 Found 151 hits with Last Name = 'bone' and Initial = 'e'
Found 151 hits with Last Name = 'bone' and Initial = 'e' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50290436
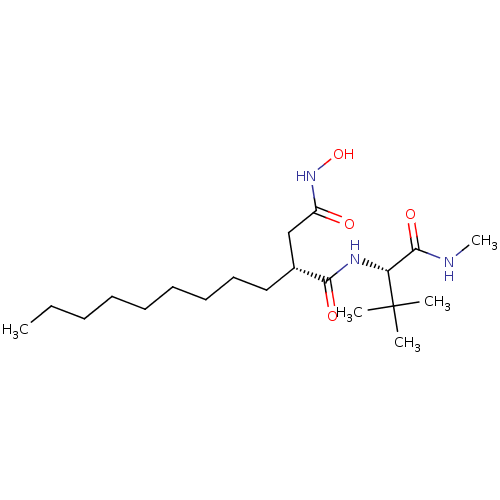
((R)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-propy...)Show SMILES CCCCCCCCC[C@H](CC(=O)NO)C(=O)N[C@H](C(=O)NC)C(C)(C)C Show InChI InChI=1S/C20H39N3O4/c1-6-7-8-9-10-11-12-13-15(14-16(24)23-27)18(25)22-17(19(26)21-5)20(2,3)4/h15,17,27H,6-14H2,1-5H3,(H,21,26)(H,22,25)(H,23,24)/t15-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | <0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound concentration for 50% inhibition of human recombinant gelatinase A (MMP-2). |
Bioorg Med Chem Lett 7: 193-198 (1997)
Article DOI: 10.1016/S0960-894X(96)00602-6
BindingDB Entry DOI: 10.7270/Q2057FXG |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50031790

((R)-N*4*-Hydroxy-N*1*-((S)-1-methylcarbamoyl-2-phe...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCCCOc1ccccc1)CC(=O)NO Show InChI InChI=1S/C25H33N3O5/c1-26-25(31)22(17-19-11-5-2-6-12-19)27-24(30)20(18-23(29)28-32)13-7-4-10-16-33-21-14-8-3-9-15-21/h2-3,5-6,8-9,11-12,14-15,20,22,32H,4,7,10,13,16-18H2,1H3,(H,26,31)(H,27,30)(H,28,29)/t20-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound concentration for 50% inhibition of human recombinant gelatinase A (MMP-2). |
Bioorg Med Chem Lett 7: 193-198 (1997)
Article DOI: 10.1016/S0960-894X(96)00602-6
BindingDB Entry DOI: 10.7270/Q2057FXG |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50290448
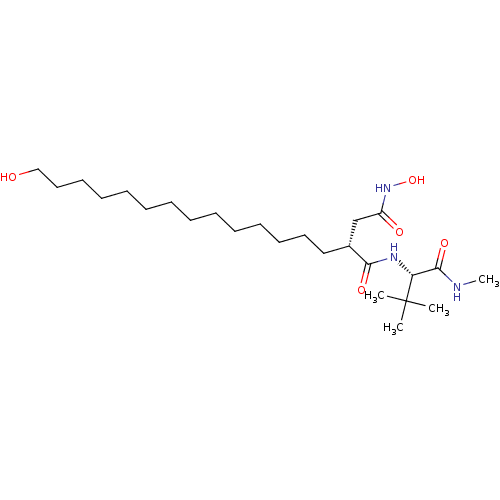
((R)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-propy...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CCCCCCCCCCCCCCO)CC(=O)NO)C(C)(C)C Show InChI InChI=1S/C25H49N3O5/c1-25(2,3)22(24(32)26-4)27-23(31)20(19-21(30)28-33)17-15-13-11-9-7-5-6-8-10-12-14-16-18-29/h20,22,29,33H,5-19H2,1-4H3,(H,26,32)(H,27,31)(H,28,30)/t20-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound concentration for 50% inhibition of human recombinant gelatinase A (MMP-2). |
Bioorg Med Chem Lett 7: 193-198 (1997)
Article DOI: 10.1016/S0960-894X(96)00602-6
BindingDB Entry DOI: 10.7270/Q2057FXG |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50290441
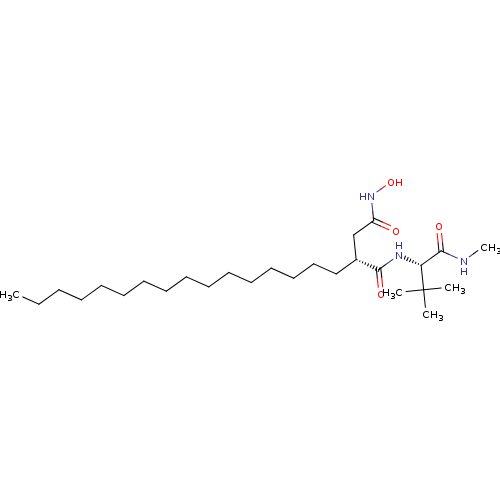
((R)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-propy...)Show SMILES CCCCCCCCCCCCCCCC[C@H](CC(=O)NO)C(=O)N[C@H](C(=O)NC)C(C)(C)C Show InChI InChI=1S/C27H53N3O4/c1-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-22(21-23(31)30-34)25(32)29-24(26(33)28-5)27(2,3)4/h22,24,34H,6-21H2,1-5H3,(H,28,33)(H,29,32)(H,30,31)/t22-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound concentration for 50% inhibition of human recombinant gelatinase A (MMP-2). |
Bioorg Med Chem Lett 7: 193-198 (1997)
Article DOI: 10.1016/S0960-894X(96)00602-6
BindingDB Entry DOI: 10.7270/Q2057FXG |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50290442
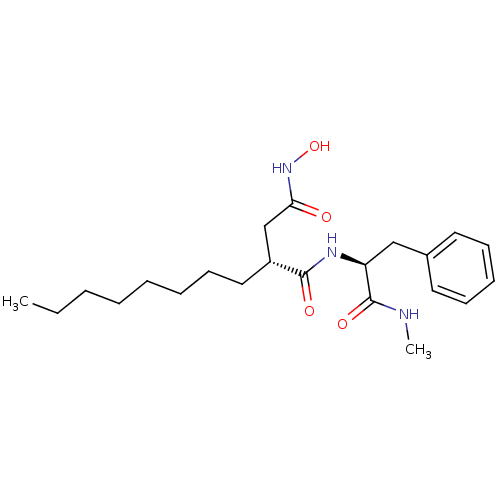
((R)-N*4*-Hydroxy-N*1*-((S)-1-methylcarbamoyl-2-phe...)Show SMILES CCCCCCCC[C@H](CC(=O)NO)C(=O)N[C@@H](Cc1ccccc1)C(=O)NC Show InChI InChI=1S/C22H35N3O4/c1-3-4-5-6-7-11-14-18(16-20(26)25-29)21(27)24-19(22(28)23-2)15-17-12-9-8-10-13-17/h8-10,12-13,18-19,29H,3-7,11,14-16H2,1-2H3,(H,23,28)(H,24,27)(H,25,26)/t18-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound concentration for 50% inhibition of human recombinant gelatinase A (MMP-2). |
Bioorg Med Chem Lett 7: 193-198 (1997)
Article DOI: 10.1016/S0960-894X(96)00602-6
BindingDB Entry DOI: 10.7270/Q2057FXG |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50290439

