 Found 115 hits with Last Name = 'bosserman' and Initial = 'm'
Found 115 hits with Last Name = 'bosserman' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carboxypeptidase B
(Homo sapiens (Human)) | BDBM50144342
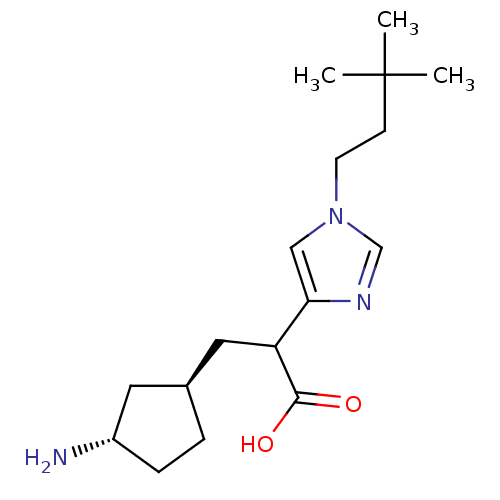
(3-((1R,3S)-3-Amino-cyclopentyl)-2-[1-(3,3-dimethyl...)Show SMILES CC(C)(C)CCn1cnc(c1)C(C[C@H]1CC[C@H](N)C1)C(O)=O Show InChI InChI=1S/C17H29N3O2/c1-17(2,3)6-7-20-10-15(19-11-20)14(16(21)22)9-12-4-5-13(18)8-12/h10-14H,4-9,18H2,1-3H3,(H,21,22)/t12-,13-,14?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human carboxypeptidase B (CPB) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B
(Homo sapiens (Human)) | BDBM50144333
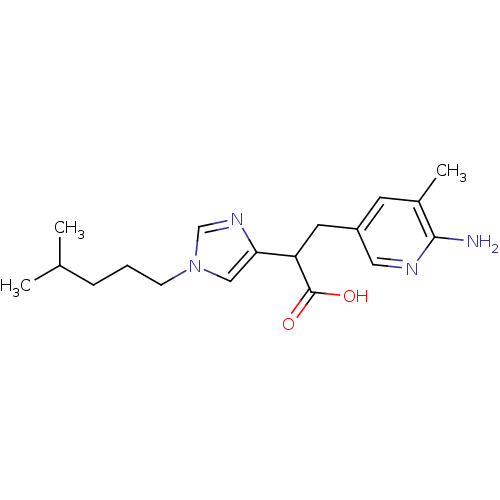
(3-(6-Amino-5-methyl-pyridin-3-yl)-2-[1-(4-methyl-p...)Show InChI InChI=1S/C18H26N4O2/c1-12(2)5-4-6-22-10-16(21-11-22)15(18(23)24)8-14-7-13(3)17(19)20-9-14/h7,9-12,15H,4-6,8H2,1-3H3,(H2,19,20)(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human carboxypeptidase B (CPB) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B
(Homo sapiens (Human)) | BDBM50144336
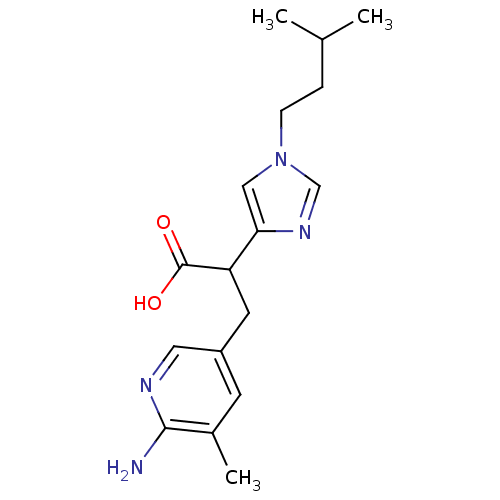
(3-(6-Amino-5-methyl-pyridin-3-yl)-2-[1-(3-methyl-b...)Show InChI InChI=1S/C17H24N4O2/c1-11(2)4-5-21-9-15(20-10-21)14(17(22)23)7-13-6-12(3)16(18)19-8-13/h6,8-11,14H,4-5,7H2,1-3H3,(H2,18,19)(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human carboxypeptidase B (CPB) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50144342

(3-((1R,3S)-3-Amino-cyclopentyl)-2-[1-(3,3-dimethyl...)Show SMILES CC(C)(C)CCn1cnc(c1)C(C[C@H]1CC[C@H](N)C1)C(O)=O Show InChI InChI=1S/C17H29N3O2/c1-17(2,3)6-7-20-10-15(19-11-20)14(16(21)22)9-12-4-5-13(18)8-12/h10-14H,4-9,18H2,1-3H3,(H,21,22)/t12-,13-,14?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50144337
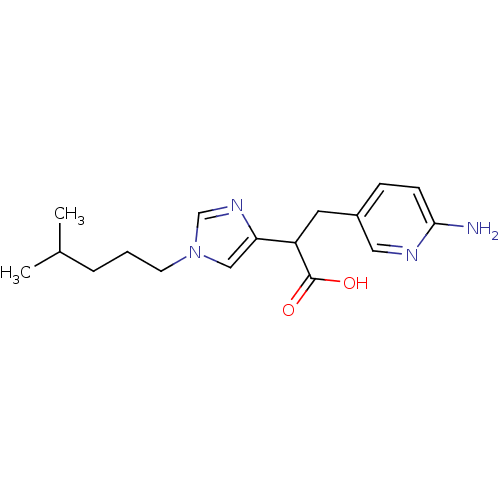
(3-(6-Amino-pyridin-3-yl)-2-[1-(4-methyl-pentyl)-1H...)Show InChI InChI=1S/C17H24N4O2/c1-12(2)4-3-7-21-10-15(20-11-21)14(17(22)23)8-13-5-6-16(18)19-9-13/h5-6,9-12,14H,3-4,7-8H2,1-2H3,(H2,18,19)(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50135934
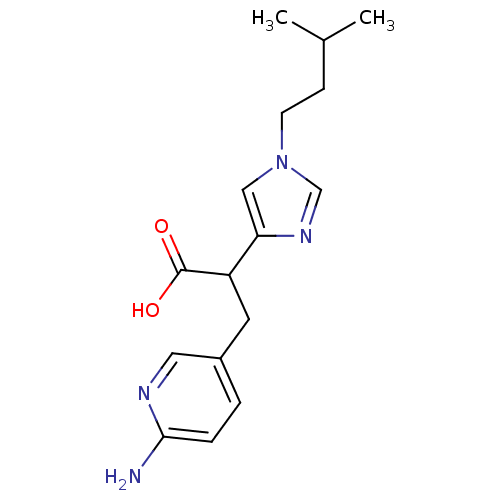
(3-(6-Amino-pyridin-3-yl)-2-[1-(3-methyl-butyl)-1H-...)Show InChI InChI=1S/C16H22N4O2/c1-11(2)5-6-20-9-14(19-10-20)13(16(21)22)7-12-3-4-15(17)18-8-12/h3-4,8-11,13H,5-7H2,1-2H3,(H2,17,18)(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of purified Carboxypeptidase B (CPB) by clot lysis assay in human plasma |
J Med Chem 46: 5294-7 (2003)
Article DOI: 10.1021/jm034141y
BindingDB Entry DOI: 10.7270/Q27H1J0Z |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B
(Homo sapiens (Human)) | BDBM50144337

