: 10.1016/j.chembiol.2010.06.007 BindingDB Entry DOI: 10.7270/Q2R20ZTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 1 (Mus musculus) | BDBM81403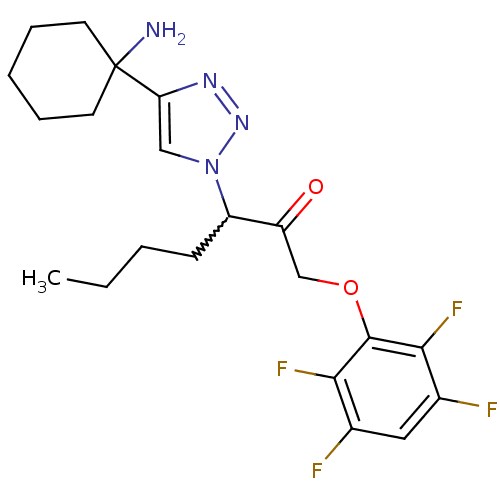 (HN3019) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford School of Medicine | Assay Description DPAP1 inhibtion was measured using DPAP1-specific fluorogenic assay. IC50 DPAP1 were determined after 30 min incubation of parasite lysates with 5 n... | Chem Biol 17: 808-19 (2010) Article DOI: 10.1016/j.chembiol.2010.06.007 BindingDB Entry DOI: 10.7270/Q2R20ZTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Legumain (Mus musculus) | BDBM50364359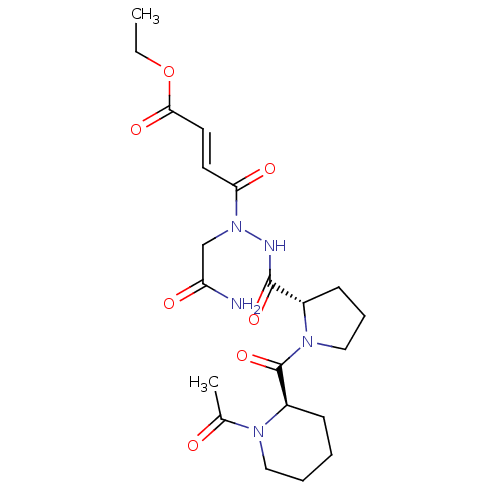 (CHEMBL1950274) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 638 | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford University School of Medicine Curated by ChEMBL | Assay Description Inhibition of mouse recombinant legumain using Cbz-Ala-Ala-Asn-AMC as substrate measured every 30 secs for 2.5 hrs by fluorimetry | Bioorg Med Chem Lett 22: 1340-3 (2012) Article DOI: 10.1016/j.bmcl.2011.12.079 BindingDB Entry DOI: 10.7270/Q2H132GV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 1 (Mus musculus) | BDBM81406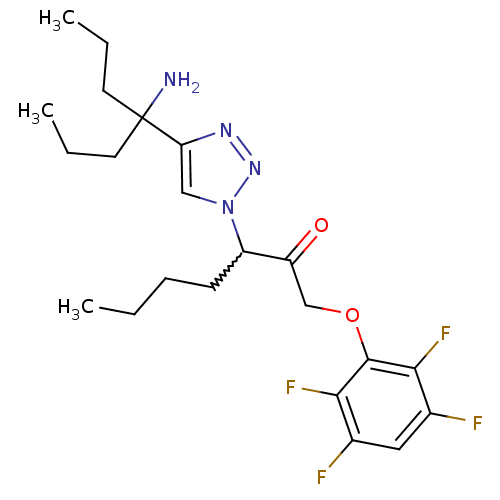 (ML4124) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford School of Medicine | Assay Description DPAP1 inhibtion was measured using DPAP1-specific fluorogenic assay. IC50 DPAP1 were determined after 30 min incubation of parasite lysates with 5 n... | Chem Biol 17: 808-19 (2010) Article DOI: 10.1016/j.chembiol.2010.06.007 BindingDB Entry DOI: 10.7270/Q2R20ZTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 1 (Mus musculus) | BDBM81397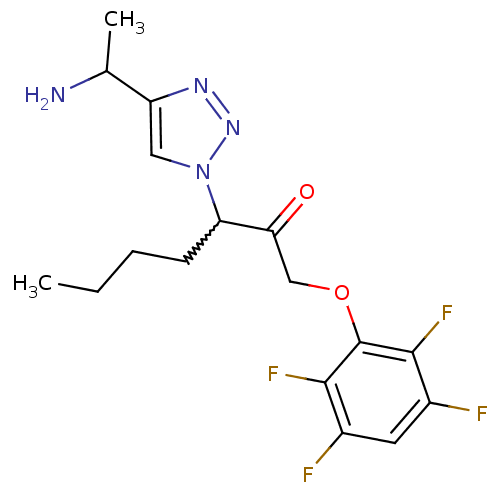 (ML4057) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford