 Found 85 hits with Last Name = 'brackley' and Initial = 'ja'
Found 85 hits with Last Name = 'brackley' and Initial = 'ja' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224496
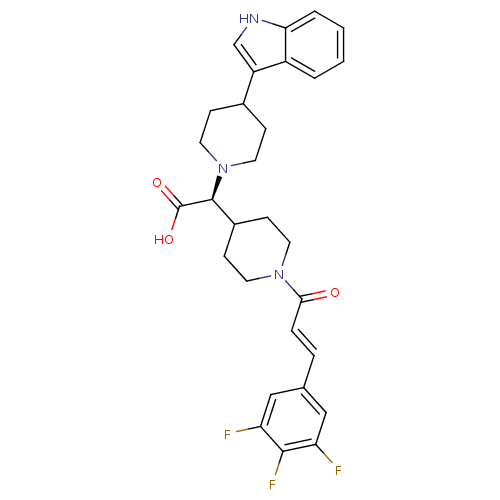
((S,E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-...)Show SMILES OC(=O)[C@H](C1CCN(CC1)C(=O)\C=C\c1cc(F)c(F)c(F)c1)N1CCC(CC1)c1c[nH]c2ccccc12 Show InChI InChI=1S/C29H30F3N3O3/c30-23-15-18(16-24(31)27(23)32)5-6-26(36)34-11-9-20(10-12-34)28(29(37)38)35-13-7-19(8-14-35)22-17-33-25-4-2-1-3-21(22)25/h1-6,15-17,19-20,28,33H,7-14H2,(H,37,38)/b6-5+/t28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Antagonist activity at CCR2 expressed in THP1 cells assessed as MCP1-induced calcium flux |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224501
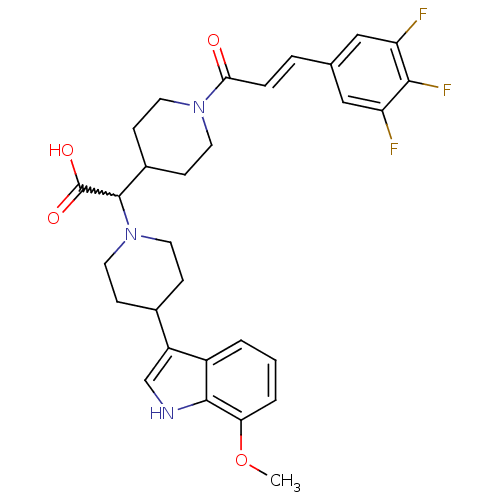
((E)-2-(4-(7-methoxy-1H-indol-3-yl)piperidin-1-yl)-...)Show SMILES COc1cccc2c(c[nH]c12)C1CCN(CC1)C(C1CCN(CC1)C(=O)\C=C\c1cc(F)c(F)c(F)c1)C(O)=O |w:17.41| Show InChI InChI=1S/C30H32F3N3O4/c1-40-25-4-2-3-21-22(17-34-28(21)25)19-7-13-36(14-8-19)29(30(38)39)20-9-11-35(12-10-20)26(37)6-5-18-15-23(31)27(33)24(32)16-18/h2-6,15-17,19-20,29,34H,7-14H2,1H3,(H,38,39)/b6-5+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224496

((S,E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-...)Show SMILES OC(=O)[C@H](C1CCN(CC1)C(=O)\C=C\c1cc(F)c(F)c(F)c1)N1CCC(CC1)c1c[nH]c2ccccc12 Show InChI InChI=1S/C29H30F3N3O3/c30-23-15-18(16-24(31)27(23)32)5-6-26(36)34-11-9-20(10-12-34)28(29(37)38)35-13-7-19(8-14-35)22-17-33-25-4-2-1-3-21(22)25/h1-6,15-17,19-20,28,33H,7-14H2,(H,37,38)/b6-5+/t28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224500