(3-(6-Amino-pyridin-3-yl)-2-[1-(4-methyl-pentyl)-1H...)Show InChI InChI=1S/C17H24N4O2/c1-12(2)4-3-7-21-10-15(20-11-21)14(17(22)23)8-13-5-6-16(18)19-9-13/h5-6,9-12,14H,3-4,7-8H2,1-2H3,(H2,18,19)(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human carboxypeptidase B (CPB) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B
(Homo sapiens (Human)) | BDBM50135934

(3-(6-Amino-pyridin-3-yl)-2-[1-(3-methyl-butyl)-1H-...)Show InChI InChI=1S/C16H22N4O2/c1-11(2)5-6-20-9-14(19-10-20)13(16(21)22)7-12-3-4-15(17)18-8-12/h3-4,8-11,13H,5-7H2,1-2H3,(H2,17,18)(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human carboxypeptidase B (CPB) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50135934

(3-(6-Amino-pyridin-3-yl)-2-[1-(3-methyl-butyl)-1H-...)Show InChI InChI=1S/C16H22N4O2/c1-11(2)5-6-20-9-14(19-10-20)13(16(21)22)7-12-3-4-15(17)18-8-12/h3-4,8-11,13H,5-7H2,1-2H3,(H2,17,18)(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50135936
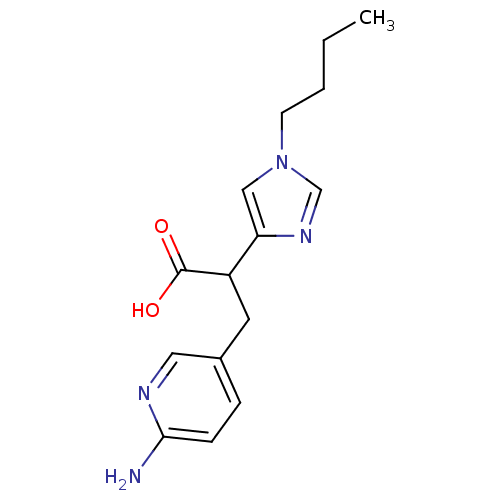
(3-(6-Amino-pyridin-3-yl)-2-(1-butyl-1H-imidazol-4-...)Show InChI InChI=1S/C15H20N4O2/c1-2-3-6-19-9-13(18-10-19)12(15(20)21)7-11-4-5-14(16)17-8-11/h4-5,8-10,12H,2-3,6-7H2,1H3,(H2,16,17)(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of purified Carboxypeptidase B (CPB) by clot lysis assay in human plasma |
J Med Chem 46: 5294-7 (2003)
Article DOI: 10.1021/jm034141y
BindingDB Entry DOI: 10.7270/Q27H1J0Z |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50135939
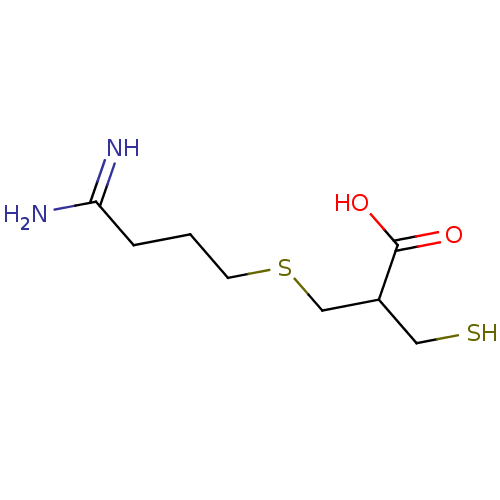
(3-(4,4-Diamino-but-3-enylsulfanyl)-2-mercaptomethy...)Show InChI InChI=1S/C8H16N2O2S2/c9-7(10)2-1-3-14-5-6(4-13)8(11)12/h6,13H,1-5H2,(H3,9,10)(H,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of purified Carboxypeptidase B (CPB) by clot lysis assay in human plasma |
J Med Chem 46: 5294-7 (2003)
Article DOI: 10.1021/jm034141y
BindingDB Entry DOI: 10.7270/Q27H1J0Z |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50144333

(3-(6-Amino-5-methyl-pyridin-3-yl)-2-[1-(4-methyl-p...)Show InChI InChI=1S/C18H26N4O2/c1-12(2)5-4-6-22-10-16(21-11-22)15(18(23)24)8-14-7-13(3)17(19)20-9-14/h7,9-12,15H,4-6,8H2,1-3H3,(H2,19,20)(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase M
(Homo sapiens (Human)) | BDBM50135939

