

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50006222 ((1S,2R,5R)-5-(6-Amino-purin-9-yl)-3-hydroxymethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Time course of inactivation of bovine liver S-adenosyl-homocysteine hydrolase | J Med Chem 31: 500-3 (1988) BindingDB Entry DOI: 10.7270/Q28P61Q3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50006222 ((1S,2R,5R)-5-(6-Amino-purin-9-yl)-3-hydroxymethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition against bovine-liver S-Adenosyl-homocysteine (AdoHcy) hydrolase | J Med Chem 31: 1729-38 (1988) BindingDB Entry DOI: 10.7270/Q2HM5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50006222 ((1S,2R,5R)-5-(6-Amino-purin-9-yl)-3-hydroxymethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibitory activity of the compound against bovine liver S-adenosyl-L-homocysteine hydrolase (AdoHcy) rate of inactivation by NpcA | J Med Chem 35: 1782-91 (1992) BindingDB Entry DOI: 10.7270/Q23J3BW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50006218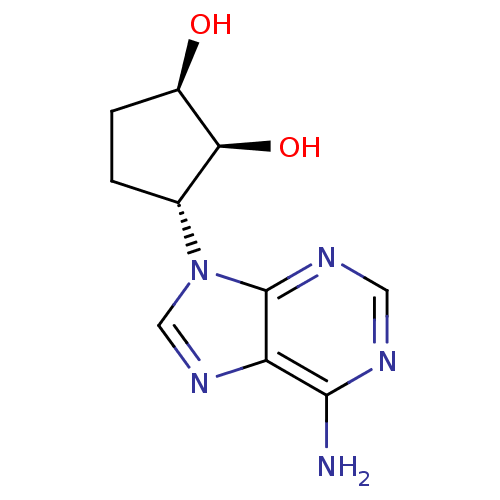 ((1R,2S,3R)-3-(6-amino-9H-purin-9-yl)cyclopentane-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibitory activity of the compound against bovine liver S-adenosyl-L-homocysteine hydrolase (AdoHcy). | J Med Chem 35: 1782-91 (1992) BindingDB Entry DOI: 10.7270/Q23J3BW0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50006220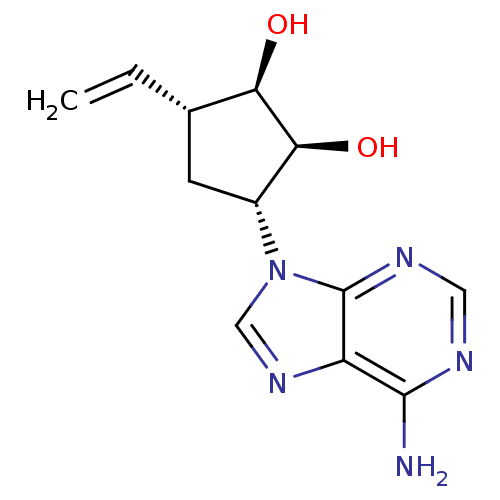 (3-(6-Amino-purin-9-yl)-5-vinyl-cyclopentane-1,2-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibitory activity of the compound against bovine liver S-adenosyl-L-homocysteine hydrolase (AdoHcy). | J Med Chem 35: 1782-91 (1992) BindingDB Entry DOI: 10.7270/Q23J3BW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50006221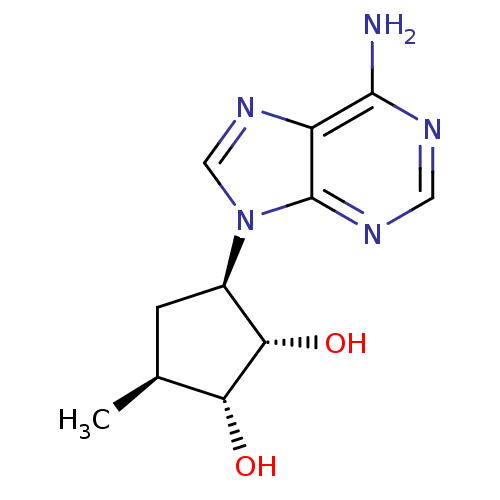 (3-(6-Amino-purin-9-yl)-5-methyl-cyclopentane-1,2-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibitory activity of the compound against bovine liver S-adenosyl-L-homocysteine hydrolase (AdoHcy). | J Med Chem 35: 1782-91 (1992) BindingDB Entry DOI: 10.7270/Q23J3BW0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50405655 (CHEMBL147260) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Time course of inactivation of bovine liver S-adenosyl-homocysteine hydrolase | J Med Chem 31: 500-3 (1988) BindingDB Entry DOI: 10.7270/Q28P61Q3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50006215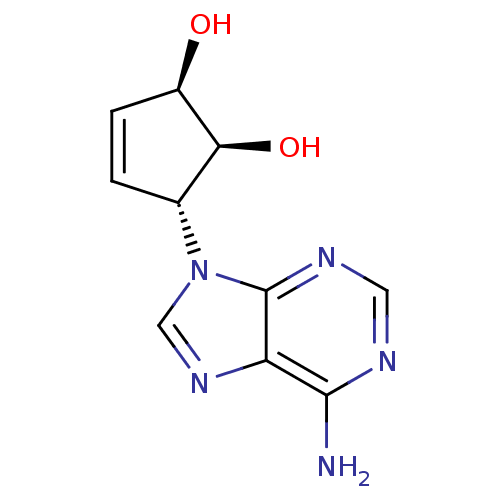 ((1'R,2'S,3'R)-9-(2',3'-dihydroxycyclopent-4'-enyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Time course of inactivation of bovine liver S-adenosyl-homocysteine hydrolase | J Med Chem 31: 500-3 (1988) BindingDB Entry DOI: 10.7270/Q28P61Q3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50006215 ((1'R,2'S,3'R)-9-(2',3'-dihydroxycyclopent-4'-enyl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 49 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibitory activity of the compound against bovine liver S-adenosyl-L-homocysteine hydrolase (AdoHcy). | J Med Chem 35: 1782-91 (1992) BindingDB Entry DOI: 10.7270/Q23J3BW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50023889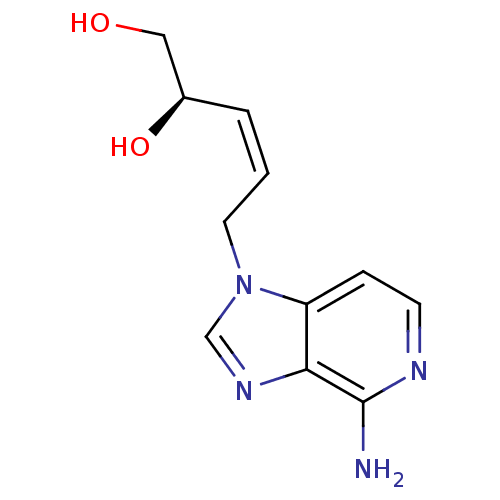 (5-(4-Amino-imidazo[4,5-c]pyridin-1-yl)-pent-3-ene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition against bovine-liver S-Adenosyl-homocysteine (AdoHcy) hydrolase | J Med Chem 31: 1729-38 (1988) BindingDB Entry DOI: 10.7270/Q2HM5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50023879 (2-[2-(6-Amino-purin-9-yl)-ethylidene]-propane-1,3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition against bovine-liver S-Adenosyl-homocysteine (AdoHcy) hydrolase | J Med Chem 31: 1729-38 (1988) BindingDB Entry DOI: 10.7270/Q2HM5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50011148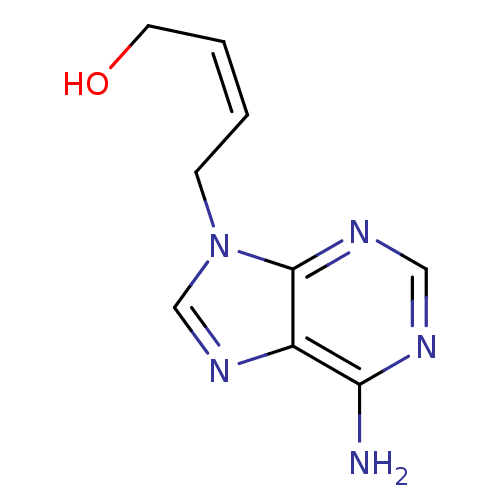 ((Z)-4-(6-Amino-purin-9-yl)-but-2-en-1-ol | 4-(6-Am...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 125 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition against bovine-liver S-Adenosyl-homocysteine (AdoHcy) hydrolase | J Med Chem 31: 1729-38 (1988) BindingDB Entry DOI: 10.7270/Q2HM5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50023885 (4-(4-Amino-imidazo[4,5-c]pyridin-1-yl)-but-2-yn-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition against bovine-liver S-Adenosyl-homocysteine (AdoHcy) hydrolase | J Med Chem 31: 1729-38 (1988) BindingDB Entry DOI: 10.7270/Q2HM5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50023886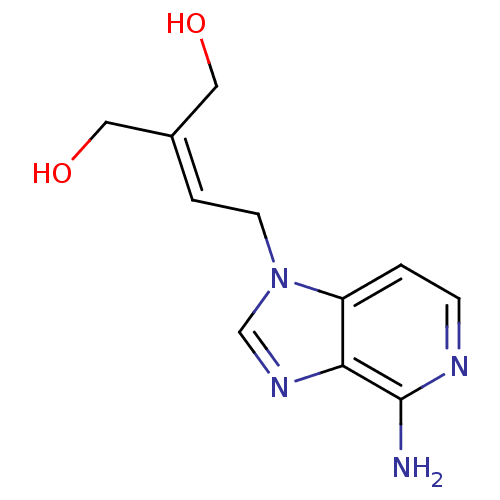 (2-[2-(4-Amino-imidazo[4,5-c]pyridin-1-yl)-ethylide...