 Found 77 hits with Last Name = 'bradley' and Initial = 'dm'
Found 77 hits with Last Name = 'bradley' and Initial = 'dm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50412939
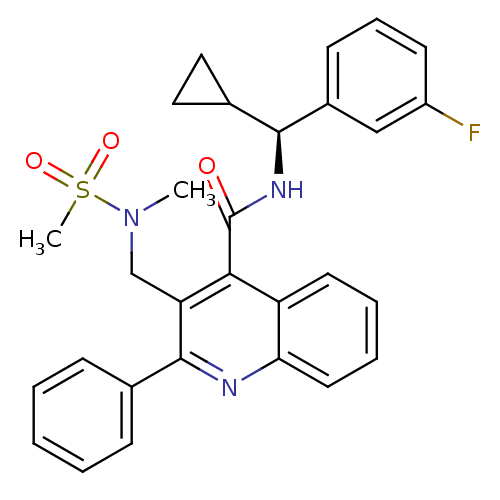
(CHEMBL479463 | GSK-256471)Show SMILES CN(Cc1c(nc2ccccc2c1C(=O)N[C@@H](C1CC1)c1cccc(F)c1)-c1ccccc1)S(C)(=O)=O |r| Show InChI InChI=1S/C29H28FN3O3S/c1-33(37(2,35)36)18-24-26(29(34)32-27(20-15-16-20)21-11-8-12-22(30)17-21)23-13-6-7-14-25(23)31-28(24)19-9-4-3-5-10-19/h3-14,17,20,27H,15-16,18H2,1-2H3,(H,32,34)/t27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscience CEDD GlaxoSmithKline Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to NK3 receptor |
Bioorg Med Chem Lett 19: 837-40 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.005
BindingDB Entry DOI: 10.7270/Q20K29S8 |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50412941
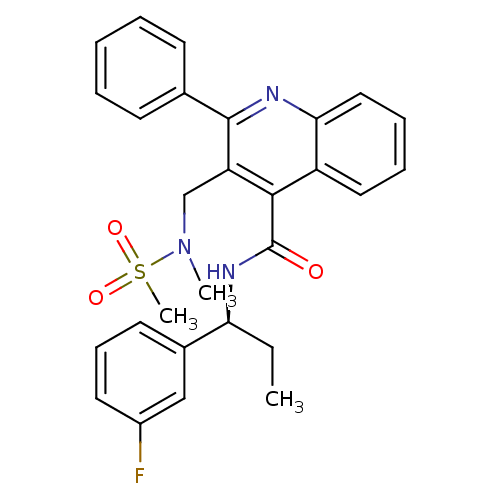
(CHEMBL521416)Show SMILES CC[C@H](NC(=O)c1c(CN(C)S(C)(=O)=O)c(nc2ccccc12)-c1ccccc1)c1cccc(F)c1 |r| Show InChI InChI=1S/C28H28FN3O3S/c1-4-24(20-13-10-14-21(29)17-20)31-28(33)26-22-15-8-9-16-25(22)30-27(19-11-6-5-7-12-19)23(26)18-32(2)36(3,34)35/h5-17,24H,4,18H2,1-3H3,(H,31,33)/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscience CEDD GlaxoSmithKline Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to NK3 receptor |
Bioorg Med Chem Lett 19: 837-40 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.005
BindingDB Entry DOI: 10.7270/Q20K29S8 |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50412940
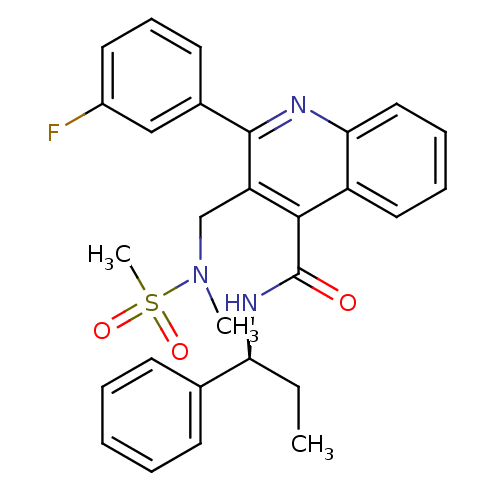
(CHEMBL519770)Show SMILES CC[C@H](NC(=O)c1c(CN(C)S(C)(=O)=O)c(nc2ccccc12)-c1cccc(F)c1)c1ccccc1 |r| Show InChI InChI=1S/C28H28FN3O3S/c1-4-24(19-11-6-5-7-12-19)31-28(33)26-22-15-8-9-16-25(22)30-27(20-13-10-14-21(29)17-20)23(26)18-32(2)36(3,34)35/h5-17,24H,4,18H2,1-3H3,(H,31,33)/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscience CEDD GlaxoSmithKline Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to NK3 receptor |
Bioorg Med Chem Lett 19: 837-40 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.005
BindingDB Entry DOI: 10.7270/Q20K29S8 |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50412942