((R)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-propy...)Show SMILES CCCCCCCC[C@H](CC(=O)NO)C(=O)N[C@H](C(=O)NC)C(C)(C)C Show InChI InChI=1S/C19H37N3O4/c1-6-7-8-9-10-11-12-14(13-15(23)22-26)17(24)21-16(18(25)20-5)19(2,3)4/h14,16,26H,6-13H2,1-5H3,(H,20,25)(H,21,24)(H,22,23)/t14-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound concentration for 50% inhibition of human recombinant gelatinase A (MMP-2). |
Bioorg Med Chem Lett 7: 193-198 (1997)
Article DOI: 10.1016/S0960-894X(96)00602-6
BindingDB Entry DOI: 10.7270/Q2057FXG |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50290449
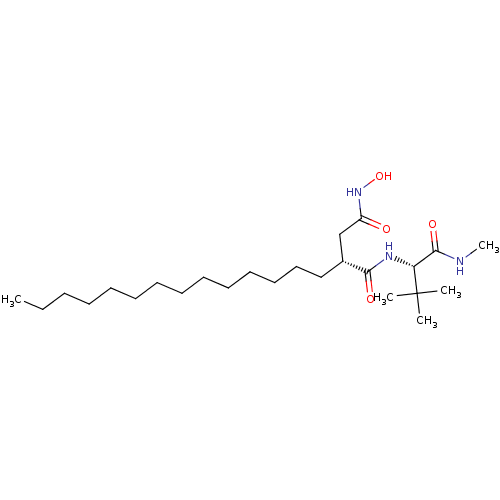
((R)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-propy...)Show SMILES CCCCCCCCCCCCCC[C@H](CC(=O)NO)C(=O)N[C@H](C(=O)NC)C(C)(C)C Show InChI InChI=1S/C25H49N3O4/c1-6-7-8-9-10-11-12-13-14-15-16-17-18-20(19-21(29)28-32)23(30)27-22(24(31)26-5)25(2,3)4/h20,22,32H,6-19H2,1-5H3,(H,26,31)(H,27,30)(H,28,29)/t20-,22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound concentration for 50% inhibition of human recombinant gelatinase A (MMP-2). |
Bioorg Med Chem Lett 7: 193-198 (1997)
Article DOI: 10.1016/S0960-894X(96)00602-6
BindingDB Entry DOI: 10.7270/Q2057FXG |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50290450

((R)-2-Dodecyl-N*4*-hydroxy-N*1*-((S)-1-methylcarba...)Show SMILES CCCCCCCCCCCC[C@H](CC(=O)NO)C(=O)N[C@@H](Cc1ccccc1)C(=O)NC Show InChI InChI=1S/C26H43N3O4/c1-3-4-5-6-7-8-9-10-11-15-18-22(20-24(30)29-33)25(31)28-23(26(32)27-2)19-21-16-13-12-14-17-21/h12-14,16-17,22-23,33H,3-11,15,18-20H2,1-2H3,(H,27,32)(H,28,31)(H,29,30)/t22-,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound concentration for 50% inhibition of human recombinant gelatinase A (MMP-2). |
Bioorg Med Chem Lett 7: 193-198 (1997)
Article DOI: 10.1016/S0960-894X(96)00602-6
BindingDB Entry DOI: 10.7270/Q2057FXG |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50290433

((R)-N*4*-Hydroxy-N*1*-((S)-1-methylcarbamoyl-2-phe...)Show SMILES CCCCCCCCC[C@H](CC(=O)NO)C(=O)N[C@@H](Cc1ccccc1)C(=O)NC Show InChI InChI=1S/C23H37N3O4/c1-3-4-5-6-7-8-12-15-19(17-21(27)26-30)22(28)25-20(23(29)24-2)16-18-13-10-9-11-14-18/h9-11,13-14,19-20,30H,3-8,12,15-17H2,1-2H3,(H,24,29)(H,25,28)(H,26,27)/t19-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound concentration for 50% inhibition of human recombinant gelatinase A (MMP-2). |
Bioorg Med Chem Lett 7: 193-198 (1997)
Article DOI: 10.1016/S0960-894X(96)00602-6
BindingDB Entry DOI: 10.7270/Q2057FXG |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50290456
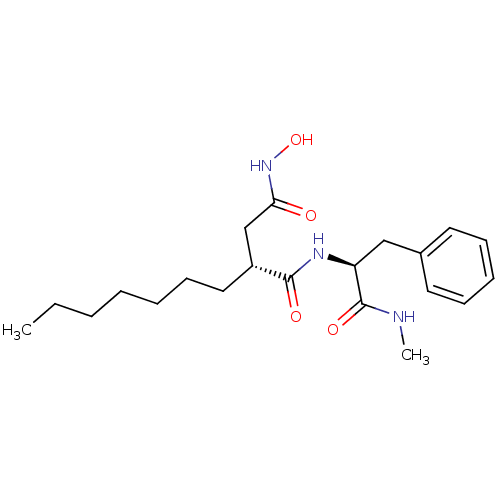
((R)-2-Heptyl-N*4*-hydroxy-N*1*-((S)-1-methylcarbam...)Show SMILES CCCCCCC[C@H](CC(=O)NO)C(=O)N[C@@H](Cc1ccccc1)C(=O)NC Show InChI InChI=1S/C21H33N3O4/c1-3-4-5-6-10-13-17(15-19(25)24-28)20(26)23-18(21(27)22-2)14-16-11-8-7-9-12-16/h7-9,11-12,17-18,28H,3-6,10,13-15H2,1-2H3,(H,22,27)(H,23,26)(H,24,25)/t17-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound concentration for 50% inhibition of human recombinant gelatinase A (MMP-2). |
Bioorg Med Chem Lett 7: 193-198 (1997)
Article DOI: 10.1016/S0960-894X(96)00602-6
BindingDB Entry DOI: 10.7270/Q2057FXG |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50290436

((R)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-propy...)Show SMILES CCCCCCCCC[C@H](CC(=O)NO)C(=O)N[C@H](C(=O)NC)C(C)(C)C Show InChI InChI=1S/C20H39N3O4/c1-6-7-8-9-10-11-12-13-15(14-16(24)23-27)18(25)22-17(19(26)21-5)20(2,3)4/h15,17,27H,6-14H2,1-5H3,(H,21,26)(H,22,25)(H,23,24)/t15-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant Gelatinase B (MMP-9) |
Bioorg Med Chem Lett 7: 193-198 (1997)
Article DOI: 10.1016/S0960-894X(96)00602-6
BindingDB Entry DOI: 10.7270/Q2057FXG |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50290453
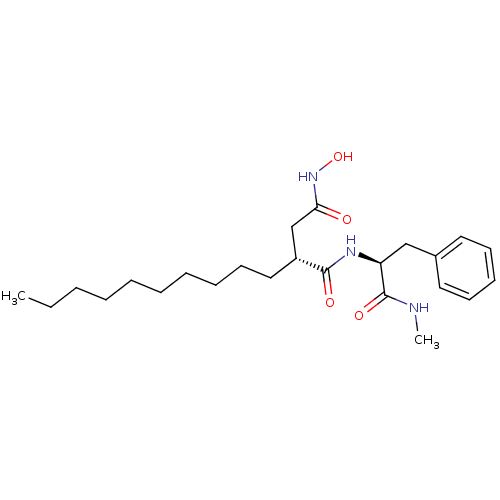
((R)-2-Decyl-N*4*-hydroxy-N*1*-((S)-1-methylcarbamo...)Show SMILES CCCCCCCCCC[C@H](CC(=O)NO)C(=O)N[C@@H](Cc1ccccc1)C(=O)NC Show InChI InChI=1S/C24H39N3O4/c1-3-4-5-6-7-8-9-13-16-20(18-22(28)27-31)23(29)26-21(24(30)25-2)17-19-14-11-10-12-15-19/h10-12,14-15,20-21,31H,3-9,13,16-18H2,1-2H3,(H,25,30)(H,26,29)(H,27,28)/t20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound concentration for 50% inhibition of human recombinant gelatinase A (MMP-2). |
Bioorg Med Chem Lett 7: 193-198 (1997)
Article DOI: 10.1016/S0960-894X(96)00602-6
BindingDB Entry DOI: 10.7270/Q2057FXG |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50455629

