 Found 69 hits with Last Name = 'bose' and Initial = 'j'
Found 69 hits with Last Name = 'bose' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50315887
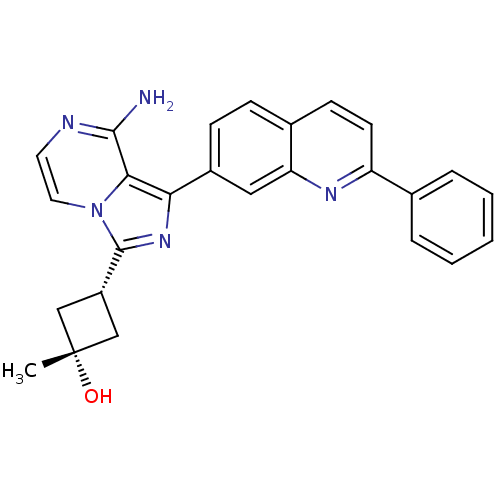
((1s,3s)-3-(8-amino-1-(2-phenylquinolin-7-yl)imidaz...)Show SMILES C[C@@]1(O)C[C@@H](C1)c1nc(-c2ccc3ccc(nc3c2)-c2ccccc2)c2c(N)nccn12 |r,wU:1.1,4.6,wD:1.0,(18.42,-37.26,;17.1,-36.49,;16.31,-37.82,;17.79,-35.11,;16.41,-34.42,;15.72,-35.8,;15.93,-32.97,;16.83,-31.71,;15.91,-30.47,;16.38,-29.01,;15.35,-27.88,;15.81,-26.41,;17.32,-26.08,;17.78,-24.62,;19.27,-24.29,;20.31,-25.42,;19.85,-26.89,;18.35,-27.21,;17.89,-28.68,;21.81,-25.09,;22.85,-26.23,;24.35,-25.9,;24.82,-24.43,;23.77,-23.29,;22.27,-23.63,;14.45,-30.96,;13.11,-30.19,;13.11,-28.65,;11.78,-30.96,;11.78,-32.51,;13.12,-33.28,;14.46,-32.5,)| Show InChI InChI=1S/C26H23N5O/c1-26(32)14-19(15-26)25-30-22(23-24(27)28-11-12-31(23)25)18-8-7-17-9-10-20(29-21(17)13-18)16-5-3-2-4-6-16/h2-13,19,32H,14-15H2,1H3,(H2,27,28)/t19-,26+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Enterprises Limited
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R (unknown origin) using poly-G1 as substrate incubated for 10 mins prior to substrate addition measured after 1 hr by time-resolve... |
Eur J Med Chem 92: 246-56 (2015)
Article DOI: 10.1016/j.ejmech.2014.12.053
BindingDB Entry DOI: 10.7270/Q2639RFV |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50071033
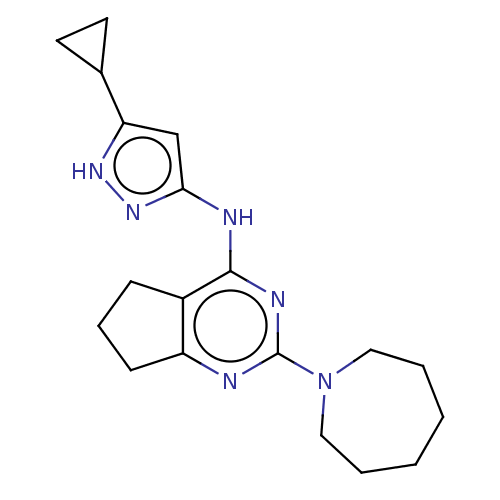
(CHEMBL3409721)Show InChI InChI=1S/C19H26N6/c1-2-4-11-25(10-3-1)19-20-15-7-5-6-14(15)18(22-19)21-17-12-16(23-24-17)13-8-9-13/h12-13H,1-11H2,(H2,20,21,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Enterprises Limited
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R (unknown origin) using poly-G1 as substrate incubated for 10 mins prior to substrate addition measured after 1 hr by time-resolve... |
Eur J Med Chem 92: 246-56 (2015)
Article DOI: 10.1016/j.ejmech.2014.12.053
BindingDB Entry DOI: 10.7270/Q2639RFV |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50005651
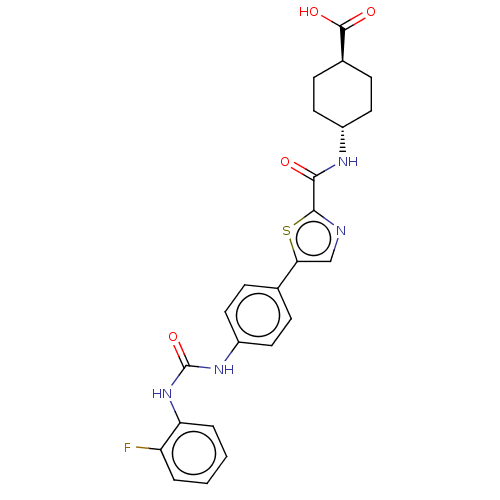
(CHEMBL3235427)Show SMILES OC(=O)[C@H]1CC[C@@H](CC1)NC(=O)c1ncc(s1)-c1ccc(NC(=O)Nc2ccccc2F)cc1 |r,wU:6.9,wD:3.2,(47.51,-36.67,;46.7,-37.98,;47.43,-39.33,;45.16,-37.93,;44.43,-36.57,;42.89,-36.52,;42.09,-37.83,;42.81,-39.19,;44.35,-39.24,;40.55,-37.79,;39.74,-39.1,;40.47,-40.46,;38.2,-39.06,;37.33,-37.79,;35.85,-38.22,;35.81,-39.76,;37.26,-40.28,;34.54,-40.64,;33.21,-39.88,;31.88,-40.65,;31.88,-42.19,;30.54,-42.96,;29.21,-42.19,;29.21,-40.65,;27.87,-42.96,;26.54,-42.19,;26.55,-40.64,;25.22,-39.87,;23.88,-40.64,;23.89,-42.19,;25.22,-42.95,;25.22,-44.49,;33.21,-42.96,;34.55,-42.19,)| Show InChI InChI=1S/C24H23FN4O4S/c25-18-3-1-2-4-19(18)29-24(33)28-17-9-5-14(6-10-17)20-13-26-22(34-20)21(30)27-16-11-7-15(8-12-16)23(31)32/h1-6,9-10,13,15-16H,7-8,11-12H2,(H,27,30)(H,31,32)(H2,28,29,33)/t15-,16- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
VIT University
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 using 1,2-diacylglycerol/[14C]-oleoyl CoA as substrate assessed as [14C]-triglyceride formation after 10 mins by scintillat... |
Eur J Med Chem 79: 203-15 (2014)
Article DOI: 10.1016/j.ejmech.2014.03.077
BindingDB Entry DOI: 10.7270/Q23R0VCD |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50005652

(CHEMBL3235428)Show SMILES OC(=O)[C@H]1CC[C@@H](CC1)NC(=O)c1ncc(s1)-c1ccc(NC(=O)Nc2ccccc2Cl)cc1 |r,wU:6.9,wD:3.2,(70.47,-38.11,;69.66,-39.41,;70.39,-40.77,;68.12,-39.37,;67.39,-38.01,;65.84,-37.96,;65.05,-39.27,;65.77,-40.63,;67.3,-40.67,;63.51,-39.23,;62.7,-40.54,;63.43,-41.9,;61.16,-40.5,;60.29,-39.23,;58.81,-39.66,;58.77,-41.2,;60.22,-41.72,;57.5,-42.08,;56.17,-41.31,;54.84,-42.08,;54.83,-43.63,;53.5,-44.4,;52.17,-43.63,;52.17,-42.09,;50.83,-44.4,;49.5,-43.62,;49.51,-42.08,;48.18,-41.31,;46.84,-42.08,;46.85,-43.63,;48.18,-44.39,;48.18,-45.93,;56.17,-44.4,;57.51,-43.63,)| Show InChI InChI=1S/C24H23ClN4O4S/c25-18-3-1-2-4-19(18)29-24(33)28-17-9-5-14(6-10-17)20-13-26-22(34-20)21(30)27-16-11-7-15(8-12-16)23(31)32/h1-6,9-10,13,15-16H,7-8,11-12H2,(H,27,30)(H,31,32)(H2,28,29,33)/t15-,16- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
VIT University
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 using 1,2-diacylglycerol/[14C]-oleoyl CoA as substrate assessed as [14C]-triglyceride formation after 10 mins by scintillat... |
Eur J Med Chem 79: 203-15 (2014)
Article DOI: 10.1016/j.ejmech.2014.03.077
BindingDB Entry DOI: 10.7270/Q23R0VCD |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50071014
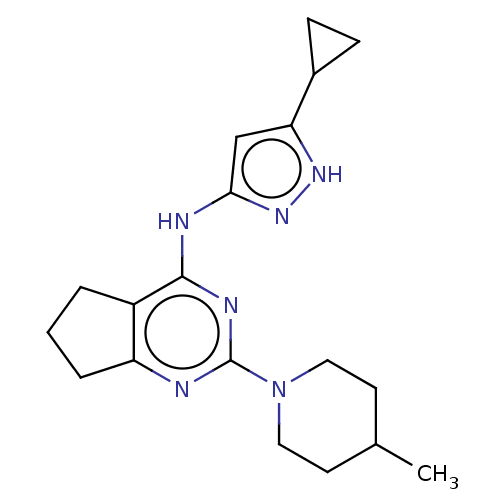
(CHEMBL3409716)Show SMILES CC1CCN(CC1)c1nc2CCCc2c(Nc2cc([nH]n2)C2CC2)n1 Show InChI InChI=1S/C19H26N6/c1-12-7-9-25(10-8-12)19-20-15-4-2-3-14(15)18(22-19)21-17-11-16(23-24-17)13-5-6-13/h11-13H,2-10H2,1H3,(H2,20,21,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Enterprises Limited
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R (unknown origin) using poly-G1 as substrate incubated for 10 mins prior to substrate addition measured after 1 hr by time-resolve... |
Eur J Med Chem 92: 246-56 (2015)
Article DOI: 10.1016/j.ejmech.2014.12.053
BindingDB Entry DOI: 10.7270/Q2639RFV |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50005654