((E)-2-(4-(5-amino-1H-indol-3-yl)piperidin-1-yl)-2-...)Show SMILES Nc1ccc2[nH]cc(C3CCN(CC3)C(C3CCN(CC3)C(=O)\C=C\c3cc(F)c(F)c(F)c3)C(O)=O)c2c1 |w:14.36| Show InChI InChI=1S/C29H31F3N4O3/c30-23-13-17(14-24(31)27(23)32)1-4-26(37)35-9-7-19(8-10-35)28(29(38)39)36-11-5-18(6-12-36)22-16-34-25-3-2-20(33)15-21(22)25/h1-4,13-16,18-19,28,34H,5-12,33H2,(H,38,39)/b4-1+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224502
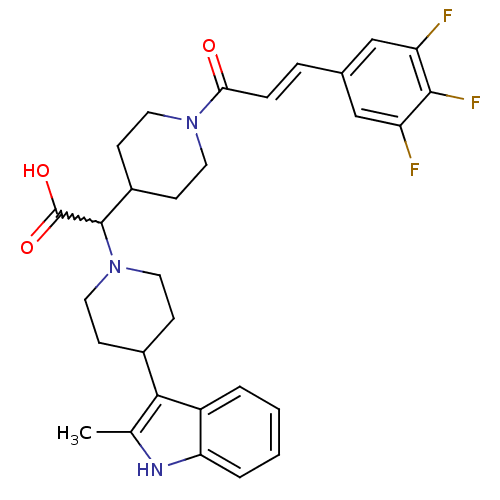
((E)-2-(4-(2-methyl-1H-indol-3-yl)piperidin-1-yl)-2...)Show SMILES Cc1[nH]c2ccccc2c1C1CCN(CC1)C(C1CCN(CC1)C(=O)\C=C\c1cc(F)c(F)c(F)c1)C(O)=O |w:16.40| Show InChI InChI=1S/C30H32F3N3O3/c1-18-27(22-4-2-3-5-25(22)34-18)20-8-14-36(15-9-20)29(30(38)39)21-10-12-35(13-11-21)26(37)7-6-19-16-23(31)28(33)24(32)17-19/h2-7,16-17,20-21,29,34H,8-15H2,1H3,(H,38,39)/b7-6+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224523
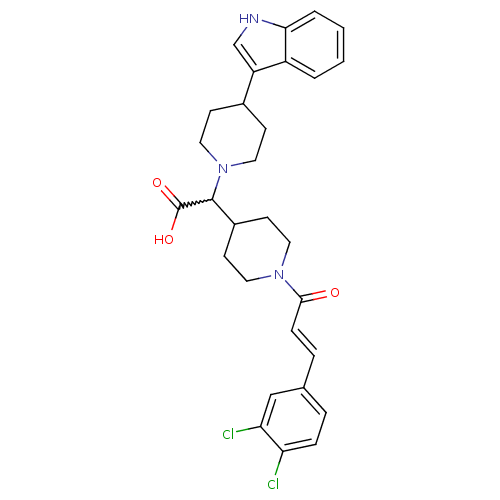
((E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-(3...)Show SMILES OC(=O)C(C1CCN(CC1)C(=O)\C=C\c1ccc(Cl)c(Cl)c1)N1CCC(CC1)c1c[nH]c2ccccc12 |w:3.2| Show InChI InChI=1S/C29H31Cl2N3O3/c30-24-7-5-19(17-25(24)31)6-8-27(35)33-13-11-21(12-14-33)28(29(36)37)34-15-9-20(10-16-34)23-18-32-26-4-2-1-3-22(23)26/h1-8,17-18,20-21,28,32H,9-16H2,(H,36,37)/b8-6+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224511
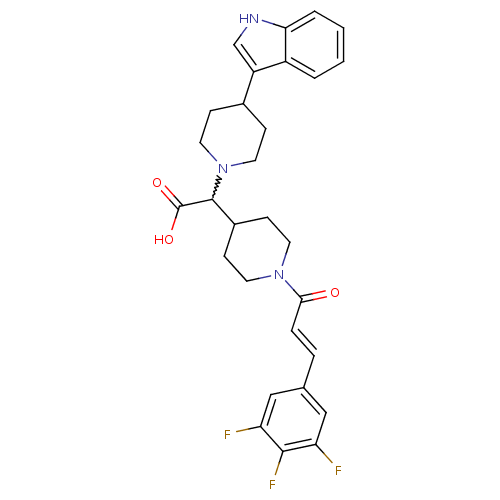
((E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-(3...)Show SMILES OC(=O)C(C1CCN(CC1)C(=O)\C=C\c1cc(F)c(F)c(F)c1)N1CCC(CC1)c1c[nH]c2ccccc12 |w:3.24| Show InChI InChI=1S/C29H30F3N3O3/c30-23-15-18(16-24(31)27(23)32)5-6-26(36)34-11-9-20(10-12-34)28(29(37)38)35-13-7-19(8-14-35)22-17-33-25-4-2-1-3-21(22)25/h1-6,15-17,19-20,28,33H,7-14H2,(H,37,38)/b6-5+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224524
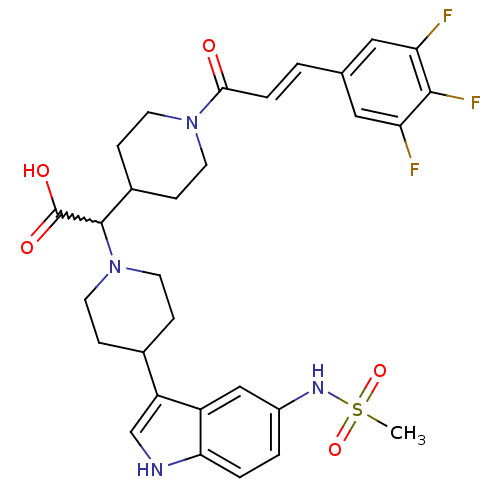
((E)-2-(4-(5-(methylsulfonamido)-1H-indol-3-yl)pipe...)Show SMILES CS(=O)(=O)Nc1ccc2[nH]cc(C3CCN(CC3)C(C3CCN(CC3)C(=O)\C=C\c3cc(F)c(F)c(F)c3)C(O)=O)c2c1 |w:18.40| Show InChI InChI=1S/C30H33F3N4O5S/c1-43(41,42)35-21-3-4-26-22(16-21)23(17-34-26)19-6-12-37(13-7-19)29(30(39)40)20-8-10-36(11-9-20)27(38)5-2-18-14-24(31)28(33)25(32)15-18/h2-5,14-17,19-20,29,34-35H,6-13H2,1H3,(H,39,40)/b5-2+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224519
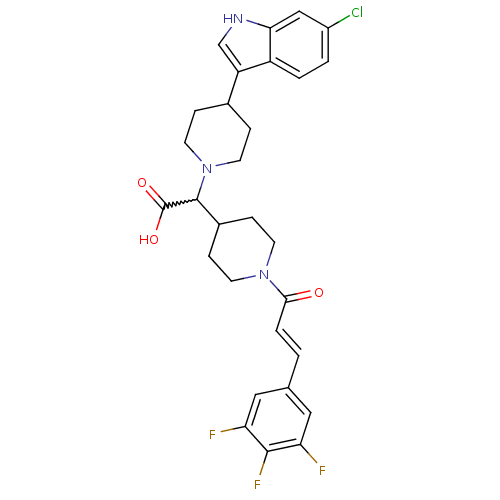
((E)-2-(4-(6-chloro-1H-indol-3-yl)piperidin-1-yl)-2...)Show SMILES OC(=O)C(C1CCN(CC1)C(=O)\C=C\c1cc(F)c(F)c(F)c1)N1CCC(CC1)c1c[nH]c2cc(Cl)ccc12 |w:3.2| Show InChI InChI=1S/C29H29ClF3N3O3/c30-20-2-3-21-22(16-34-25(21)15-20)18-5-11-36(12-6-18)28(29(38)39)19-7-9-35(10-8-19)26(37)4-1-17-13-23(31)27(33)24(32)14-17/h1-4,13-16,18-19,28,34H,5-12H2,(H,38,39)/b4-1+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224512
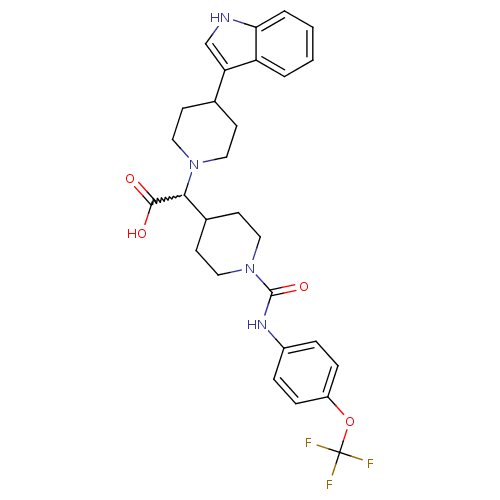
(2-(1-((4-(trifluoromethoxy)phenyl)carbamoyl)piperi...)Show SMILES OC(=O)C(C1CCN(CC1)C(=O)Nc1ccc(OC(F)(F)F)cc1)N1CCC(CC1)c1c[nH]c2ccccc12 |w:3.2| Show InChI InChI=1S/C28H31F3N4O4/c29-28(30,31)39-21-7-5-20(6-8-21)33-27(38)35-15-11-19(12-16-35)25(26(36)37)34-13-9-18(10-14-34)23-17-32-24-4-2-1-3-22(23)24/h1-8,17-19,25,32H,9-16H2,(H,33,38)(H,36,37) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224510
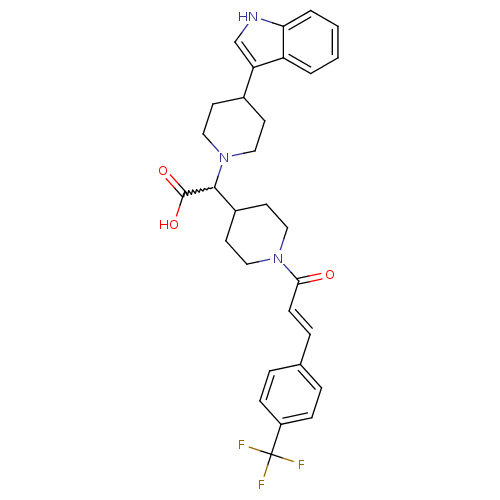
((E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-(4...)Show SMILES OC(=O)C(C1CCN(CC1)C(=O)\C=C\c1ccc(cc1)C(F)(F)F)N1CCC(CC1)c1c[nH]c2ccccc12 |w:3.2| Show InChI InChI=1S/C30H32F3N3O3/c31-30(32,33)23-8-5-20(6-9-23)7-10-27(37)35-15-13-22(14-16-35)28(29(38)39)36-17-11-21(12-18-36)25-19-34-26-4-2-1-3-24(25)26/h1-10,19,21-22,28,34H,11-18H2,(H,38,39)/b10-7+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224508
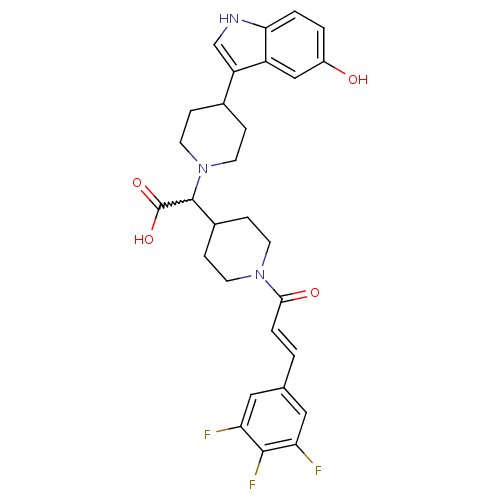
((E)-2-(4-(5-hydroxy-1H-indol-3-yl)piperidin-1-yl)-...)Show SMILES OC(=O)C(C1CCN(CC1)C(=O)\C=C\c1cc(F)c(F)c(F)c1)N1CCC(CC1)c1c[nH]c2ccc(O)cc12 |w:3.2| Show InChI InChI=1S/C29H30F3N3O4/c30-23-13-17(14-24(31)27(23)32)1-4-26(37)34-9-7-19(8-10-34)28(29(38)39)35-11-5-18(6-12-35)22-16-33-25-3-2-20(36)15-21(22)25/h1-4,13-16,18-19,28,33,36H,5-12H2,(H,38,39)/b4-1+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224521