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 166 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition against bovine-liver S-Adenosyl-homocysteine (AdoHcy) hydrolase | J Med Chem 31: 1729-38 (1988) BindingDB Entry DOI: 10.7270/Q2HM5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50023887 (4-(6-Amino-purin-9-yl)-2-methyl-but-2-en-1-ol | CH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition against bovine-liver S-Adenosyl-homocysteine (AdoHcy) hydrolase | J Med Chem 31: 1729-38 (1988) BindingDB Entry DOI: 10.7270/Q2HM5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50023882 (5-(6-Amino-purin-9-yl)-pent-3-ene-1,2-diol | CHEMB...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition against bovine-liver S-Adenosyl-homocysteine (AdoHcy) hydrolase | J Med Chem 31: 1729-38 (1988) BindingDB Entry DOI: 10.7270/Q2HM5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50023877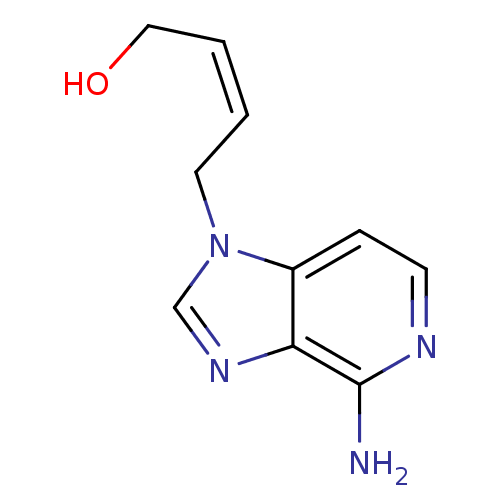 (4-(4-Amino-imidazo[4,5-c]pyridin-1-yl)-but-2-en-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition against bovine-liver S-Adenosyl-homocysteine (AdoHcy) hydrolase | J Med Chem 31: 1729-38 (1988) BindingDB Entry DOI: 10.7270/Q2HM5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50011180 (4-(6-Amino-purin-9-yl)-but-2-en-1-ol | CHEMBL49917) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition against bovine-liver S-Adenosyl-homocysteine (AdoHcy) hydrolase | J Med Chem 31: 1729-38 (1988) BindingDB Entry DOI: 10.7270/Q2HM5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50023884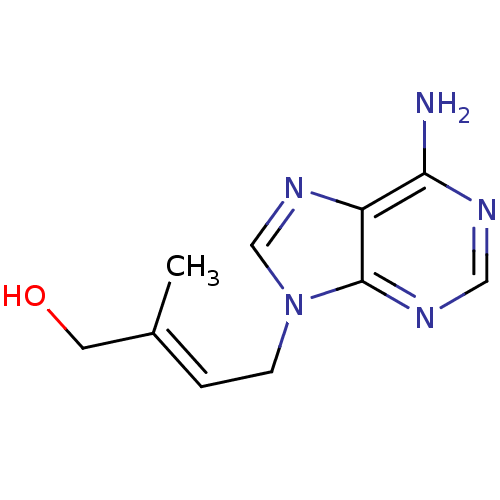 (4-(6-Amino-purin-9-yl)-2-methyl-but-2-en-1-ol | CH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition against bovine-liver S-Adenosyl-homocysteine (AdoHcy) hydrolase | J Med Chem 31: 1729-38 (1988) BindingDB Entry DOI: 10.7270/Q2HM5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50006217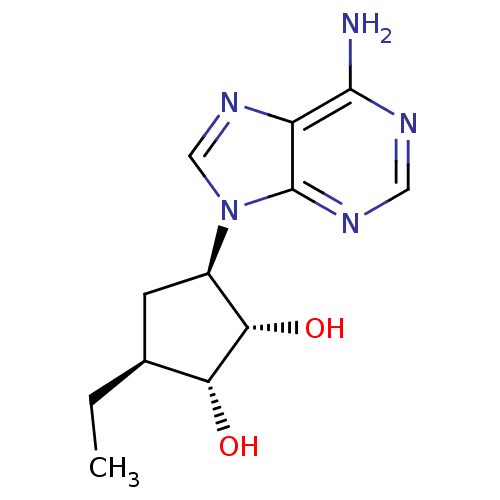 (3-(6-Amino-purin-9-yl)-5-ethyl-cyclopentane-1,2-di...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 586 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibitory activity of the compound against bovine liver S-adenosyl-L-homocysteine hydrolase (AdoHcy). | J Med Chem 35: 1782-91 (1992) BindingDB Entry DOI: 10.7270/Q23J3BW0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50367765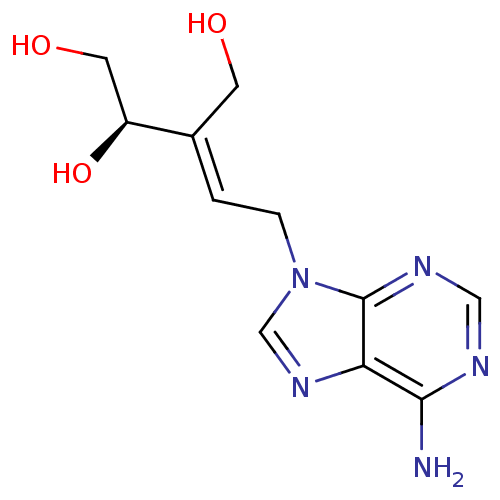 (CHEMBL1794978) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibition against bovine-liver S-Adenosyl-homocysteine (AdoHcy) hydrolase | J Med Chem 31: 1729-38 (1988) BindingDB Entry DOI: 10.7270/Q2HM5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50006219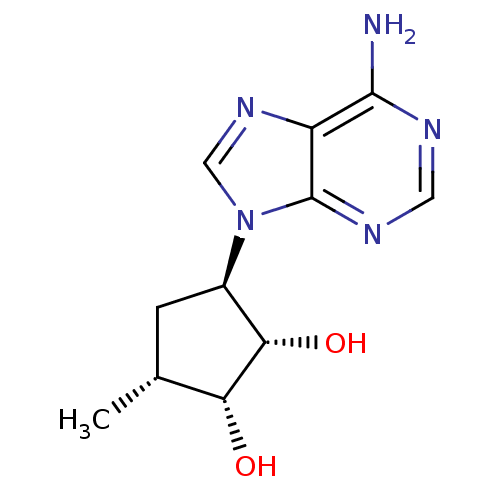 (3-(6-Amino-purin-9-yl)-5-methyl-cyclopentane-1,2-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibitory activity of the compound against bovine liver S-adenosyl-L-homocysteine hydrolase (AdoHcy). | J Med Chem 35: 1782-91 (1992) BindingDB Entry DOI: 10.7270/Q23J3BW0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50006223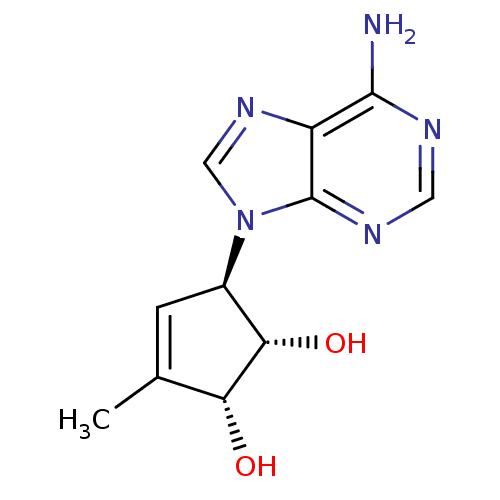 (5-(6-Amino-purin-9-yl)-3-methyl-cyclopent-3-ene-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 9.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibitory activity of the compound against bovine liver S-adenosyl-L-homocysteine hydrolase (AdoHcy). | J Med Chem 35: 1782-91 (1992) BindingDB Entry DOI: 10.7270/Q23J3BW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50006216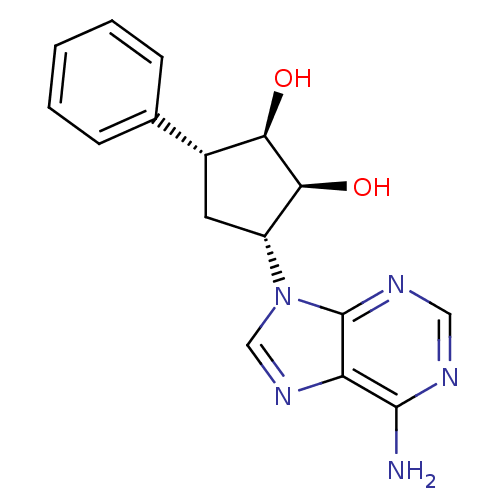 (3-(6-Amino-purin-9-yl)-5-phenyl-cyclopentane-1,2-d...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Inhibitory activity of the compound against bovine liver S-adenosyl-L-homocysteine hydrolase (AdoHcy). | J Med Chem 35: 1782-91 (1992) BindingDB Entry DOI: 10.7270/Q23J3BW0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM11459 (2,6,9-trisubstituted adenine derivative | 2-N-(4-a...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Lawrence Berkeley National Laboratory | Assay Description The enzyme was assayed with substrate GST-Rb in the presence of 10 uM ATP/[gamma-33P] ATP, and capturing the 33-P labeled reaction products on GSH-Se... | J Med Chem 44: 524-30 (2001) Article DOI: 10.1021/jm001043t BindingDB Entry DOI: 10.7270/Q2Q23XGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM11460 (2,6,9-trisubstituted adenine derivative | 2-N-(4-a...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Lawrence Berkeley National Laboratory | Assay Description The enzyme was assayed with substrate GST-Rb in the presence of 10 uM ATP/[gamma-33P] ATP, and capturing the 33-P labeled reaction products on GSH-Se... | J Med Chem 44: 524-30 (2001) Article DOI: 10.1021/jm001043t BindingDB Entry DOI: 10.7270/Q2Q23XGD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM11458 (2,6,9-trisubstituted adenine derivative | 2-N-(4-a...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Lawrence Berkeley National Laboratory | Assay Description The enzyme was assayed with substrate GST-Rb in the presence of 10 uM ATP/[gamma-33P] ATP, and capturing the 33-P labeled reaction products on GSH-Se... | J Med Chem 44: 524-30 (2001) Article DOI: 10.1021/jm001043t BindingDB Entry DOI: 10.7270/Q2Q23XGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Mus