(CHEMBL487720)Show SMILES [Na+].[H][C@@](O)(CC[C@@]1([H])[C@@H](C)C=CC2=C[C@H](C)C[C@H](OC(=O)C(C)(C)CC)[C@]12[H])C[C@@H](O)CC([O-])=O |r,c:9,t:11| Show InChI InChI=1S/C25H40O6.Na/c1-6-25(4,5)24(30)31-21-12-15(2)11-17-8-7-16(3)20(23(17)21)10-9-18(26)13-19(27)14-22(28)29;/h7-8,11,15-16,18-21,23,26-27H,6,9-10,12-14H2,1-5H3,(H,28,29);/q;+1/p-1/t15-,16-,18+,19+,20-,21-,23-;/m0./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
British Bio-technology Limited
Curated by ChEMBL
| Assay Description
Inhibitory activity against HMG-CoA reductase from rat liver microsomal preparation |
J Med Chem 35: 3388-93 (1992)
BindingDB Entry DOI: 10.7270/Q2HD7W9V |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50290440
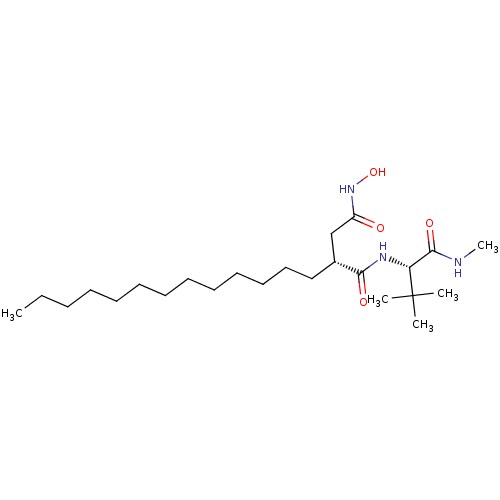
((R)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-propy...)Show SMILES CCCCCCCCCCCCC[C@H](CC(=O)NO)C(=O)N[C@H](C(=O)NC)C(C)(C)C Show InChI InChI=1S/C24H47N3O4/c1-6-7-8-9-10-11-12-13-14-15-16-17-19(18-20(28)27-31)22(29)26-21(23(30)25-5)24(2,3)4/h19,21,31H,6-18H2,1-5H3,(H,25,30)(H,26,29)(H,27,28)/t19-,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound concentration for 50% inhibition of human recombinant gelatinase A (MMP-2). |
Bioorg Med Chem Lett 7: 193-198 (1997)
Article DOI: 10.1016/S0960-894X(96)00602-6
BindingDB Entry DOI: 10.7270/Q2057FXG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50347385
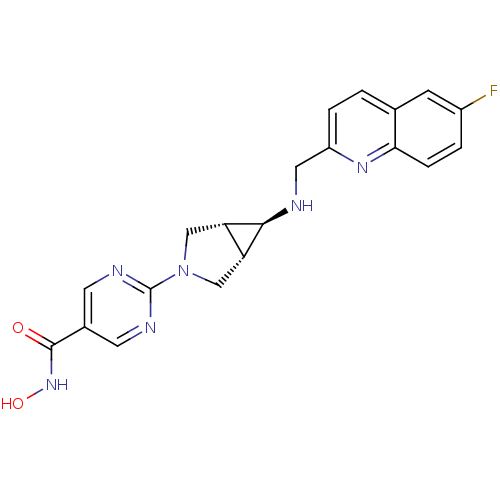
(CHEMBL1801250)Show SMILES ONC(=O)c1cnc(nc1)N1C[C@H]2[C@@H](C1)[C@@H]2NCc1ccc2cc(F)ccc2n1 |r| Show InChI InChI=1S/C20H19FN6O2/c21-13-2-4-17-11(5-13)1-3-14(25-17)8-22-18-15-9-27(10-16(15)18)20-23-6-12(7-24-20)19(28)26-29/h1-7,15-16,18,22,29H,8-10H2,(H,26,28)/t15-,16+,18+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Chroma Therapeutics Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using fluor de Lys as substrate by fluorometric analysis |
J Med Chem 53: 8663-78 (2010)
Article DOI: 10.1021/jm101177s
BindingDB Entry DOI: 10.7270/Q2G1616P |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50280355

(2,2-Dimethyl-butyric acid (1S,3S,4aR,7S,8S,8aS)-8-...)Show SMILES CCC(C)(C)C(=O)O[C@H]1C[C@H](C[C@@H]2C=C[C@H](C)[C@H](CC[C@@H]3C[C@@H](O)CC(=O)O3)[C@@H]12)\C=C\C |c:13| Show InChI InChI=1S/C27H42O5/c1-6-8-18-13-19-10-9-17(3)22(12-11-21-15-20(28)16-24(29)31-21)25(19)23(14-18)32-26(30)27(4,5)7-2/h6,8-10,17-23,25,28H,7,11-16H2,1-5H3/b8-6+/t17-,18-,19-,20+,21+,22-,23-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of microsomal rat liver HMG-CoA reductase |
Bioorg Med Chem Lett 2: 223-228 (1992)
Article DOI: 10.1016/S0960-894X(01)81069-6
BindingDB Entry DOI: 10.7270/Q28C9W59 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50063918

((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-1-methyl...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)[C@H](CSc1cccs1)C(=O)NO Show InChI InChI=1S/C23H31N3O4S2/c1-15(2)12-17(18(22(28)26-30)14-32-20-10-7-11-31-20)21(27)25-19(23(29)24-3)13-16-8-5-4-6-9-16/h4-11,15,17-19,30H,12-14H2,1-3H3,(H,24,29)(H,25,27)(H,26,28)/t17-,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound concentration for 50% inhibition of human recombinant gelatinase A (MMP-2). |
Bioorg Med Chem Lett 7: 193-198 (1997)
Article DOI: 10.1016/S0960-894X(96)00602-6
BindingDB Entry DOI: 10.7270/Q2057FXG |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50280362
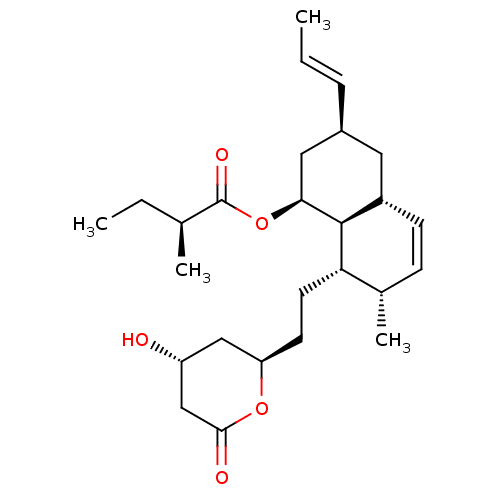
((S)-2-Methyl-butyric acid (1S,3S,4aR,7S,8S,8aS)-8-...)Show SMILES CC[C@H](C)C(=O)O[C@H]1C[C@H](C[C@@H]2C=C[C@H](C)[C@H](CC[C@@H]3C[C@@H](O)CC(=O)O3)[C@@H]12)\C=C\C |c:12| Show InChI InChI=1S/C26H40O5/c1-5-7-18-12-19-9-8-17(4)22(11-10-21-14-20(27)15-24(28)30-21)25(19)23(13-18)31-26(29)16(3)6-2/h5,7-9,16-23,25,27H,6,10-15H2,1-4H3/b7-5+/t16-,17-,18-,19-,20+,21+,22-,23-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of microsomal rat liver HMG-CoA reductase |
Bioorg Med Chem Lett 2: 223-228 (1992)
Article DOI: 10.1016/S0960-894X(01)81069-6
BindingDB Entry DOI: 10.7270/Q28C9W59 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50347385

