 Found 73 hits with Last Name = 'bora' and Initial = 'e'
Found 73 hits with Last Name = 'bora' and Initial = 'e' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
NAD-dependent protein deacetylase sirtuin-2
(Homo sapiens (Human)) | BDBM50513317
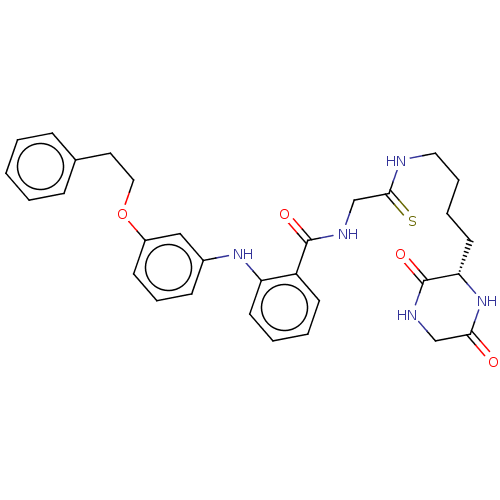
(CHEMBL4465620)Show SMILES O=C(NCC(=S)NCCCC[C@@H]1NC(=O)CNC1=O)c1ccccc1Nc1cccc(OCCc2ccccc2)c1 |r| Show InChI InChI=1S/C31H35N5O4S/c37-28-20-33-31(39)27(36-28)15-6-7-17-32-29(41)21-34-30(38)25-13-4-5-14-26(25)35-23-11-8-12-24(19-23)40-18-16-22-9-2-1-3-10-22/h1-5,8-14,19,27,35H,6-7,15-18,20-21H2,(H,32,41)(H,33,39)(H,34,38)(H,36,37)/t27-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged SIRT2 expressed in Escherichia coli using p53 (Gln-Pro-Lys-Lys(Ac)) (317 to 320 residues) as substrate incu... |
J Med Chem 62: 5844-5862 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00255
BindingDB Entry DOI: 10.7270/Q2JH3QJR |
More data for this
Ligand-Target Pair | |
NAD-dependent protein deacetylase sirtuin-2
(Homo sapiens (Human)) | BDBM50513318
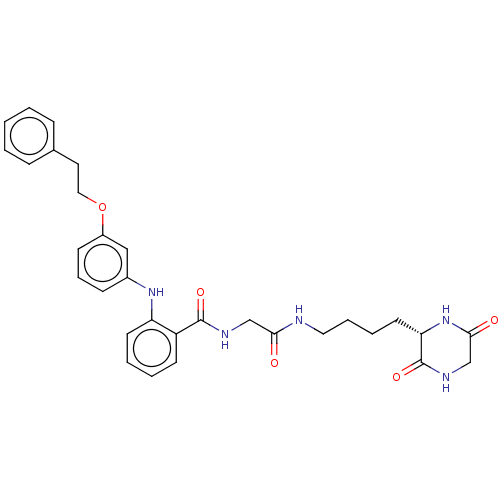
(CHEMBL4516553)Show SMILES O=C(CNC(=O)c1ccccc1Nc1cccc(OCCc2ccccc2)c1)NCCCC[C@@H]1NC(=O)CNC1=O |r| Show InChI InChI=1S/C31H35N5O5/c37-28(32-17-7-6-15-27-31(40)34-21-29(38)36-27)20-33-30(39)25-13-4-5-14-26(25)35-23-11-8-12-24(19-23)41-18-16-22-9-2-1-3-10-22/h1-5,8-14,19,27,35H,6-7,15-18,20-21H2,(H,32,37)(H,33,39)(H,34,40)(H,36,38)/t27-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged SIRT2 expressed in Escherichia coli using p53 (Gln-Pro-Lys-Lys(Ac)) (317 to 320 residues) as substrate incu... |
J Med Chem 62: 5844-5862 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00255
BindingDB Entry DOI: 10.7270/Q2JH3QJR |
More data for this
Ligand-Target Pair | |
Alpha-ketoglutarate-dependent dioxygenase FTO
(Homo sapiens (Human)) | BDBM50596052
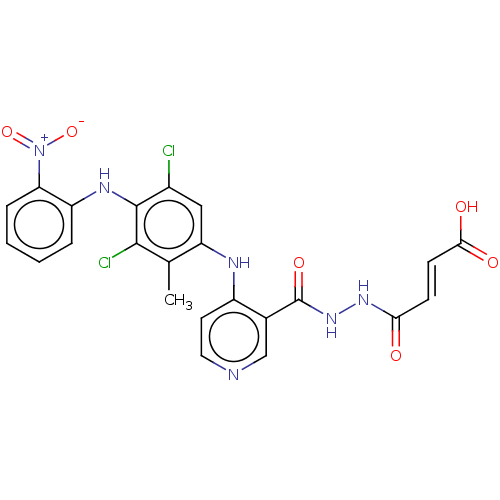
(CHEMBL5196484)Show SMILES Cc1c(Nc2ccncc2C(=O)NNC(=O)\C=C\C(O)=O)cc(Cl)c(Nc2ccccc2[N+]([O-])=O)c1Cl | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01107
BindingDB Entry DOI: 10.7270/Q2QF8XWG |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50255048
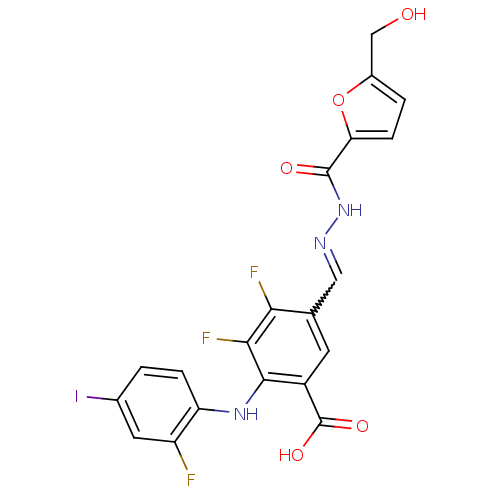
(3,4-Difluoro-2-(2-fluoro-4-iodo-phenylamino)-5-[(5...)Show SMILES OCc1ccc(o1)C(=O)NN=Cc1cc(C(O)=O)c(Nc2ccc(I)cc2F)c(F)c1F |w:11.12| Show InChI InChI=1S/C20H13F3IN3O5/c21-13-6-10(24)1-3-14(13)26-18-12(20(30)31)5-9(16(22)17(18)23)7-25-27-19(29)15-4-2-11(8-28)32-15/h1-7,26,28H,8H2,(H,27,29)(H,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to SRC (unknown origin) |
Bioorg Med Chem Lett 19: 226-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.108
BindingDB Entry DOI: 10.7270/Q22F7N98 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50595758
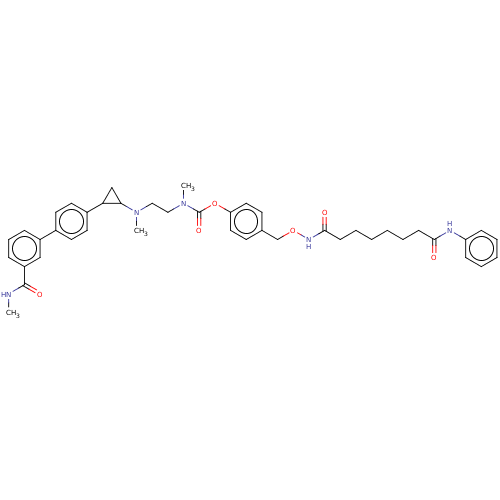
(CHEMBL5207682)Show SMILES CNC(=O)c1cccc(c1)-c1ccc(cc1)C1CC1N(C)CCN(C)C(=O)Oc1ccc(CONC(=O)CCCCCCC(=O)Nc2ccccc2)cc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00126
BindingDB Entry DOI: 10.7270/Q2BC43JF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50255048