((E)-2-(4-(5-methoxy-1H-indol-3-yl)piperidin-1-yl)-...)Show SMILES COc1ccc2[nH]cc(C3CCN(CC3)C(C3CCN(CC3)C(=O)\C=C\c3cc(F)c(F)c(F)c3)C(O)=O)c2c1 |w:15.37| Show InChI InChI=1S/C30H32F3N3O4/c1-40-21-3-4-26-22(16-21)23(17-34-26)19-6-12-36(13-7-19)29(30(38)39)20-8-10-35(11-9-20)27(37)5-2-18-14-24(31)28(33)25(32)15-18/h2-5,14-17,19-20,29,34H,6-13H2,1H3,(H,38,39)/b5-2+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224499
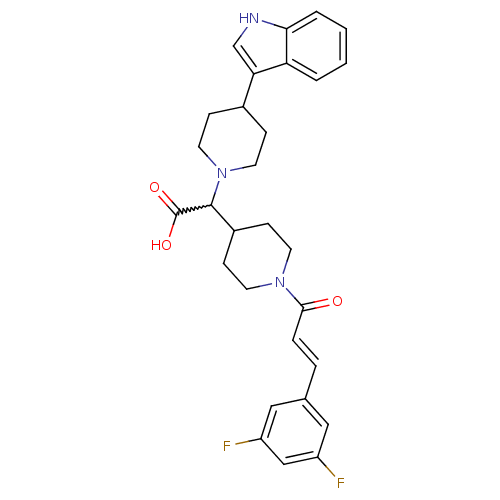
((E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-(3...)Show SMILES OC(=O)C(C1CCN(CC1)C(=O)\C=C\c1cc(F)cc(F)c1)N1CCC(CC1)c1c[nH]c2ccccc12 |w:3.2| Show InChI InChI=1S/C29H31F2N3O3/c30-22-15-19(16-23(31)17-22)5-6-27(35)33-11-9-21(10-12-33)28(29(36)37)34-13-7-20(8-14-34)25-18-32-26-4-2-1-3-24(25)26/h1-6,15-18,20-21,28,32H,7-14H2,(H,36,37)/b6-5+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224506
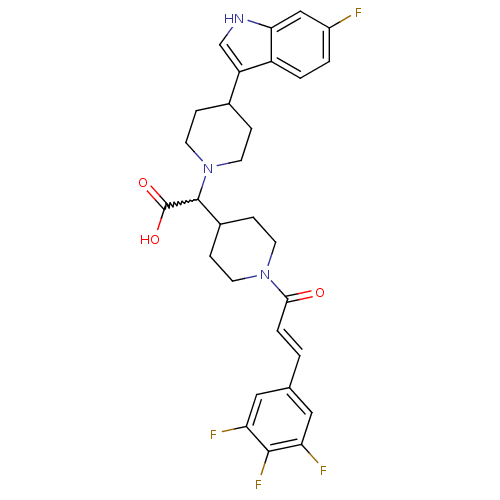
((E)-2-(4-(6-fluoro-1H-indol-3-yl)piperidin-1-yl)-2...)Show SMILES OC(=O)C(C1CCN(CC1)C(=O)\C=C\c1cc(F)c(F)c(F)c1)N1CCC(CC1)c1c[nH]c2cc(F)ccc12 |w:3.2| Show InChI InChI=1S/C29H29F4N3O3/c30-20-2-3-21-22(16-34-25(21)15-20)18-5-11-36(12-6-18)28(29(38)39)19-7-9-35(10-8-19)26(37)4-1-17-13-23(31)27(33)24(32)14-17/h1-4,13-16,18-19,28,34H,5-12H2,(H,38,39)/b4-1+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224516

