

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vascular endothelial growth factor receptor 1 (Homo sapiens (Human)) | BDBM4810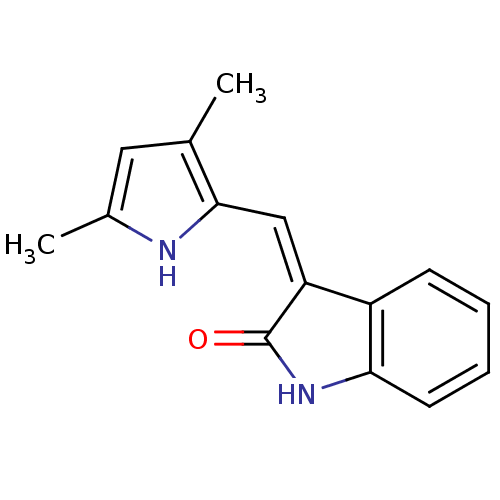 ((3Z)-3-[(3,5-dimethyl-1H-pyrrol-2-yl)methylidene]-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description The in vitro kinase assays were performed in 96-well plates using the recombinant GST-fused kinase domains expressed in baculovirus and purified over... | J Med Chem 43: 2310-23 (2000) Article DOI: 10.1021/jm9909443 BindingDB Entry DOI: 10.7270/Q24M92R5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066461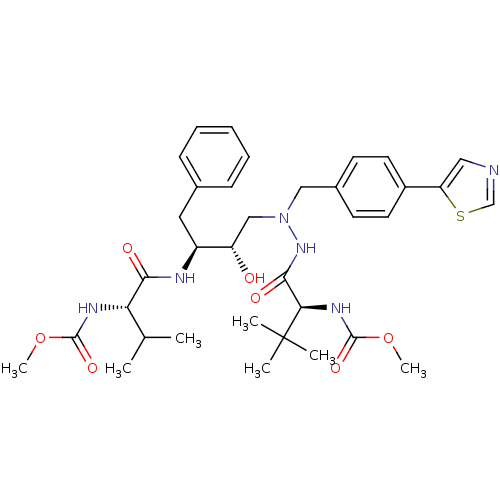 (CHEMBL326408 | {(S)-1-[N'-[(2S,3S)-2-Hydroxy-3-((S...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy AG Curated by ChEMBL | Assay Description The percent reduction of the reverse transcriptase (RT) activity in HIV-1/MN-infected MT-2 cells. | J Med Chem 41: 3387-401 (1998) Article DOI: 10.1021/jm970873c BindingDB Entry DOI: 10.7270/Q2H1315D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066474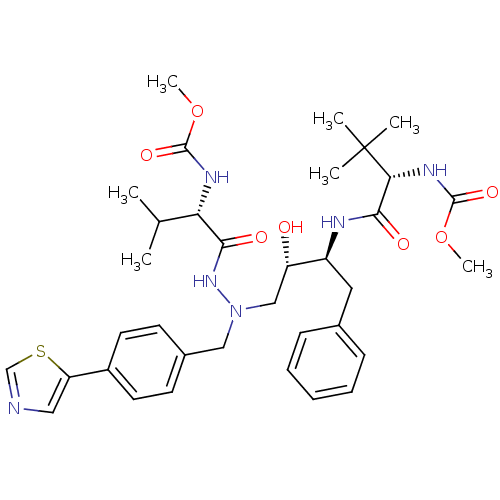 (((S)-1-{(1S,2S)-1-Benzyl-2-hydroxy-3-[N'-((S)-2-me...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy AG Curated by ChEMBL | Assay Description The percent reduction of the reverse transcriptase (RT) activity in HIV-1/MN-infected MT-2 cells. | J Med Chem 41: 3387-401 (1998) Article DOI: 10.1021/jm970873c BindingDB Entry DOI: 10.7270/Q2H1315D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066462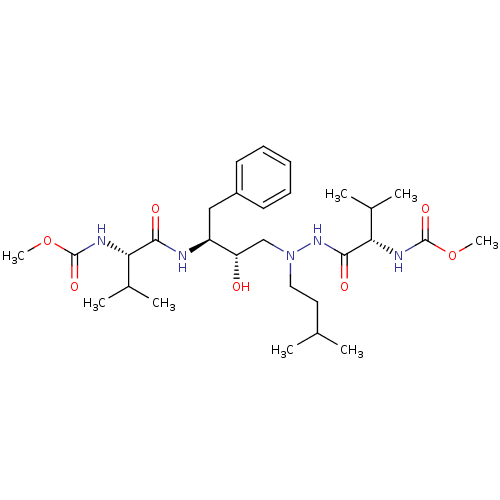 (CHEMBL326347 | {(S)-1-[N'-[(2S,3S)-2-Hydroxy-3-((S...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy AG Curated by ChEMBL | Assay Description The percent reduction of the reverse transcriptase (RT) activity in HIV-1/MN-infected MT-2 cells. | J Med Chem 41: 3387-401 (1998) Article DOI: 10.1021/jm970873c BindingDB Entry DOI: 10.7270/Q2H1315D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066473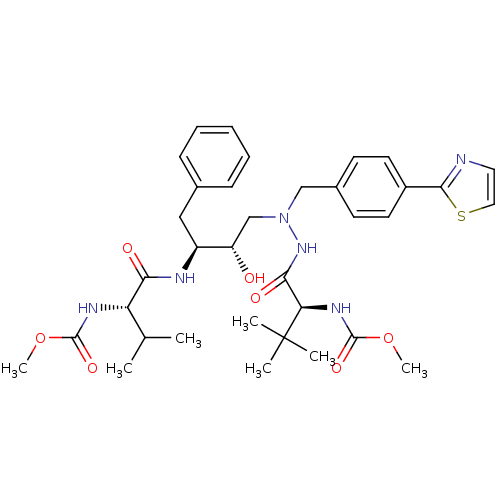 (CHEMBL325900 | {(S)-1-[N'-[(2S,3S)-2-Hydroxy-3-((S...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy AG Curated by ChEMBL | Assay Description The percent reduction of the reverse transcriptase (RT) activity in HIV-1/MN-infected MT-2 cells. | J Med Chem 41: 3387-401 (1998) Article DOI: 10.1021/jm970873c BindingDB Entry DOI: 10.7270/Q2H1315D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066476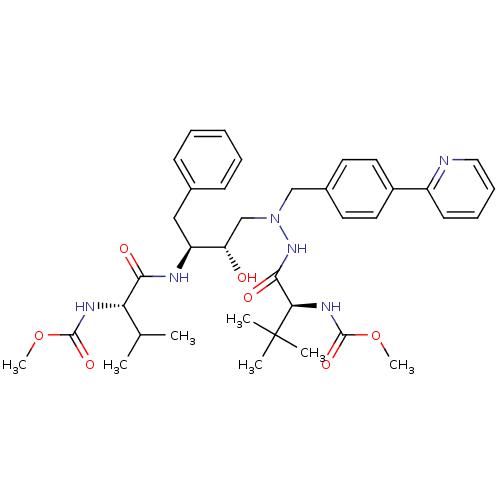 (CHEMBL333386 | {(S)-1-[N'-[(2S,3S)-2-Hydroxy-3-((S...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy AG Curated by ChEMBL | Assay Description The percent reduction of the reverse transcriptase (RT) activity in HIV-1/MN-infected MT-2 cells. | J Med Chem 41: 3387-401 (1998) Article DOI: 10.1021/jm970873c BindingDB Entry DOI: 10.7270/Q2H1315D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066459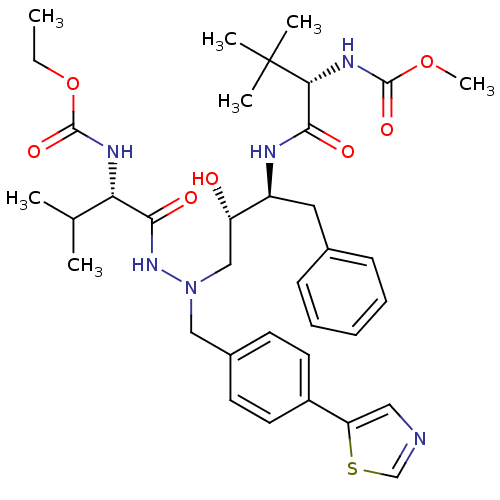 (((S)-1-{(1S,2S)-1-Benzyl-3-[N'-((S)-2-ethoxycarbon...