(CHEMBL3235430)Show SMILES OC(=O)[C@H]1CC[C@@H](CC1)NC(=O)c1ncc(s1)-c1ccc(NC(=O)Nc2cc(F)c(F)cc2F)cc1 |r,wU:6.9,wD:3.2,(48.29,-50.11,;47.48,-51.42,;48.2,-52.78,;45.94,-51.37,;45.21,-50.02,;43.66,-49.97,;42.86,-51.28,;43.59,-52.64,;45.12,-52.68,;41.33,-51.24,;40.52,-52.55,;41.25,-53.9,;38.98,-52.51,;38.11,-51.24,;36.63,-51.67,;36.59,-53.21,;38.04,-53.73,;35.32,-54.09,;33.98,-53.32,;32.66,-54.09,;32.65,-55.64,;31.32,-56.41,;29.99,-55.64,;29.99,-54.1,;28.65,-56.4,;27.32,-55.63,;27.33,-54.09,;26,-53.32,;26,-51.78,;24.66,-54.09,;23.33,-53.32,;24.67,-55.64,;26,-56.4,;26,-57.94,;33.99,-56.41,;35.32,-55.64,)| Show InChI InChI=1S/C24H21F3N4O4S/c25-16-9-18(27)19(10-17(16)26)31-24(35)30-15-5-1-12(2-6-15)20-11-28-22(36-20)21(32)29-14-7-3-13(4-8-14)23(33)34/h1-2,5-6,9-11,13-14H,3-4,7-8H2,(H,29,32)(H,33,34)(H2,30,31,35)/t13-,14- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
VIT University
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 using 1,2-diacylglycerol/[14C]-oleoyl CoA as substrate assessed as [14C]-triglyceride formation after 10 mins by scintillat... |
Eur J Med Chem 79: 203-15 (2014)
Article DOI: 10.1016/j.ejmech.2014.03.077
BindingDB Entry DOI: 10.7270/Q23R0VCD |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50071057
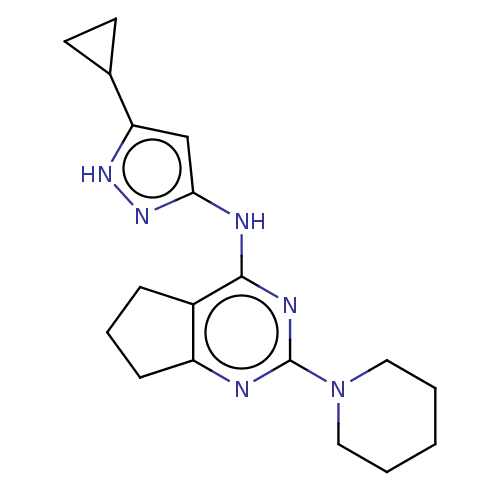
(CHEMBL3409713)Show InChI InChI=1S/C18H24N6/c1-2-9-24(10-3-1)18-19-14-6-4-5-13(14)17(21-18)20-16-11-15(22-23-16)12-7-8-12/h11-12H,1-10H2,(H2,19,20,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Enterprises Limited
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R (unknown origin) using poly-G1 as substrate incubated for 10 mins prior to substrate addition measured after 1 hr by time-resolve... |
Eur J Med Chem 92: 246-56 (2015)
Article DOI: 10.1016/j.ejmech.2014.12.053
BindingDB Entry DOI: 10.7270/Q2639RFV |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50005649

(CHEMBL3235425)Show SMILES OC(=O)[C@H]1CC[C@@H](CC1)NC(=O)c1ncc(s1)-c1ccc(NC(=O)Nc2cc(F)cc(F)c2)cc1 |r,wU:6.9,wD:3.2,(47.51,-36.67,;46.7,-37.98,;47.43,-39.33,;45.16,-37.93,;44.43,-36.57,;42.89,-36.52,;42.09,-37.83,;42.81,-39.19,;44.35,-39.24,;40.55,-37.79,;39.74,-39.1,;40.47,-40.46,;38.2,-39.06,;37.33,-37.79,;35.85,-38.22,;35.81,-39.76,;37.26,-40.28,;34.54,-40.64,;33.21,-39.88,;31.88,-40.65,;31.88,-42.19,;30.54,-42.96,;29.21,-42.19,;29.21,-40.65,;27.87,-42.96,;26.54,-42.19,;25.22,-42.95,;23.89,-42.19,;22.54,-42.97,;23.88,-40.64,;25.22,-39.87,;25.22,-38.32,;26.55,-40.64,;33.21,-42.96,;34.55,-42.19,)| Show InChI InChI=1S/C24H22F2N4O4S/c25-15-9-16(26)11-19(10-15)30-24(34)29-18-5-1-13(2-6-18)20-12-27-22(35-20)21(31)28-17-7-3-14(4-8-17)23(32)33/h1-2,5-6,9-12,14,17H,3-4,7-8H2,(H,28,31)(H,32,33)(H2,29,30,34)/t14-,17- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
VIT University
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 using 1,2-diacylglycerol/[14C]-oleoyl CoA as substrate assessed as [14C]-triglyceride formation after 10 mins by scintillat... |
Eur J Med Chem 79: 203-15 (2014)
Article DOI: 10.1016/j.ejmech.2014.03.077
BindingDB Entry DOI: 10.7270/Q23R0VCD |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50071036
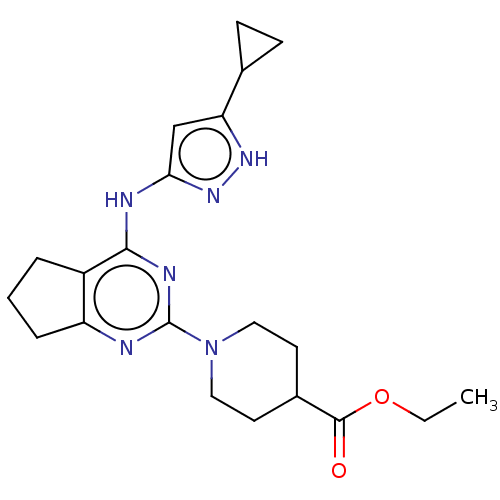
(CHEMBL3409718)Show SMILES CCOC(=O)C1CCN(CC1)c1nc2CCCc2c(Nc2cc([nH]n2)C2CC2)n1 Show InChI InChI=1S/C21H28N6O2/c1-2-29-20(28)14-8-10-27(11-9-14)21-22-16-5-3-4-15(16)19(24-21)23-18-12-17(25-26-18)13-6-7-13/h12-14H,2-11H2,1H3,(H2,22,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Enterprises Limited
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R (unknown origin) using poly-G1 as substrate incubated for 10 mins prior to substrate addition measured after 1 hr by time-resolve... |
Eur J Med Chem 92: 246-56 (2015)
Article DOI: 10.1016/j.ejmech.2014.12.053
BindingDB Entry DOI: 10.7270/Q2639RFV |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50005653