(3,4-Difluoro-2-(2-fluoro-4-iodo-phenylamino)-5-[(5...)Show SMILES OCc1ccc(o1)C(=O)NN=Cc1cc(C(O)=O)c(Nc2ccc(I)cc2F)c(F)c1F |w:11.12| Show InChI InChI=1S/C20H13F3IN3O5/c21-13-6-10(24)1-3-14(13)26-18-12(20(30)31)5-9(16(22)17(18)23)7-25-27-19(29)15-4-2-11(8-28)32-15/h1-7,26,28H,8H2,(H,27,29)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to ABL (unknown origin) |
Bioorg Med Chem Lett 19: 226-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.108
BindingDB Entry DOI: 10.7270/Q22F7N98 |
More data for this
Ligand-Target Pair | |
NAD-dependent protein deacetylase sirtuin-2
(Homo sapiens (Human)) | BDBM50148781

(CHEMBL3770903)Show SMILES Cc1cc(C)nc(SCC(=O)Nc2ncc(Cc3cccc4ccccc34)s2)n1 Show InChI InChI=1S/C22H20N4OS2/c1-14-10-15(2)25-22(24-14)28-13-20(27)26-21-23-12-18(29-21)11-17-8-5-7-16-6-3-4-9-19(16)17/h3-10,12H,11,13H2,1-2H3,(H,23,26,27) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged SIRT2 expressed in Escherichia coli using p53 (Gln-Pro-Lys-Lys(Ac)) (317 to 320 residues) as substrate incu... |
J Med Chem 62: 5844-5862 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00255
BindingDB Entry DOI: 10.7270/Q2JH3QJR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
NAD-dependent protein deacetylase sirtuin-2
(Homo sapiens (Human)) | BDBM50513317

(CHEMBL4465620)Show SMILES O=C(NCC(=S)NCCCC[C@@H]1NC(=O)CNC1=O)c1ccccc1Nc1cccc(OCCc2ccccc2)c1 |r| Show InChI InChI=1S/C31H35N5O4S/c37-28-20-33-31(39)27(36-28)15-6-7-17-32-29(41)21-34-30(38)25-13-4-5-14-26(25)35-23-11-8-12-24(19-23)40-18-16-22-9-2-1-3-10-22/h1-5,8-14,19,27,35H,6-7,15-18,20-21H2,(H,32,41)(H,33,39)(H,34,38)(H,36,37)/t27-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged SIRT2 expressed in Escherichia coli using p53 (Gln-Pro-Lys-Lys(Ac)) (317 to 320 residues) as substrate incu... |
J Med Chem 62: 5844-5862 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00255
BindingDB Entry DOI: 10.7270/Q2JH3QJR |
More data for this
Ligand-Target Pair | |
NAD-dependent protein deacetylase sirtuin-2
(Homo sapiens (Human)) | BDBM50513320

(CHEMBL4471909)Show SMILES C[C@H](NC(=O)[C@H](CCCCNC(=O)CNC(=O)c1ccccc1Nc1cccc(OCCc2ccccc2)c1)NC(=O)OCc1ccccc1)C(N)=O |r| Show InChI InChI=1S/C40H46N6O7/c1-28(37(41)48)44-39(50)35(46-40(51)53-27-30-15-6-3-7-16-30)21-10-11-23-42-36(47)26-43-38(49)33-19-8-9-20-34(33)45-31-17-12-18-32(25-31)52-24-22-29-13-4-2-5-14-29/h2-9,12-20,25,28,35,45H,10-11,21-24,26-27H2,1H3,(H2,41,48)(H,42,47)(H,43,49)(H,44,50)(H,46,51)/t28-,35-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged SIRT2 expressed in Escherichia coli using p53 (Gln-Pro-Lys-Lys(Ac)) (317 to 320 residues) as substrate incu... |
J Med Chem 62: 5844-5862 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00255
BindingDB Entry DOI: 10.7270/Q2JH3QJR |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50595759