(CHEMBL1801250)Show SMILES ONC(=O)c1cnc(nc1)N1C[C@H]2[C@@H](C1)[C@@H]2NCc1ccc2cc(F)ccc2n1 |r| Show InChI InChI=1S/C20H19FN6O2/c21-13-2-4-17-11(5-13)1-3-14(25-17)8-22-18-15-9-27(10-16(15)18)20-23-6-12(7-24-20)19(28)26-29/h1-7,15-16,18,22,29H,8-10H2,(H,26,28)/t15-,16+,18+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Chroma Therapeutics Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 using fluor de Lys as substrate by fluorometric analysis |
J Med Chem 53: 8663-78 (2010)
Article DOI: 10.1021/jm101177s
BindingDB Entry DOI: 10.7270/Q2G1616P |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50063917

((2S,3R)-N(4)-[(2S)-3,3-dimethyl-1-(methylamino)-1-...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)[C@H](O)C(=O)NO)C(C)(C)C |r| Show InChI InChI=1S/C15H29N3O5/c1-8(2)7-9(10(19)13(21)18-23)12(20)17-11(14(22)16-6)15(3,4)5/h8-11,19,23H,7H2,1-6H3,(H,16,22)(H,17,20)(H,18,21)/t9-,10+,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
50% inhibition of human recombinant fibroblast collagenase (MMP-1, HFC) |
Bioorg Med Chem Lett 7: 193-198 (1997)
Article DOI: 10.1016/S0960-894X(96)00602-6
BindingDB Entry DOI: 10.7270/Q2057FXG |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50280356
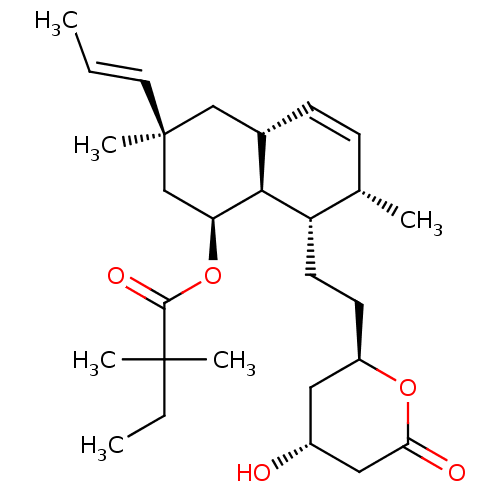
(2,2-Dimethyl-butyric acid (1S,3S,4aR,7S,8S,8aS)-8-...)Show SMILES CCC(C)(C)C(=O)O[C@H]1C[C@](C)(C[C@@H]2C=C[C@H](C)[C@H](CC[C@@H]3C[C@@H](O)CC(=O)O3)[C@@H]12)\C=C\C |c:14| Show InChI InChI=1S/C28H44O5/c1-7-13-28(6)16-19-10-9-18(3)22(12-11-21-14-20(29)15-24(30)32-21)25(19)23(17-28)33-26(31)27(4,5)8-2/h7,9-10,13,18-23,25,29H,8,11-12,14-17H2,1-6H3/b13-7+/t18-,19-,20+,21+,22-,23-,25-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of microsomal rat liver HMG-CoA reductase |
Bioorg Med Chem Lett 2: 223-228 (1992)
Article DOI: 10.1016/S0960-894X(01)81069-6
BindingDB Entry DOI: 10.7270/Q28C9W59 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50063918

((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-1-methyl...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)[C@H](CSc1cccs1)C(=O)NO Show InChI InChI=1S/C23H31N3O4S2/c1-15(2)12-17(18(22(28)26-30)14-32-20-10-7-11-31-20)21(27)25-19(23(29)24-3)13-16-8-5-4-6-9-16/h4-11,15,17-19,30H,12-14H2,1-3H3,(H,24,29)(H,25,27)(H,26,28)/t17-,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
50% inhibition of human recombinant fibroblast collagenase (MMP-1, HFC) |
Bioorg Med Chem Lett 7: 193-198 (1997)
Article DOI: 10.1016/S0960-894X(96)00602-6
BindingDB Entry DOI: 10.7270/Q2057FXG |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50063917

((2S,3R)-N(4)-[(2S)-3,3-dimethyl-1-(methylamino)-1-...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)[C@H](O)C(=O)NO)C(C)(C)C |r| Show InChI InChI=1S/C15H29N3O5/c1-8(2)7-9(10(19)13(21)18-23)12(20)17-11(14(22)16-6)15(3,4)5/h8-11,19,23H,7H2,1-6H3,(H,16,22)(H,17,20)(H,18,21)/t9-,10+,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound concentration for 50% inhibition of human recombinant gelatinase A (MMP-2). |
Bioorg Med Chem Lett 7: 193-198 (1997)
Article DOI: 10.1016/S0960-894X(96)00602-6
BindingDB Entry DOI: 10.7270/Q2057FXG |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50063918

((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-1-methyl...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)[C@H](CSc1cccs1)C(=O)NO Show InChI InChI=1S/C23H31N3O4S2/c1-15(2)12-17(18(22(28)26-30)14-32-20-10-7-11-31-20)21(27)25-19(23(29)24-3)13-16-8-5-4-6-9-16/h4-11,15,17-19,30H,12-14H2,1-3H3,(H,24,29)(H,25,27)(H,26,28)/t17-,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
50% inhibition of human recombinant Matrilysin |
Bioorg Med Chem Lett 7: 193-198 (1997)
Article DOI: 10.1016/S0960-894X(96)00602-6
BindingDB Entry DOI: 10.7270/Q2057FXG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50347384
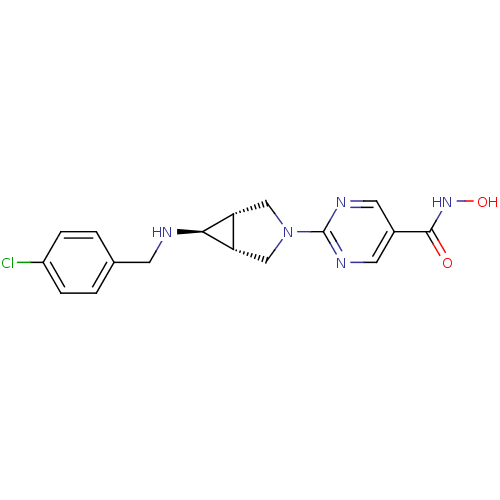
(CHEMBL1801238)Show SMILES ONC(=O)c1cnc(nc1)N1C[C@H]2[C@@H](C1)[C@@H]2NCc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C17H18ClN5O2/c18-12-3-1-10(2-4-12)5-19-15-13-8-23(9-14(13)15)17-20-6-11(7-21-17)16(24)22-25/h1-4,6-7,13-15,19,25H,5,8-9H2,(H,22,24)/t13-,14+,15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Chroma Therapeutics Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 using fluor de Lys as substrate by fluorometric analysis |
J Med Chem 53: 8663-78 (2010)
Article DOI: 10.1021/jm101177s
BindingDB Entry DOI: 10.7270/Q2G1616P |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50347385