(CHEMBL3235429)Show SMILES OC(=O)[C@H]1CC[C@@H](CC1)NC(=O)c1ncc(s1)-c1ccc(NC(=O)Nc2ccc(F)cc2F)cc1 |r,wU:6.9,wD:3.2,(25.13,-47.8,;24.31,-49.11,;25.04,-50.47,;22.78,-49.06,;22.05,-47.71,;20.5,-47.66,;19.7,-48.97,;20.43,-50.33,;21.96,-50.37,;18.16,-48.93,;17.36,-50.24,;18.09,-51.59,;15.82,-50.2,;14.95,-48.93,;13.47,-49.36,;13.43,-50.9,;14.88,-51.42,;12.16,-51.78,;10.82,-51.01,;9.49,-51.78,;9.49,-53.33,;8.16,-54.1,;6.82,-53.33,;6.83,-51.79,;5.49,-54.09,;4.16,-53.32,;4.17,-51.78,;2.84,-51.01,;1.5,-51.78,;.17,-51.01,;1.5,-53.33,;2.84,-54.09,;2.84,-55.63,;10.83,-54.1,;12.16,-53.33,)| Show InChI InChI=1S/C24H22F2N4O4S/c25-15-5-10-19(18(26)11-15)30-24(34)29-17-6-1-13(2-7-17)20-12-27-22(35-20)21(31)28-16-8-3-14(4-9-16)23(32)33/h1-2,5-7,10-12,14,16H,3-4,8-9H2,(H,28,31)(H,32,33)(H2,29,30,34)/t14-,16- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
VIT University
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 using 1,2-diacylglycerol/[14C]-oleoyl CoA as substrate assessed as [14C]-triglyceride formation after 10 mins by scintillat... |
Eur J Med Chem 79: 203-15 (2014)
Article DOI: 10.1016/j.ejmech.2014.03.077
BindingDB Entry DOI: 10.7270/Q23R0VCD |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50071031
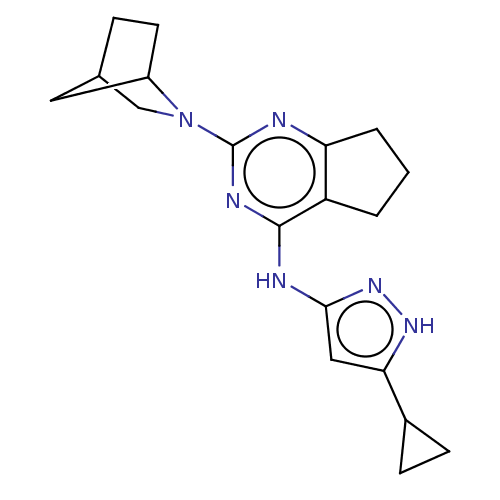
(CHEMBL3409723)Show SMILES C1CC1c1cc(Nc2nc(nc3CCCc23)N2CC3CCC2C3)n[nH]1 Show InChI InChI=1S/C19H24N6/c1-2-14-15(3-1)20-19(25-10-11-4-7-13(25)8-11)22-18(14)21-17-9-16(23-24-17)12-5-6-12/h9,11-13H,1-8,10H2,(H2,20,21,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Enterprises Limited
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R (unknown origin) using poly-G1 as substrate incubated for 10 mins prior to substrate addition measured after 1 hr by time-resolve... |
Eur J Med Chem 92: 246-56 (2015)
Article DOI: 10.1016/j.ejmech.2014.12.053
BindingDB Entry DOI: 10.7270/Q2639RFV |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50005655

(CHEMBL3235431)Show SMILES OC(=O)[C@H]1CC[C@@H](CC1)NC(=O)c1ncc(s1)-c1ccc(NC(=O)Nc2ccccc2)cc1 |r,wU:6.9,wD:3.2,(71.08,-51.02,;70.26,-52.33,;70.99,-53.69,;68.73,-52.28,;68,-50.93,;66.45,-50.88,;65.65,-52.19,;66.38,-53.55,;67.91,-53.59,;64.11,-52.15,;63.31,-53.46,;64.04,-54.81,;61.77,-53.42,;60.9,-52.15,;59.42,-52.58,;59.38,-54.12,;60.83,-54.64,;58.11,-55,;56.77,-54.23,;55.44,-55,;55.44,-56.55,;54.11,-57.32,;52.77,-56.55,;52.78,-55.01,;51.44,-57.31,;50.11,-56.54,;50.12,-55,;48.79,-54.23,;47.45,-55,;47.45,-56.55,;48.79,-57.31,;56.78,-57.32,;58.11,-56.55,)| Show InChI InChI=1S/C24H24N4O4S/c29-21(26-18-12-8-16(9-13-18)23(30)31)22-25-14-20(33-22)15-6-10-19(11-7-15)28-24(32)27-17-4-2-1-3-5-17/h1-7,10-11,14,16,18H,8-9,12-13H2,(H,26,29)(H,30,31)(H2,27,28,32)/t16-,18- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
VIT University
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 using 1,2-diacylglycerol/[14C]-oleoyl CoA as substrate assessed as [14C]-triglyceride formation after 10 mins by scintillat... |
Eur J Med Chem 79: 203-15 (2014)
Article DOI: 10.1016/j.ejmech.2014.03.077
BindingDB Entry DOI: 10.7270/Q23R0VCD |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50071032
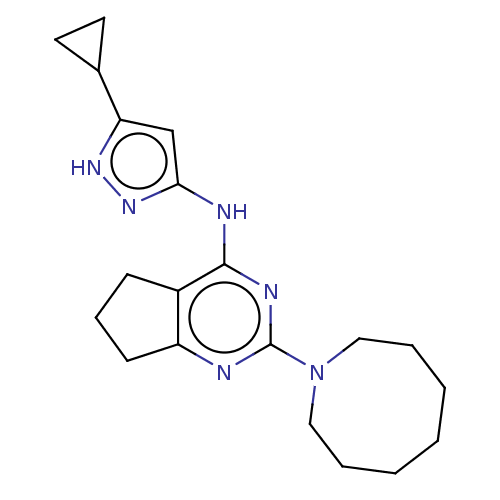
(CHEMBL3409722)Show SMILES C1CC1c1cc(Nc2nc(nc3CCCc23)N2CCCCCCC2)n[nH]1 Show InChI InChI=1S/C20H28N6/c1-2-4-11-26(12-5-3-1)20-21-16-8-6-7-15(16)19(23-20)22-18-13-17(24-25-18)14-9-10-14/h13-14H,1-12H2,(H2,21,22,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Enterprises Limited
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R (unknown origin) using poly-G1 as substrate incubated for 10 mins prior to substrate addition measured after 1 hr by time-resolve... |
Eur J Med Chem 92: 246-56 (2015)
Article DOI: 10.1016/j.ejmech.2014.12.053
BindingDB Entry DOI: 10.7270/Q2639RFV |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50354643
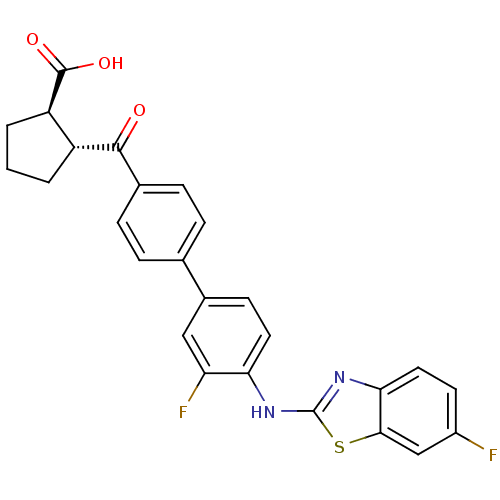
(CHEMBL1834206)Show SMILES OC(=O)[C@@H]1CCC[C@H]1C(=O)c1ccc(cc1)-c1ccc(Nc2nc3ccc(F)cc3s2)c(F)c1 |r| Show InChI InChI=1S/C26H20F2N2O3S/c27-17-9-11-22-23(13-17)34-26(30-22)29-21-10-8-16(12-20(21)28)14-4-6-15(7-5-14)24(31)18-2-1-3-19(18)25(32)33/h4-13,18-19H,1-3H2,(H,29,30)(H,32,33)/t18-,19-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
VIT University
Curated by ChEMBL
| Assay Description
Inhibition of DGAT1 (unknown origin) |
Eur J Med Chem 79: 203-15 (2014)
Article DOI: 10.1016/j.ejmech.2014.03.077
BindingDB Entry DOI: 10.7270/Q23R0VCD |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50005647