musculus) | BDBM50006222 ((1S,2R,5R)-5-(6-Amino-purin-9-yl)-3-hydroxymethyl-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Evaluated for the 50% inhibition of S-Adenosyl-homocysteine (AdoHcy) hydrolase L929 lysate from murine L-929 cells | J Med Chem 31: 1729-38 (1988) BindingDB Entry DOI: 10.7270/Q2HM5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1 (Homo sapiens (Human)) | BDBM11460 (2,6,9-trisubstituted adenine derivative | 2-N-(4-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Lawrence Berkeley National Laboratory | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 0.5 uM ATP/ [gamma-33P] ATP. Af... | J Med Chem 44: 524-30 (2001) Article DOI: 10.1021/jm001043t BindingDB Entry DOI: 10.7270/Q2Q23XGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1 (Homo sapiens (Human)) | BDBM11459 (2,6,9-trisubstituted adenine derivative | 2-N-(4-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Lawrence Berkeley National Laboratory | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 0.5 uM ATP/ [gamma-33P] ATP. Af... | J Med Chem 44: 524-30 (2001) Article DOI: 10.1021/jm001043t BindingDB Entry DOI: 10.7270/Q2Q23XGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1 (Homo sapiens (Human)) | BDBM11458 (2,6,9-trisubstituted adenine derivative | 2-N-(4-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 226 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Lawrence Berkeley National Laboratory | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 0.5 uM ATP/ [gamma-33P] ATP. Af... | J Med Chem 44: 524-30 (2001) Article DOI: 10.1021/jm001043t BindingDB Entry DOI: 10.7270/Q2Q23XGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 2/G1/S-specific cyclin-E1 (Homo sapiens (Human)) | BDBM11461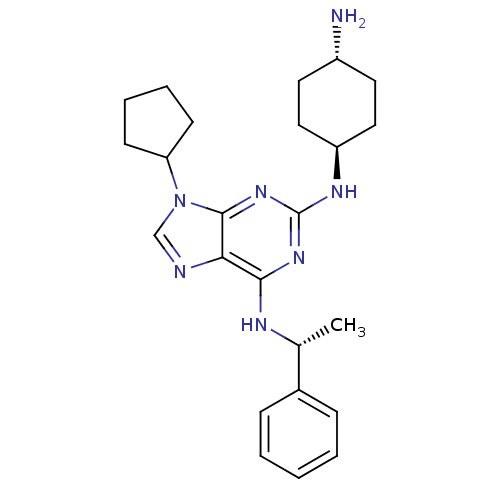 (2,6,9-trisubstituted adenine derivative | 2-N-(4-a...) | PDB UniProtKB/SwissProt antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Lawrence Berkeley National Laboratory | Assay Description The enzyme was assayed with substrate GST-Rb in the presence of 10 uM ATP/[gamma-33P] ATP, and capturing the 33-P labeled reaction products on GSH-Se... | J Med Chem 44: 524-30 (2001) Article DOI: 10.1021/jm001043t BindingDB Entry DOI: 10.7270/Q2Q23XGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 (Homo sapiens (Human)) | BDBM11459 (2,6,9-trisubstituted adenine derivative | 2-N-(4-a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 780 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Lawrence