(2-(1-((3,4-dichlorophenyl)carbamoyl)piperidin-4-yl...)Show SMILES OC(=O)C(C1CCN(CC1)C(=O)Nc1ccc(Cl)c(Cl)c1)N1CCC(CC1)c1c[nH]c2ccccc12 |w:3.3| Show InChI InChI=1S/C27H30Cl2N4O3/c28-22-6-5-19(15-23(22)29)31-27(36)33-13-9-18(10-14-33)25(26(34)35)32-11-7-17(8-12-32)21-16-30-24-4-2-1-3-20(21)24/h1-6,15-18,25,30H,7-14H2,(H,31,36)(H,34,35) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224498
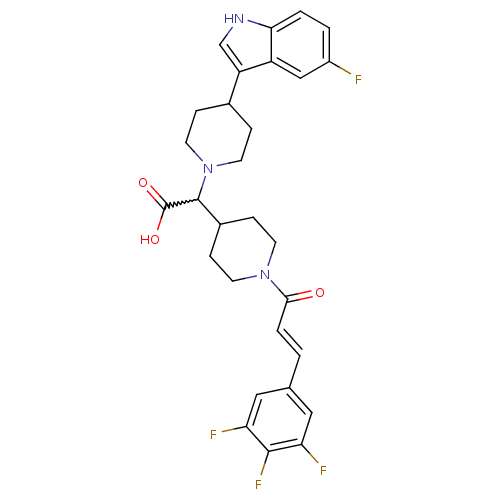
((E)-2-(4-(5-fluoro-1H-indol-3-yl)piperidin-1-yl)-2...)Show SMILES OC(=O)C(C1CCN(CC1)C(=O)\C=C\c1cc(F)c(F)c(F)c1)N1CCC(CC1)c1c[nH]c2ccc(F)cc12 |w:3.2| Show InChI InChI=1S/C29H29F4N3O3/c30-20-2-3-25-21(15-20)22(16-34-25)18-5-11-36(12-6-18)28(29(38)39)19-7-9-35(10-8-19)26(37)4-1-17-13-23(31)27(33)24(32)14-17/h1-4,13-16,18-19,28,34H,5-12H2,(H,38,39)/b4-1+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50400781
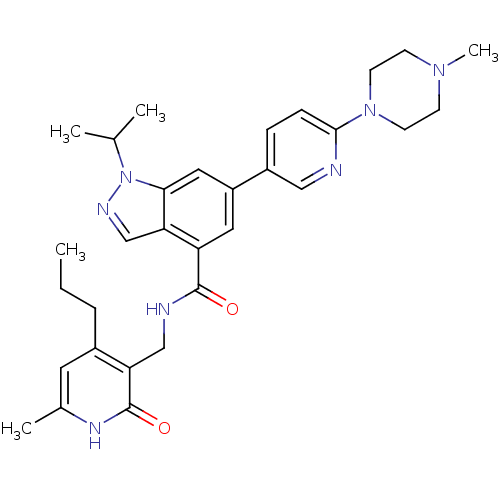
(CHEMBL2204997)Show SMILES CCCc1cc(C)[nH]c(=O)c1CNC(=O)c1cc(cc2n(ncc12)C(C)C)-c1ccc(nc1)N1CCN(C)CC1 Show InChI InChI=1S/C31H39N7O2/c1-6-7-22-14-21(4)35-31(40)26(22)18-33-30(39)25-15-24(16-28-27(25)19-34-38(28)20(2)3)23-8-9-29(32-17-23)37-12-10-36(5)11-13-37/h8-9,14-17,19-20H,6-7,10-13,18H2,1-5H3,(H,33,39)(H,35,40) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of EZH2-mediated proliferation of human LNCaP cells after 6 days by chemiluminescence analysis |
ACS Med Chem Lett 3: 1091-1096 (2012)
Article DOI: 10.1021/ml3003346
BindingDB Entry DOI: 10.7270/Q2NK3G60 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224515

((E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-cinna...)Show SMILES OC(=O)C(C1CCN(CC1)C(=O)\C=C\c1ccccc1)N1CCC(CC1)c1c[nH]c2ccccc12 |w:3.3| Show InChI InChI=1S/C29H33N3O3/c33-27(11-10-21-6-2-1-3-7-21)31-16-14-23(15-17-31)28(29(34)35)32-18-12-22(13-19-32)25-20-30-26-9-5-4-8-24(25)26/h1-11,20,22-23,28,30H,12-19H2,(H,34,35)/b11-10+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224505
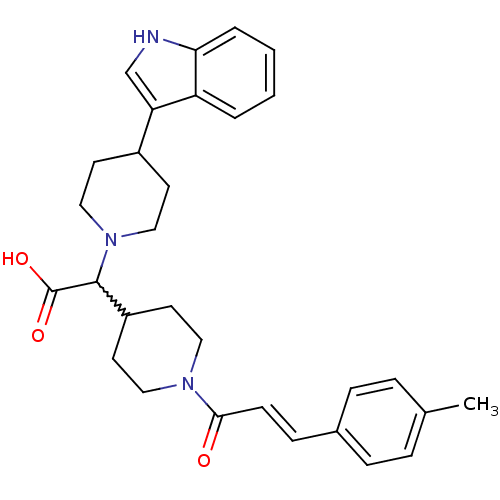
((E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-p-...)Show SMILES Cc1ccc(\C=C\C(=O)N2CCC(CC2)C(N2CCC(CC2)c2c[nH]c3ccccc23)C(O)=O)cc1 |w:15.15| Show InChI InChI=1S/C30H35N3O3/c1-21-6-8-22(9-7-21)10-11-28(34)32-16-14-24(15-17-32)29(30(35)36)33-18-12-23(13-19-33)26-20-31-27-5-3-2-4-25(26)27/h2-11,20,23-24,29,31H,12-19H2,1H3,(H,35,36)/b11-10+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 115 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224517

(2-(1-((4-(trifluoromethyl)phenyl)carbamoyl)piperid...)Show SMILES OC(=O)C(C1CCN(CC1)C(=O)Nc1ccc(cc1)C(F)(F)F)N1CCC(CC1)c1c[nH]c2ccccc12 |w:3.2| Show InChI InChI=1S/C28H31F3N4O3/c29-28(30,31)20-5-7-21(8-6-20)33-27(38)35-15-11-19(12-16-35)25(26(36)37)34-13-9-18(10-14-34)23-17-32-24-4-2-1-3-22(23)24/h1-8,17-19,25,32H,9-16H2,(H,33,38)(H,36,37) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50400778

(CHEMBL2204995)Show SMILES CCCc1cc(C)[nH]c(=O)c1CNC(=O)c1cc(cc2n(ncc12)C(C)C)-c1ccnc(c1)N1CCN(C)CC1 Show InChI InChI=1S/C31H39N7O2/c1-6-7-23-14-21(4)35-31(40)26(23)18-33-30(39)25-15-24(16-28-27(25)19-34-38(28)20(2)3)22-8-9-32-29(17-22)37-12-10-36(5)11-13-37/h8-9,14-17,19-20H,6-7,10-13,18H2,1-5H3,(H,33,39)(H,35,40) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 174 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of EZH2-mediated proliferation of human LNCaP cells after 6 days by chemiluminescence analysis |
ACS Med Chem Lett 3: 1091-1096 (2012)
Article DOI: 10.1021/ml3003346
BindingDB Entry DOI: 10.7270/Q2NK3G60 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224514
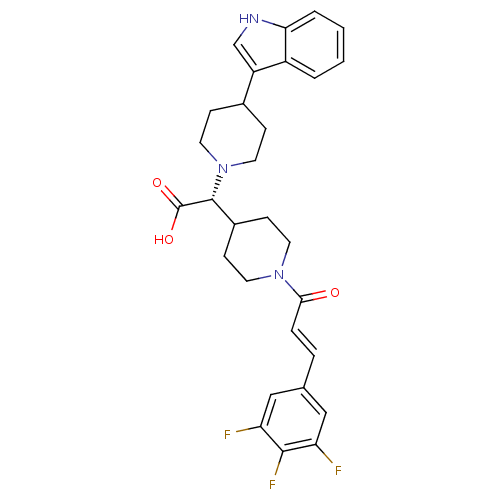
((R,E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-...)Show SMILES OC(=O)[C@@H](C1CCN(CC1)C(=O)\C=C\c1cc(F)c(F)c(F)c1)N1CCC(CC1)c1c[nH]c2ccccc12 Show InChI InChI=1S/C29H30F3N3O3/c30-23-15-18(16-24(31)27(23)32)5-6-26(36)34-11-9-20(10-12-34)28(29(37)38)35-13-7-19(8-14-35)22-17-33-25-4-2-1-3-21(22)25/h1-6,15-17,19-20,28,33H,7-14H2,(H,37,38)/b6-5+/t28-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224507