School of Medicine | Assay Description DPAP1 inhibtion was measured using DPAP1-specific fluorogenic assay. IC50 DPAP1 were determined after 30 min incubation of parasite lysates with 5 n... | Chem Biol 17: 808-19 (2010) Article DOI: 10.1016/j.chembiol.2010.06.007 BindingDB Entry DOI: 10.7270/Q2R20ZTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dipeptidyl peptidase 1 (Mus musculus) | BDBM81399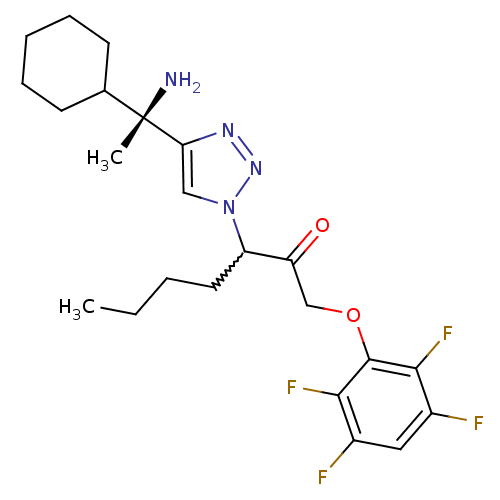 (ML4046A) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Stanford School of Medicine | Assay Description DPAP1 inhibtion was measured using DPAP1-specific fluorogenic assay. IC50 DPAP1 were determined after 30 min incubation of parasite lysates with 5 n... | Chem Biol 17: 808-19 (2010) Article DOI: 10.1016/j.chembiol.2010.06.007 BindingDB Entry DOI: 10.7270/Q2R20ZTG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Legumain (Mus musculus) | BDBM36333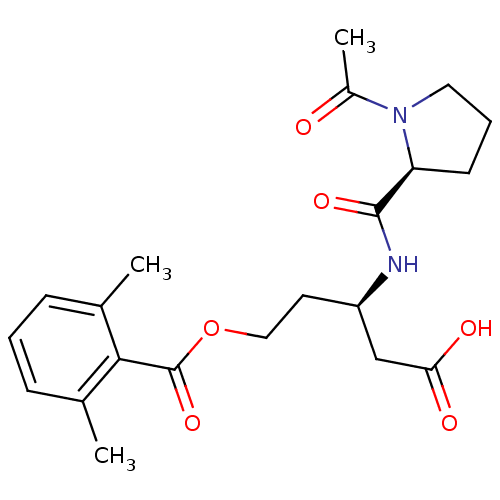 (LI-0 | Legumain Inhibitor -0) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 704 | n/a | n/a | n/a | n/a | 5.8 | 25 |
Stanford University | Assay Description IC50 measurements and enzyme kinetics assays were performed on a Spectramax M5 fluorescent plate reader (Molecular Devices). | ACS Chem Biol 5: 233-43 (2010) Article DOI: 10.1021/cb900232a BindingDB Entry DOI: 10.7270/Q2Z31X0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Legumain (Homo sapiens (Human)) | BDBM228617 (US9345789, LI-0) | PDB MMDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 704 | n/a | n/a | n/a | n/a | 5.8 | n/a |