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy AG Curated by ChEMBL | Assay Description The percent reduction of the reverse transcriptase (RT) activity in HIV-1/MN-infected MT-2 cells. | J Med Chem 41: 3387-401 (1998) Article DOI: 10.1021/jm970873c BindingDB Entry DOI: 10.7270/Q2H1315D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM13934 (Atazanavir | BMS 232632 | CGP 73547 | CHEMBL1163 |...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy AG Curated by ChEMBL | Assay Description The percent reduction of the reverse transcriptase (RT) activity in HIV-1/MN-infected MT-2 cells. | J Med Chem 41: 3387-401 (1998) Article DOI: 10.1021/jm970873c BindingDB Entry DOI: 10.7270/Q2H1315D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066475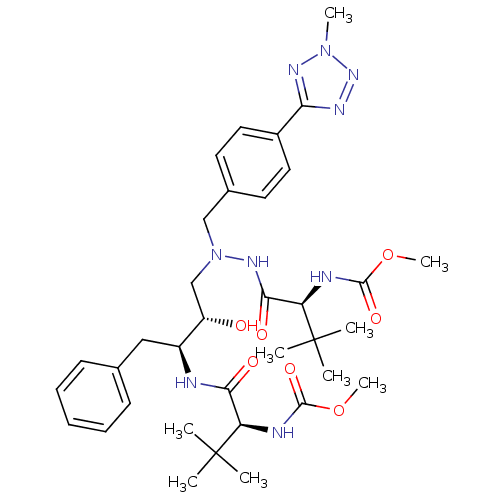 (((S)-1-{N'-[(2S,3S)-2-Hydroxy-3-((S)-2-methoxycarb...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy AG Curated by ChEMBL | Assay Description The percent reduction of the reverse transcriptase (RT) activity in HIV-1/MN-infected MT-2 cells. | J Med Chem 41: 3387-401 (1998) Article DOI: 10.1021/jm970873c BindingDB Entry DOI: 10.7270/Q2H1315D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066472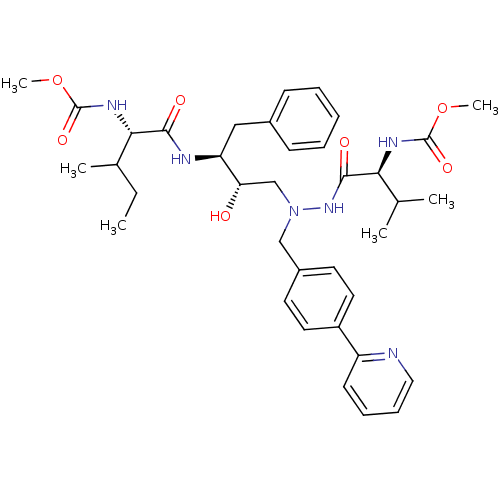 (((S)-1-{(1S,2S)-1-Benzyl-2-hydroxy-3-[N'-((S)-2-me...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy AG Curated by ChEMBL | Assay Description The percent reduction of the reverse transcriptase (RT) activity in HIV-1/MN-infected MT-2 cells. | J Med Chem 41: 3387-401 (1998) Article DOI: 10.1021/jm970873c BindingDB Entry DOI: 10.7270/Q2H1315D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066468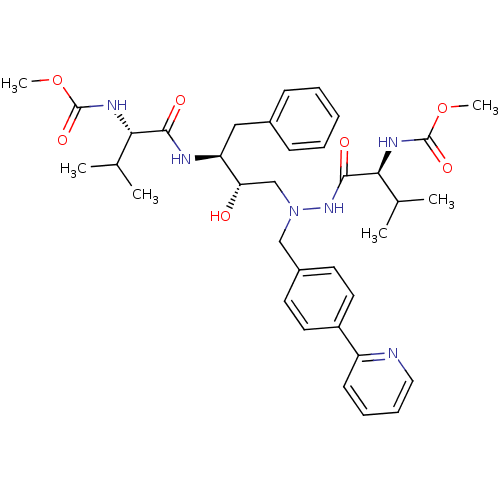 (CHEMBL324521 | {(S)-1-[N'-[(2S,3S)-2-Hydroxy-3-((S...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy AG Curated by ChEMBL | Assay Description The percent reduction of the reverse transcriptase (RT) activity in HIV-1/MN-infected MT-2 cells. | J Med Chem 41: 3387-401 (1998) Article DOI: 10.1021/jm970873c BindingDB Entry DOI: 10.7270/Q2H1315D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM4876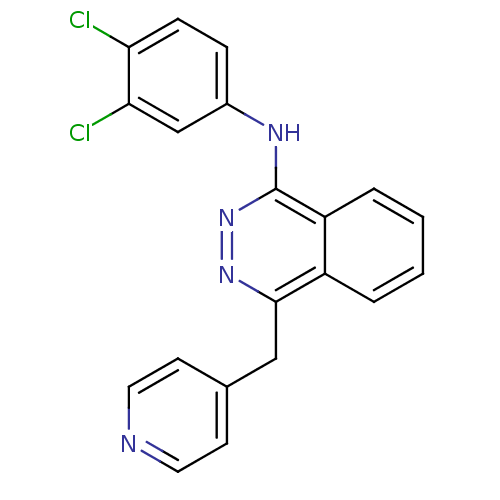 (1-(3,4-Dichloroanilino)-4-(4-pyridylmethyl)phthala...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description The in vitro kinase assays were performed in 96-well plates using the recombinant GST-fused kinase domains expressed in baculovirus and purified over... | J Med Chem 43: 2310-23 (2000) Article DOI: 10.1021/jm9909443 BindingDB Entry DOI: 10.7270/Q24M92R5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066463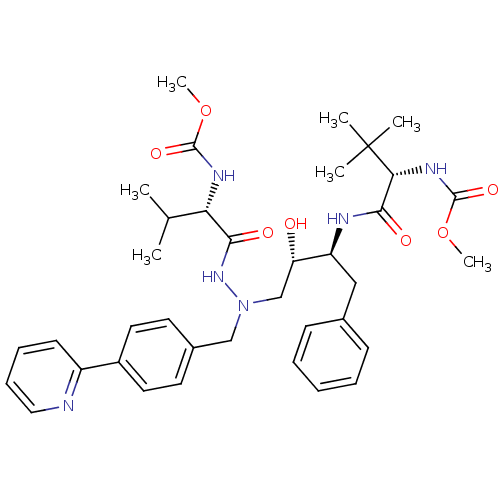 (((S)-1-{(1S,2S)-1-Benzyl-2-hydroxy-3-[N'-((S)-2-me...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy AG Curated by ChEMBL | Assay Description The percent reduction of the reverse transcriptase (RT) activity in HIV-1/MN-infected MT-2 cells. | J Med Chem 41: 3387-401 (1998) Article DOI: 10.1021/jm970873c BindingDB Entry DOI: 10.7270/Q2H1315D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066465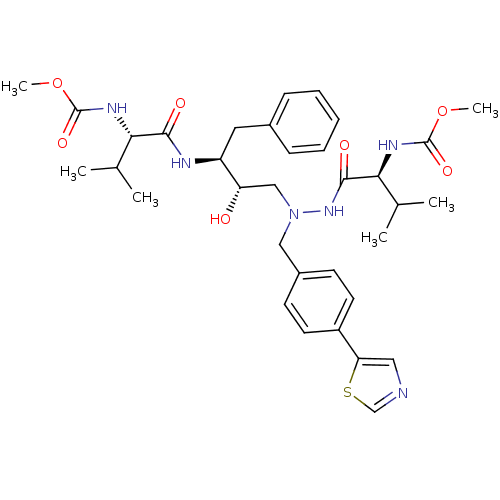 (CHEMBL442013 | {(S)-1-[N'-[(2S,3S)-2-Hydroxy-3-((S...