(CHEMBL5175995)Show SMILES CNC(=O)c1cccc(c1)-c1ccc(cc1)[C@@H]1C[C@H]1N(C)CCN(C)C(=O)Oc1ccc(CONC(=O)CCCCCCC(=O)Nc2ccccc2)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 444 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00126
BindingDB Entry DOI: 10.7270/Q2BC43JF |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50595760
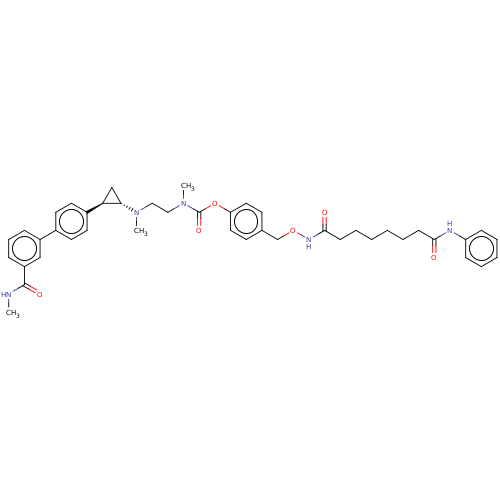
(CHEMBL5177373)Show SMILES CNC(=O)c1cccc(c1)-c1ccc(cc1)[C@H]1C[C@@H]1N(C)CCN(C)C(=O)Oc1ccc(CONC(=O)CCCCCCC(=O)Nc2ccccc2)cc1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 457 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00126
BindingDB Entry DOI: 10.7270/Q2BC43JF |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50255048

(3,4-Difluoro-2-(2-fluoro-4-iodo-phenylamino)-5-[(5...)Show SMILES OCc1ccc(o1)C(=O)NN=Cc1cc(C(O)=O)c(Nc2ccc(I)cc2F)c(F)c1F |w:11.12| Show InChI InChI=1S/C20H13F3IN3O5/c21-13-6-10(24)1-3-14(13)26-18-12(20(30)31)5-9(16(22)17(18)23)7-25-27-19(29)15-4-2-11(8-28)32-15/h1-7,26,28H,8H2,(H,27,29)(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to LCK (unknown origin) |
Bioorg Med Chem Lett 19: 226-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.108
BindingDB Entry DOI: 10.7270/Q22F7N98 |
More data for this
Ligand-Target Pair | |
Alpha-ketoglutarate-dependent dioxygenase FTO
(Homo sapiens (Human)) | BDBM50596051
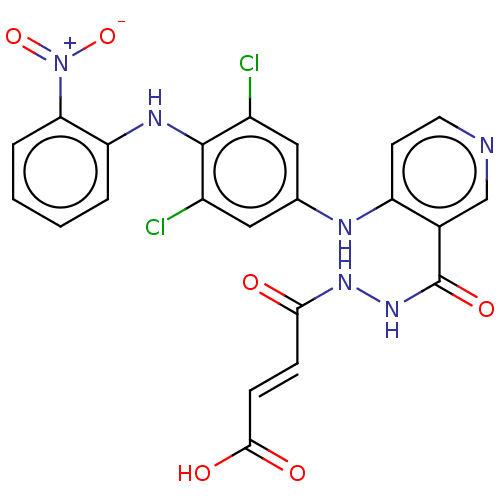
(CHEMBL5183243)Show SMILES OC(=O)\C=C\C(=O)NNC(=O)c1cnccc1Nc1cc(Cl)c(Nc2ccccc2[N+]([O-])=O)c(Cl)c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01107
BindingDB Entry DOI: 10.7270/Q2QF8XWG |
More data for this
Ligand-Target Pair | |
NAD-dependent protein deacetylase sirtuin-2
(Homo sapiens (Human)) | BDBM50513318