(CHEMBL1801250)Show SMILES ONC(=O)c1cnc(nc1)N1C[C@H]2[C@@H](C1)[C@@H]2NCc1ccc2cc(F)ccc2n1 |r| Show InChI InChI=1S/C20H19FN6O2/c21-13-2-4-17-11(5-13)1-3-14(25-17)8-22-18-15-9-27(10-16(15)18)20-23-6-12(7-24-20)19(28)26-29/h1-7,15-16,18,22,29H,8-10H2,(H,26,28)/t15-,16+,18+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Chroma Therapeutics Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3 using fluor de Lys as substrate by fluorometric analysis |
J Med Chem 53: 8663-78 (2010)
Article DOI: 10.1021/jm101177s
BindingDB Entry DOI: 10.7270/Q2G1616P |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50280350
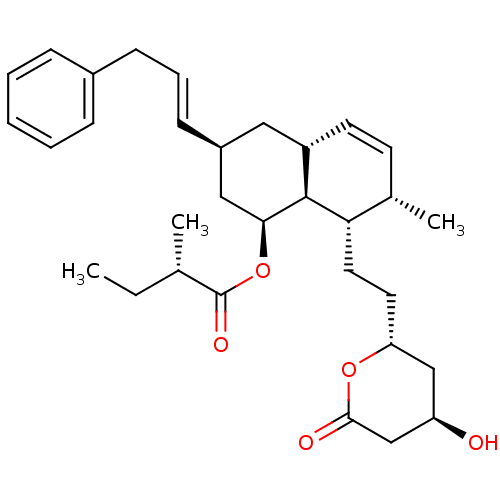
((S)-2-Methyl-butyric acid (1S,3S,4aR,7S,8S,8aS)-8-...)Show SMILES CC[C@H](C)C(=O)O[C@H]1C[C@H](C[C@@H]2C=C[C@H](C)[C@H](CC[C@@H]3C[C@@H](O)CC(=O)O3)[C@@H]12)\C=C\Cc1ccccc1 |c:12| Show InChI InChI=1S/C32H44O5/c1-4-21(2)32(35)37-29-18-24(12-8-11-23-9-6-5-7-10-23)17-25-14-13-22(3)28(31(25)29)16-15-27-19-26(33)20-30(34)36-27/h5-10,12-14,21-22,24-29,31,33H,4,11,15-20H2,1-3H3/b12-8+/t21-,22-,24-,25-,26+,27+,28-,29-,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of microsomal rat liver HMG-CoA reductase |
Bioorg Med Chem Lett 2: 223-228 (1992)
Article DOI: 10.1016/S0960-894X(01)81069-6
BindingDB Entry DOI: 10.7270/Q28C9W59 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50280364

(2,2-Dimethyl-butyric acid (1S,3S,4aR,7S,8S,8aS)-8-...)Show SMILES CCC\C(C)=C\[C@@H]1C[C@H](OC(=O)C(C)(C)CC)[C@H]2[C@H](C1)C=C[C@H](C)[C@@H]2CC[C@@H]1C[C@@H](O)CC(=O)O1 |c:21| Show InChI InChI=1S/C30H48O5/c1-7-9-19(3)14-21-15-22-11-10-20(4)25(13-12-24-17-23(31)18-27(32)34-24)28(22)26(16-21)35-29(33)30(5,6)8-2/h10-11,14,20-26,28,31H,7-9,12-13,15-18H2,1-6H3/b19-14+/t20-,21-,22-,23+,24+,25-,26-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of microsomal rat liver HMG-CoA reductase |
Bioorg Med Chem Lett 2: 223-228 (1992)
Article DOI: 10.1016/S0960-894X(01)81069-6
BindingDB Entry DOI: 10.7270/Q28C9W59 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50347384

(CHEMBL1801238)Show SMILES ONC(=O)c1cnc(nc1)N1C[C@H]2[C@@H](C1)[C@@H]2NCc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C17H18ClN5O2/c18-12-3-1-10(2-4-12)5-19-15-13-8-23(9-14(13)15)17-20-6-11(7-21-17)16(24)22-25/h1-4,6-7,13-15,19,25H,5,8-9H2,(H,22,24)/t13-,14+,15+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Chroma Therapeutics Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC3 using fluor de Lys as substrate by fluorometric analysis |
J Med Chem 53: 8663-78 (2010)
Article DOI: 10.1021/jm101177s
BindingDB Entry DOI: 10.7270/Q2G1616P |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50290438
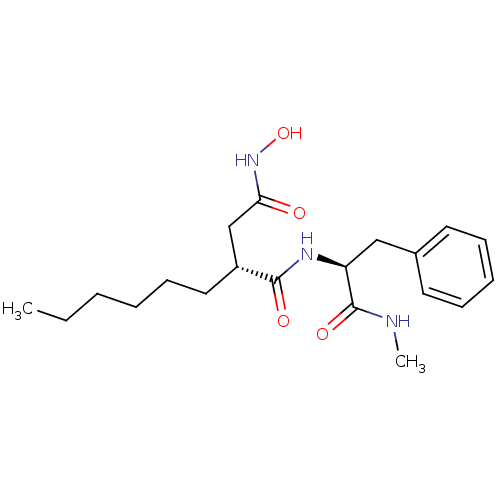
((R)-2-Hexyl-N*4*-hydroxy-N*1*-((S)-1-methylcarbamo...)Show SMILES CCCCCC[C@H](CC(=O)NO)C(=O)N[C@@H](Cc1ccccc1)C(=O)NC Show InChI InChI=1S/C20H31N3O4/c1-3-4-5-9-12-16(14-18(24)23-27)19(25)22-17(20(26)21-2)13-15-10-7-6-8-11-15/h6-8,10-11,16-17,27H,3-5,9,12-14H2,1-2H3,(H,21,26)(H,22,25)(H,23,24)/t16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound concentration for 50% inhibition of human recombinant gelatinase A (MMP-2). |
Bioorg Med Chem Lett 7: 193-198 (1997)
Article DOI: 10.1016/S0960-894X(96)00602-6
BindingDB Entry DOI: 10.7270/Q2057FXG |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50004774
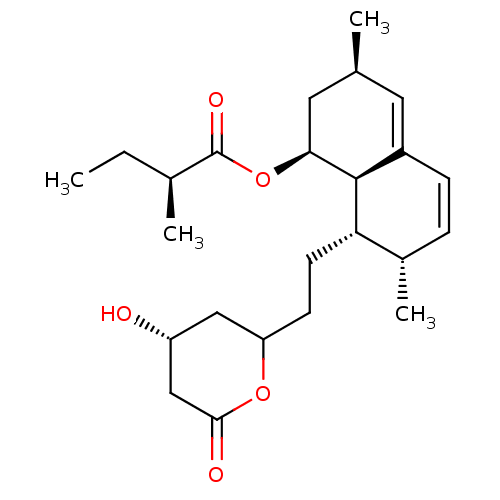
((S)-2-Methyl-butyric acid (1S,3R,7S,8S,8aR)-8-[2-(...)Show SMILES CC[C@H](C)C(=O)O[C@H]1C[C@@H](C)C=C2C=C[C@H](C)[C@H](CCC3C[C@@H](O)CC(=O)O3)[C@@H]12 |c:13,t:11| Show InChI InChI=1S/C24H36O5/c1-5-15(3)24(27)29-21-11-14(2)10-17-7-6-16(4)20(23(17)21)9-8-19-12-18(25)13-22(26)28-19/h6-7,10,14-16,18-21,23,25H,5,8-9,11-13H2,1-4H3/t14-,15-,16-,18+,19?,20-,21-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of microsomal rat liver HMG-CoA reductase |
Bioorg Med Chem Lett 2: 223-228 (1992)
Article DOI: 10.1016/S0960-894X(01)81069-6
BindingDB Entry DOI: 10.7270/Q28C9W59 |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Homo sapiens (Human)) | BDBM50455629