(3-(4,4-Diamino-but-3-enylsulfanyl)-2-mercaptomethy...)Show InChI InChI=1S/C8H16N2O2S2/c9-7(10)2-1-3-14-5-6(4-13)8(11)12/h6,13H,1-5H2,(H3,9,10)(H,11,12) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of purified Carboxypeptidase M (CPM) by clot lysis assay in human plasma |
J Med Chem 46: 5294-7 (2003)
Article DOI: 10.1021/jm034141y
BindingDB Entry DOI: 10.7270/Q27H1J0Z |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B
(Homo sapiens (Human)) | BDBM50144316
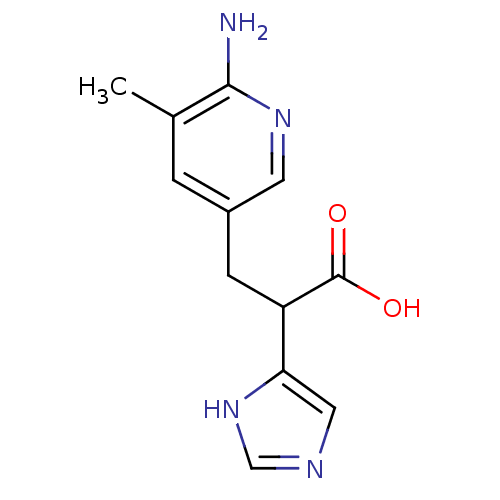
(3-(6-Amino-5-methyl-pyridin-3-yl)-2-(1H-imidazol-4...)Show InChI InChI=1S/C12H14N4O2/c1-7-2-8(4-15-11(7)13)3-9(12(17)18)10-5-14-6-16-10/h2,4-6,9H,3H2,1H3,(H2,13,15)(H,14,16)(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human carboxypeptidase B (CPB) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50135935
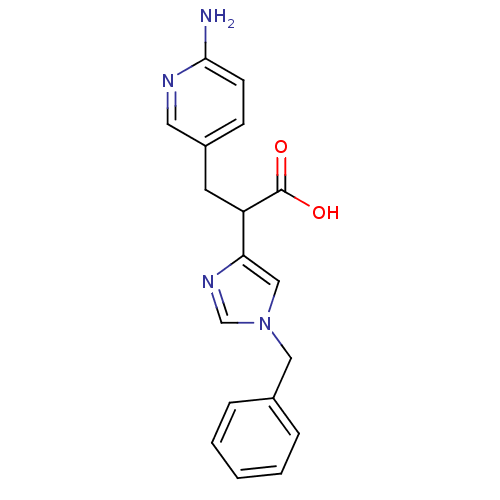
(3-(6-Amino-pyridin-3-yl)-2-(1-benzyl-1H-imidazol-4...)Show InChI InChI=1S/C18H18N4O2/c19-17-7-6-14(9-20-17)8-15(18(23)24)16-11-22(12-21-16)10-13-4-2-1-3-5-13/h1-7,9,11-12,15H,8,10H2,(H2,19,20)(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of purified Carboxypeptidase B (CPB) by clot lysis assay in human plasma |
J Med Chem 46: 5294-7 (2003)
Article DOI: 10.1021/jm034141y
BindingDB Entry DOI: 10.7270/Q27H1J0Z |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50144336

(3-(6-Amino-5-methyl-pyridin-3-yl)-2-[1-(3-methyl-b...)Show InChI InChI=1S/C17H24N4O2/c1-11(2)4-5-21-9-15(20-10-21)14(17(22)23)7-13-6-12(3)16(18)19-8-13/h6,8-11,14H,4-5,7H2,1-3H3,(H2,18,19)(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50144317
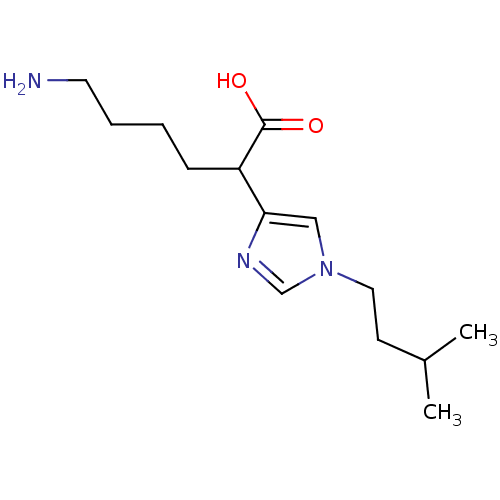
(6-Amino-2-[1-(3-methyl-butyl)-1H-imidazol-4-yl]-he...)Show InChI InChI=1S/C14H25N3O2/c1-11(2)6-8-17-9-13(16-10-17)12(14(18)19)5-3-4-7-15/h9-12H,3-8,15H2,1-2H3,(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50135940
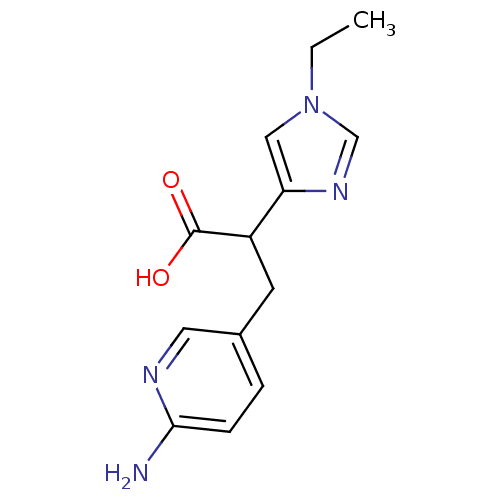
(3-(6-Amino-pyridin-3-yl)-2-(1-ethyl-1H-imidazol-4-...)Show InChI InChI=1S/C13H16N4O2/c1-2-17-7-11(16-8-17)10(13(18)19)5-9-3-4-12(14)15-6-9/h3-4,6-8,10H,2,5H2,1H3,(H2,14,15)(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of purified Carboxypeptidase B (CPB) by clot lysis assay in human plasma |
J Med Chem 46: 5294-7 (2003)
Article DOI: 10.1021/jm034141y
BindingDB Entry DOI: 10.7270/Q27H1J0Z |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50135937
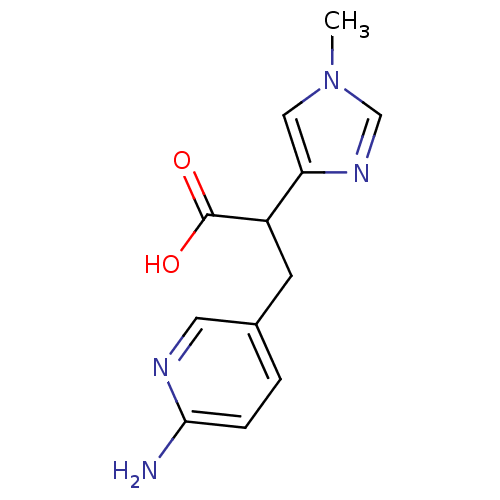
(3-(6-Amino-pyridin-3-yl)-2-(1-methyl-1H-imidazol-4...)Show InChI InChI=1S/C12H14N4O2/c1-16-6-10(15-7-16)9(12(17)18)4-8-2-3-11(13)14-5-8/h2-3,5-7,9H,4H2,1H3,(H2,13,14)(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of purified Carboxypeptidase B (CPB) by clot lysis assay in human plasma |
J Med Chem 46: 5294-7 (2003)
Article DOI: 10.1021/jm034141y
BindingDB Entry DOI: 10.7270/Q27H1J0Z |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50135938
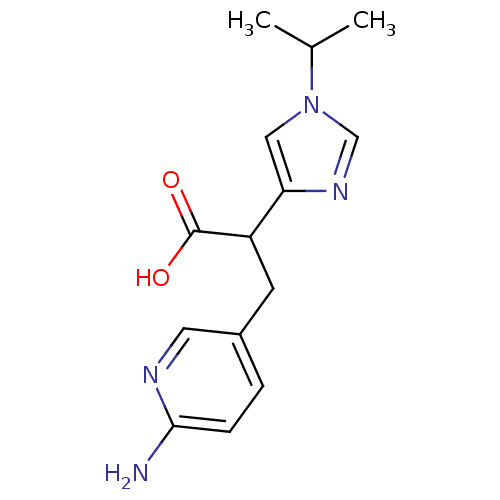
(3-(6-Amino-pyridin-3-yl)-2-(1-isopropyl-1H-imidazo...)Show InChI InChI=1S/C14H18N4O2/c1-9(2)18-7-12(17-8-18)11(14(19)20)5-10-3-4-13(15)16-6-10/h3-4,6-9,11H,5H2,1-2H3,(H2,15,16)(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of purified Carboxypeptidase B (CPB) by clot lysis assay in human plasma |
J Med Chem 46: 5294-7 (2003)
Article DOI: 10.1021/jm034141y
BindingDB Entry DOI: 10.7270/Q27H1J0Z |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B
(Homo sapiens (Human)) | BDBM50135933
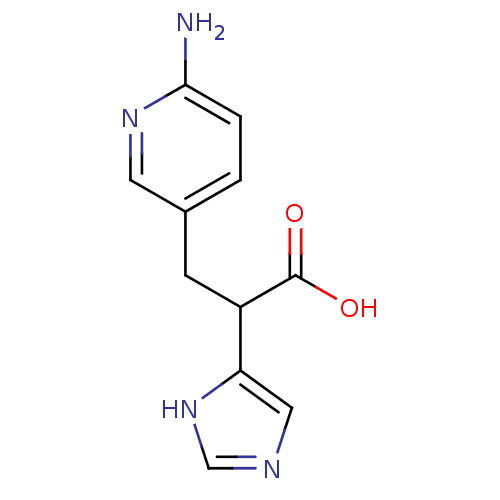
(3-(6-Amino-pyridin-3-yl)-2-(1H-imidazol-4-yl)-prop...)Show InChI InChI=1S/C11H12N4O2/c12-10-2-1-7(4-14-10)3-8(11(16)17)9-5-13-6-15-9/h1-2,4-6,8H,3H2,(H2,12,14)(H,13,15)(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human carboxypeptidase B (CPB) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50135933