(CHEMBL4516553)Show SMILES O=C(CNC(=O)c1ccccc1Nc1cccc(OCCc2ccccc2)c1)NCCCC[C@@H]1NC(=O)CNC1=O |r| Show InChI InChI=1S/C31H35N5O5/c37-28(32-17-7-6-15-27-31(40)34-21-29(38)36-27)20-33-30(39)25-13-4-5-14-26(25)35-23-11-8-12-24(19-23)41-18-16-22-9-2-1-3-10-22/h1-5,8-14,19,27,35H,6-7,15-18,20-21H2,(H,32,37)(H,33,39)(H,34,40)(H,36,38)/t27-/m0/s1 | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged SIRT2 expressed in Escherichia coli using p53 (Gln-Pro-Lys-Lys(Ac)) (317 to 320 residues) as substrate incu... |
J Med Chem 62: 5844-5862 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00255
BindingDB Entry DOI: 10.7270/Q2JH3QJR |
More data for this
Ligand-Target Pair | |
Alpha-ketoglutarate-dependent dioxygenase FTO
(Homo sapiens (Human)) | BDBM50596053
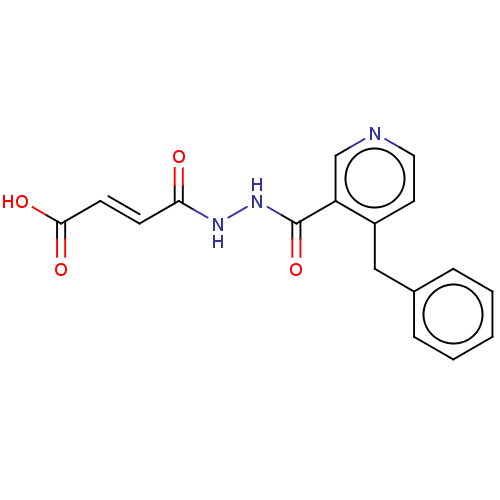
(CHEMBL5173876) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01107
BindingDB Entry DOI: 10.7270/Q2QF8XWG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50255012
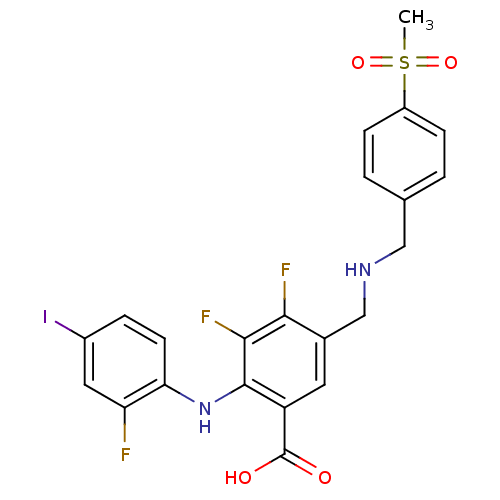
(5-((4-(methylsulfonyl)benzylamino)methyl)-3,4-difl...)Show SMILES CS(=O)(=O)c1ccc(CNCc2cc(C(O)=O)c(Nc3ccc(I)cc3F)c(F)c2F)cc1 Show InChI InChI=1S/C22H18F3IN2O4S/c1-33(31,32)15-5-2-12(3-6-15)10-27-11-13-8-16(22(29)30)21(20(25)19(13)24)28-18-7-4-14(26)9-17(18)23/h2-9,27-28H,10-11H2,1H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to Aurora A (unknown origin) |
Bioorg Med Chem Lett 19: 226-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.108
BindingDB Entry DOI: 10.7270/Q22F7N98 |
More data for this
Ligand-Target Pair | |
NAD-dependent protein deacetylase sirtuin-2
(Homo sapiens (Human)) | BDBM50513319
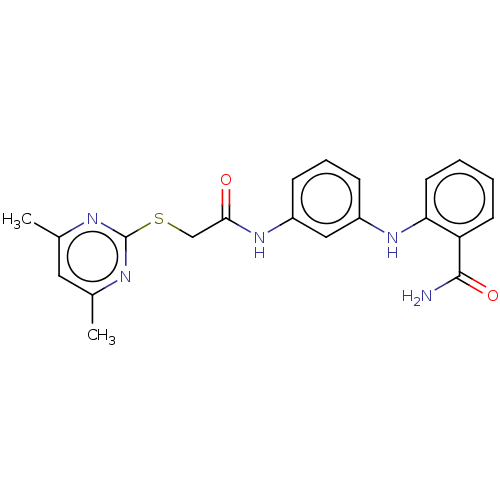
(CHEMBL4589851)Show SMILES Cc1cc(C)nc(SCC(=O)Nc2cccc(Nc3ccccc3C(N)=O)c2)n1 Show InChI InChI=1S/C21H21N5O2S/c1-13-10-14(2)24-21(23-13)29-12-19(27)26-16-7-5-6-15(11-16)25-18-9-4-3-8-17(18)20(22)28/h3-11,25H,12H2,1-2H3,(H2,22,28)(H,26,27) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged SIRT2 expressed in Escherichia coli using p53 (Gln-Pro-Lys-Lys(Ac)) (317 to 320 residues) as substrate incu... |
J Med Chem 62: 5844-5862 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00255
BindingDB Entry DOI: 10.7270/Q2JH3QJR |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50595757
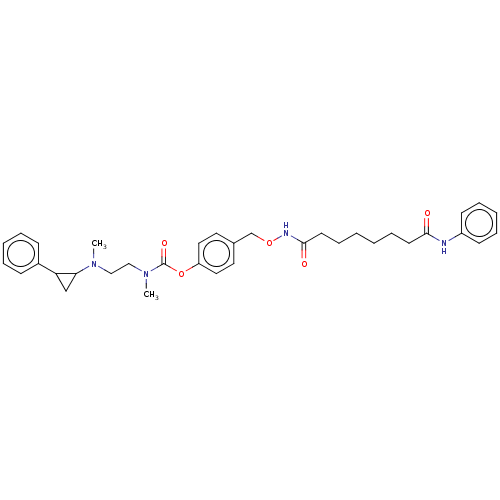
(CHEMBL5174314)Show SMILES CN(CCN(C)C(=O)Oc1ccc(CONC(=O)CCCCCCC(=O)Nc2ccccc2)cc1)C1CC1c1ccccc1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00126
BindingDB Entry DOI: 10.7270/Q2BC43JF |
More data for this
Ligand-Target Pair | |
NAD-dependent protein deacetylase sirtuin-2
(Homo sapiens (Human)) | BDBM50392111
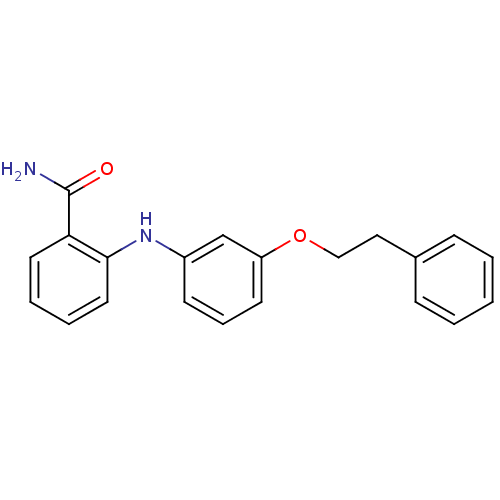
(CHEMBL2152613)Show InChI InChI=1S/C21H20N2O2/c22-21(24)19-11-4-5-12-20(19)23-17-9-6-10-18(15-17)25-14-13-16-7-2-1-3-8-16/h1-12,15,23H,13-14H2,(H2,22,24) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.74E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Prefectural University of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of human N-terminal His-tagged SIRT2 expressed in Escherichia coli using p53 (Gln-Pro-Lys-Lys(Ac)) (317 to 320 residues) as substrate incu... |
J Med Chem 62: 5844-5862 (2019)
Article DOI: 10.1021/acs.jmedchem.9b00255
BindingDB Entry DOI: 10.7270/Q2JH3QJR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50255013
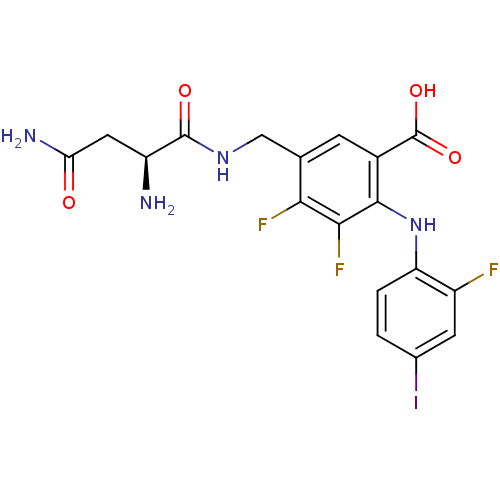
((S)-5-((2,4-diamino-4-oxobutanamido)methyl)-3,4-di...)Show SMILES N[C@@H](CC(N)=O)C(=O)NCc1cc(C(O)=O)c(Nc2ccc(I)cc2F)c(F)c1F |r| Show InChI InChI=1S/C18H16F3IN4O4/c19-10-4-8(22)1-2-12(10)26-16-9(18(29)30)3-7(14(20)15(16)21)6-25-17(28)11(23)5-13(24)27/h1-4,11,26H,5-6,23H2,(H2,24,27)(H,25,28)(H,29,30)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to Aurora A (unknown origin) |
Bioorg Med Chem Lett 19: 226-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.108
BindingDB Entry DOI: 10.7270/Q22F7N98 |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor receptor superfamily member 1A
(Homo sapiens (Human)) | BDBM50255012