((E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-(4...)Show SMILES OC(=O)C(C1CCN(CC1)C(=O)\C=C\c1ccc(cc1)[N+]([O-])=O)N1CCC(CC1)c1c[nH]c2ccccc12 |w:3.2| Show InChI InChI=1S/C29H32N4O5/c34-27(10-7-20-5-8-23(9-6-20)33(37)38)31-15-13-22(14-16-31)28(29(35)36)32-17-11-21(12-18-32)25-19-30-26-4-2-1-3-24(25)26/h1-10,19,21-22,28,30H,11-18H2,(H,35,36)/b10-7+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224503
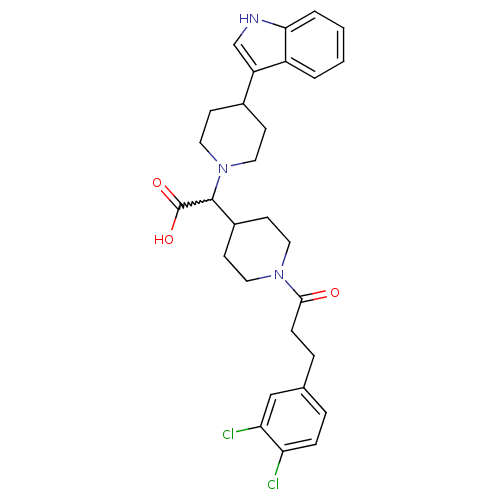
(2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-(3,4-d...)Show SMILES OC(=O)C(C1CCN(CC1)C(=O)CCc1ccc(Cl)c(Cl)c1)N1CCC(CC1)c1c[nH]c2ccccc12 |w:3.2| Show InChI InChI=1S/C29H33Cl2N3O3/c30-24-7-5-19(17-25(24)31)6-8-27(35)33-13-11-21(12-14-33)28(29(36)37)34-15-9-20(10-16-34)23-18-32-26-4-2-1-3-22(23)26/h1-5,7,17-18,20-21,28,32H,6,8-16H2,(H,36,37) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50400779
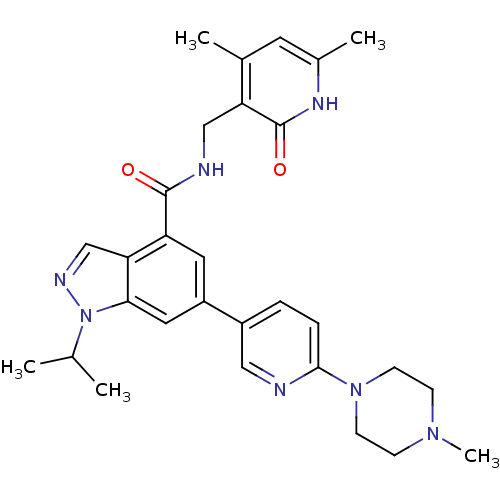
(CHEMBL2204998)Show SMILES CC(C)n1ncc2c(cc(cc12)-c1ccc(nc1)N1CCN(C)CC1)C(=O)NCc1c(C)cc(C)[nH]c1=O Show InChI InChI=1S/C29H35N7O2/c1-18(2)36-26-14-22(21-6-7-27(30-15-21)35-10-8-34(5)9-11-35)13-23(25(26)17-32-36)28(37)31-16-24-19(3)12-20(4)33-29(24)38/h6-7,12-15,17-18H,8-11,16H2,1-5H3,(H,31,37)(H,33,38) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 324 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of EZH2-mediated proliferation of human LNCaP cells after 6 days by chemiluminescence analysis |
ACS Med Chem Lett 3: 1091-1096 (2012)
Article DOI: 10.1021/ml3003346
BindingDB Entry DOI: 10.7270/Q2NK3G60 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224520

((E)-1-(4-((4-(1H-indol-3-yl)piperidin-1-yl)methyl)...)Show SMILES Fc1cc(\C=C\C(=O)N2CCC(CN3CCC(CC3)c3c[nH]c4ccccc34)CC2)cc(F)c1F Show InChI InChI=1S/C28H30F3N3O/c29-24-15-20(16-25(30)28(24)31)5-6-27(35)34-13-7-19(8-14-34)18-33-11-9-21(10-12-33)23-17-32-26-4-2-1-3-22(23)26/h1-6,15-17,19,21,32H,7-14,18H2/b6-5+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224513

((E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-(4...)Show SMILES COc1ccc(\C=C\C(=O)N2CCC(CC2)C(N2CCC(CC2)c2c[nH]c3ccccc23)C(O)=O)cc1 |w:16.35| Show InChI InChI=1S/C30H35N3O4/c1-37-24-9-6-21(7-10-24)8-11-28(34)32-16-14-23(15-17-32)29(30(35)36)33-18-12-22(13-19-33)26-20-31-27-5-3-2-4-25(26)27/h2-11,20,22-23,29,31H,12-19H2,1H3,(H,35,36)/b11-8+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 660 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224495
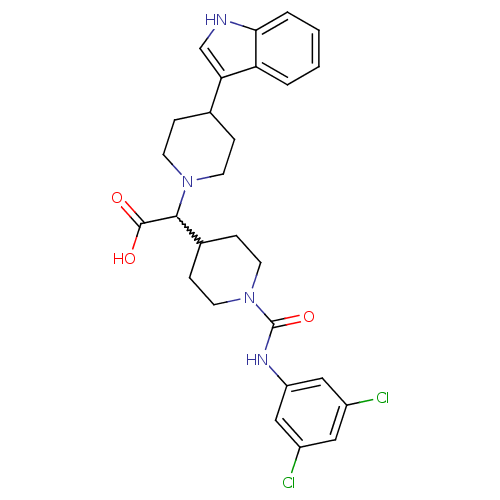
(2-(1-((3,5-dichlorophenyl)carbamoyl)piperidin-4-yl...)Show SMILES OC(=O)C(C1CCN(CC1)C(=O)Nc1cc(Cl)cc(Cl)c1)N1CCC(CC1)c1c[nH]c2ccccc12 |w:3.3| Show InChI InChI=1S/C27H30Cl2N4O3/c28-19-13-20(29)15-21(14-19)31-27(36)33-11-7-18(8-12-33)25(26(34)35)32-9-5-17(6-10-32)23-16-30-24-4-2-1-3-22(23)24/h1-4,13-18,25,30H,5-12H2,(H,31,36)(H,34,35) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224522
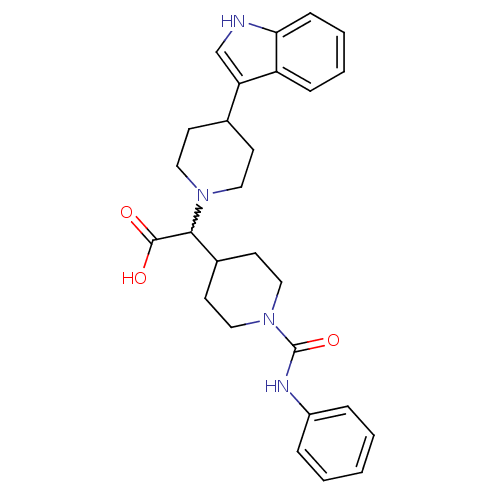
(2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(phenylca...)Show SMILES OC(=O)C(C1CCN(CC1)C(=O)Nc1ccccc1)N1CCC(CC1)c1c[nH]c2ccccc12 |w:3.20| Show InChI InChI=1S/C27H32N4O3/c32-26(33)25(20-12-16-31(17-13-20)27(34)29-21-6-2-1-3-7-21)30-14-10-19(11-15-30)23-18-28-24-9-5-4-8-22(23)24/h1-9,18-20,25,28H,10-17H2,(H,29,34)(H,32,33) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224504