(CHEMBL3235422)Show SMILES OC(=O)[C@H]1CC[C@@H](CC1)NC(=O)c1ncc(s1)-c1ccc(NC(=O)c2nc(oc2C(F)(F)F)-c2ccccc2)cc1 |r,wU:6.9,wD:3.2,(28.27,-23.6,;27.46,-24.91,;28.18,-26.27,;25.92,-24.86,;25.19,-23.5,;23.64,-23.45,;22.84,-24.77,;23.57,-26.12,;25.1,-26.17,;21.31,-24.72,;20.5,-26.04,;21.23,-27.39,;18.96,-25.99,;18.09,-24.72,;16.61,-25.16,;16.57,-26.7,;18.02,-27.21,;15.3,-27.57,;13.96,-26.81,;12.64,-27.58,;12.63,-29.12,;11.3,-29.89,;9.97,-29.12,;9.97,-27.58,;8.51,-29.61,;7.25,-28.72,;6.01,-29.63,;6.5,-31.09,;8.04,-31.08,;8.96,-32.32,;8.34,-33.73,;10.49,-32.14,;9.71,-33.65,;4.55,-29.18,;4.22,-27.67,;2.75,-27.21,;1.61,-28.25,;1.95,-29.76,;3.42,-30.21,;13.97,-29.89,;15.31,-29.12,)| Show InChI InChI=1S/C28H23F3N4O5S/c29-28(30,31)22-21(35-25(40-22)16-4-2-1-3-5-16)23(36)33-18-10-6-15(7-11-18)20-14-32-26(41-20)24(37)34-19-12-8-17(9-13-19)27(38)39/h1-7,10-11,14,17,19H,8-9,12-13H2,(H,33,36)(H,34,37)(H,38,39)/t17-,19- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 73 | n/a | n/a | n/a | n/a | n/a | n/a |
VIT University
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 using 1,2-diacylglycerol/[14C]-oleoyl CoA as substrate assessed as [14C]-triglyceride formation after 10 mins by scintillat... |
Eur J Med Chem 79: 203-15 (2014)
Article DOI: 10.1016/j.ejmech.2014.03.077
BindingDB Entry DOI: 10.7270/Q23R0VCD |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50005648
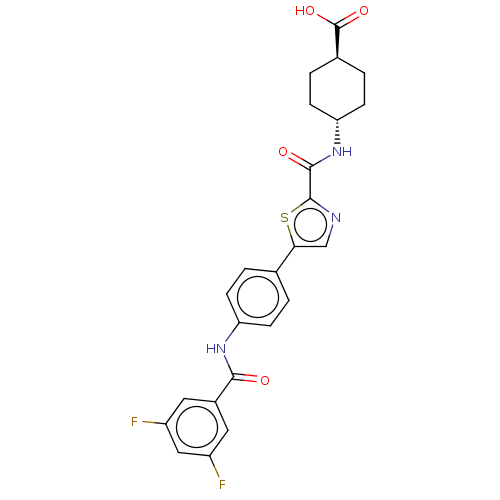
(CHEMBL3235423)Show SMILES OC(=O)[C@H]1CC[C@@H](CC1)NC(=O)c1ncc(s1)-c1ccc(NC(=O)c2cc(F)cc(F)c2)cc1 |r,wU:6.9,wD:3.2,(51.34,-23.3,;50.53,-24.61,;51.25,-25.96,;48.99,-24.56,;48.26,-23.2,;46.71,-23.15,;45.91,-24.46,;46.64,-25.82,;48.17,-25.87,;44.37,-24.42,;43.57,-25.73,;44.3,-27.09,;42.03,-25.69,;41.16,-24.42,;39.68,-24.86,;39.64,-26.39,;41.09,-26.91,;38.37,-27.27,;37.03,-26.51,;35.7,-27.28,;35.7,-28.82,;34.37,-29.59,;33.03,-28.82,;33.04,-27.28,;31.7,-29.59,;31.7,-31.12,;30.38,-31.89,;30.38,-33.43,;29.03,-31.12,;29.04,-29.58,;27.7,-28.81,;30.37,-28.81,;37.04,-29.59,;38.37,-28.82,)| Show InChI InChI=1S/C24H21F2N3O4S/c25-16-9-15(10-17(26)11-16)21(30)28-18-5-1-13(2-6-18)20-12-27-23(34-20)22(31)29-19-7-3-14(4-8-19)24(32)33/h1-2,5-6,9-12,14,19H,3-4,7-8H2,(H,28,30)(H,29,31)(H,32,33)/t14-,19- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
VIT University
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 using 1,2-diacylglycerol/[14C]-oleoyl CoA as substrate assessed as [14C]-triglyceride formation after 10 mins by scintillat... |
Eur J Med Chem 79: 203-15 (2014)
Article DOI: 10.1016/j.ejmech.2014.03.077
BindingDB Entry DOI: 10.7270/Q23R0VCD |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50071056
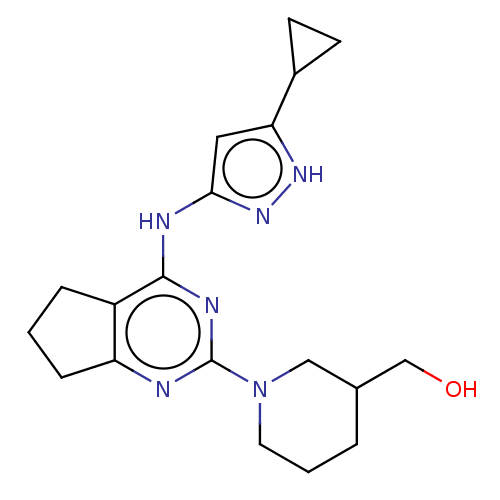
(CHEMBL3409714)Show SMILES OCC1CCCN(C1)c1nc2CCCc2c(Nc2cc([nH]n2)C2CC2)n1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Enterprises Limited
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R (unknown origin) using poly-G1 as substrate incubated for 10 mins prior to substrate addition measured after 1 hr by time-resolve... |
Eur J Med Chem 92: 246-56 (2015)
Article DOI: 10.1016/j.ejmech.2014.12.053
BindingDB Entry DOI: 10.7270/Q2639RFV |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50071026
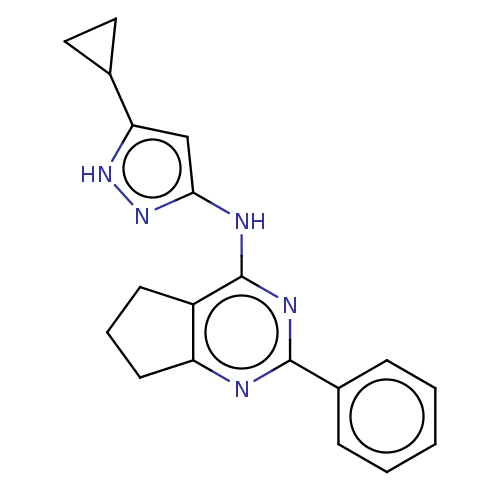
(CHEMBL3409728)Show InChI InChI=1S/C19H19N5/c1-2-5-13(6-3-1)18-20-15-8-4-7-14(15)19(22-18)21-17-11-16(23-24-17)12-9-10-12/h1-3,5-6,11-12H,4,7-10H2,(H2,20,21,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Enterprises Limited
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R (unknown origin) using poly-G1 as substrate incubated for 10 mins prior to substrate addition measured after 1 hr by time-resolve... |
Eur J Med Chem 92: 246-56 (2015)
Article DOI: 10.1016/j.ejmech.2014.12.053
BindingDB Entry DOI: 10.7270/Q2639RFV |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50071019
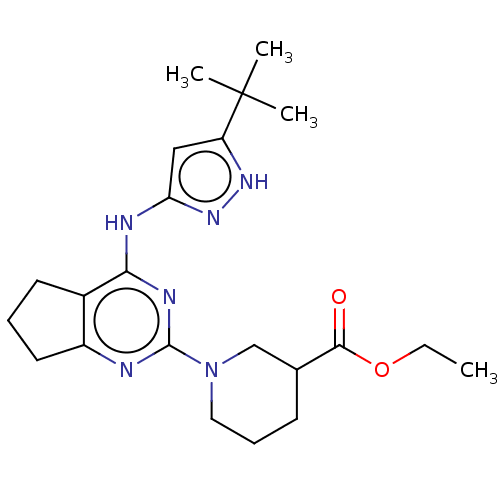
(CHEMBL3409735)Show SMILES CCOC(=O)C1CCCN(C1)c1nc2CCCc2c(Nc2cc([nH]n2)C(C)(C)C)n1 Show InChI InChI=1S/C22H32N6O2/c1-5-30-20(29)14-8-7-11-28(13-14)21-23-16-10-6-9-15(16)19(25-21)24-18-12-17(26-27-18)22(2,3)4/h12,14H,5-11,13H2,1-4H3,(H2,23,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 108 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Enterprises Limited
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R (unknown origin) using poly-G1 as substrate incubated for 10 mins prior to substrate addition measured after 1 hr by time-resolve... |
Eur J Med Chem 92: 246-56 (2015)
Article DOI: 10.1016/j.ejmech.2014.12.053
BindingDB Entry DOI: 10.7270/Q2639RFV |
More data for this
Ligand-Target Pair | |
Insulin receptor
(Homo sapiens (Human)) | BDBM50071014

(CHEMBL3409716)Show SMILES CC1CCN(CC1)c1nc2CCCc2c(Nc2cc([nH]n2)C2CC2)n1 Show InChI InChI=1S/C19H26N6/c1-12-7-9-25(10-8-12)19-20-15-4-2-3-14(15)18(22-19)21-17-11-16(23-24-17)13-5-6-13/h11-13H,2-10H2,1H3,(H2,20,21,22,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Enterprises Limited
Curated by ChEMBL
| Assay Description
Inhibition of IR (unknown origin) using poly-G1 as substrate incubated for 10 mins prior to substrate addition measured after 1 hr by time-resolved f... |
Eur J Med Chem 92: 246-56 (2015)
Article DOI: 10.1016/j.ejmech.2014.12.053
BindingDB Entry DOI: 10.7270/Q2639RFV |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50341782