(5-((4-(methylsulfonyl)benzylamino)methyl)-3,4-difl...)Show SMILES CS(=O)(=O)c1ccc(CNCc2cc(C(O)=O)c(Nc3ccc(I)cc3F)c(F)c2F)cc1 Show InChI InChI=1S/C22H18F3IN2O4S/c1-33(31,32)15-5-2-12(3-6-15)10-27-11-13-8-16(22(29)30)21(20(25)19(13)24)28-18-7-4-14(26)9-17(18)23/h2-9,27-28H,10-11H2,1H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to TRAK-A (unknown origin) |
Bioorg Med Chem Lett 19: 226-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.108
BindingDB Entry DOI: 10.7270/Q22F7N98 |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM50255013

((S)-5-((2,4-diamino-4-oxobutanamido)methyl)-3,4-di...)Show SMILES N[C@@H](CC(N)=O)C(=O)NCc1cc(C(O)=O)c(Nc2ccc(I)cc2F)c(F)c1F |r| Show InChI InChI=1S/C18H16F3IN4O4/c19-10-4-8(22)1-2-12(10)26-16-9(18(29)30)3-7(14(20)15(16)21)6-25-17(28)11(23)5-13(24)27/h1-4,11,26H,5-6,23H2,(H2,24,27)(H,25,28)(H,29,30)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to PKC beta2 (unknown origin) |
Bioorg Med Chem Lett 19: 226-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.108
BindingDB Entry DOI: 10.7270/Q22F7N98 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50255048

(3,4-Difluoro-2-(2-fluoro-4-iodo-phenylamino)-5-[(5...)Show SMILES OCc1ccc(o1)C(=O)NN=Cc1cc(C(O)=O)c(Nc2ccc(I)cc2F)c(F)c1F |w:11.12| Show InChI InChI=1S/C20H13F3IN3O5/c21-13-6-10(24)1-3-14(13)26-18-12(20(30)31)5-9(16(22)17(18)23)7-25-27-19(29)15-4-2-11(8-28)32-15/h1-7,26,28H,8H2,(H,27,29)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to FGFR1 (unknown origin) |
Bioorg Med Chem Lett 19: 226-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.108
BindingDB Entry DOI: 10.7270/Q22F7N98 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50255048

(3,4-Difluoro-2-(2-fluoro-4-iodo-phenylamino)-5-[(5...)Show SMILES OCc1ccc(o1)C(=O)NN=Cc1cc(C(O)=O)c(Nc2ccc(I)cc2F)c(F)c1F |w:11.12| Show InChI InChI=1S/C20H13F3IN3O5/c21-13-6-10(24)1-3-14(13)26-18-12(20(30)31)5-9(16(22)17(18)23)7-25-27-19(29)15-4-2-11(8-28)32-15/h1-7,26,28H,8H2,(H,27,29)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to ECK (unknown origin) |
Bioorg Med Chem Lett 19: 226-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.108
BindingDB Entry DOI: 10.7270/Q22F7N98 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK1
(Homo sapiens (Human)) | BDBM50255049
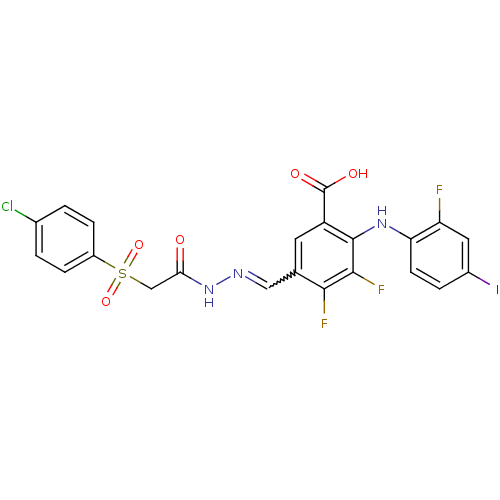
(5-{[2-(4-Chloro-benzenesulfonyl)-acetyl]-hydrazono...)Show SMILES OC(=O)c1cc(C=NNC(=O)CS(=O)(=O)c2ccc(Cl)cc2)c(F)c(F)c1Nc1ccc(I)cc1F |w:6.5| Show InChI InChI=1S/C22H14ClF3IN3O5S/c23-12-1-4-14(5-2-12)36(34,35)10-18(31)30-28-9-11-7-15(22(32)33)21(20(26)19(11)25)29-17-6-3-13(27)8-16(17)24/h1-9,29H,10H2,(H,30,31)(H,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to CLK1 (unknown origin) |
Bioorg Med Chem Lett 19: 226-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.108
BindingDB Entry DOI: 10.7270/Q22F7N98 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50255048

(3,4-Difluoro-2-(2-fluoro-4-iodo-phenylamino)-5-[(5...)Show SMILES OCc1ccc(o1)C(=O)NN=Cc1cc(C(O)=O)c(Nc2ccc(I)cc2F)c(F)c1F |w:11.12| Show InChI InChI=1S/C20H13F3IN3O5/c21-13-6-10(24)1-3-14(13)26-18-12(20(30)31)5-9(16(22)17(18)23)7-25-27-19(29)15-4-2-11(8-28)32-15/h1-7,26,28H,8H2,(H,27,29)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to Aurora A (unknown origin) |
Bioorg Med Chem Lett 19: 226-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.108
BindingDB Entry DOI: 10.7270/Q22F7N98 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50255049