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy AG Curated by ChEMBL | Assay Description The percent reduction of the reverse transcriptase (RT) activity in HIV-1/MN-infected MT-2 cells. | J Med Chem 41: 3387-401 (1998) Article DOI: 10.1021/jm970873c BindingDB Entry DOI: 10.7270/Q2H1315D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066467 (CHEMBL114039 | {(S)-1-[N'-[(2S,3S)-2-Hydroxy-3-((S...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy AG Curated by ChEMBL | Assay Description The percent reduction of the reverse transcriptase (RT) activity in HIV-1/MN-infected MT-2 cells. | J Med Chem 41: 3387-401 (1998) Article DOI: 10.1021/jm970873c BindingDB Entry DOI: 10.7270/Q2H1315D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066469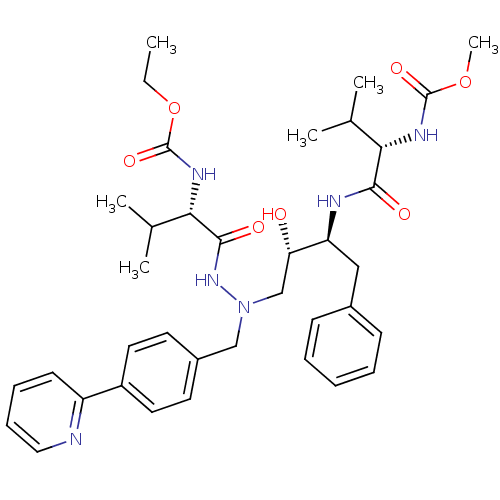 (CHEMBL113943 | {(S)-1-[N'-[(2S,3S)-2-Hydroxy-3-((S...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy AG Curated by ChEMBL | Assay Description The percent reduction of the reverse transcriptase (RT) activity in HIV-1/MN-infected MT-2 cells. | J Med Chem 41: 3387-401 (1998) Article DOI: 10.1021/jm970873c BindingDB Entry DOI: 10.7270/Q2H1315D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066471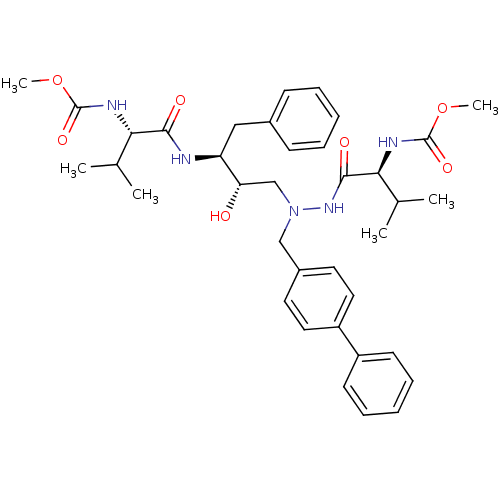 (((S)-1-{N'-Biphenyl-4-ylmethyl-N'-[(2S,3S)-2-hydro...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy AG Curated by ChEMBL | Assay Description The percent reduction of the reverse transcriptase (RT) activity in HIV-1/MN-infected MT-2 cells. | J Med Chem 41: 3387-401 (1998) Article DOI: 10.1021/jm970873c BindingDB Entry DOI: 10.7270/Q2H1315D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM4851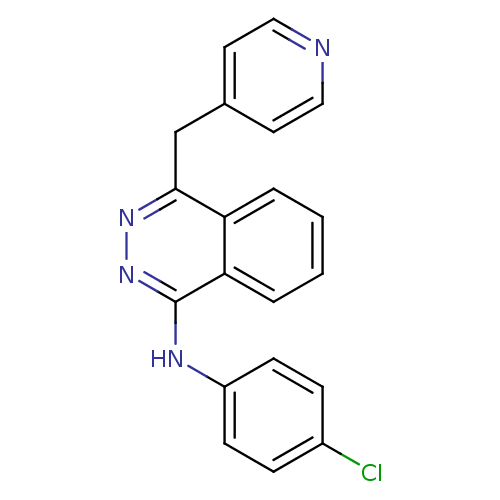 ((4-chlorophenyl)-[4-(4-pyridylmethyl)phthalazin-1-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description The in vitro kinase assays were performed in 96-well plates using the recombinant GST-fused kinase domains expressed in baculovirus and purified over... | J Med Chem 43: 2310-23 (2000) Article DOI: 10.1021/jm9909443 BindingDB Entry DOI: 10.7270/Q24M92R5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 1 (Homo sapiens (Human)) | BDBM4871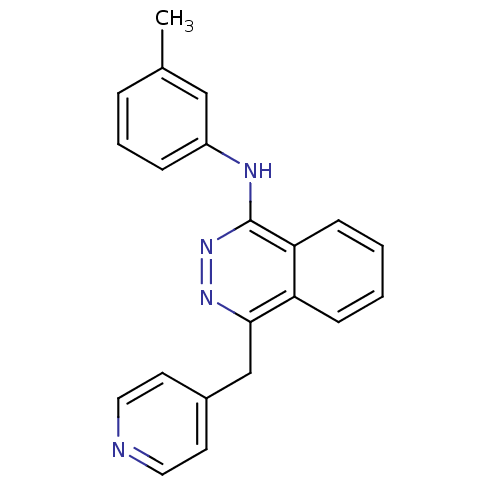 (1-(3-Methylanilino)-4-(4-pyridylmethyl)phthalazine...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description The in vitro kinase assays were performed in 96-well plates using the recombinant GST-fused kinase domains expressed in baculovirus and purified over... | J Med Chem 43: 2310-23 (2000) Article DOI: 10.1021/jm9909443 BindingDB Entry DOI: 10.7270/Q24M92R5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM4878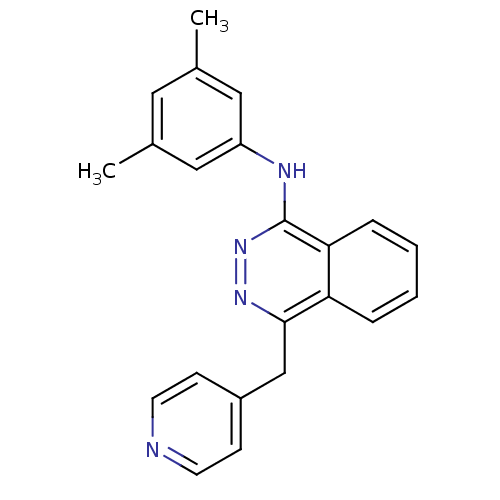 (1-(3,5-Dimethylanilino)-4-(4-pyridylmethyl)phthala...