(CHEMBL487720)Show SMILES [Na+].[H][C@@](O)(CC[C@@]1([H])[C@@H](C)C=CC2=C[C@H](C)C[C@H](OC(=O)C(C)(C)CC)[C@]12[H])C[C@@H](O)CC([O-])=O |r,c:9,t:11| Show InChI InChI=1S/C25H40O6.Na/c1-6-25(4,5)24(30)31-21-12-15(2)11-17-8-7-16(3)20(23(17)21)10-9-18(26)13-19(27)14-22(28)29;/h7-8,11,15-16,18-21,23,26-27H,6,9-10,12-14H2,1-5H3,(H,28,29);/q;+1/p-1/t15-,16-,18+,19+,20-,21-,23-;/m0./s1 | PDB
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
British Bio-technology Limited
Curated by ChEMBL
| Assay Description
Concentration required to inhibit HMG-CoA reductase by 50% was determined in Hep G2 cell line |
J Med Chem 35: 3388-93 (1992)
BindingDB Entry DOI: 10.7270/Q2HD7W9V |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50406129

(CHEMBL175236)Show SMILES CC[C@H](C)C(=O)O[C@H]1C[C@@H](C)C=C2C=C[C@H](C)[C@H](CC[C@@H](O)C[C@@H](O)CC([O-])=O)[C@@H]12 |c:13,t:11| Show InChI InChI=1S/C24H38O6/c1-5-15(3)24(29)30-21-11-14(2)10-17-7-6-16(4)20(23(17)21)9-8-18(25)12-19(26)13-22(27)28/h6-7,10,14-16,18-21,23,25-26H,5,8-9,11-13H2,1-4H3,(H,27,28)/p-1/t14-,15-,16-,18+,19+,20-,21-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
British Bio-technology Limited
Curated by ChEMBL
| Assay Description
Inhibitory activity against HMG-CoA reductase from rat liver microsomal preparation |
J Med Chem 35: 3388-93 (1992)
BindingDB Entry DOI: 10.7270/Q2HD7W9V |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50031790

((R)-N*4*-Hydroxy-N*1*-((S)-1-methylcarbamoyl-2-phe...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCCCOc1ccccc1)CC(=O)NO Show InChI InChI=1S/C25H33N3O5/c1-26-25(31)22(17-19-11-5-2-6-12-19)27-24(30)20(18-23(29)28-32)13-7-4-10-16-33-21-14-8-3-9-15-21/h2-3,5-6,8-9,11-12,14-15,20,22,32H,4,7,10,13,16-18H2,1H3,(H,26,31)(H,27,30)(H,28,29)/t20-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant stromelysin (MMP-3, HFS) |
Bioorg Med Chem Lett 7: 193-198 (1997)
Article DOI: 10.1016/S0960-894X(96)00602-6
BindingDB Entry DOI: 10.7270/Q2057FXG |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50347384

(CHEMBL1801238)Show SMILES ONC(=O)c1cnc(nc1)N1C[C@H]2[C@@H](C1)[C@@H]2NCc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C17H18ClN5O2/c18-12-3-1-10(2-4-12)5-19-15-13-8-23(9-14(13)15)17-20-6-11(7-21-17)16(24)22-25/h1-4,6-7,13-15,19,25H,5,8-9H2,(H,22,24)/t13-,14+,15+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Chroma Therapeutics Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 using fluor de Lys as substrate by fluorometric analysis |
J Med Chem 53: 8663-78 (2010)
Article DOI: 10.1021/jm101177s
BindingDB Entry DOI: 10.7270/Q2G1616P |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50004776

(CHEMBL323880 | Sodium; 3,5-dihydroxy-7-[6-hydroxy-...)Show SMILES CC[C@H](C)C(=O)O[C@H]1C[C@H](O)C[C@@H]2C=C[C@H](C)[C@H](CC[C@H](O)C[C@H](O)CC([O-])=O)[C@@H]12 |c:13| Show InChI InChI=1S/C23H38O7/c1-4-13(2)23(29)30-20-11-17(25)9-15-6-5-14(3)19(22(15)20)8-7-16(24)10-18(26)12-21(27)28/h5-6,13-20,22,24-26H,4,7-12H2,1-3H3,(H,27,28)/p-1/t13-,14-,15-,16-,17+,18-,19-,20-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
British Bio-technology Limited
Curated by ChEMBL
| Assay Description
Inhibitory activity against HMG-CoA reductase from rat liver microsomal preparation |
J Med Chem 35: 3388-93 (1992)
BindingDB Entry DOI: 10.7270/Q2HD7W9V |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50280363
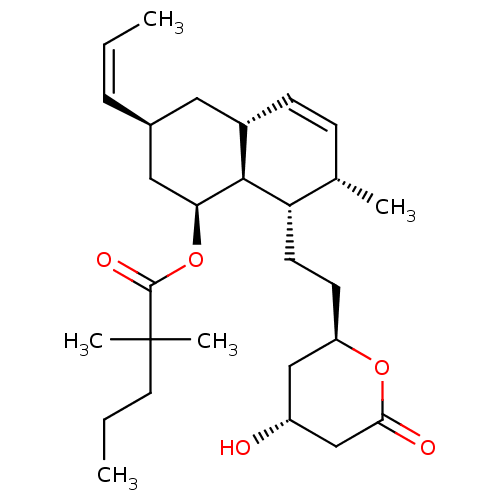
(2,2-Dimethyl-pentanoic acid (1S,3S,4aR,7S,8S,8aS)-...)Show SMILES CCCC(C)(C)C(=O)O[C@H]1C[C@H](C[C@@H]2C=C[C@H](C)[C@H](CC[C@@H]3C[C@@H](O)CC(=O)O3)[C@@H]12)\C=C/C |c:14| Show InChI InChI=1S/C28H44O5/c1-6-8-19-14-20-10-9-18(3)23(12-11-22-16-21(29)17-25(30)32-22)26(20)24(15-19)33-27(31)28(4,5)13-7-2/h6,8-10,18-24,26,29H,7,11-17H2,1-5H3/b8-6-/t18-,19-,20-,21+,22+,23-,24-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of microsomal rat liver HMG-CoA reductase |
Bioorg Med Chem Lett 2: 223-228 (1992)
Article DOI: 10.1016/S0960-894X(01)81069-6
BindingDB Entry DOI: 10.7270/Q28C9W59 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50290446
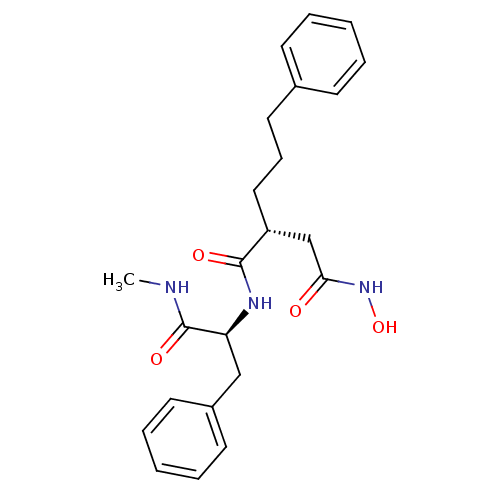
((R)-N*4*-Hydroxy-N*1*-((S)-1-methylcarbamoyl-2-phe...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCc1ccccc1)CC(=O)NO Show InChI InChI=1S/C23H29N3O4/c1-24-23(29)20(15-18-11-6-3-7-12-18)25-22(28)19(16-21(27)26-30)14-8-13-17-9-4-2-5-10-17/h2-7,9-12,19-20,30H,8,13-16H2,1H3,(H,24,29)(H,25,28)(H,26,27)/t19-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound concentration for 50% inhibition of human recombinant gelatinase A (MMP-2). |
Bioorg Med Chem Lett 7: 193-198 (1997)
Article DOI: 10.1016/S0960-894X(96)00602-6
BindingDB Entry DOI: 10.7270/Q2057FXG |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50280357
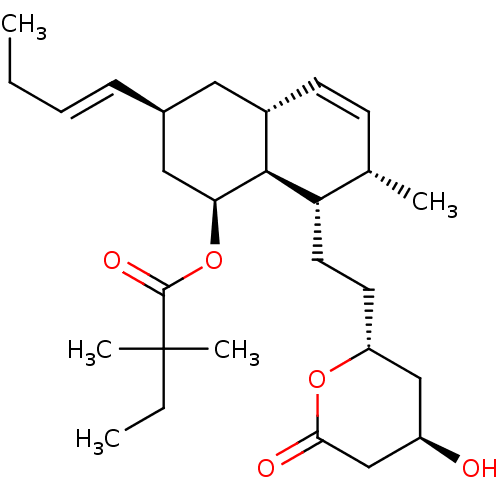
(2,2-Dimethyl-butyric acid (1S,3S,4aR,7S,8S,8aS)-3-...)Show SMILES CC\C=C\[C@@H]1C[C@H](OC(=O)C(C)(C)CC)[C@H]2[C@H](C1)C=C[C@H](C)[C@@H]2CC[C@@H]1C[C@@H](O)CC(=O)O1 |c:19| Show InChI InChI=1S/C28H44O5/c1-6-8-9-19-14-20-11-10-18(3)23(13-12-22-16-21(29)17-25(30)32-22)26(20)24(15-19)33-27(31)28(4,5)7-2/h8-11,18-24,26,29H,6-7,12-17H2,1-5H3/b9-8+/t18-,19-,20-,21+,22+,23-,24-,26-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of microsomal rat liver HMG-CoA reductase |
Bioorg Med Chem Lett 2: 223-228 (1992)
Article DOI: 10.1016/S0960-894X(01)81069-6
BindingDB Entry DOI: 10.7270/Q28C9W59 |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50290436