Berkeley National Laboratory | Assay Description The enzyme was assayed with substrate GST-Rb in the presence of 10 uM ATP/[gamma-33P] ATP, and capturing the 33-P labeled reaction products on GSH-Se... | J Med Chem 44: 524-30 (2001) Article DOI: 10.1021/jm001043t BindingDB Entry DOI: 10.7270/Q2Q23XGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50023880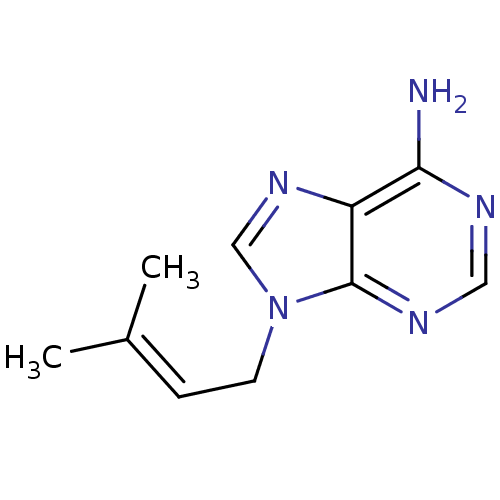 (9-(3-Methyl-but-2-enyl)-9H-purin-6-ylamine | CHEMB...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Evaluated for the 50% inhibition of bovine-liver S-Adenosyl-homocysteine (AdoHcy) hydrolase | J Med Chem 31: 1729-38 (1988) BindingDB Entry DOI: 10.7270/Q2HM5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50023888 (5-(6-Amino-purin-9-yl)-pent-3-ene-1,2-diol | CHEMB...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 950 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Evaluated for the 50% inhibition of bovine-liver S-Adenosyl-homocysteine (AdoHcy) hydrolase | J Med Chem 31: 1729-38 (1988) BindingDB Entry DOI: 10.7270/Q2HM5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50011163 (4-(6-Amino-purin-9-yl)-but-2-yn-1-ol | CHEMBL46348) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Evaluated for the 50% inhibition of bovine-liver S-Adenosyl-homocysteine (AdoHcy) hydrolase | J Med Chem 31: 1729-38 (1988) BindingDB Entry DOI: 10.7270/Q2HM5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Homo sapiens (Human)) | BDBM50023883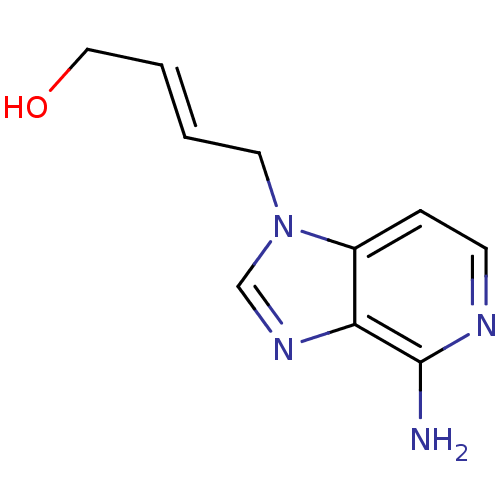 (4-(4-Amino-imidazo[4,5-c]pyridin-1-yl)-but-2-en-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Evaluated for the 50% inhibition of bovine-liver S-Adenosyl-homocysteine (AdoHcy) hydrolase | J Med Chem 31: 1729-38 (1988) BindingDB Entry DOI: 10.7270/Q2HM5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase/G2/mitotic-specific cyclin- 1 (Homo sapiens (Human)) | BDBM11461 (2,6,9-trisubstituted adenine derivative | 2-N-(4-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | 8.0 | 30 |
Lawrence Berkeley National Laboratory | Assay Description The biochemical activity of compounds was determined by incubation with specific enzyme and substrate in the presence 0.5 uM ATP/ [gamma-33P] ATP. Af... | J Med Chem 44: 524-30 (2001) Article DOI: 10.1021/jm001043t BindingDB Entry DOI: 10.7270/Q2Q23XGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 (Homo sapiens (Human)) | BDBM11458 (2,6,9-trisubstituted adenine derivative | 