(3-(6-Amino-pyridin-3-yl)-2-(1H-imidazol-4-yl)-prop...)Show InChI InChI=1S/C11H12N4O2/c12-10-2-1-7(4-14-10)3-8(11(16)17)9-5-13-6-15-9/h1-2,4-6,8H,3H2,(H2,12,14)(H,13,15)(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50144316

(3-(6-Amino-5-methyl-pyridin-3-yl)-2-(1H-imidazol-4...)Show InChI InChI=1S/C12H14N4O2/c1-7-2-8(4-15-11(7)13)3-9(12(17)18)10-5-14-6-16-10/h2,4-6,9H,3H2,1H3,(H2,13,15)(H,14,16)(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50144325
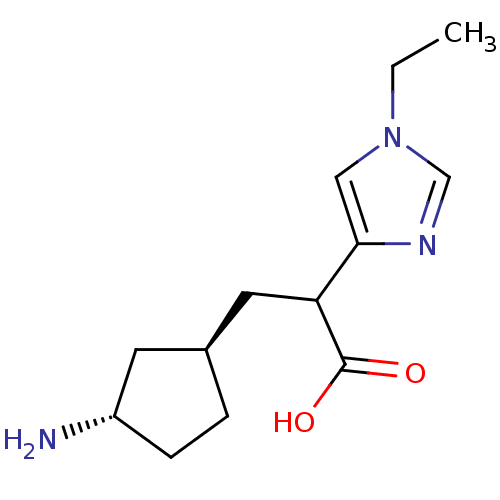
(3-((1R,3S)-3-Amino-cyclopentyl)-2-(1-ethyl-1H-imid...)Show InChI InChI=1S/C13H21N3O2/c1-2-16-7-12(15-8-16)11(13(17)18)6-9-3-4-10(14)5-9/h7-11H,2-6,14H2,1H3,(H,17,18)/t9-,10-,11?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50144942
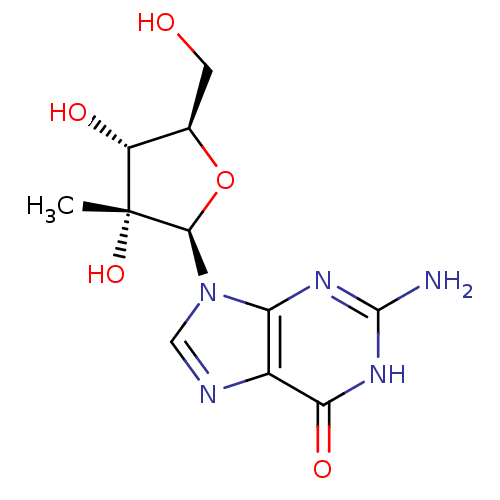
(2'-C-Me-guanosine | 2'-C-methyl-guanosine | 2'-C-m...)Show SMILES C[C@@]1(O)[C@H](O)[C@@H](CO)O[C@H]1n1cnc2c1nc(N)[nH]c2=O Show InChI InChI=1S/C11H15N5O5/c1-11(20)6(18)4(2-17)21-9(11)16-3-13-5-7(16)14-10(12)15-8(5)19/h3-4,6,9,17-18,20H,2H2,1H3,(H3,12,14,15,19)/t4-,6-,9-,11-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Isis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition HCV NS5B-mediated RNA synthesis |
J Med Chem 47: 2283-95 (2004)
Article DOI: 10.1021/jm030424e
BindingDB Entry DOI: 10.7270/Q2QZ2BQ3 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B
(Homo sapiens (Human)) | BDBM50144325