((E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-(3...)Show SMILES NC(=O)C(C1CCN(CC1)C(=O)\C=C\c1cc(F)c(F)c(F)c1)N1CCC(CC1)c1c[nH]c2ccccc12 |w:3.24| Show InChI InChI=1S/C29H31F3N4O2/c30-23-15-18(16-24(31)27(23)32)5-6-26(37)35-11-9-20(10-12-35)28(29(33)38)36-13-7-19(8-14-36)22-17-34-25-4-2-1-3-21(22)25/h1-6,15-17,19-20,28,34H,7-14H2,(H2,33,38)/b6-5+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50400780
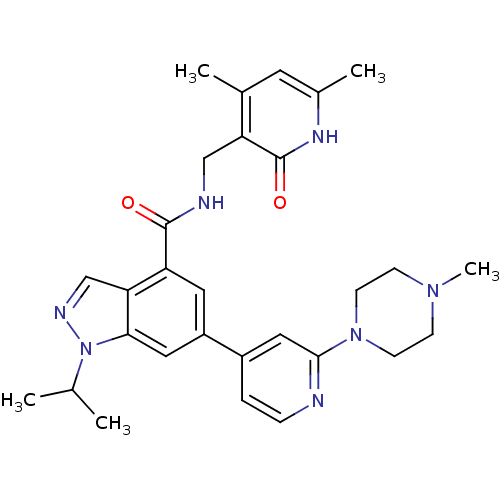
(CHEMBL2204996)Show SMILES CC(C)n1ncc2c(cc(cc12)-c1ccnc(c1)N1CCN(C)CC1)C(=O)NCc1c(C)cc(C)[nH]c1=O Show InChI InChI=1S/C29H35N7O2/c1-18(2)36-26-14-22(21-6-7-30-27(15-21)35-10-8-34(5)9-11-35)13-23(25(26)17-32-36)28(37)31-16-24-19(3)12-20(4)33-29(24)38/h6-7,12-15,17-18H,8-11,16H2,1-5H3,(H,31,37)(H,33,38) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of EZH2-mediated proliferation of human LNCaP cells after 6 days by chemiluminescence analysis |
ACS Med Chem Lett 3: 1091-1096 (2012)
Article DOI: 10.1021/ml3003346
BindingDB Entry DOI: 10.7270/Q2NK3G60 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Mus musculus) | BDBM50224496

((S,E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-...)Show SMILES OC(=O)[C@H](C1CCN(CC1)C(=O)\C=C\c1cc(F)c(F)c(F)c1)N1CCC(CC1)c1c[nH]c2ccccc12 Show InChI InChI=1S/C29H30F3N3O3/c30-23-15-18(16-24(31)27(23)32)5-6-26(36)34-11-9-20(10-12-34)28(29(37)38)35-13-7-19(8-14-35)22-17-33-25-4-2-1-3-21(22)25/h1-6,15-17,19-20,28,33H,7-14H2,(H,37,38)/b6-5+/t28-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-mouse MCP1 from CCR2 in mouse WEHI265.1 cells |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Mus musculus) | BDBM50224496

((S,E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-...)Show SMILES OC(=O)[C@H](C1CCN(CC1)C(=O)\C=C\c1cc(F)c(F)c(F)c1)N1CCC(CC1)c1c[nH]c2ccccc12 Show InChI InChI=1S/C29H30F3N3O3/c30-23-15-18(16-24(31)27(23)32)5-6-26(36)34-11-9-20(10-12-34)28(29(37)38)35-13-7-19(8-14-35)22-17-33-25-4-2-1-3-21(22)25/h1-6,15-17,19-20,28,33H,7-14H2,(H,37,38)/b6-5+/t28-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-rat MCP1 from rat CCR2 receptor in monocytes |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Mus musculus) | BDBM50224496

((S,E)-2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(1-(3-...)Show SMILES OC(=O)[C@H](C1CCN(CC1)C(=O)\C=C\c1cc(F)c(F)c(F)c1)N1CCC(CC1)c1c[nH]c2ccccc12 Show InChI InChI=1S/C29H30F3N3O3/c30-23-15-18(16-24(31)27(23)32)5-6-26(36)34-11-9-20(10-12-34)28(29(37)38)35-13-7-19(8-14-35)22-17-33-25-4-2-1-3-21(22)25/h1-6,15-17,19-20,28,33H,7-14H2,(H,37,38)/b6-5+/t28-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Displacement of [125I]-mouse MCP1 from CCR2 in mouse peripheral blood monocytes |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50400782
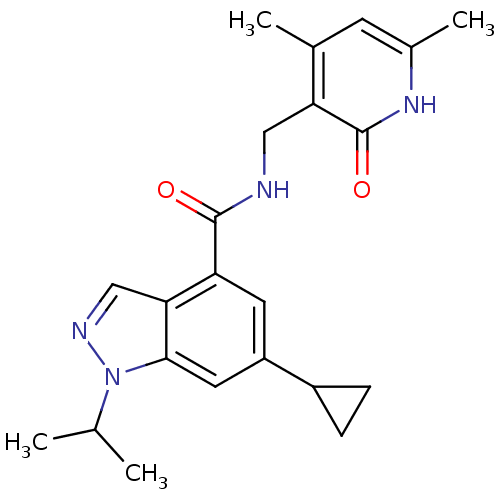
(CHEMBL2204999)Show SMILES CC(C)n1ncc2c(cc(cc12)C1CC1)C(=O)NCc1c(C)cc(C)[nH]c1=O Show InChI InChI=1S/C22H26N4O2/c1-12(2)26-20-9-16(15-5-6-15)8-17(19(20)11-24-26)21(27)23-10-18-13(3)7-14(4)25-22(18)28/h7-9,11-12,15H,5-6,10H2,1-4H3,(H,23,27)(H,25,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of EZH2-mediated proliferation of human LNCaP cells after 6 days by chemiluminescence analysis |
ACS Med Chem Lett 3: 1091-1096 (2012)
Article DOI: 10.1021/ml3003346
BindingDB Entry DOI: 10.7270/Q2NK3G60 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50400778