(5-{[2-(4-Chloro-benzenesulfonyl)-acetyl]-hydrazono...)Show SMILES OC(=O)c1cc(C=NNC(=O)CS(=O)(=O)c2ccc(Cl)cc2)c(F)c(F)c1Nc1ccc(I)cc1F |w:6.5| Show InChI InChI=1S/C22H14ClF3IN3O5S/c23-12-1-4-14(5-2-12)36(34,35)10-18(31)30-28-9-11-7-15(22(32)33)21(20(26)19(11)25)29-17-6-3-13(27)8-16(17)24/h1-9,29H,10H2,(H,30,31)(H,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to SRC (unknown origin) |
Bioorg Med Chem Lett 19: 226-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.108
BindingDB Entry DOI: 10.7270/Q22F7N98 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50255048

(3,4-Difluoro-2-(2-fluoro-4-iodo-phenylamino)-5-[(5...)Show SMILES OCc1ccc(o1)C(=O)NN=Cc1cc(C(O)=O)c(Nc2ccc(I)cc2F)c(F)c1F |w:11.12| Show InChI InChI=1S/C20H13F3IN3O5/c21-13-6-10(24)1-3-14(13)26-18-12(20(30)31)5-9(16(22)17(18)23)7-25-27-19(29)15-4-2-11(8-28)32-15/h1-7,26,28H,8H2,(H,27,29)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to VEGFR2 (unknown origin) |
Bioorg Med Chem Lett 19: 226-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.108
BindingDB Entry DOI: 10.7270/Q22F7N98 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ABL1
(Homo sapiens (Human)) | BDBM50255049

(5-{[2-(4-Chloro-benzenesulfonyl)-acetyl]-hydrazono...)Show SMILES OC(=O)c1cc(C=NNC(=O)CS(=O)(=O)c2ccc(Cl)cc2)c(F)c(F)c1Nc1ccc(I)cc1F |w:6.5| Show InChI InChI=1S/C22H14ClF3IN3O5S/c23-12-1-4-14(5-2-12)36(34,35)10-18(31)30-28-9-11-7-15(22(32)33)21(20(26)19(11)25)29-17-6-3-13(27)8-16(17)24/h1-9,29H,10H2,(H,30,31)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to ABL (unknown origin) |
Bioorg Med Chem Lett 19: 226-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.108
BindingDB Entry DOI: 10.7270/Q22F7N98 |
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM50255049

(5-{[2-(4-Chloro-benzenesulfonyl)-acetyl]-hydrazono...)Show SMILES OC(=O)c1cc(C=NNC(=O)CS(=O)(=O)c2ccc(Cl)cc2)c(F)c(F)c1Nc1ccc(I)cc1F |w:6.5| Show InChI InChI=1S/C22H14ClF3IN3O5S/c23-12-1-4-14(5-2-12)36(34,35)10-18(31)30-28-9-11-7-15(22(32)33)21(20(26)19(11)25)29-17-6-3-13(27)8-16(17)24/h1-9,29H,10H2,(H,30,31)(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to Aurora A (unknown origin) |
Bioorg Med Chem Lett 19: 226-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.108
BindingDB Entry DOI: 10.7270/Q22F7N98 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase Src
(Homo sapiens (Human)) | BDBM50255013

((S)-5-((2,4-diamino-4-oxobutanamido)methyl)-3,4-di...)Show SMILES N[C@@H](CC(N)=O)C(=O)NCc1cc(C(O)=O)c(Nc2ccc(I)cc2F)c(F)c1F |r| Show InChI InChI=1S/C18H16F3IN4O4/c19-10-4-8(22)1-2-12(10)26-16-9(18(29)30)3-7(14(20)15(16)21)6-25-17(28)11(23)5-13(24)27/h1-4,11,26H,5-6,23H2,(H2,24,27)(H,25,28)(H,29,30)/t11-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to SRC (unknown origin) |
Bioorg Med Chem Lett 19: 226-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.108
BindingDB Entry DOI: 10.7270/Q22F7N98 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 9
(Homo sapiens (Human)) | BDBM50255048

(3,4-Difluoro-2-(2-fluoro-4-iodo-phenylamino)-5-[(5...)Show SMILES OCc1ccc(o1)C(=O)NN=Cc1cc(C(O)=O)c(Nc2ccc(I)cc2F)c(F)c1F |w:11.12| Show InChI InChI=1S/C20H13F3IN3O5/c21-13-6-10(24)1-3-14(13)26-18-12(20(30)31)5-9(16(22)17(18)23)7-25-27-19(29)15-4-2-11(8-28)32-15/h1-7,26,28H,8H2,(H,27,29)(H,30,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to MAP3K9 (unknown origin) |
Bioorg Med Chem Lett 19: 226-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.108
BindingDB Entry DOI: 10.7270/Q22F7N98 |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor receptor superfamily member 1A
(Homo sapiens (Human)) | BDBM50255049

(5-{[2-(4-Chloro-benzenesulfonyl)-acetyl]-hydrazono...)Show SMILES OC(=O)c1cc(C=NNC(=O)CS(=O)(=O)c2ccc(Cl)cc2)c(F)c(F)c1Nc1ccc(I)cc1F |w:6.5| Show InChI InChI=1S/C22H14ClF3IN3O5S/c23-12-1-4-14(5-2-12)36(34,35)10-18(31)30-28-9-11-7-15(22(32)33)21(20(26)19(11)25)29-17-6-3-13(27)8-16(17)24/h1-9,29H,10H2,(H,30,31)(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to TRAK-A (unknown origin) |
Bioorg Med Chem Lett 19: 226-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.108
BindingDB Entry DOI: 10.7270/Q22F7N98 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 9
(Homo sapiens (Human)) | BDBM50255012

(5-((4-(methylsulfonyl)benzylamino)methyl)-3,4-difl...)Show SMILES CS(=O)(=O)c1ccc(CNCc2cc(C(O)=O)c(Nc3ccc(I)cc3F)c(F)c2F)cc1 Show InChI InChI=1S/C22H18F3IN2O4S/c1-33(31,32)15-5-2-12(3-6-15)10-27-11-13-8-16(22(29)30)21(20(25)19(13)24)28-18-7-4-14(26)9-17(18)23/h2-9,27-28H,10-11H2,1H3,(H,29,30) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to MAP3K9 (unknown origin) |
Bioorg Med Chem Lett 19: 226-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.108
BindingDB Entry DOI: 10.7270/Q22F7N98 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase 3
(Homo sapiens (Human)) | BDBM50255013