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description The in vitro kinase assays were performed in 96-well plates using the recombinant GST-fused kinase domains expressed in baculovirus and purified over... | J Med Chem 43: 2310-23 (2000) Article DOI: 10.1021/jm9909443 BindingDB Entry DOI: 10.7270/Q24M92R5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066477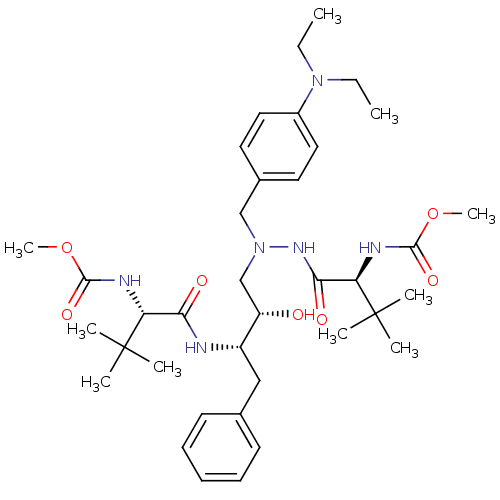 (((S)-1-{N'-(4-Diethylamino-benzyl)-N'-[(2S,3S)-2-h...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy AG Curated by ChEMBL | Assay Description The percent reduction of the reverse transcriptase (RT) activity in HIV-1/MN-infected MT-2 cells. | J Med Chem 41: 3387-401 (1998) Article DOI: 10.1021/jm970873c BindingDB Entry DOI: 10.7270/Q2H1315D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066464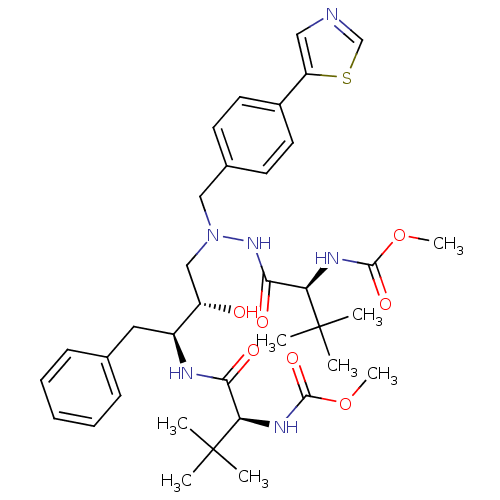 (CHEMBL116165 | {(S)-1-[N'-[(2S,3S)-2-Hydroxy-3-((S...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy AG Curated by ChEMBL | Assay Description The percent reduction of the reverse transcriptase (RT) activity in HIV-1/MN-infected MT-2 cells. | J Med Chem 41: 3387-401 (1998) Article DOI: 10.1021/jm970873c BindingDB Entry DOI: 10.7270/Q2H1315D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066460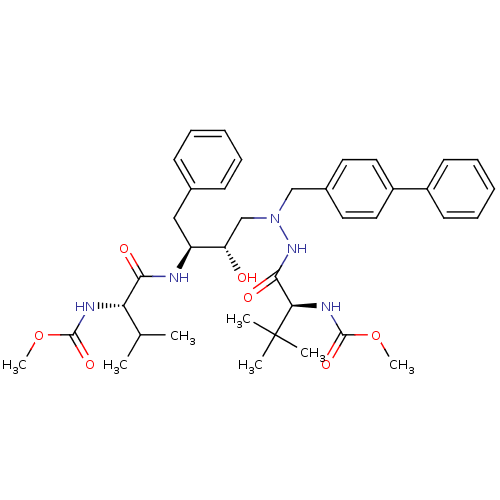 (((S)-1-{N'-Biphenyl-4-ylmethyl-N'-[(2S,3S)-2-hydro...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy AG Curated by ChEMBL | Assay Description The percent reduction of the reverse transcriptase (RT) activity in HIV-1/MN-infected MT-2 cells. | J Med Chem 41: 3387-401 (1998) Article DOI: 10.1021/jm970873c BindingDB Entry DOI: 10.7270/Q2H1315D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066466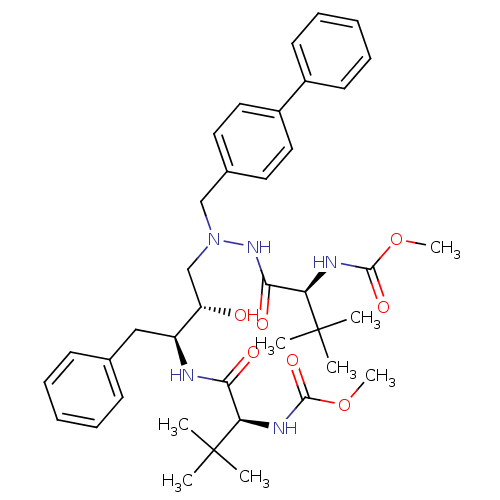 (((S)-1-{N'-Biphenyl-4-ylmethyl-N'-[(2S,3S)-2-hydro...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy AG Curated by ChEMBL | Assay Description The percent reduction of the reverse transcriptase (RT) activity in HIV-1/MN-infected MT-2 cells. | J Med Chem 41: 3387-401 (1998) Article DOI: 10.1021/jm970873c BindingDB Entry DOI: 10.7270/Q2H1315D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 1 (Homo sapiens (Human)) | BDBM4870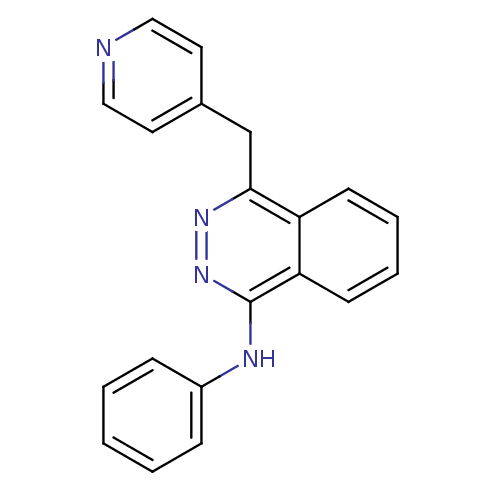 (1-Anilino-4-(4-pyridylmethyl)phthalazine Dihydroch...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description The in vitro kinase assays were performed in 96-well plates using the recombinant GST-fused kinase domains expressed in baculovirus and purified over... | J Med Chem 43: 2310-23 (2000) Article DOI: 10.1021/jm9909443 BindingDB Entry DOI: 10.7270/Q24M92R5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 1 (Homo sapiens (Human)) | BDBM4853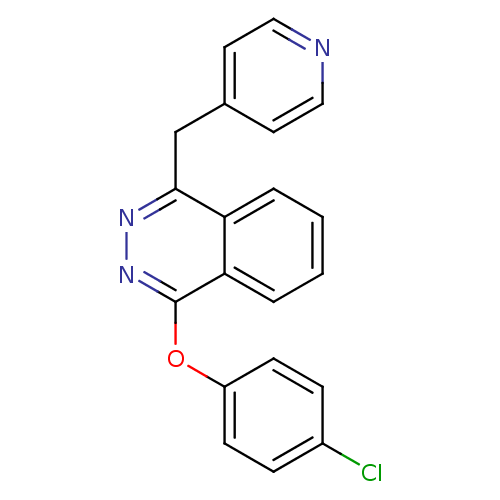 (1-(4-Chlorophenoxy)-4-(4-pyridylmethyl)phthalazine...