(4-(4-{4-[(2-Phenyl-5-trifluoromethyloxazole-4-carb...)Show SMILES OC(=O)[C@H]1CC[C@@H](CC1)C(=O)N1CCC(CC1)c1ccc(NC(=O)c2nc(oc2C(F)(F)F)-c2ccccc2)cc1 |r,wU:6.9,wD:3.2,(20.61,-31.2,;19.27,-30.45,;19.25,-28.91,;17.95,-31.23,;16.6,-30.48,;15.28,-31.27,;15.31,-32.8,;16.65,-33.55,;17.96,-32.77,;13.99,-33.59,;14.01,-35.12,;12.65,-32.84,;11.32,-33.63,;9.99,-32.88,;9.97,-31.35,;11.27,-30.55,;12.62,-31.3,;8.62,-30.6,;7.3,-31.4,;5.96,-30.65,;5.92,-29.1,;4.57,-28.36,;3.27,-29.17,;3.32,-30.7,;1.9,-28.44,;.69,-29.39,;-.59,-28.53,;-.17,-27.05,;1.38,-26.98,;2.14,-25.65,;3.68,-25.64,;1.37,-24.32,;2.76,-24.25,;-1.94,-29.27,;-3.26,-28.47,;-4.61,-29.21,;-4.65,-30.75,;-3.32,-31.55,;-1.97,-30.81,;7.25,-28.31,;8.6,-29.06,)| Show InChI InChI=1S/C30H30F3N3O5/c31-30(32,33)25-24(35-27(41-25)20-4-2-1-3-5-20)26(37)34-23-12-10-18(11-13-23)19-14-16-36(17-15-19)28(38)21-6-8-22(9-7-21)29(39)40/h1-5,10-13,19,21-22H,6-9,14-17H2,(H,34,37)(H,39,40)/t21-,22- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 114 | n/a | n/a | n/a | n/a | n/a | n/a |
VIT University
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 using 1,2-diacylglycerol/[14C]-oleoyl CoA as substrate assessed as [14C]-triglyceride formation after 10 mins by scintillat... |
Eur J Med Chem 79: 203-15 (2014)
Article DOI: 10.1016/j.ejmech.2014.03.077
BindingDB Entry DOI: 10.7270/Q23R0VCD |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
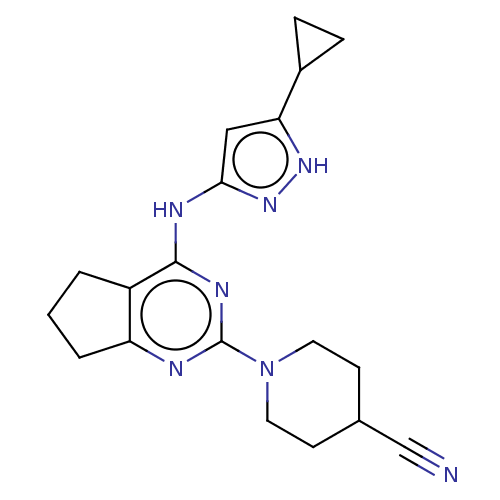
(Homo sapiens (Human)) | BDBM50071035
(CHEMBL3409719)Show SMILES N#CC1CCN(CC1)c1nc2CCCc2c(Nc2cc([nH]n2)C2CC2)n1 Show InChI InChI=1S/C19H23N7/c20-11-12-6-8-26(9-7-12)19-21-15-3-1-2-14(15)18(23-19)22-17-10-16(24-25-17)13-4-5-13/h10,12-13H,1-9H2,(H2,21,22,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 124 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Enterprises Limited
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R (unknown origin) using poly-G1 as substrate incubated for 10 mins prior to substrate addition measured after 1 hr by time-resolve... |
Eur J Med Chem 92: 246-56 (2015)
Article DOI: 10.1016/j.ejmech.2014.12.053
BindingDB Entry DOI: 10.7270/Q2639RFV |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50071029
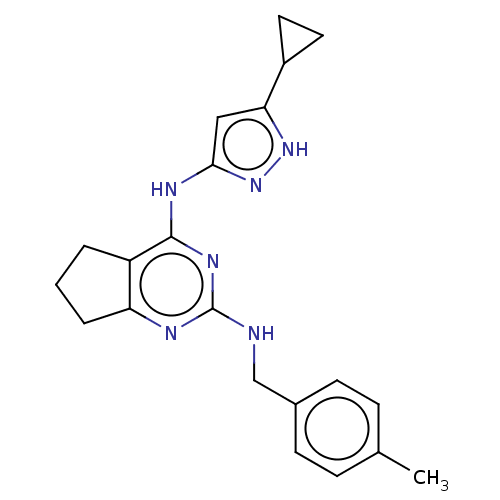
(CHEMBL3409725)Show SMILES Cc1ccc(CNc2nc3CCCc3c(Nc3cc([nH]n3)C3CC3)n2)cc1 Show InChI InChI=1S/C21H24N6/c1-13-5-7-14(8-6-13)12-22-21-23-17-4-2-3-16(17)20(25-21)24-19-11-18(26-27-19)15-9-10-15/h5-8,11,15H,2-4,9-10,12H2,1H3,(H3,22,23,24,25,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 149 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Enterprises Limited
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R (unknown origin) using poly-G1 as substrate incubated for 10 mins prior to substrate addition measured after 1 hr by time-resolve... |
Eur J Med Chem 92: 246-56 (2015)
Article DOI: 10.1016/j.ejmech.2014.12.053
BindingDB Entry DOI: 10.7270/Q2639RFV |
More data for this
Ligand-Target Pair | |
Diacylglycerol O-acyltransferase 1
(Homo sapiens (Human)) | BDBM50005650

(CHEMBL3235426)Show SMILES COc1ccccc1NC(=O)Nc1ccc(cc1)-c1cnc(s1)C(=O)N[C@H]1CC[C@@H](CC1)C(O)=O |r,wU:26.28,wD:29.35,(1.32,-43.95,;2.65,-43.18,;2.64,-41.64,;1.31,-40.88,;1.31,-39.33,;2.64,-38.56,;3.97,-39.33,;3.96,-40.87,;5.3,-41.64,;6.63,-40.87,;6.63,-39.33,;7.96,-41.64,;9.3,-40.88,;9.3,-39.33,;10.63,-38.56,;11.96,-39.32,;11.97,-40.87,;10.63,-41.65,;13.23,-38.45,;13.28,-36.91,;14.75,-36.47,;15.62,-37.75,;14.68,-38.97,;17.16,-37.79,;17.9,-39.14,;17.97,-36.48,;19.51,-36.52,;20.31,-35.21,;21.85,-35.26,;22.58,-36.61,;21.77,-37.92,;20.23,-37.88,;24.12,-36.66,;24.93,-35.35,;24.85,-38.02,)| Show InChI InChI=1S/C25H26N4O5S/c1-34-20-5-3-2-4-19(20)29-25(33)28-18-10-6-15(7-11-18)21-14-26-23(35-21)22(30)27-17-12-8-16(9-13-17)24(31)32/h2-7,10-11,14,16-17H,8-9,12-13H2,1H3,(H,27,30)(H,31,32)(H2,28,29,33)/t16-,17- | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
VIT University
Curated by ChEMBL
| Assay Description
Inhibition of human DGAT1 using 1,2-diacylglycerol/[14C]-oleoyl CoA as substrate assessed as [14C]-triglyceride formation after 10 mins by scintillat... |
Eur J Med Chem 79: 203-15 (2014)
Article DOI: 10.1016/j.ejmech.2014.03.077
BindingDB Entry DOI: 10.7270/Q23R0VCD |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
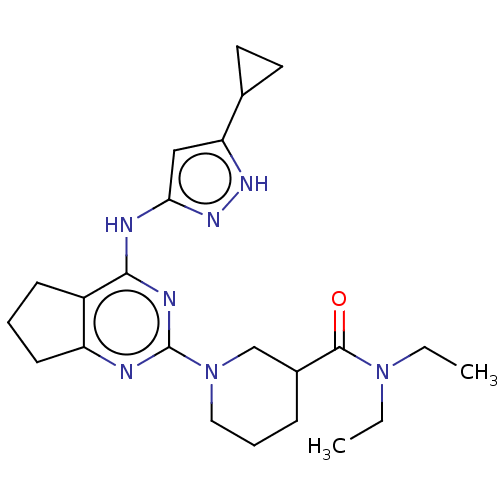
(Homo sapiens (Human)) | BDBM50071038
(CHEMBL3409715)Show SMILES CCN(CC)C(=O)C1CCCN(C1)c1nc2CCCc2c(Nc2cc([nH]n2)C2CC2)n1 Show InChI InChI=1S/C23H33N7O/c1-3-29(4-2)22(31)16-7-6-12-30(14-16)23-24-18-9-5-8-17(18)21(26-23)25-20-13-19(27-28-20)15-10-11-15/h13,15-16H,3-12,14H2,1-2H3,(H2,24,25,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 184 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Enterprises Limited
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R (unknown origin) using poly-G1 as substrate incubated for 10 mins prior to substrate addition measured after 1 hr by time-resolve... |
Eur J Med Chem 92: 246-56 (2015)
Article DOI: 10.1016/j.ejmech.2014.12.053
BindingDB Entry DOI: 10.7270/Q2639RFV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50402299

(CHEMBL2206064)Show SMILES CNc1nc(N)nc2nc(ccc12)-c1c(Oc2ccccc2)cccc1C(F)(F)F Show InChI InChI=1S/C21H16F3N5O/c1-26-18-13-10-11-15(27-19(13)29-20(25)28-18)17-14(21(22,23)24)8-5-9-16(17)30-12-6-3-2-4-7-12/h2-11H,1H3,(H3,25,26,27,28,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human PTP1B (1 to 321 amino acid residues) expressed in Escherichia coli using TRDI(pY)E as substrate incubated for 15 mins prior to su... |
Bioorg Med Chem Lett 22: 7518-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.035
BindingDB Entry DOI: 10.7270/Q2VQ33V6 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50071037
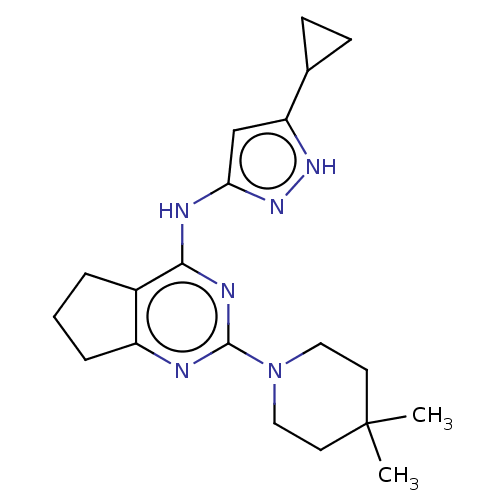
(CHEMBL3409717)Show SMILES CC1(C)CCN(CC1)c1nc2CCCc2c(Nc2cc([nH]n2)C2CC2)n1 Show InChI InChI=1S/C20H28N6/c1-20(2)8-10-26(11-9-20)19-21-15-5-3-4-14(15)18(23-19)22-17-12-16(24-25-17)13-6-7-13/h12-13H,3-11H2,1-2H3,(H2,21,22,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 203 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Enterprises Limited
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R (unknown origin) using poly-G1 as substrate incubated for 10 mins prior to substrate addition measured after 1 hr by time-resolve... |
Eur J Med Chem 92: 246-56 (2015)
Article DOI: 10.1016/j.ejmech.2014.12.053
BindingDB Entry DOI: 10.7270/Q2639RFV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50402291