((S)-5-((2,4-diamino-4-oxobutanamido)methyl)-3,4-di...)Show SMILES N[C@@H](CC(N)=O)C(=O)NCc1cc(C(O)=O)c(Nc2ccc(I)cc2F)c(F)c1F |r| Show InChI InChI=1S/C18H16F3IN4O4/c19-10-4-8(22)1-2-12(10)26-16-9(18(29)30)3-7(14(20)15(16)21)6-25-17(28)11(23)5-13(24)27/h1-4,11,26H,5-6,23H2,(H2,24,27)(H,25,28)(H,29,30)/t11-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to MST2 (unknown origin) |
Bioorg Med Chem Lett 19: 226-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.108
BindingDB Entry DOI: 10.7270/Q22F7N98 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Lck
(Homo sapiens (Human)) | BDBM50255049

(5-{[2-(4-Chloro-benzenesulfonyl)-acetyl]-hydrazono...)Show SMILES OC(=O)c1cc(C=NNC(=O)CS(=O)(=O)c2ccc(Cl)cc2)c(F)c(F)c1Nc1ccc(I)cc1F |w:6.5| Show InChI InChI=1S/C22H14ClF3IN3O5S/c23-12-1-4-14(5-2-12)36(34,35)10-18(31)30-28-9-11-7-15(22(32)33)21(20(26)19(11)25)29-17-6-3-13(27)8-16(17)24/h1-9,29H,10H2,(H,30,31)(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to LCK (unknown origin) |
Bioorg Med Chem Lett 19: 226-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.108
BindingDB Entry DOI: 10.7270/Q22F7N98 |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase kinase kinase 9
(Homo sapiens (Human)) | BDBM50255049

(5-{[2-(4-Chloro-benzenesulfonyl)-acetyl]-hydrazono...)Show SMILES OC(=O)c1cc(C=NNC(=O)CS(=O)(=O)c2ccc(Cl)cc2)c(F)c(F)c1Nc1ccc(I)cc1F |w:6.5| Show InChI InChI=1S/C22H14ClF3IN3O5S/c23-12-1-4-14(5-2-12)36(34,35)10-18(31)30-28-9-11-7-15(22(32)33)21(20(26)19(11)25)29-17-6-3-13(27)8-16(17)24/h1-9,29H,10H2,(H,30,31)(H,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to MAP3K9 (unknown origin) |
Bioorg Med Chem Lett 19: 226-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.108
BindingDB Entry DOI: 10.7270/Q22F7N98 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase BTK
(Homo sapiens (Human)) | BDBM50255048

(3,4-Difluoro-2-(2-fluoro-4-iodo-phenylamino)-5-[(5...)Show SMILES OCc1ccc(o1)C(=O)NN=Cc1cc(C(O)=O)c(Nc2ccc(I)cc2F)c(F)c1F |w:11.12| Show InChI InChI=1S/C20H13F3IN3O5/c21-13-6-10(24)1-3-14(13)26-18-12(20(30)31)5-9(16(22)17(18)23)7-25-27-19(29)15-4-2-11(8-28)32-15/h1-7,26,28H,8H2,(H,27,29)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to BTK (unknown origin) |
Bioorg Med Chem Lett 19: 226-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.108
BindingDB Entry DOI: 10.7270/Q22F7N98 |
More data for this
Ligand-Target Pair | |
RNA demethylase ALKBH5
(Homo sapiens) | BDBM50596051

(CHEMBL5183243)Show SMILES OC(=O)\C=C\C(=O)NNC(=O)c1cnccc1Nc1cc(Cl)c(Nc2ccccc2[N+]([O-])=O)c(Cl)c1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01107
BindingDB Entry DOI: 10.7270/Q2QF8XWG |
More data for this
Ligand-Target Pair | |
RNA demethylase ALKBH5
(Homo sapiens) | BDBM50596052

(CHEMBL5196484)Show SMILES Cc1c(Nc2ccncc2C(=O)NNC(=O)\C=C\C(O)=O)cc(Cl)c(Nc2ccccc2[N+]([O-])=O)c1Cl | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01107
BindingDB Entry DOI: 10.7270/Q2QF8XWG |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor receptor superfamily member 1A
(Homo sapiens (Human)) | BDBM50255013

((S)-5-((2,4-diamino-4-oxobutanamido)methyl)-3,4-di...)Show SMILES N[C@@H](CC(N)=O)C(=O)NCc1cc(C(O)=O)c(Nc2ccc(I)cc2F)c(F)c1F |r| Show InChI InChI=1S/C18H16F3IN4O4/c19-10-4-8(22)1-2-12(10)26-16-9(18(29)30)3-7(14(20)15(16)21)6-25-17(28)11(23)5-13(24)27/h1-4,11,26H,5-6,23H2,(H2,24,27)(H,25,28)(H,29,30)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to TRAK-A (unknown origin) |
Bioorg Med Chem Lett 19: 226-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.108
BindingDB Entry DOI: 10.7270/Q22F7N98 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase CLK1
(Homo sapiens (Human)) | BDBM50255048

(3,4-Difluoro-2-(2-fluoro-4-iodo-phenylamino)-5-[(5...)Show SMILES OCc1ccc(o1)C(=O)NN=Cc1cc(C(O)=O)c(Nc2ccc(I)cc2F)c(F)c1F |w:11.12| Show InChI InChI=1S/C20H13F3IN3O5/c21-13-6-10(24)1-3-14(13)26-18-12(20(30)31)5-9(16(22)17(18)23)7-25-27-19(29)15-4-2-11(8-28)32-15/h1-7,26,28H,8H2,(H,27,29)(H,30,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to CLK1 (unknown origin) |
Bioorg Med Chem Lett 19: 226-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.108
BindingDB Entry DOI: 10.7270/Q22F7N98 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50255012