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis Pharmaceuticals | Assay Description The in vitro kinase assays were performed in 96-well plates using the recombinant GST-fused kinase domains expressed in baculovirus and purified over... | J Med Chem 43: 2310-23 (2000) Article DOI: 10.1021/jm9909443 BindingDB Entry DOI: 10.7270/Q24M92R5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 1 (Homo sapiens (Human)) | BDBM4876 (1-(3,4-Dichloroanilino)-4-(4-pyridylmethyl)phthala...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description The in vitro kinase assays were performed in 96-well plates using the recombinant GST-fused kinase domains expressed in baculovirus and purified over... | J Med Chem 43: 2310-23 (2000) Article DOI: 10.1021/jm9909443 BindingDB Entry DOI: 10.7270/Q24M92R5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 1 (Homo sapiens (Human)) | BDBM4854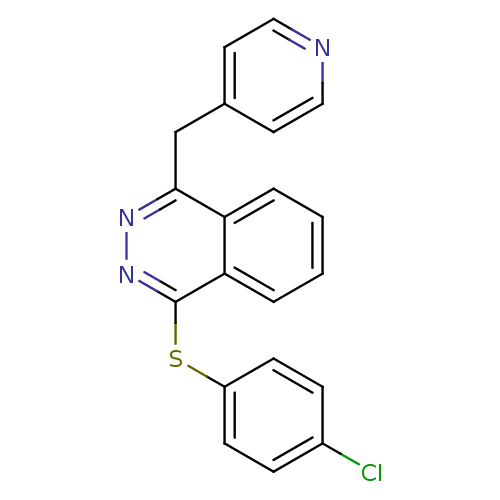 (1-(4-Chlorophenylsulfanyl)-4-(4-pyridylmethyl)phth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis Pharmaceuticals | Assay Description The in vitro kinase assays were performed in 96-well plates using the recombinant GST-fused kinase domains expressed in baculovirus and purified over... | J Med Chem 43: 2310-23 (2000) Article DOI: 10.1021/jm9909443 BindingDB Entry DOI: 10.7270/Q24M92R5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066470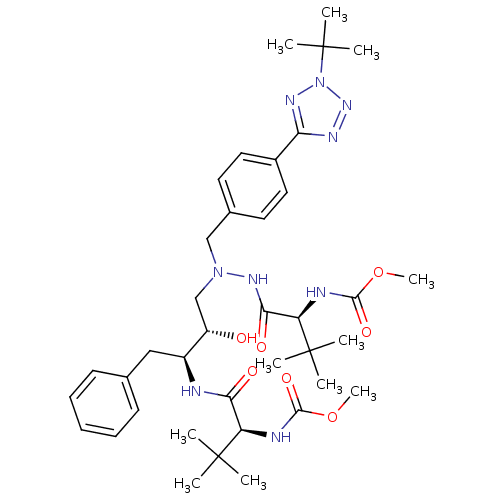 (((S)-1-{N'-[4-(2-tert-Butyl-2H-tetrazol-5-yl)-benz...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy AG Curated by ChEMBL | Assay Description The percent reduction of the reverse transcriptase (RT) activity in HIV-1/MN-infected MT-2 cells. | J Med Chem 41: 3387-401 (1998) Article DOI: 10.1021/jm970873c BindingDB Entry DOI: 10.7270/Q2H1315D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 1 (Homo sapiens (Human)) | BDBM4851 ((4-chlorophenyl)-[4-(4-pyridylmethyl)phthalazin-1-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 77 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis Pharmaceuticals | Assay Description The in vitro kinase assays were performed in 96-well plates using the recombinant GST-fused kinase domains expressed in baculovirus and purified over... | J Med Chem 43: 2310-23 (2000) Article DOI: 10.1021/jm9909443 BindingDB Entry DOI: 10.7270/Q24M92R5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 1 (Homo sapiens (Human)) | BDBM4875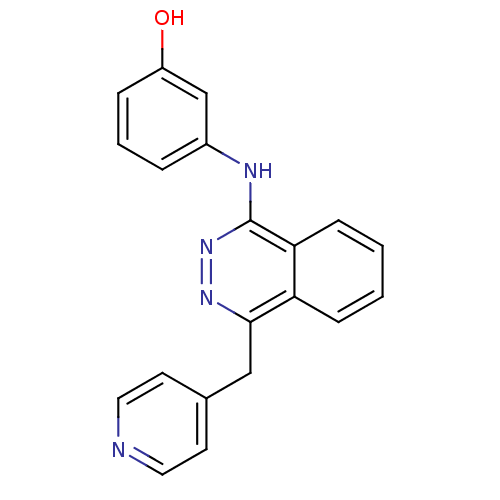 (1-(3-Hydroxyanilino)-4-(4-pyridylmethyl)phthalazin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description The in vitro kinase assays were performed in 96-well plates using the recombinant GST-fused kinase domains expressed in baculovirus and purified over... | J Med Chem 43: 2310-23 (2000) Article DOI: 10.1021/jm9909443 BindingDB Entry DOI: 10.7270/Q24M92R5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM4871 (1-(3-Methylanilino)-4-(4-pyridylmethyl)phthalazine...