(3-((1R,3S)-3-Amino-cyclopentyl)-2-(1-ethyl-1H-imid...)Show InChI InChI=1S/C13H21N3O2/c1-2-16-7-12(15-8-16)11(13(17)18)6-9-3-4-10(14)5-9/h7-11H,2-6,14H2,1H3,(H,17,18)/t9-,10-,11?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human carboxypeptidase B (CPB) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B
(Homo sapiens (Human)) | BDBM50144317

(6-Amino-2-[1-(3-methyl-butyl)-1H-imidazol-4-yl]-he...)Show InChI InChI=1S/C14H25N3O2/c1-11(2)6-8-17-9-13(16-10-17)12(14(18)19)5-3-4-7-15/h9-12H,3-8,15H2,1-2H3,(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human carboxypeptidase B (CPB) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50144322
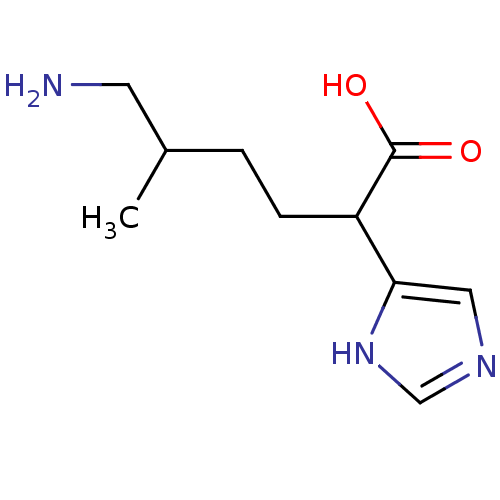
(6-Amino-2-(1H-imidazol-4-yl)-5-methyl-hexanoic aci...)Show InChI InChI=1S/C10H17N3O2/c1-7(4-11)2-3-8(10(14)15)9-5-12-6-13-9/h5-8H,2-4,11H2,1H3,(H,12,13)(H,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50144320
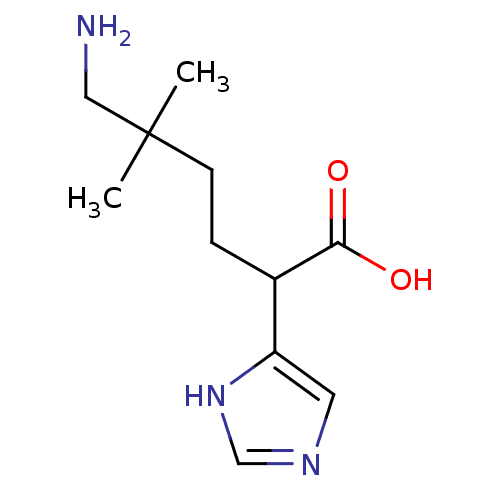
(6-Amino-2-(1H-imidazol-4-yl)-5,5-dimethyl-hexanoic...)Show InChI InChI=1S/C11H19N3O2/c1-11(2,6-12)4-3-8(10(15)16)9-5-13-7-14-9/h5,7-8H,3-4,6,12H2,1-2H3,(H,13,14)(H,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase A1
(Homo sapiens (Human)) | BDBM50121929
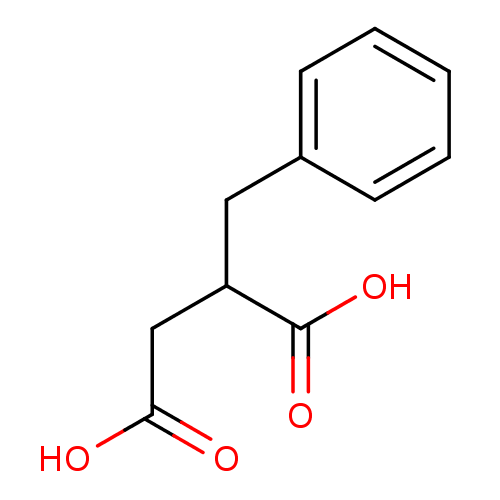
((phenylmethyl)butanedioic acid | 2-benzylbutanedio...)Show InChI InChI=1S/C11H12O4/c12-10(13)7-9(11(14)15)6-8-4-2-1-3-5-8/h1-5,9H,6-7H2,(H,12,13)(H,14,15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of Carboxypeptidase A (CPA) was determined by clot lysis assay using human plasma |
J Med Chem 46: 5294-7 (2003)
Article DOI: 10.1021/jm034141y
BindingDB Entry DOI: 10.7270/Q27H1J0Z |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50144334
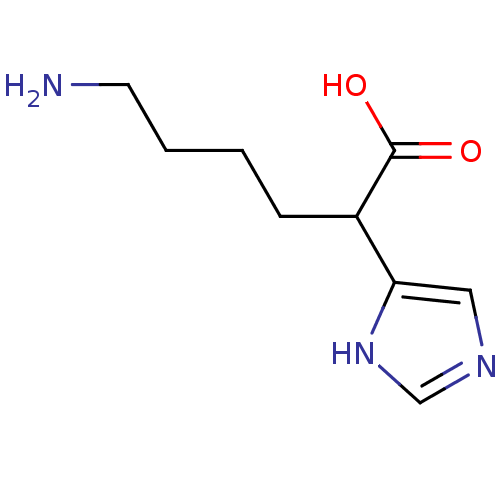
(6-Amino-2-(1H-imidazol-4-yl)-hexanoic acid | CHEMB...)Show InChI InChI=1S/C9H15N3O2/c10-4-2-1-3-7(9(13)14)8-5-11-6-12-8/h5-7H,1-4,10H2,(H,11,12)(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B
(Homo sapiens (Human)) | BDBM50144320