2-N-(4-a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Lawrence Berkeley National Laboratory | Assay Description The enzyme was assayed with substrate GST-Rb in the presence of 10 uM ATP/[gamma-33P] ATP, and capturing the 33-P labeled reaction products on GSH-Se... | J Med Chem 44: 524-30 (2001) Article DOI: 10.1021/jm001043t BindingDB Entry DOI: 10.7270/Q2Q23XGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 (Homo sapiens (Human)) | BDBM11460 (2,6,9-trisubstituted adenine derivative | 2-N-(4-a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Lawrence Berkeley National Laboratory | Assay Description The enzyme was assayed with substrate GST-Rb in the presence of 10 uM ATP/[gamma-33P] ATP, and capturing the 33-P labeled reaction products on GSH-Se... | J Med Chem 44: 524-30 (2001) Article DOI: 10.1021/jm001043t BindingDB Entry DOI: 10.7270/Q2Q23XGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cyclin-dependent kinase 4/G1/S-specific cyclin-D1 (Homo sapiens (Human)) | BDBM11461 (2,6,9-trisubstituted adenine derivative | 2-N-(4-a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | 7.5 | 30 |
Lawrence Berkeley National Laboratory | Assay Description The enzyme was assayed with substrate GST-Rb in the presence of 10 uM ATP/[gamma-33P] ATP, and capturing the 33-P labeled reaction products on GSH-Se... | J Med Chem 44: 524-30 (2001) Article DOI: 10.1021/jm001043t BindingDB Entry DOI: 10.7270/Q2Q23XGD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Mus musculus) | BDBM50023889 (5-(4-Amino-imidazo[4,5-c]pyridin-1-yl)-pent-3-ene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Evaluated for the 50% inhibition of S-Adenosyl-homocysteine (AdoHcy) hydrolase L929 lysate from murine L-929 cells | J Med Chem 31: 1729-38 (1988) BindingDB Entry DOI: 10.7270/Q2HM5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Mus musculus) | BDBM50023879 (2-[2-(6-Amino-purin-9-yl)-ethylidene]-propane-1,3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Evaluated for the 50% inhibition of S-Adenosyl-homocysteine (AdoHcy) hydrolase L929 lysate from murine L-929 cells | J Med Chem 31: 1729-38 (1988) BindingDB Entry DOI: 10.7270/Q2HM5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Mus musculus) | BDBM50023882 (5-(6-Amino-purin-9-yl)-pent-3-ene-1,2-diol | CHEMB...