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description The in vitro kinase assays were performed in 96-well plates using the recombinant GST-fused kinase domains expressed in baculovirus and purified over... | J Med Chem 43: 2310-23 (2000) Article DOI: 10.1021/jm9909443 BindingDB Entry DOI: 10.7270/Q24M92R5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50066458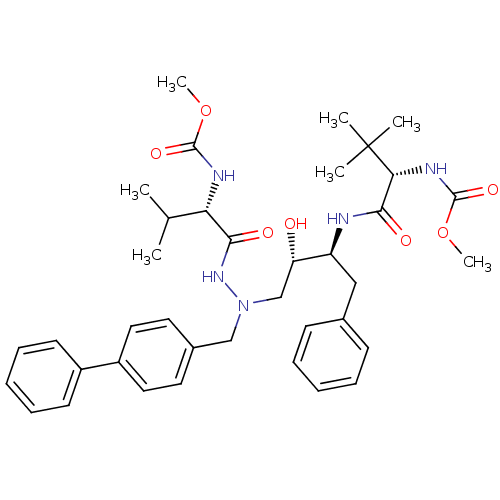 (((S)-1-{(1S,2S)-1-Benzyl-3-[N-biphenyl-4-ylmethyl-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy AG Curated by ChEMBL | Assay Description The percent reduction of the reverse transcriptase (RT) activity in HIV-1/MN-infected MT-2 cells. | J Med Chem 41: 3387-401 (1998) Article DOI: 10.1021/jm970873c BindingDB Entry DOI: 10.7270/Q2H1315D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM4867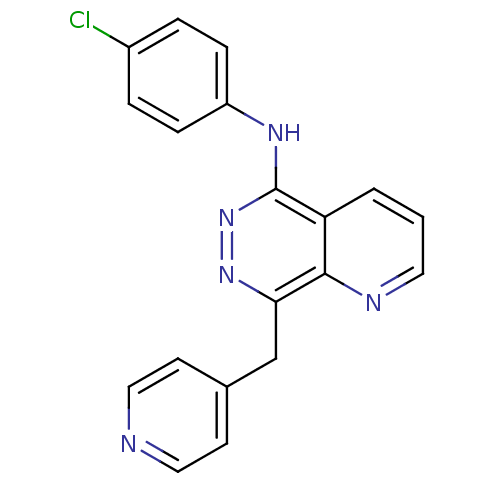 (CGP79787 Analog 50 | CHEMBL75077 | N-(4-chlorophen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description The in vitro kinase assays were performed in 96-well plates using the recombinant GST-fused kinase domains expressed in baculovirus and purified over... | J Med Chem 43: 2310-23 (2000) Article DOI: 10.1021/jm9909443 BindingDB Entry DOI: 10.7270/Q24M92R5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM4877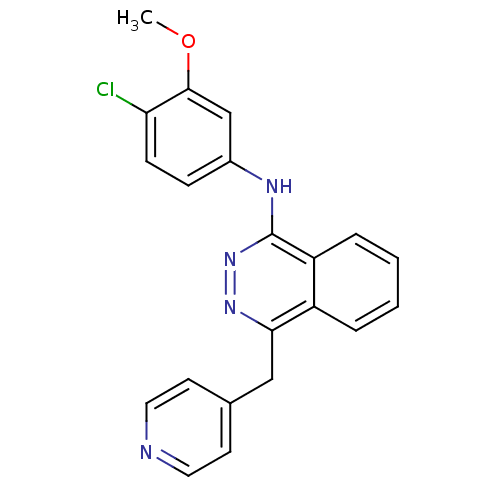 (1-(3-Methoxy-4-chloroanilino)-4-(4-pyridylmethyl)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description The in vitro kinase assays were performed in 96-well plates using the recombinant GST-fused kinase domains expressed in baculovirus and purified over... | J Med Chem 43: 2310-23 (2000) Article DOI: 10.1021/jm9909443 BindingDB Entry DOI: 10.7270/Q24M92R5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 1 (Homo sapiens (Human)) | BDBM4878 (1-(3,5-Dimethylanilino)-4-(4-pyridylmethyl)phthala...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description The in vitro kinase assays were performed in 96-well plates using the recombinant GST-fused kinase domains expressed in baculovirus and purified over... | J Med Chem 43: 2310-23 (2000) Article DOI: 10.1021/jm9909443 BindingDB Entry DOI: 10.7270/Q24M92R5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM202 (CGP 53820 analog | CHEMBL324572 | ethyl N-[(1S)-1-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 177 | n/a | n/a | n/a | n/a | n/a | n/a |
Ciba-Geigy AG Curated by ChEMBL | Assay Description The percent reduction of the reverse transcriptase (RT) activity in HIV-1/MN-infected MT-2 cells. | J Med Chem 41: 3387-401 (1998) Article DOI: 10.1021/jm970873c BindingDB Entry DOI: 10.7270/Q2H1315D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM4881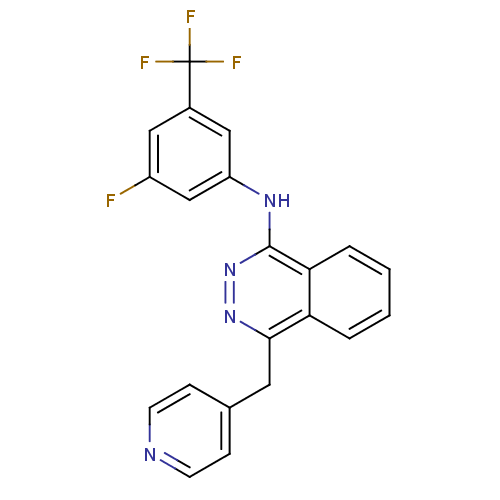 (1-(3-Trifluormethyl-5-fluoroanilino)-4-(4-pyridylm...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description The in vitro kinase assays were performed in 96-well plates using the recombinant GST-fused kinase domains expressed in baculovirus and purified over... | J Med Chem 43: 2310-23 (2000) Article DOI: 10.1021/jm9909443 BindingDB Entry DOI: 10.7270/Q24M92R5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM4873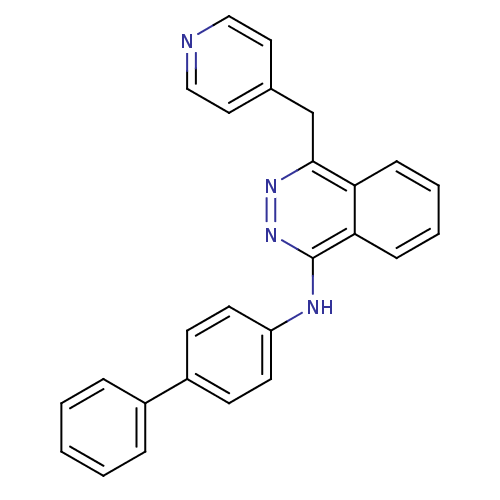 (1-(4-Biphenylamino)-4-(4-pyridylmethyl)phthalazine...