(6-Amino-2-(1H-imidazol-4-yl)-5,5-dimethyl-hexanoic...)Show InChI InChI=1S/C11H19N3O2/c1-11(2,6-12)4-3-8(10(15)16)9-5-13-7-14-9/h5,7-8H,3-4,6,12H2,1-2H3,(H,13,14)(H,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human carboxypeptidase B (CPB) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50144937
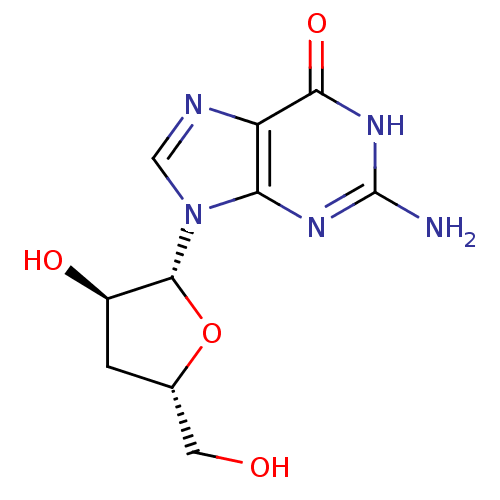
(2-Amino-9-((2R,3R,5S)-3-hydroxy-5-hydroxymethyl-te...)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1O[C@H](CO)C[C@H]1O Show InChI InChI=1S/C10H13N5O4/c11-10-13-7-6(8(18)14-10)12-3-15(7)9-5(17)1-4(2-16)19-9/h3-5,9,16-17H,1-2H2,(H3,11,13,14,18)/t4-,5+,9+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Isis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition HCV NS5B-mediated RNA synthesis |
J Med Chem 47: 2283-95 (2004)
Article DOI: 10.1021/jm030424e
BindingDB Entry DOI: 10.7270/Q2QZ2BQ3 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50135933

(3-(6-Amino-pyridin-3-yl)-2-(1H-imidazol-4-yl)-prop...)Show InChI InChI=1S/C11H12N4O2/c12-10-2-1-7(4-14-10)3-8(11(16)17)9-5-13-6-15-9/h1-2,4-6,8H,3H2,(H2,12,14)(H,13,15)(H,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of purified Carboxypeptidase B (CPB) by clot lysis assay in human plasma |
J Med Chem 46: 5294-7 (2003)
Article DOI: 10.1021/jm034141y
BindingDB Entry DOI: 10.7270/Q27H1J0Z |
More data for this
Ligand-Target Pair | |
Carboxypeptidase A1
(Homo sapiens (Human)) | BDBM50135934

(3-(6-Amino-pyridin-3-yl)-2-[1-(3-methyl-butyl)-1H-...)Show InChI InChI=1S/C16H22N4O2/c1-11(2)5-6-20-9-14(19-10-20)13(16(21)22)7-12-3-4-15(17)18-8-12/h3-4,8-11,13H,5-7H2,1-2H3,(H2,17,18)(H,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of Carboxypeptidase A (CPA) was determined by clot lysis assay using human plasma |
J Med Chem 46: 5294-7 (2003)
Article DOI: 10.1021/jm034141y
BindingDB Entry DOI: 10.7270/Q27H1J0Z |
More data for this
Ligand-Target Pair | |
Carboxypeptidase A1
(Homo sapiens (Human)) | BDBM50135934

(3-(6-Amino-pyridin-3-yl)-2-[1-(3-methyl-butyl)-1H-...)Show InChI InChI=1S/C16H22N4O2/c1-11(2)5-6-20-9-14(19-10-20)13(16(21)22)7-12-3-4-15(17)18-8-12/h3-4,8-11,13H,5-7H2,1-2H3,(H2,17,18)(H,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of Carboxypeptidase A (CPA) was determined by clot lysis assay using human plasma |
J Med Chem 46: 5294-7 (2003)
Article DOI: 10.1021/jm034141y
BindingDB Entry DOI: 10.7270/Q27H1J0Z |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50144323
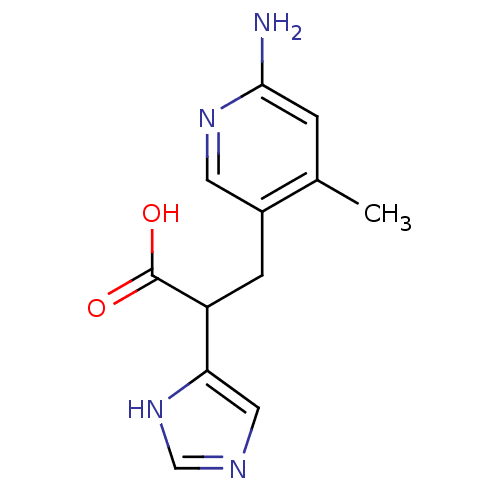
(3-(6-Amino-4-methyl-pyridin-3-yl)-2-(1H-imidazol-4...)Show InChI InChI=1S/C12H14N4O2/c1-7-2-11(13)15-4-8(7)3-9(12(17)18)10-5-14-6-16-10/h2,4-6,9H,3H2,1H3,(H2,13,15)(H,14,16)(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B
(Homo sapiens (Human)) | BDBM50144322

(6-Amino-2-(1H-imidazol-4-yl)-5-methyl-hexanoic aci...)Show InChI InChI=1S/C10H17N3O2/c1-7(4-11)2-3-8(10(14)15)9-5-12-6-13-9/h5-8H,2-4,11H2,1H3,(H,12,13)(H,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human carboxypeptidase B (CPB) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50121929

((phenylmethyl)butanedioic acid | 2-benzylbutanedio...)Show InChI InChI=1S/C11H12O4/c12-10(13)7-9(11(14)15)6-8-4-2-1-3-5-8/h1-5,9H,6-7H2,(H,12,13)(H,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of purified Carboxypeptidase B (CPB) by clot lysis assay in human plasma |
J Med Chem 46: 5294-7 (2003)
Article DOI: 10.1021/jm034141y
BindingDB Entry DOI: 10.7270/Q27H1J0Z |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50144947