((R)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-propy...)Show SMILES CCCCCCCCC[C@H](CC(=O)NO)C(=O)N[C@H](C(=O)NC)C(C)(C)C Show InChI InChI=1S/C20H39N3O4/c1-6-7-8-9-10-11-12-13-15(14-16(24)23-27)18(25)22-17(19(26)21-5)20(2,3)4/h15,17,27H,6-14H2,1-5H3,(H,21,26)(H,22,25)(H,23,24)/t15-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant stromelysin (MMP-3, HFS) |
Bioorg Med Chem Lett 7: 193-198 (1997)
Article DOI: 10.1016/S0960-894X(96)00602-6
BindingDB Entry DOI: 10.7270/Q2057FXG |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50290457
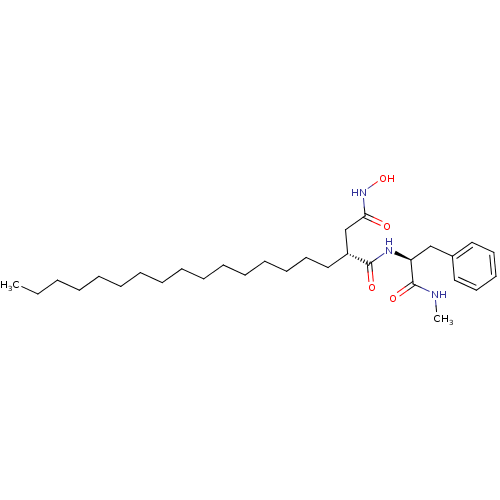
((R)-2-Hexadecyl-N*4*-hydroxy-N*1*-((S)-1-methylcar...)Show SMILES CCCCCCCCCCCCCCCC[C@H](CC(=O)NO)C(=O)N[C@@H](Cc1ccccc1)C(=O)NC Show InChI InChI=1S/C30H51N3O4/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-19-22-26(24-28(34)33-37)29(35)32-27(30(36)31-2)23-25-20-17-16-18-21-25/h16-18,20-21,26-27,37H,3-15,19,22-24H2,1-2H3,(H,31,36)(H,32,35)(H,33,34)/t26-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound concentration for 50% inhibition of human recombinant gelatinase A (MMP-2). |
Bioorg Med Chem Lett 7: 193-198 (1997)
Article DOI: 10.1016/S0960-894X(96)00602-6
BindingDB Entry DOI: 10.7270/Q2057FXG |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50290438

((R)-2-Hexyl-N*4*-hydroxy-N*1*-((S)-1-methylcarbamo...)Show SMILES CCCCCC[C@H](CC(=O)NO)C(=O)N[C@@H](Cc1ccccc1)C(=O)NC Show InChI InChI=1S/C20H31N3O4/c1-3-4-5-9-12-16(14-18(24)23-27)19(25)22-17(20(26)21-2)13-15-10-7-6-8-11-15/h6-8,10-11,16-17,27H,3-5,9,12-14H2,1-2H3,(H,21,26)(H,22,25)(H,23,24)/t16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
50% inhibition of human recombinant fibroblast collagenase (MMP-1, HFC) |
Bioorg Med Chem Lett 7: 193-198 (1997)
Article DOI: 10.1016/S0960-894X(96)00602-6
BindingDB Entry DOI: 10.7270/Q2057FXG |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50280349
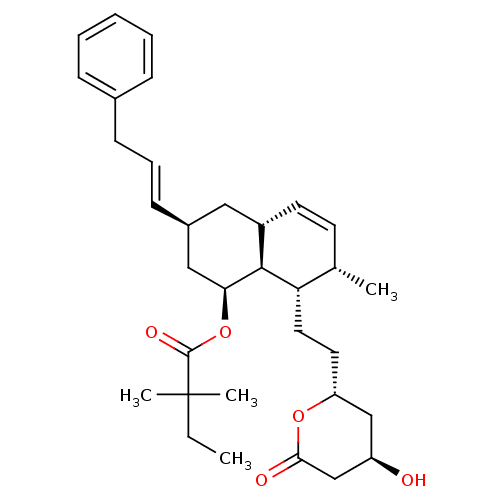
(2,2-Dimethyl-butyric acid (1S,3S,4aR,7S,8S,8aS)-8-...)Show SMILES CCC(C)(C)C(=O)O[C@H]1C[C@H](C[C@@H]2C=C[C@H](C)[C@H](CC[C@@H]3C[C@@H](O)CC(=O)O3)[C@@H]12)\C=C\Cc1ccccc1 |c:13| Show InChI InChI=1S/C33H46O5/c1-5-33(3,4)32(36)38-29-19-24(13-9-12-23-10-7-6-8-11-23)18-25-15-14-22(2)28(31(25)29)17-16-27-20-26(34)21-30(35)37-27/h6-11,13-15,22,24-29,31,34H,5,12,16-21H2,1-4H3/b13-9+/t22-,24-,25-,26+,27+,28-,29-,31-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of microsomal rat liver HMG-CoA reductase |
Bioorg Med Chem Lett 2: 223-228 (1992)
Article DOI: 10.1016/S0960-894X(01)81069-6
BindingDB Entry DOI: 10.7270/Q28C9W59 |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50290457