The Board of Trustees of the Leland Stanford Junior University US Patent | Assay Description Assay buffers consist of 20 mM citric acid, 60 mM disodium hydrogen orthophosphate, 1 mM EDTA, 0.1% CHAPS, 4 mM DTT, pH 5.8 for legumain, 50 mM dihyd... | US Patent US9345789 (2016) BindingDB Entry DOI: 10.7270/Q2610Z63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Mus musculus) | BDBM36334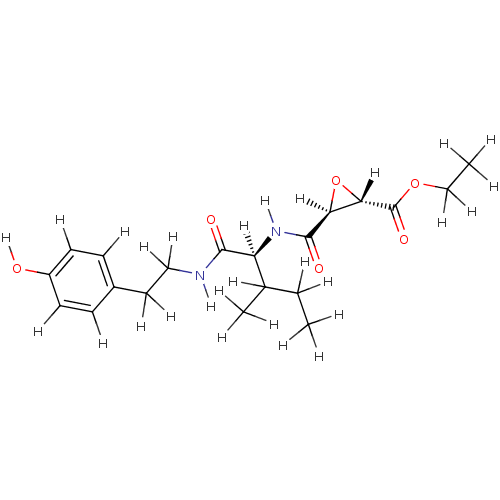 (CID644294 | JPM 565 | JPM-OEt | US9345789, JPM-Oet) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 780 | n/a | n/a | n/a | n/a | 6.25 | 25 |
Stanford University | Assay Description IC50 measurements and enzyme kinetics assays were performed on a Spectramax M5 fluorescent plate reader (Molecular Devices). | ACS Chem Biol 5: 233-43 (2010) Article DOI: 10.1021/cb900232a BindingDB Entry DOI: 10.7270/Q2Z31X0W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cathepsin B (Homo sapiens (Human)) | BDBM36334 (CID644294 | JPM 565 | JPM-OEt | US9345789, JPM-Oet) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 780 | n/a | n/a | n/a | n/a | 5.8 | n/a |
The Board of Trustees of the Leland Stanford Junior University US Patent | Assay Description Assay buffers consist of 20 mM citric acid, 60 mM disodium hydrogen orthophosphate, 1 mM EDTA, 0.1% CHAPS, 4 mM DTT, pH 5.8 for legumain, 50 mM dihyd... | US Patent US9345789 (2016) BindingDB Entry DOI: 10.7270/Q2610Z63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sentrin-specific protease 2 (Homo sapiens (Human)) | BDBM81516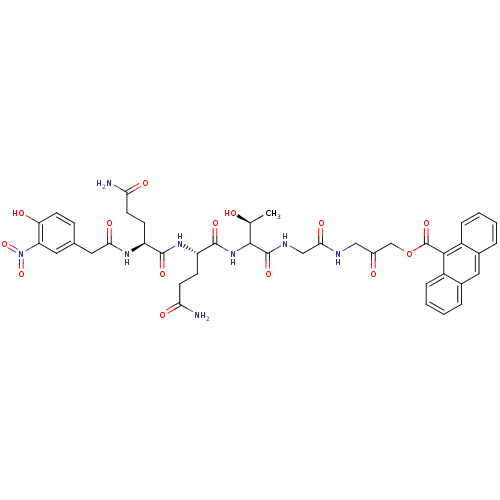 (VEA-500) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 860 | n/a | n/a | n/a | n/a | 8.0 | 37 |
Stanford University | Assay Description The fluorogenic substrate library screen was performed with hSENP1 in low salt tris buffer at final concentration of 2uM. Fluorophore release (AFC) ... | Chem Biol 18: 722-32 (2011) Article DOI: 10.1016/j.chembiol.2011.05.008 BindingDB Entry DOI: 10.7270/Q2542M2N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 125 total ) | Next | Last >> |