(CHEMBL2204995)Show SMILES CCCc1cc(C)[nH]c(=O)c1CNC(=O)c1cc(cc2n(ncc12)C(C)C)-c1ccnc(c1)N1CCN(C)CC1 Show InChI InChI=1S/C31H39N7O2/c1-6-7-23-14-21(4)35-31(40)26(23)18-33-30(39)25-15-24(16-28-27(25)19-34-38(28)20(2)3)22-8-9-32-29(17-22)37-12-10-36(5)11-13-37/h8-9,14-17,19-20H,6-7,10-13,18H2,1-5H3,(H,33,39)(H,35,40) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of EZH2-mediated proliferation of human LNCaP cells after 6 days by chemiluminescence analysis |
ACS Med Chem Lett 3: 1091-1096 (2012)
Article DOI: 10.1021/ml3003346
BindingDB Entry DOI: 10.7270/Q2NK3G60 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224509
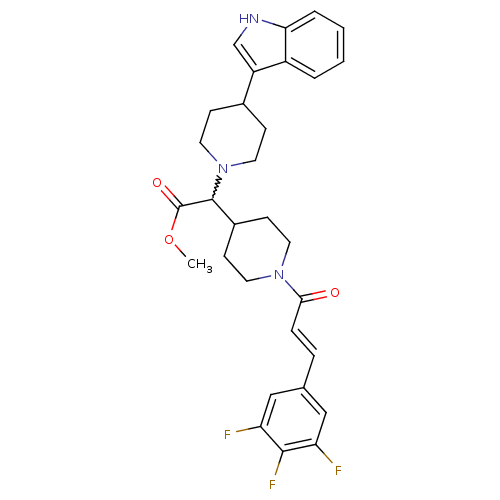
((E)-methyl 2-(4-(1H-indol-3-yl)piperidin-1-yl)-2-(...)Show SMILES COC(=O)C(C1CCN(CC1)C(=O)\C=C\c1cc(F)c(F)c(F)c1)N1CCC(CC1)c1c[nH]c2ccccc12 |w:4.25| Show InChI InChI=1S/C30H32F3N3O3/c1-39-30(38)29(36-14-8-20(9-15-36)23-18-34-26-5-3-2-4-22(23)26)21-10-12-35(13-11-21)27(37)7-6-19-16-24(31)28(33)25(32)17-19/h2-7,16-18,20-21,29,34H,8-15H2,1H3/b7-6+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224518

((E)-2-(4-(1-acetyl-1H-indol-3-yl)piperidin-1-yl)-2...)Show SMILES CC(=O)n1cc(C2CCN(CC2)C(C2CCN(CC2)C(=O)\C=C\c2cc(F)cc(F)c2)C(O)=O)c2ccccc12 |w:12.33| Show InChI InChI=1S/C31H33F2N3O4/c1-20(37)36-19-27(26-4-2-3-5-28(26)36)22-8-14-35(15-9-22)30(31(39)40)23-10-12-34(13-11-23)29(38)7-6-21-16-24(32)18-25(33)17-21/h2-7,16-19,22-23,30H,8-15H2,1H3,(H,39,40)/b7-6+ | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50400779

(CHEMBL2204998)Show SMILES CC(C)n1ncc2c(cc(cc12)-c1ccc(nc1)N1CCN(C)CC1)C(=O)NCc1c(C)cc(C)[nH]c1=O Show InChI InChI=1S/C29H35N7O2/c1-18(2)36-26-14-22(21-6-7-27(30-15-21)35-10-8-34(5)9-11-35)13-23(25(26)17-32-36)28(37)31-16-24-19(3)12-20(4)33-29(24)38/h6-7,12-15,17-18H,8-11,16H2,1-5H3,(H,31,37)(H,33,38) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of EZH2-mediated proliferation of human LNCaP cells after 6 days by chemiluminescence analysis |
ACS Med Chem Lett 3: 1091-1096 (2012)
Article DOI: 10.1021/ml3003346
BindingDB Entry DOI: 10.7270/Q2NK3G60 |
More data for this
Ligand-Target Pair | |
C-C chemokine receptor type 2
(Homo sapiens (Human)) | BDBM50224497
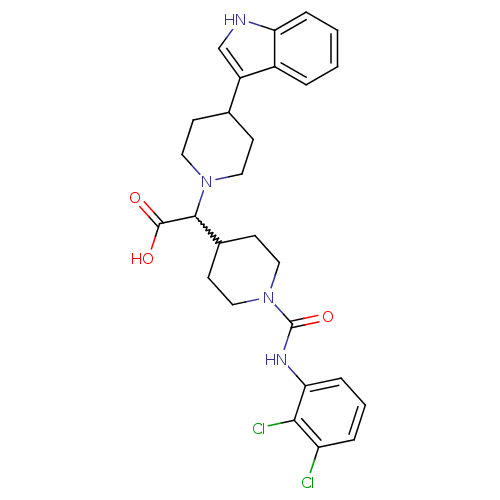
(2-(1-((2,3-dichlorophenyl)carbamoyl)piperidin-4-yl...)Show SMILES OC(=O)C(C1CCN(CC1)C(=O)Nc1cccc(Cl)c1Cl)N1CCC(CC1)c1c[nH]c2ccccc12 |w:3.3| Show InChI InChI=1S/C27H30Cl2N4O3/c28-21-5-3-7-23(24(21)29)31-27(36)33-14-10-18(11-15-33)25(26(34)35)32-12-8-17(9-13-32)20-16-30-22-6-2-1-4-19(20)22/h1-7,16-18,25,30H,8-15H2,(H,31,36)(H,34,35) | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human CCR2 |
J Med Chem 50: 5561-3 (2007)
Article DOI: 10.1021/jm070902b
BindingDB Entry DOI: 10.7270/Q24M948Q |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase EZH2
(Homo sapiens (Human)) | BDBM50400783
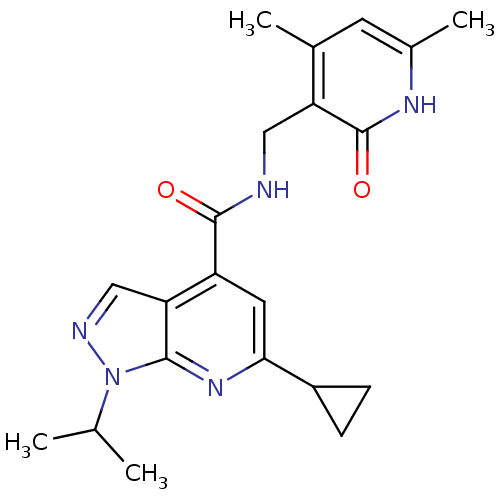
(CHEMBL1608462)Show SMILES CC(C)n1ncc2c(cc(nc12)C1CC1)C(=O)NCc1c(C)cc(C)[nH]c1=O Show InChI InChI=1S/C21H25N5O2/c1-11(2)26-19-17(10-23-26)15(8-18(25-19)14-5-6-14)20(27)22-9-16-12(3)7-13(4)24-21(16)28/h7-8,10-11,14H,5-6,9H2,1-4H3,(H,22,27)(H,24,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of EZH2-mediated proliferation of human LNCaP cells after 6 days by chemiluminescence analysis |
ACS Med Chem Lett 3: 1091-1096 (2012)
Article DOI: 10.1021/ml3003346
BindingDB Entry DOI: 10.7270/Q2NK3G60 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase SETD7
(Homo sapiens (Human)) | BDBM50400781

(CHEMBL2204997)Show SMILES CCCc1cc(C)[nH]c(=O)c1CNC(=O)c1cc(cc2n(ncc12)C(C)C)-c1ccc(nc1)N1CCN(C)CC1 Show InChI InChI=1S/C31H39N7O2/c1-6-7-22-14-21(4)35-31(40)26(22)18-33-30(39)25-15-24(16-28-27(25)19-34-38(28)20(2)3)23-8-9-29(32-17-23)37-12-10-36(5)11-13-37/h8-9,14-17,19-20H,6-7,10-13,18H2,1-5H3,(H,33,39)(H,35,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of SET7 using histone H3 as substrate preincubated for 10 mins followed by addition of [3H]SAM and incubated for 60 mins |
ACS Med Chem Lett 3: 1091-1096 (2012)
Article DOI: 10.1021/ml3003346
BindingDB Entry DOI: 10.7270/Q2NK3G60 |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 3 [N508S]
(Homo sapiens (Human)) | BDBM50400781