((R)-2-Hexadecyl-N*4*-hydroxy-N*1*-((S)-1-methylcar...)Show SMILES CCCCCCCCCCCCCCCC[C@H](CC(=O)NO)C(=O)N[C@@H](Cc1ccccc1)C(=O)NC Show InChI InChI=1S/C30H51N3O4/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-19-22-26(24-28(34)33-37)29(35)32-27(30(36)31-2)23-25-20-17-16-18-21-25/h16-18,20-21,26-27,37H,3-15,19,22-24H2,1-2H3,(H,31,36)(H,32,35)(H,33,34)/t26-,27+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
50% inhibition of human recombinant Matrilysin |
Bioorg Med Chem Lett 7: 193-198 (1997)
Article DOI: 10.1016/S0960-894X(96)00602-6
BindingDB Entry DOI: 10.7270/Q2057FXG |
More data for this
Ligand-Target Pair | |
Matrilysin
(Homo sapiens (Human)) | BDBM50063917

((2S,3R)-N(4)-[(2S)-3,3-dimethyl-1-(methylamino)-1-...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)[C@H](O)C(=O)NO)C(C)(C)C |r| Show InChI InChI=1S/C15H29N3O5/c1-8(2)7-9(10(19)13(21)18-23)12(20)17-11(14(22)16-6)15(3,4)5/h8-11,19,23H,7H2,1-6H3,(H,16,22)(H,17,20)(H,18,21)/t9-,10+,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
50% inhibition of human recombinant Matrilysin |
Bioorg Med Chem Lett 7: 193-198 (1997)
Article DOI: 10.1016/S0960-894X(96)00602-6
BindingDB Entry DOI: 10.7270/Q2057FXG |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50290453

((R)-2-Decyl-N*4*-hydroxy-N*1*-((S)-1-methylcarbamo...)Show SMILES CCCCCCCCCC[C@H](CC(=O)NO)C(=O)N[C@@H](Cc1ccccc1)C(=O)NC Show InChI InChI=1S/C24H39N3O4/c1-3-4-5-6-7-8-9-13-16-20(18-22(28)27-31)23(29)26-21(24(30)25-2)17-19-14-11-10-12-15-19/h10-12,14-15,20-21,31H,3-9,13,16-18H2,1-2H3,(H,25,30)(H,26,29)(H,27,28)/t20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
50% inhibition of human recombinant fibroblast collagenase (MMP-1, HFC) |
Bioorg Med Chem Lett 7: 193-198 (1997)
Article DOI: 10.1016/S0960-894X(96)00602-6
BindingDB Entry DOI: 10.7270/Q2057FXG |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50063918

((2R,3S)-N*4*-Hydroxy-2-isobutyl-N*1*-((S)-1-methyl...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CC(C)C)[C@H](CSc1cccs1)C(=O)NO Show InChI InChI=1S/C23H31N3O4S2/c1-15(2)12-17(18(22(28)26-30)14-32-20-10-7-11-31-20)21(27)25-19(23(29)24-3)13-16-8-5-4-6-9-16/h4-11,15,17-19,30H,12-14H2,1-3H3,(H,24,29)(H,25,27)(H,26,28)/t17-,18+,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
50% inhibition of human recombinant stromelysin (MMP-3, HFS) |
Bioorg Med Chem Lett 7: 193-198 (1997)
Article DOI: 10.1016/S0960-894X(96)00602-6
BindingDB Entry DOI: 10.7270/Q2057FXG |
More data for this
Ligand-Target Pair | |
3-hydroxy-3-methylglutaryl-coenzyme A reductase
(Rattus norvegicus (rat)) | BDBM50280352
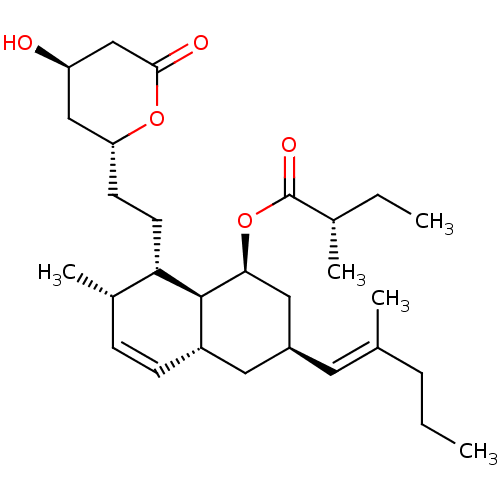
((S)-2-Methyl-butyric acid (1S,3S,4aR,7S,8S,8aS)-8-...)Show SMILES CCC\C(C)=C\[C@@H]1C[C@H](OC(=O)[C@@H](C)CC)[C@H]2[C@H](C1)C=C[C@H](C)[C@@H]2CC[C@@H]1C[C@@H](O)CC(=O)O1 |c:20| Show InChI InChI=1S/C29H46O5/c1-6-8-18(3)13-21-14-22-10-9-20(5)25(12-11-24-16-23(30)17-27(31)33-24)28(22)26(15-21)34-29(32)19(4)7-2/h9-10,13,19-26,28,30H,6-8,11-12,14-17H2,1-5H3/b18-13+/t19-,20-,21-,22-,23+,24+,25-,26-,28-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of microsomal rat liver HMG-CoA reductase |
Bioorg Med Chem Lett 2: 223-228 (1992)
Article DOI: 10.1016/S0960-894X(01)81069-6
BindingDB Entry DOI: 10.7270/Q28C9W59 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM19149

(CHEMBL98 | N-hydroxy-N'-phenyloctanediamide | SAHA...)Show InChI InChI=1S/C14H20N2O3/c17-13(15-12-8-4-3-5-9-12)10-6-1-2-7-11-14(18)16-19/h3-5,8-9,19H,1-2,6-7,10-11H2,(H,15,17)(H,16,18) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Chroma Therapeutics Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 using fluor de Lys as substrate by fluorometric analysis |
J Med Chem 53: 8663-78 (2010)
Article DOI: 10.1021/jm101177s
BindingDB Entry DOI: 10.7270/Q2G1616P |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50031790

((R)-N*4*-Hydroxy-N*1*-((S)-1-methylcarbamoyl-2-phe...)Show SMILES CNC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CCCCCOc1ccccc1)CC(=O)NO Show InChI InChI=1S/C25H33N3O5/c1-26-25(31)22(17-19-11-5-2-6-12-19)27-24(30)20(18-23(29)28-32)13-7-4-10-16-33-21-14-8-3-9-15-21/h2-3,5-6,8-9,11-12,14-15,20,22,32H,4,7,10,13,16-18H2,1H3,(H,26,31)(H,27,30)(H,28,29)/t20-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant fibroblast collagenase (MMP-1, HFC) |
Bioorg Med Chem Lett 7: 193-198 (1997)
Article DOI: 10.1016/S0960-894X(96)00602-6
BindingDB Entry DOI: 10.7270/Q2057FXG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data