(CHEMBL2206071)Show SMILES CNc1nc(N)nc2nc(c(cc12)C(O)=O)-c1ccccc1C(F)(F)F Show InChI InChI=1S/C16H12F3N5O2/c1-21-12-9-6-8(14(25)26)11(22-13(9)24-15(20)23-12)7-4-2-3-5-10(7)16(17,18)19/h2-6H,1H3,(H,25,26)(H3,20,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human PTP1B (1 to 321 amino acid residues) expressed in Escherichia coli using TRDI(pY)E as substrate incubated for 15 mins prior to su... |
Bioorg Med Chem Lett 22: 7518-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.035
BindingDB Entry DOI: 10.7270/Q2VQ33V6 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50071022
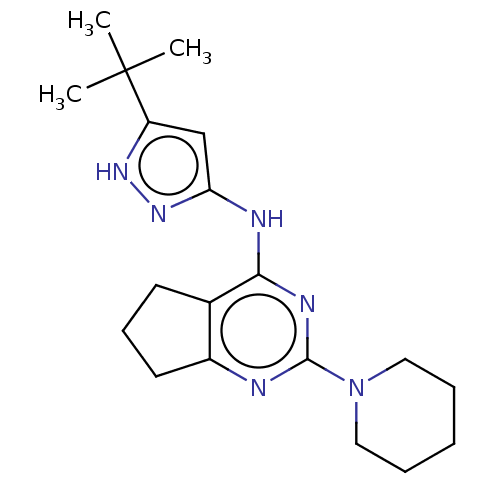
(CHEMBL3409732)Show SMILES CC(C)(C)c1cc(Nc2nc(nc3CCCc23)N2CCCCC2)n[nH]1 Show InChI InChI=1S/C19H28N6/c1-19(2,3)15-12-16(24-23-15)21-17-13-8-7-9-14(13)20-18(22-17)25-10-5-4-6-11-25/h12H,4-11H2,1-3H3,(H2,20,21,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Enterprises Limited
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R (unknown origin) using poly-G1 as substrate incubated for 10 mins prior to substrate addition measured after 1 hr by time-resolve... |
Eur J Med Chem 92: 246-56 (2015)
Article DOI: 10.1016/j.ejmech.2014.12.053
BindingDB Entry DOI: 10.7270/Q2639RFV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50402302
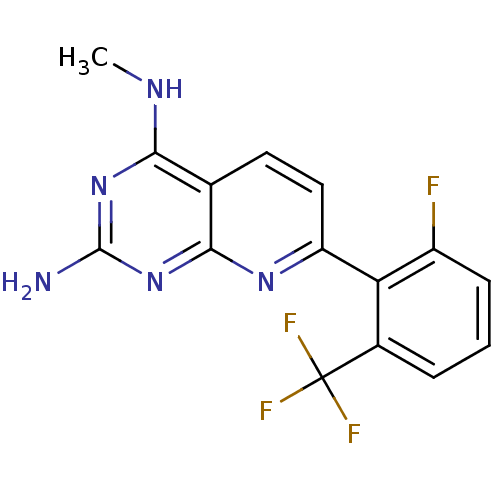
(CHEMBL2205521)Show InChI InChI=1S/C15H11F4N5/c1-21-12-7-5-6-10(22-13(7)24-14(20)23-12)11-8(15(17,18)19)3-2-4-9(11)16/h2-6H,1H3,(H3,20,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human PTP1B (1 to 321 amino acid residues) expressed in Escherichia coli using TRDI(pY)E as substrate incubated for 15 mins prior to su... |
Bioorg Med Chem Lett 22: 7518-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.035
BindingDB Entry DOI: 10.7270/Q2VQ33V6 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50071030
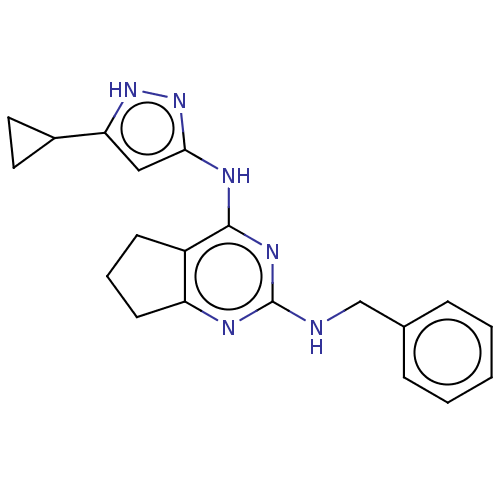
(CHEMBL3409724)Show SMILES C(Nc1nc2CCCc2c(Nc2cc([nH]n2)C2CC2)n1)c1ccccc1 Show InChI InChI=1S/C20H22N6/c1-2-5-13(6-3-1)12-21-20-22-16-8-4-7-15(16)19(24-20)23-18-11-17(25-26-18)14-9-10-14/h1-3,5-6,11,14H,4,7-10,12H2,(H3,21,22,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 267 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Enterprises Limited
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R (unknown origin) using poly-G1 as substrate incubated for 10 mins prior to substrate addition measured after 1 hr by time-resolve... |
Eur J Med Chem 92: 246-56 (2015)
Article DOI: 10.1016/j.ejmech.2014.12.053
BindingDB Entry DOI: 10.7270/Q2639RFV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50402292
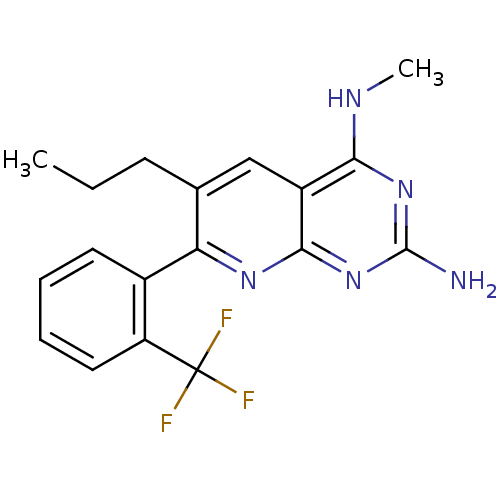
(CHEMBL2206070)Show InChI InChI=1S/C18H18F3N5/c1-3-6-10-9-12-15(23-2)25-17(22)26-16(12)24-14(10)11-7-4-5-8-13(11)18(19,20)21/h4-5,7-9H,3,6H2,1-2H3,(H3,22,23,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human PTP1B (1 to 321 amino acid residues) expressed in Escherichia coli using TRDI(pY)E as substrate incubated for 15 mins prior to su... |
Bioorg Med Chem Lett 22: 7518-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.035
BindingDB Entry DOI: 10.7270/Q2VQ33V6 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50071021
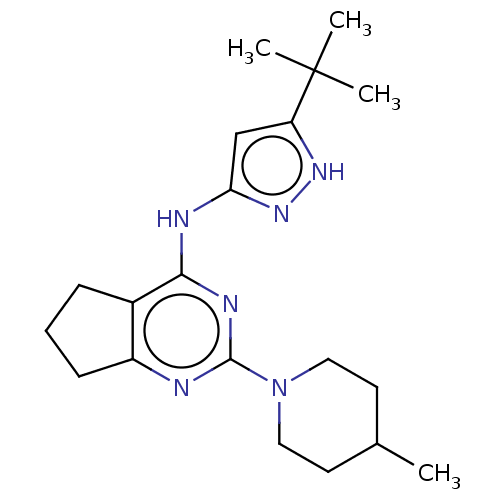
(CHEMBL3409733)Show SMILES CC1CCN(CC1)c1nc2CCCc2c(Nc2cc([nH]n2)C(C)(C)C)n1 Show InChI InChI=1S/C20H30N6/c1-13-8-10-26(11-9-13)19-21-15-7-5-6-14(15)18(23-19)22-17-12-16(24-25-17)20(2,3)4/h12-13H,5-11H2,1-4H3,(H2,21,22,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Enterprises Limited
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R (unknown origin) using poly-G1 as substrate incubated for 10 mins prior to substrate addition measured after 1 hr by time-resolve... |
Eur J Med Chem 92: 246-56 (2015)
Article DOI: 10.1016/j.ejmech.2014.12.053
BindingDB Entry DOI: 10.7270/Q2639RFV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50402300
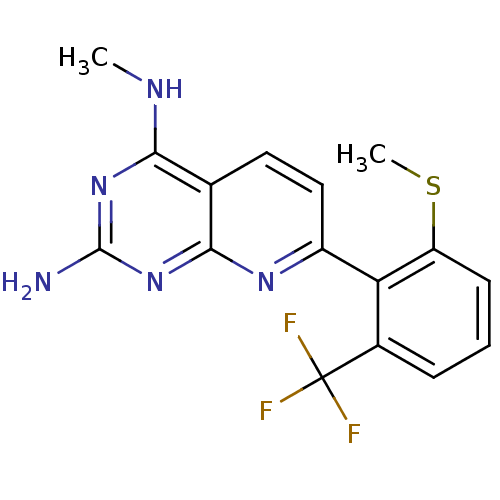
(CHEMBL2206063)Show InChI InChI=1S/C16H14F3N5S/c1-21-13-8-6-7-10(22-14(8)24-15(20)23-13)12-9(16(17,18)19)4-3-5-11(12)25-2/h3-7H,1-2H3,(H3,20,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human PTP1B (1 to 321 amino acid residues) expressed in Escherichia coli using TRDI(pY)E as substrate incubated for 15 mins prior to su... |
Bioorg Med Chem Lett 22: 7518-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.035
BindingDB Entry DOI: 10.7270/Q2VQ33V6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50402296
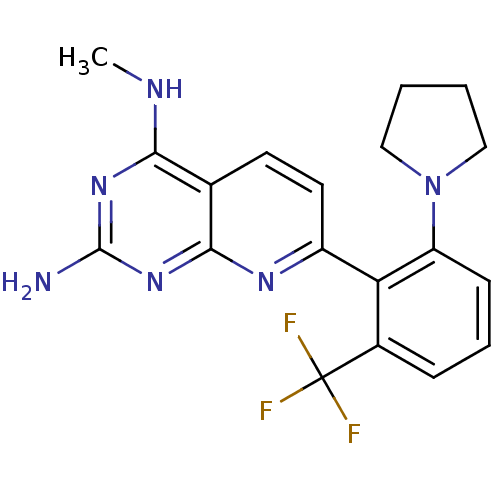
(CHEMBL2206067)Show SMILES CNc1nc(N)nc2nc(ccc12)-c1c(cccc1C(F)(F)F)N1CCCC1 Show InChI InChI=1S/C19H19F3N6/c1-24-16-11-7-8-13(25-17(11)27-18(23)26-16)15-12(19(20,21)22)5-4-6-14(15)28-9-2-3-10-28/h4-8H,2-3,9-10H2,1H3,(H3,23,24,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human PTP1B (1 to 321 amino acid residues) expressed in Escherichia coli using TRDI(pY)E as substrate incubated for 15 mins prior to su... |
Bioorg Med Chem Lett 22: 7518-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.035
BindingDB Entry DOI: 10.7270/Q2VQ33V6 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50071025
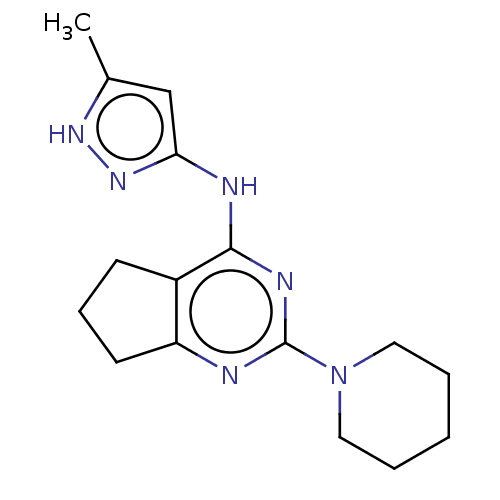
(CHEMBL3409729)Show InChI InChI=1S/C16H22N6/c1-11-10-14(21-20-11)18-15-12-6-5-7-13(12)17-16(19-15)22-8-3-2-4-9-22/h10H,2-9H2,1H3,(H2,17,18,19,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 442 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Enterprises Limited
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R (unknown origin) using poly-G1 as substrate incubated for 10 mins prior to substrate addition measured after 1 hr by time-resolve... |
Eur J Med Chem 92: 246-56 (2015)
Article DOI: 10.1016/j.ejmech.2014.12.053
BindingDB Entry DOI: 10.7270/Q2639RFV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50402316