(5-((4-(methylsulfonyl)benzylamino)methyl)-3,4-difl...)Show SMILES CS(=O)(=O)c1ccc(CNCc2cc(C(O)=O)c(Nc3ccc(I)cc3F)c(F)c2F)cc1 Show InChI InChI=1S/C22H18F3IN2O4S/c1-33(31,32)15-5-2-12(3-6-15)10-27-11-13-8-16(22(29)30)21(20(25)19(13)24)28-18-7-4-14(26)9-17(18)23/h2-9,27-28H,10-11H2,1H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to VEGFR2 (unknown origin) |
Bioorg Med Chem Lett 19: 226-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.108
BindingDB Entry DOI: 10.7270/Q22F7N98 |
More data for this
Ligand-Target Pair | |
Tumor necrosis factor receptor superfamily member 1A
(Homo sapiens (Human)) | BDBM50255048

(3,4-Difluoro-2-(2-fluoro-4-iodo-phenylamino)-5-[(5...)Show SMILES OCc1ccc(o1)C(=O)NN=Cc1cc(C(O)=O)c(Nc2ccc(I)cc2F)c(F)c1F |w:11.12| Show InChI InChI=1S/C20H13F3IN3O5/c21-13-6-10(24)1-3-14(13)26-18-12(20(30)31)5-9(16(22)17(18)23)7-25-27-19(29)15-4-2-11(8-28)32-15/h1-7,26,28H,8H2,(H,27,29)(H,30,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to TRAK-A (unknown origin) |
Bioorg Med Chem Lett 19: 226-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.108
BindingDB Entry DOI: 10.7270/Q22F7N98 |
More data for this
Ligand-Target Pair | |
Lysine-specific histone demethylase 1A
(Homo sapiens (Human)) | BDBM50113851

((+/-)-Tranylcypromine | 2-PCPA | 2-Phenyl-cyclopro...)Show InChI InChI=1S/C9H11N/c10-9-6-8(9)7-4-2-1-3-5-7/h1-5,8-9H,6,10H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.2c00126
BindingDB Entry DOI: 10.7270/Q2BC43JF |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50255012

(5-((4-(methylsulfonyl)benzylamino)methyl)-3,4-difl...)Show SMILES CS(=O)(=O)c1ccc(CNCc2cc(C(O)=O)c(Nc3ccc(I)cc3F)c(F)c2F)cc1 Show InChI InChI=1S/C22H18F3IN2O4S/c1-33(31,32)15-5-2-12(3-6-15)10-27-11-13-8-16(22(29)30)21(20(25)19(13)24)28-18-7-4-14(26)9-17(18)23/h2-9,27-28H,10-11H2,1H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to FGFR1 (unknown origin) |
Bioorg Med Chem Lett 19: 226-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.108
BindingDB Entry DOI: 10.7270/Q22F7N98 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50255013

((S)-5-((2,4-diamino-4-oxobutanamido)methyl)-3,4-di...)Show SMILES N[C@@H](CC(N)=O)C(=O)NCc1cc(C(O)=O)c(Nc2ccc(I)cc2F)c(F)c1F |r| Show InChI InChI=1S/C18H16F3IN4O4/c19-10-4-8(22)1-2-12(10)26-16-9(18(29)30)3-7(14(20)15(16)21)6-25-17(28)11(23)5-13(24)27/h1-4,11,26H,5-6,23H2,(H2,24,27)(H,25,28)(H,29,30)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to FGFR1 (unknown origin) |
Bioorg Med Chem Lett 19: 226-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.108
BindingDB Entry DOI: 10.7270/Q22F7N98 |
More data for this
Ligand-Target Pair | |
Fibroblast growth factor receptor 1
(Homo sapiens (Human)) | BDBM50255049

(5-{[2-(4-Chloro-benzenesulfonyl)-acetyl]-hydrazono...)Show SMILES OC(=O)c1cc(C=NNC(=O)CS(=O)(=O)c2ccc(Cl)cc2)c(F)c(F)c1Nc1ccc(I)cc1F |w:6.5| Show InChI InChI=1S/C22H14ClF3IN3O5S/c23-12-1-4-14(5-2-12)36(34,35)10-18(31)30-28-9-11-7-15(22(32)33)21(20(26)19(11)25)29-17-6-3-13(27)8-16(17)24/h1-9,29H,10H2,(H,30,31)(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to FGFR1 (unknown origin) |
Bioorg Med Chem Lett 19: 226-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.108
BindingDB Entry DOI: 10.7270/Q22F7N98 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50255012

(5-((4-(methylsulfonyl)benzylamino)methyl)-3,4-difl...)Show SMILES CS(=O)(=O)c1ccc(CNCc2cc(C(O)=O)c(Nc3ccc(I)cc3F)c(F)c2F)cc1 Show InChI InChI=1S/C22H18F3IN2O4S/c1-33(31,32)15-5-2-12(3-6-15)10-27-11-13-8-16(22(29)30)21(20(25)19(13)24)28-18-7-4-14(26)9-17(18)23/h2-9,27-28H,10-11H2,1H3,(H,29,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to ECK (unknown origin) |
Bioorg Med Chem Lett 19: 226-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.108
BindingDB Entry DOI: 10.7270/Q22F7N98 |
More data for this
Ligand-Target Pair | |
Ephrin type-A receptor 2
(Homo sapiens (Human)) | BDBM50255013

((S)-5-((2,4-diamino-4-oxobutanamido)methyl)-3,4-di...)Show SMILES N[C@@H](CC(N)=O)C(=O)NCc1cc(C(O)=O)c(Nc2ccc(I)cc2F)c(F)c1F |r| Show InChI InChI=1S/C18H16F3IN4O4/c19-10-4-8(22)1-2-12(10)26-16-9(18(29)30)3-7(14(20)15(16)21)6-25-17(28)11(23)5-13(24)27/h1-4,11,26H,5-6,23H2,(H2,24,27)(H,25,28)(H,29,30)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Binding affinity to ECK (unknown origin) |
Bioorg Med Chem Lett 19: 226-9 (2008)
Article DOI: 10.1016/j.bmcl.2008.10.108
BindingDB Entry DOI: 10.7270/Q22F7N98 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data