(2-Amino-9-((2R,3R,4R,5R)-4-hydroxy-5-hydroxymethyl...)Show SMILES CO[C@@H]1[C@H](O)[C@@H](CO)O[C@H]1n1cnc2c1nc(N)[nH]c2=O Show InChI InChI=1S/C11H15N5O5/c1-20-7-6(18)4(2-17)21-10(7)16-3-13-5-8(16)14-11(12)15-9(5)19/h3-4,6-7,10,17-18H,2H2,1H3,(H3,12,14,15,19)/t4-,6-,7-,10-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Isis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition HCV NS5B-mediated RNA synthesis |
J Med Chem 47: 2283-95 (2004)
Article DOI: 10.1021/jm030424e
BindingDB Entry DOI: 10.7270/Q2QZ2BQ3 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase A1
(Homo sapiens (Human)) | BDBM50100009
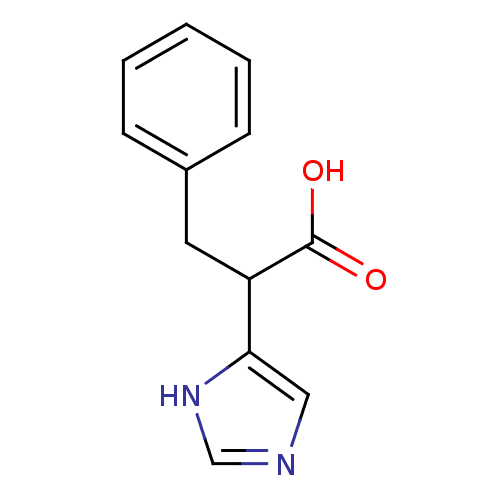
(2-(1H-Imidazol-4-yl)-3-phenyl-propionic acid | 2-(...)Show InChI InChI=1S/C12H12N2O2/c15-12(16)10(11-7-13-8-14-11)6-9-4-2-1-3-5-9/h1-5,7-8,10H,6H2,(H,13,14)(H,15,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of Carboxypeptidase A (CPA) was determined by clot lysis assay using human plasma |
J Med Chem 46: 5294-7 (2003)
Article DOI: 10.1021/jm034141y
BindingDB Entry DOI: 10.7270/Q27H1J0Z |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50144938
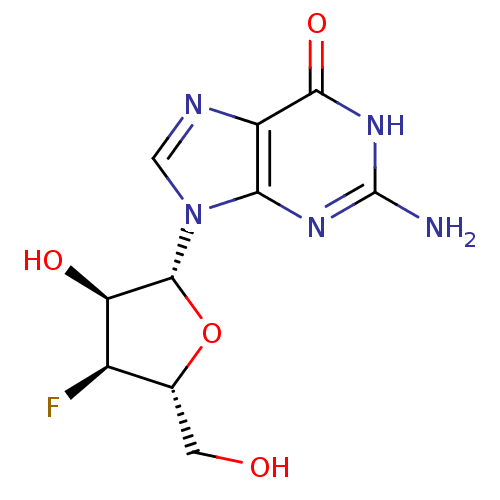
(2-Amino-9-((2R,3S,4S,5R)-4-fluoro-3-hydroxy-5-hydr...)Show SMILES Nc1nc2n(cnc2c(=O)[nH]1)[C@@H]1O[C@H](CO)[C@@H](F)[C@H]1O Show InChI InChI=1S/C10H12FN5O4/c11-4-3(1-17)20-9(6(4)18)16-2-13-5-7(16)14-10(12)15-8(5)19/h2-4,6,9,17-18H,1H2,(H3,12,14,15,19)/t3-,4-,6-,9-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Isis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition HCV NS5B-mediated RNA synthesis |
J Med Chem 47: 2283-95 (2004)
Article DOI: 10.1021/jm030424e
BindingDB Entry DOI: 10.7270/Q2QZ2BQ3 |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50144948

((2R,3R,4R,5R)-2-(6-amino-9H-purin-9-yl)-5-(hydroxy...)Show SMILES C[C@@]1(O)[C@H](O)[C@@H](CO)O[C@H]1n1cnc2c(N)ncnc12 |r| Show InChI InChI=1S/C11H15N5O4/c1-11(19)7(18)5(2-17)20-10(11)16-4-15-6-8(12)13-3-14-9(6)16/h3-5,7,10,17-19H,2H2,1H3,(H2,12,13,14)/t5-,7-,10-,11-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Isis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition HCV NS5B-mediated RNA synthesis |
J Med Chem 47: 2283-95 (2004)
Article DOI: 10.1021/jm030424e
BindingDB Entry DOI: 10.7270/Q2QZ2BQ3 |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50144949
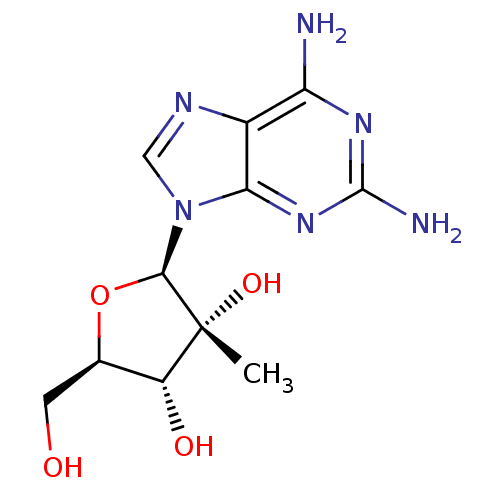
((2R,3R,4R,5R)-2-(2,6-diamino-9H-purin-9-yl)-5-(hyd...)Show SMILES C[C@@]1(O)[C@H](O)[C@@H](CO)O[C@H]1n1cnc2c(N)nc(N)nc12 Show InChI InChI=1S/C11H16N6O4/c1-11(20)6(19)4(2-18)21-9(11)17-3-14-5-7(12)15-10(13)16-8(5)17/h3-4,6,9,18-20H,2H2,1H3,(H4,12,13,15,16)/t4-,6-,9-,11-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Isis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition HCV NS5B-mediated RNA synthesis |
J Med Chem 47: 2283-95 (2004)
Article DOI: 10.1021/jm030424e
BindingDB Entry DOI: 10.7270/Q2QZ2BQ3 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50144329
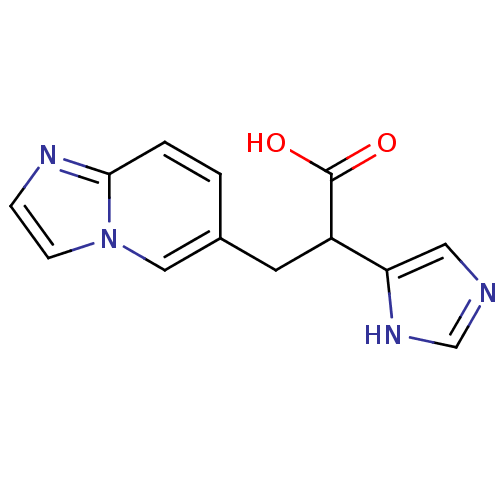
(3-Imidazo[1,2-a]pyridin-6-yl-2-(1H-imidazol-4-yl)-...)Show InChI InChI=1S/C13H12N4O2/c18-13(19)10(11-6-14-8-16-11)5-9-1-2-12-15-3-4-17(12)7-9/h1-4,6-8,10H,5H2,(H,14,16)(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B
(Homo sapiens (Human)) | BDBM50144334