(CHEMBL2206073)Show InChI InChI=1S/C15H12F3N5/c1-20-12-9-6-7-11(21-13(9)23-14(19)22-12)8-4-2-3-5-10(8)15(16,17)18/h2-7H,1H3,(H3,19,20,21,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human PTP1B (1 to 321 amino acid residues) expressed in Escherichia coli using TRDI(pY)E as substrate incubated for 15 mins prior to su... |
Bioorg Med Chem Lett 22: 7518-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.035
BindingDB Entry DOI: 10.7270/Q2VQ33V6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50402293

(CHEMBL2203315)Show InChI InChI=1S/C16H14F3N5/c1-8-7-10-13(21-2)23-15(20)24-14(10)22-12(8)9-5-3-4-6-11(9)16(17,18)19/h3-7H,1-2H3,(H3,20,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human PTP1B (1 to 321 amino acid residues) expressed in Escherichia coli using TRDI(pY)E as substrate incubated for 15 mins prior to su... |
Bioorg Med Chem Lett 22: 7518-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.035
BindingDB Entry DOI: 10.7270/Q2VQ33V6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50402303
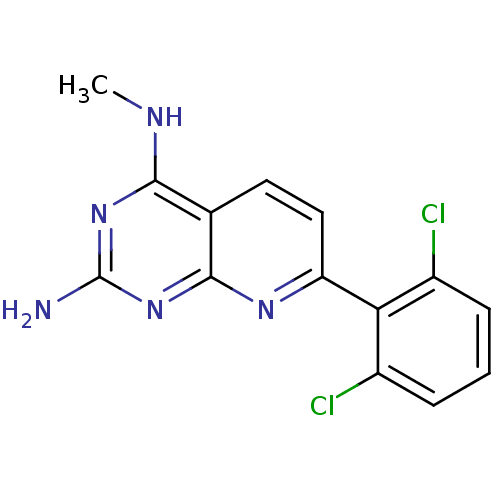
(CHEMBL2205520)Show InChI InChI=1S/C14H11Cl2N5/c1-18-12-7-5-6-10(19-13(7)21-14(17)20-12)11-8(15)3-2-4-9(11)16/h2-6H,1H3,(H3,17,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human PTP1B (1 to 321 amino acid residues) expressed in Escherichia coli using TRDI(pY)E as substrate incubated for 15 mins prior to su... |
Bioorg Med Chem Lett 22: 7518-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.035
BindingDB Entry DOI: 10.7270/Q2VQ33V6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50402308

(CHEMBL2205515)Show InChI InChI=1S/C14H12BrN5/c1-17-12-9-6-7-11(8-4-2-3-5-10(8)15)18-13(9)20-14(16)19-12/h2-7H,1H3,(H3,16,17,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 530 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human PTP1B (1 to 321 amino acid residues) expressed in Escherichia coli using TRDI(pY)E as substrate incubated for 15 mins prior to su... |
Bioorg Med Chem Lett 22: 7518-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.035
BindingDB Entry DOI: 10.7270/Q2VQ33V6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50402301
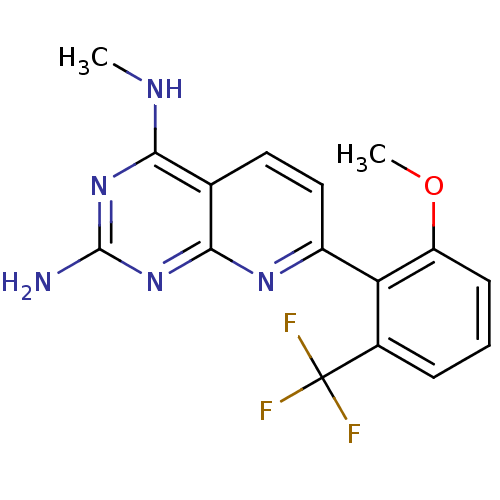
(CHEMBL2205522)Show InChI InChI=1S/C16H14F3N5O/c1-21-13-8-6-7-10(22-14(8)24-15(20)23-13)12-9(16(17,18)19)4-3-5-11(12)25-2/h3-7H,1-2H3,(H3,20,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 560 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human PTP1B (1 to 321 amino acid residues) expressed in Escherichia coli using TRDI(pY)E as substrate incubated for 15 mins prior to su... |
Bioorg Med Chem Lett 22: 7518-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.035
BindingDB Entry DOI: 10.7270/Q2VQ33V6 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50071020
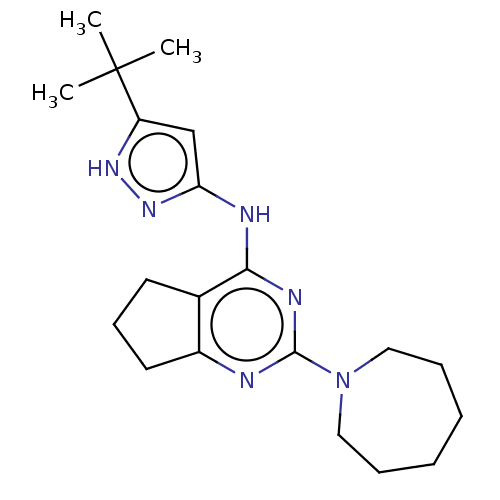
(CHEMBL3409734)Show SMILES CC(C)(C)c1cc(Nc2nc(nc3CCCc23)N2CCCCCC2)n[nH]1 Show InChI InChI=1S/C20H30N6/c1-20(2,3)16-13-17(25-24-16)22-18-14-9-8-10-15(14)21-19(23-18)26-11-6-4-5-7-12-26/h13H,4-12H2,1-3H3,(H2,21,22,23,24,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 568 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Enterprises Limited
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R (unknown origin) using poly-G1 as substrate incubated for 10 mins prior to substrate addition measured after 1 hr by time-resolve... |
Eur J Med Chem 92: 246-56 (2015)
Article DOI: 10.1016/j.ejmech.2014.12.053
BindingDB Entry DOI: 10.7270/Q2639RFV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50402305
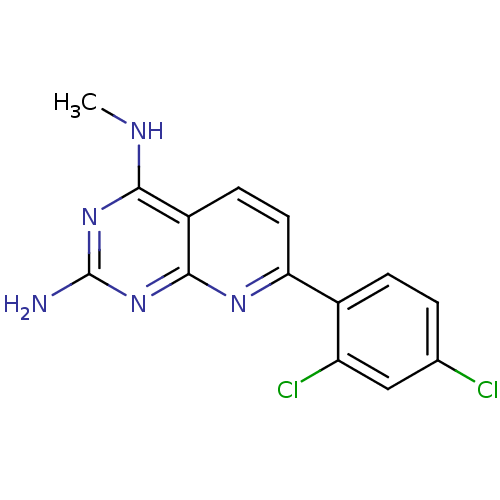
(CHEMBL2205518)Show InChI InChI=1S/C14H11Cl2N5/c1-18-12-9-4-5-11(19-13(9)21-14(17)20-12)8-3-2-7(15)6-10(8)16/h2-6H,1H3,(H3,17,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human PTP1B (1 to 321 amino acid residues) expressed in Escherichia coli using TRDI(pY)E as substrate incubated for 15 mins prior to su... |
Bioorg Med Chem Lett 22: 7518-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.035
BindingDB Entry DOI: 10.7270/Q2VQ33V6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50402294