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Evaluated for the 50% inhibition of S-Adenosyl-homocysteine (AdoHcy) hydrolase L929 lysate from murine L-929 cells | J Med Chem 31: 1729-38 (1988) BindingDB Entry DOI: 10.7270/Q2HM5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Mus musculus) | BDBM50023886 (2-[2-(4-Amino-imidazo[4,5-c]pyridin-1-yl)-ethylide...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Evaluated for the 50% inhibition of S-Adenosyl-homocysteine (AdoHcy) hydrolase L929 lysate from murine L-929 cells | J Med Chem 31: 1729-38 (1988) BindingDB Entry DOI: 10.7270/Q2HM5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Mus musculus) | BDBM50011148 ((Z)-4-(6-Amino-purin-9-yl)-but-2-en-1-ol | 4-(6-Am...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.45E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Evaluated for the 50% inhibition of S-Adenosyl-homocysteine (AdoHcy) hydrolase L929 lysate from murine L-929 cells | J Med Chem 31: 1729-38 (1988) BindingDB Entry DOI: 10.7270/Q2HM5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Mus musculus) | BDBM50023877 (4-(4-Amino-imidazo[4,5-c]pyridin-1-yl)-but-2-en-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Evaluated for the 50% inhibition of S-Adenosyl-homocysteine (AdoHcy) hydrolase L929 lysate from murine L-929 cells | J Med Chem 31: 1729-38 (1988) BindingDB Entry DOI: 10.7270/Q2HM5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Mus musculus) | BDBM50023887 (4-(6-Amino-purin-9-yl)-2-methyl-but-2-en-1-ol | CH...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Evaluated for the 50% inhibition of S-Adenosyl-homocysteine (AdoHcy) hydrolase L929 lysate from murine L-929 cells | J Med Chem 31: 1729-38 (1988) BindingDB Entry DOI: 10.7270/Q2HM5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Mus musculus) | BDBM50023885 (4-(4-Amino-imidazo[4,5-c]pyridin-1-yl)-but-2-yn-1-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Evaluated for the 50% inhibition of S-Adenosyl-homocysteine (AdoHcy) hydrolase L929 lysate from murine L-929 cells | J Med Chem 31: 1729-38 (1988) BindingDB Entry DOI: 10.7270/Q2HM5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosylhomocysteinase (Mus musculus) | BDBM50011180 (4-(6-Amino-purin-9-yl)-but-2-en-1-ol | CHEMBL49917) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kansas Curated by ChEMBL | Assay Description Evaluated for the 50% inhibition of S-Adenosyl-homocysteine (AdoHcy) hydrolase L929 lysate from murine L-929 cells | J Med Chem 31: 1729-38 (1988) BindingDB Entry DOI: 10.7270/Q2HM5920 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||