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description The in vitro kinase assays were performed in 96-well plates using the recombinant GST-fused kinase domains expressed in baculovirus and purified over... | J Med Chem 43: 2310-23 (2000) Article DOI: 10.1021/jm9909443 BindingDB Entry DOI: 10.7270/Q24M92R5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM4861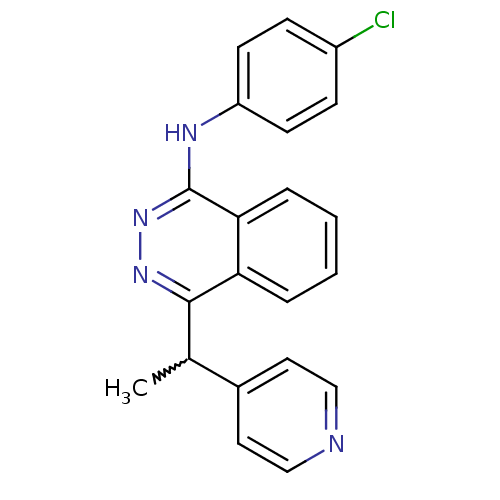 (CGP79787 Analog 52 | N-(4-chlorophenyl)-4-[1-(pyri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description The in vitro kinase assays were performed in 96-well plates using the recombinant GST-fused kinase domains expressed in baculovirus and purified over... | J Med Chem 43: 2310-23 (2000) Article DOI: 10.1021/jm9909443 BindingDB Entry DOI: 10.7270/Q24M92R5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM4872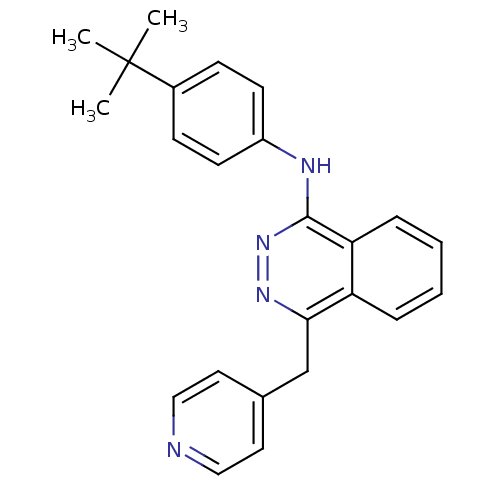 (1-(4-tert-Butylanilino)-4-(4-pyridylmethyl)phthala...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description The in vitro kinase assays were performed in 96-well plates using the recombinant GST-fused kinase domains expressed in baculovirus and purified over... | J Med Chem 43: 2310-23 (2000) Article DOI: 10.1021/jm9909443 BindingDB Entry DOI: 10.7270/Q24M92R5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 1 (Homo sapiens (Human)) | BDBM4866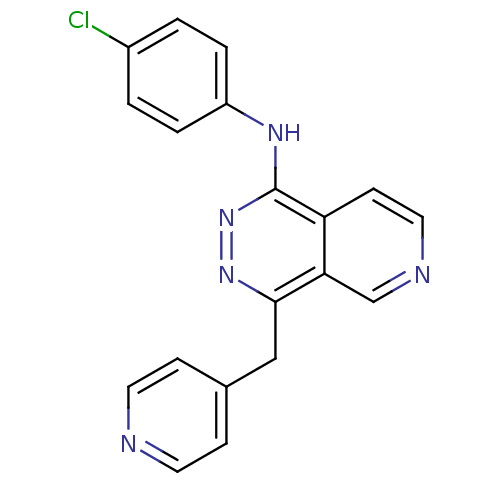 (CGP79787 Analog 49 | N-(4-chlorophenyl)-4-(pyridin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis Pharmaceuticals | Assay Description The in vitro kinase assays were performed in 96-well plates using the recombinant GST-fused kinase domains expressed in baculovirus and purified over... | J Med Chem 43: 2310-23 (2000) Article DOI: 10.1021/jm9909443 BindingDB Entry DOI: 10.7270/Q24M92R5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 1 (Homo sapiens (Human)) | BDBM4873 (1-(4-Biphenylamino)-4-(4-pyridylmethyl)phthalazine...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description The in vitro kinase assays were performed in 96-well plates using the recombinant GST-fused kinase domains expressed in baculovirus and purified over... | J Med Chem 43: 2310-23 (2000) Article DOI: 10.1021/jm9909443 BindingDB Entry DOI: 10.7270/Q24M92R5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM4858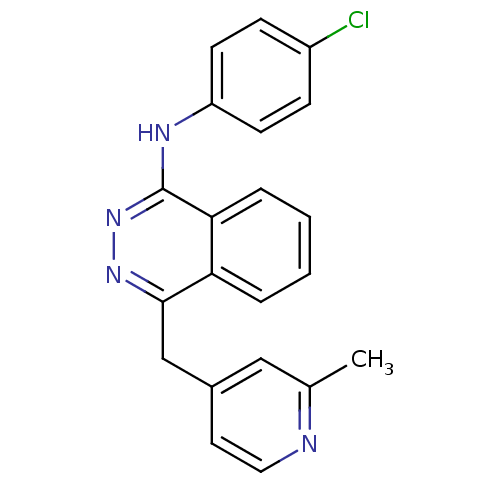 (1-(4-Chloroanilino)-4-[2-methylpyridin-4-ylmethyl]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description The in vitro kinase assays were performed in 96-well plates using the recombinant GST-fused kinase domains expressed in baculovirus and purified over... | J Med Chem 43: 2310-23 (2000) Article DOI: 10.1021/jm9909443 BindingDB Entry DOI: 10.7270/Q24M92R5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 1 (Homo sapiens (Human)) | BDBM4867 (CGP79787 Analog 50 | CHEMBL75077 | N-(4-chlorophen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis Pharmaceuticals | Assay Description The in vitro kinase assays were performed in 96-well plates using the recombinant GST-fused kinase domains expressed in baculovirus and purified over... | J Med Chem 43: 2310-23 (2000) Article DOI: 10.1021/jm9909443 BindingDB Entry DOI: 10.7270/Q24M92R5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 1 (Homo sapiens (Human)) | BDBM4872 (1-(4-tert-Butylanilino)-4-(4-pyridylmethyl)phthala...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description The in vitro kinase assays were performed in 96-well plates using the recombinant GST-fused kinase domains expressed in baculovirus and purified over... | J Med Chem 43: 2310-23 (2000) Article DOI: 10.1021/jm9909443 BindingDB Entry DOI: 10.7270/Q24M92R5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM4874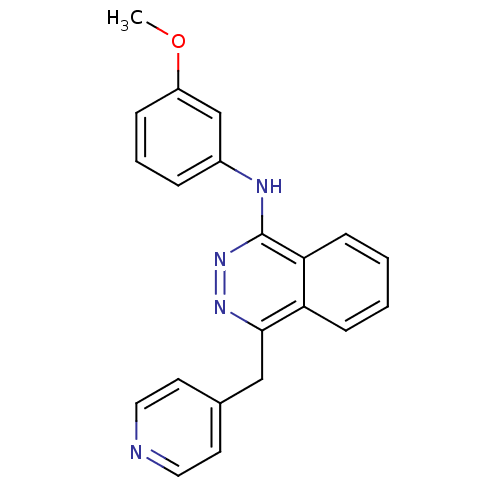 (1-(3-Methoxyanilino)-4-(4-pyridylmethyl)phthalazin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description The in vitro kinase assays were performed in 96-well plates using the recombinant GST-fused kinase domains expressed in baculovirus and purified over... | J Med Chem 43: 2310-23 (2000) Article DOI: 10.1021/jm9909443 BindingDB Entry DOI: 10.7270/Q24M92R5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 1 (Homo sapiens (Human)) | BDBM4869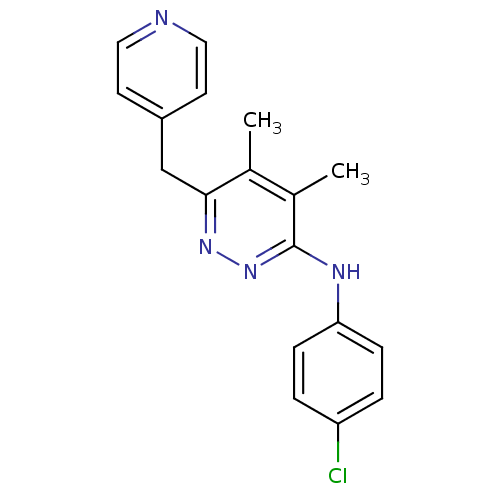 (3-(4-Chloroanilino)-4,5-dimethyl-6-(4-pyridylmethy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | 7.5 | 22 |
Novartis Pharmaceuticals | Assay Description The in vitro kinase assays were performed in 96-well plates using the recombinant GST-fused kinase domains expressed in baculovirus and purified over... | J Med Chem 43: 2310-23 (2000) Article DOI: 10.1021/jm9909443 BindingDB Entry DOI: 10.7270/Q24M92R5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 1 (Homo sapiens (Human)) | BDBM4877 (1-(3-Methoxy-4-chloroanilino)-4-(4-pyridylmethyl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description The in vitro kinase assays were performed in 96-well plates using the recombinant GST-fused kinase domains expressed in baculovirus and purified over... | J Med Chem 43: 2310-23 (2000) Article DOI: 10.1021/jm9909443 BindingDB Entry DOI: 10.7270/Q24M92R5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vascular endothelial growth factor receptor 2 (Homo sapiens (Human)) | BDBM4879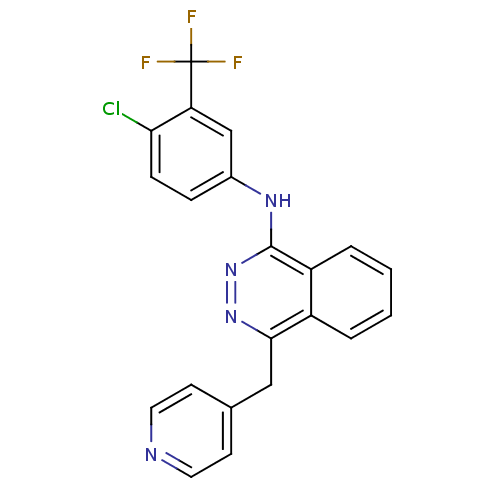 (1-(3-Trifluormethyl-4-chloroanilino)-4-(4-pyridylm...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Pharmaceuticals | Assay Description The in vitro kinase assays were performed in 96-well plates using the recombinant GST-fused kinase domains expressed in baculovirus and purified over... | J Med Chem 43: 2310-23 (2000) Article DOI: 10.1021/jm9909443 BindingDB Entry DOI: 10.7270/Q24M92R5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 109 total ) | Next | Last >> |