(6-Amino-2-(1H-imidazol-4-yl)-hexanoic acid | CHEMB...)Show InChI InChI=1S/C9H15N3O2/c10-4-2-1-3-7(9(13)14)8-5-11-6-12-8/h5-7H,1-4,10H2,(H,11,12)(H,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human carboxypeptidase B (CPB) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
RNA-directed RNA polymerase
(Hepatitis C virus) | BDBM50144934

((2R,3R,4R,5R)-2-(6-hydroxy-9H-purin-9-yl)-5-(hydro...)Show SMILES C[C@@]1(O)[C@H](O)[C@@H](CO)O[C@H]1n1cnc2c1nc[nH]c2=O Show InChI InChI=1S/C11H14N4O5/c1-11(19)7(17)5(2-16)20-10(11)15-4-14-6-8(15)12-3-13-9(6)18/h3-5,7,10,16-17,19H,2H2,1H3,(H,12,13,18)/t5-,7-,10-,11-/m1/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Isis Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition HCV NS5B-mediated RNA synthesis |
J Med Chem 47: 2283-95 (2004)
Article DOI: 10.1021/jm030424e
BindingDB Entry DOI: 10.7270/Q2QZ2BQ3 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50144324
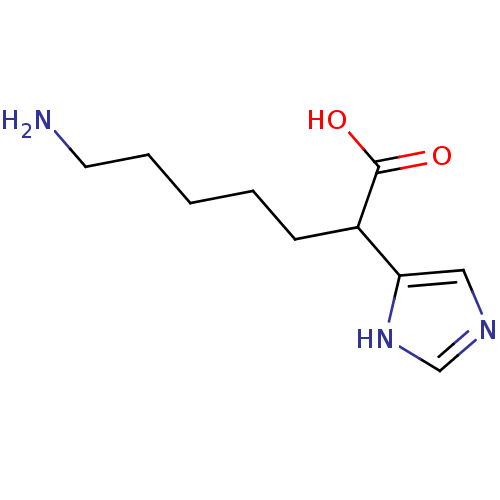
(7-Amino-2-(1H-imidazol-4-yl)-heptanoic acid | CHEM...)Show InChI InChI=1S/C10H17N3O2/c11-5-3-1-2-4-8(10(14)15)9-6-12-7-13-9/h6-8H,1-5,11H2,(H,12,13)(H,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50144332
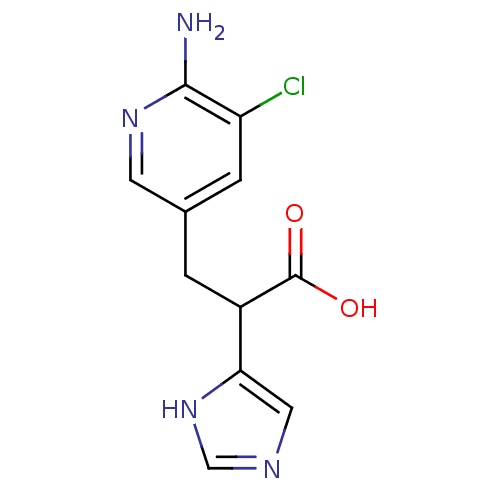
(3-(6-Amino-5-chloro-pyridin-3-yl)-2-(1H-imidazol-4...)Show InChI InChI=1S/C11H11ClN4O2/c12-8-2-6(3-15-10(8)13)1-7(11(17)18)9-4-14-5-16-9/h2-5,7H,1H2,(H2,13,15)(H,14,16)(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50144339
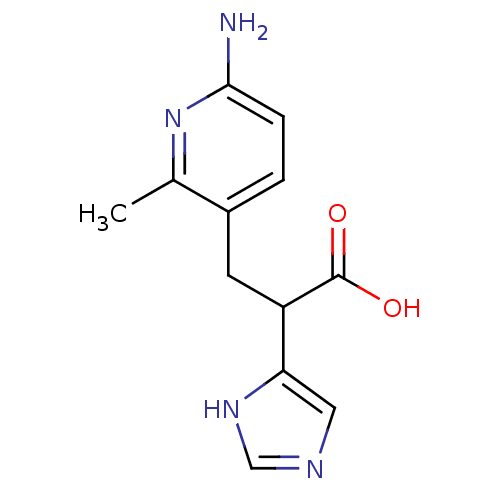
(3-(6-Amino-2-methyl-pyridin-3-yl)-2-(1H-imidazol-4...)Show InChI InChI=1S/C12H14N4O2/c1-7-8(2-3-11(13)16-7)4-9(12(17)18)10-5-14-6-15-10/h2-3,5-6,9H,4H2,1H3,(H2,13,16)(H,14,15)(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibitory potency against human TAFIa (thrombin-activatable fibrinolysis inhibitor) |
Bioorg Med Chem Lett 14: 2141-5 (2004)
Article DOI: 10.1016/j.bmcl.2004.02.033
BindingDB Entry DOI: 10.7270/Q2N8797S |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data