(CHEMBL2206069)Show InChI InChI=1S/C18H19FN6/c1-21-16-11-7-8-13(22-17(11)24-18(20)23-16)15-12(19)5-4-6-14(15)25-9-2-3-10-25/h4-8H,2-3,9-10H2,1H3,(H3,20,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 620 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human PTP1B (1 to 321 amino acid residues) expressed in Escherichia coli using TRDI(pY)E as substrate incubated for 15 mins prior to su... |
Bioorg Med Chem Lett 22: 7518-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.035
BindingDB Entry DOI: 10.7270/Q2VQ33V6 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50071017

(CHEMBL3409737)Show SMILES CC1CCN(CC1)c1nc2c(CCC2(F)F)c(Nc2cc([nH]n2)C2CC2)n1 Show InChI InChI=1S/C19H24F2N6/c1-11-5-8-27(9-6-11)18-23-16-13(4-7-19(16,20)21)17(24-18)22-15-10-14(25-26-15)12-2-3-12/h10-12H,2-9H2,1H3,(H2,22,23,24,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 786 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Enterprises Limited
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R (unknown origin) using poly-G1 as substrate incubated for 10 mins prior to substrate addition measured after 1 hr by time-resolve... |
Eur J Med Chem 92: 246-56 (2015)
Article DOI: 10.1016/j.ejmech.2014.12.053
BindingDB Entry DOI: 10.7270/Q2639RFV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50402295
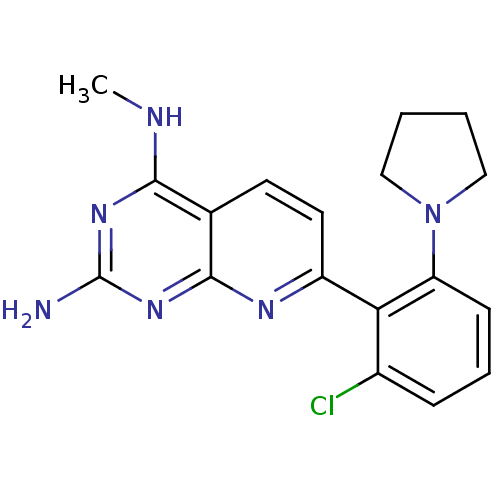
(CHEMBL2206068)Show InChI InChI=1S/C18H19ClN6/c1-21-16-11-7-8-13(22-17(11)24-18(20)23-16)15-12(19)5-4-6-14(15)25-9-2-3-10-25/h4-8H,2-3,9-10H2,1H3,(H3,20,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human PTP1B (1 to 321 amino acid residues) expressed in Escherichia coli using TRDI(pY)E as substrate incubated for 15 mins prior to su... |
Bioorg Med Chem Lett 22: 7518-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.035
BindingDB Entry DOI: 10.7270/Q2VQ33V6 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50402304

(CHEMBL2205519)Show InChI InChI=1S/C14H11Cl2N5/c1-18-12-8-3-5-11(19-13(8)21-14(17)20-12)9-6-7(15)2-4-10(9)16/h2-6H,1H3,(H3,17,18,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human PTP1B (1 to 321 amino acid residues) expressed in Escherichia coli using TRDI(pY)E as substrate incubated for 15 mins prior to su... |
Bioorg Med Chem Lett 22: 7518-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.035
BindingDB Entry DOI: 10.7270/Q2VQ33V6 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50071023
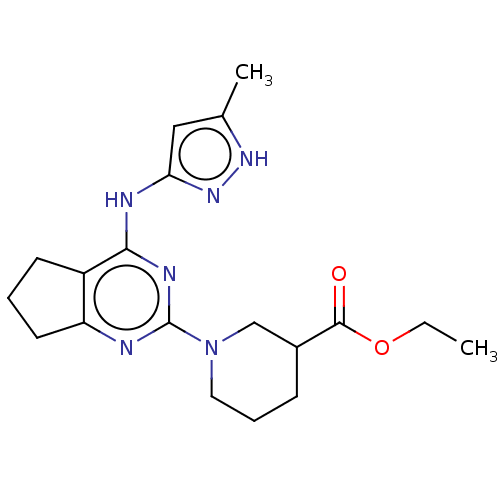
(CHEMBL3409731)Show SMILES CCOC(=O)C1CCCN(C1)c1nc2CCCc2c(Nc2cc(C)[nH]n2)n1 Show InChI InChI=1S/C19H26N6O2/c1-3-27-18(26)13-6-5-9-25(11-13)19-20-15-8-4-7-14(15)17(22-19)21-16-10-12(2)23-24-16/h10,13H,3-9,11H2,1-2H3,(H2,20,21,22,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Enterprises Limited
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R (unknown origin) using poly-G1 as substrate incubated for 10 mins prior to substrate addition measured after 1 hr by time-resolve... |
Eur J Med Chem 92: 246-56 (2015)
Article DOI: 10.1016/j.ejmech.2014.12.053
BindingDB Entry DOI: 10.7270/Q2639RFV |
More data for this
Ligand-Target Pair | |
Tyrosine-protein phosphatase non-receptor type 1
(Homo sapiens (Human)) | BDBM50402290

(CHEMBL2206072)Show SMILES CNc1nc(N)nc2nc(-c3ccccc3C(F)(F)F)c(cc12)S(C)(=O)=O Show InChI InChI=1S/C16H14F3N5O2S/c1-21-13-9-7-11(27(2,25)26)12(22-14(9)24-15(20)23-13)8-5-3-4-6-10(8)16(17,18)19/h3-7H,1-2H3,(H3,20,21,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche Research Center
Curated by ChEMBL
| Assay Description
Inhibition of human PTP1B (1 to 321 amino acid residues) expressed in Escherichia coli using TRDI(pY)E as substrate incubated for 15 mins prior to su... |
Bioorg Med Chem Lett 22: 7518-22 (2012)
Article DOI: 10.1016/j.bmcl.2012.10.035
BindingDB Entry DOI: 10.7270/Q2VQ33V6 |
More data for this
Ligand-Target Pair | |
Insulin-like growth factor 1 receptor
(Homo sapiens (Human)) | BDBM50071059
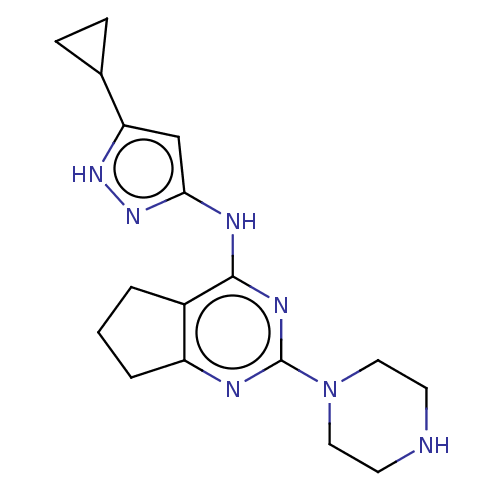
(CHEMBL3409712)Show InChI InChI=1S/C17H23N7/c1-2-12-13(3-1)19-17(24-8-6-18-7-9-24)21-16(12)20-15-10-14(22-23-15)11-4-5-11/h10-11,18H,1-9H2,(H2,19,20,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Piramal Enterprises Limited
Curated by ChEMBL
| Assay Description
Inhibition of IGF1R (unknown origin) using poly-G1 as substrate incubated for 10 mins prior to substrate addition measured after 1 hr by time-resolve... |
Eur J Med Chem 92: 246-56 (2015)
Article DOI: 10.1016/j.ejmech.2014.12.053
BindingDB Entry DOI: 10.7270/Q2639RFV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data