(CHEMBL2204997)Show SMILES CCCc1cc(C)[nH]c(=O)c1CNC(=O)c1cc(cc2n(ncc12)C(C)C)-c1ccc(nc1)N1CCN(C)CC1 Show InChI InChI=1S/C31H39N7O2/c1-6-7-22-14-21(4)35-31(40)26(22)18-33-30(39)25-15-24(16-28-27(25)19-34-38(28)20(2)3)23-8-9-29(32-17-23)37-12-10-36(5)11-13-37/h8-9,14-17,19-20H,6-7,10-13,18H2,1-5H3,(H,33,39)(H,35,40) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PRMT3 using histone H3 as substrate preincubated for 10 mins followed by addition of [3H]SAM and incubated for 60 mins |
ACS Med Chem Lett 3: 1091-1096 (2012)
Article DOI: 10.1021/ml3003346
BindingDB Entry DOI: 10.7270/Q2NK3G60 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase SETD7
(Homo sapiens (Human)) | BDBM50400778

(CHEMBL2204995)Show SMILES CCCc1cc(C)[nH]c(=O)c1CNC(=O)c1cc(cc2n(ncc12)C(C)C)-c1ccnc(c1)N1CCN(C)CC1 Show InChI InChI=1S/C31H39N7O2/c1-6-7-23-14-21(4)35-31(40)26(23)18-33-30(39)25-15-24(16-28-27(25)19-34-38(28)20(2)3)22-8-9-32-29(17-22)37-12-10-36(5)11-13-37/h8-9,14-17,19-20H,6-7,10-13,18H2,1-5H3,(H,33,39)(H,35,40) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of SET7 using histone H3 as substrate preincubated for 10 mins followed by addition of [3H]SAM and incubated for 60 mins |
ACS Med Chem Lett 3: 1091-1096 (2012)
Article DOI: 10.1021/ml3003346
BindingDB Entry DOI: 10.7270/Q2NK3G60 |
More data for this
Ligand-Target Pair | |
Protein arginine N-methyltransferase 3 [N508S]
(Homo sapiens (Human)) | BDBM50400778

(CHEMBL2204995)Show SMILES CCCc1cc(C)[nH]c(=O)c1CNC(=O)c1cc(cc2n(ncc12)C(C)C)-c1ccnc(c1)N1CCN(C)CC1 Show InChI InChI=1S/C31H39N7O2/c1-6-7-23-14-21(4)35-31(40)26(23)18-33-30(39)25-15-24(16-28-27(25)19-34-38(28)20(2)3)22-8-9-32-29(17-22)37-12-10-36(5)11-13-37/h8-9,14-17,19-20H,6-7,10-13,18H2,1-5H3,(H,33,39)(H,35,40) | PDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PRMT3 using histone H3 as substrate preincubated for 10 mins followed by addition of [3H]SAM and incubated for 60 mins |
ACS Med Chem Lett 3: 1091-1096 (2012)
Article DOI: 10.1021/ml3003346
BindingDB Entry DOI: 10.7270/Q2NK3G60 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase SUV39H1
(Homo sapiens (Human)) | BDBM50400781

(CHEMBL2204997)Show SMILES CCCc1cc(C)[nH]c(=O)c1CNC(=O)c1cc(cc2n(ncc12)C(C)C)-c1ccc(nc1)N1CCN(C)CC1 Show InChI InChI=1S/C31H39N7O2/c1-6-7-22-14-21(4)35-31(40)26(22)18-33-30(39)25-15-24(16-28-27(25)19-34-38(28)20(2)3)23-8-9-29(32-17-23)37-12-10-36(5)11-13-37/h8-9,14-17,19-20H,6-7,10-13,18H2,1-5H3,(H,33,39)(H,35,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of SUV39H1 using histone H3 as substrate preincubated for 10 mins followed by addition of [3H]SAM and incubated for 60 mins |
ACS Med Chem Lett 3: 1091-1096 (2012)
Article DOI: 10.1021/ml3003346
BindingDB Entry DOI: 10.7270/Q2NK3G60 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase SUV39H1
(Homo sapiens (Human)) | BDBM50400779

(CHEMBL2204998)Show SMILES CC(C)n1ncc2c(cc(cc12)-c1ccc(nc1)N1CCN(C)CC1)C(=O)NCc1c(C)cc(C)[nH]c1=O Show InChI InChI=1S/C29H35N7O2/c1-18(2)36-26-14-22(21-6-7-27(30-15-21)35-10-8-34(5)9-11-35)13-23(25(26)17-32-36)28(37)31-16-24-19(3)12-20(4)33-29(24)38/h6-7,12-15,17-18H,8-11,16H2,1-5H3,(H,31,37)(H,33,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of SUV39H1 using histone H3 as substrate preincubated for 10 mins followed by addition of [3H]SAM and incubated for 60 mins |
ACS Med Chem Lett 3: 1091-1096 (2012)
Article DOI: 10.1021/ml3003346
BindingDB Entry DOI: 10.7270/Q2NK3G60 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase SETMAR
(Homo sapiens (Human)) | BDBM50400778

(CHEMBL2204995)Show SMILES CCCc1cc(C)[nH]c(=O)c1CNC(=O)c1cc(cc2n(ncc12)C(C)C)-c1ccnc(c1)N1CCN(C)CC1 Show InChI InChI=1S/C31H39N7O2/c1-6-7-23-14-21(4)35-31(40)26(23)18-33-30(39)25-15-24(16-28-27(25)19-34-38(28)20(2)3)22-8-9-32-29(17-22)37-12-10-36(5)11-13-37/h8-9,14-17,19-20H,6-7,10-13,18H2,1-5H3,(H,33,39)(H,35,40) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of SETMAR using histone H3 as substrate preincubated for 10 mins followed by addition of [3H]SAM and incubated for 60 mins |
ACS Med Chem Lett 3: 1091-1096 (2012)
Article DOI: 10.1021/ml3003346
BindingDB Entry DOI: 10.7270/Q2NK3G60 |
More data for this
Ligand-Target Pair | |
Histone-lysine N-methyltransferase SETMAR
(Homo sapiens (Human)) | BDBM50400781

(CHEMBL2204997)Show SMILES CCCc1cc(C)[nH]c(=O)c1CNC(=O)c1cc(cc2n(ncc12)C(C)C)-c1ccc(nc1)N1CCN(C)CC1 Show InChI InChI=1S/C31H39N7O2/c1-6-7-22-14-21(4)35-31(40)26(22)18-33-30(39)25-15-24(16-28-27(25)19-34-38(28)20(2)3)23-8-9-29(32-17-23)37-12-10-36(5)11-13-37/h8-9,14-17,19-20H,6-7,10-13,18H2,1-5H3,(H,33,39)(H,35,40) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of SETMAR using histone H3 as substrate preincubated for 10 mins followed by addition of [3H]SAM and incubated for 60 mins |
ACS Med Chem Lett 3: 1091-1096 (2012)
Article DOI: 10.1021/ml3003346
BindingDB Entry DOI: 10.7270/Q2NK3G60 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data