(CHEMBL480249)Show SMILES CN(Cc1c(nc2ccccc2c1C(=O)N[C@@H](C1CC1)c1ccccc1)-c1ccccc1)S(C)(=O)=O |r| Show InChI InChI=1S/C29H29N3O3S/c1-32(36(2,34)35)19-24-26(29(33)31-27(22-17-18-22)20-11-5-3-6-12-20)23-15-9-10-16-25(23)30-28(24)21-13-7-4-8-14-21/h3-16,22,27H,17-19H2,1-2H3,(H,31,33)/t27-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscience CEDD GlaxoSmithKline Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to NK3 receptor |
Bioorg Med Chem Lett 19: 837-40 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.005
BindingDB Entry DOI: 10.7270/Q20K29S8 |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50412943

(CHEMBL480818)Show SMILES CC[C@H](NC(=O)c1c(N)c(nc2ccccc12)-c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C25H23N3O/c1-2-20(17-11-5-3-6-12-17)28-25(29)22-19-15-9-10-16-21(19)27-24(23(22)26)18-13-7-4-8-14-18/h3-16,20H,2,26H2,1H3,(H,28,29)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscience CEDD GlaxoSmithKline Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to NK3 receptor |
Bioorg Med Chem Lett 19: 837-40 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.005
BindingDB Entry DOI: 10.7270/Q20K29S8 |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50412944
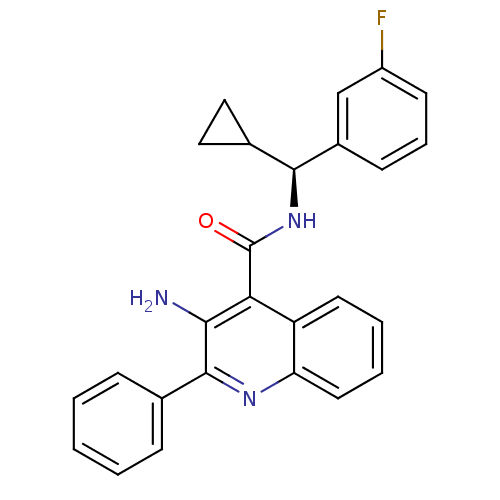
(CHEMBL479652)Show SMILES Nc1c(nc2ccccc2c1C(=O)N[C@@H](C1CC1)c1cccc(F)c1)-c1ccccc1 |r| Show InChI InChI=1S/C26H22FN3O/c27-19-10-6-9-18(15-19)24(17-13-14-17)30-26(31)22-20-11-4-5-12-21(20)29-25(23(22)28)16-7-2-1-3-8-16/h1-12,15,17,24H,13-14,28H2,(H,30,31)/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscience CEDD GlaxoSmithKline Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to NK3 receptor |
Bioorg Med Chem Lett 19: 837-40 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.005
BindingDB Entry DOI: 10.7270/Q20K29S8 |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50412945
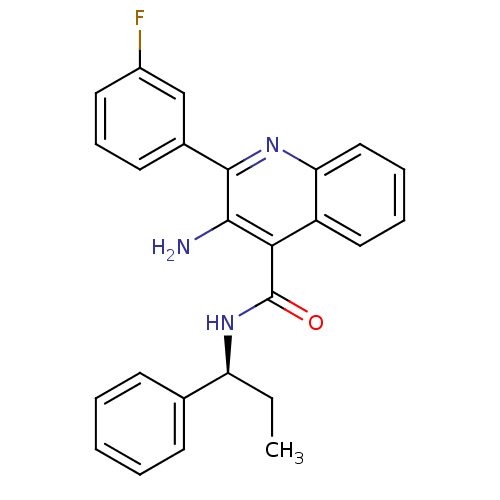
(CHEMBL516441)Show SMILES CC[C@H](NC(=O)c1c(N)c(nc2ccccc12)-c1cccc(F)c1)c1ccccc1 |r| Show InChI InChI=1S/C25H22FN3O/c1-2-20(16-9-4-3-5-10-16)29-25(30)22-19-13-6-7-14-21(19)28-24(23(22)27)17-11-8-12-18(26)15-17/h3-15,20H,2,27H2,1H3,(H,29,30)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscience CEDD GlaxoSmithKline Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to NK3 receptor |
Bioorg Med Chem Lett 19: 837-40 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.005
BindingDB Entry DOI: 10.7270/Q20K29S8 |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50412947
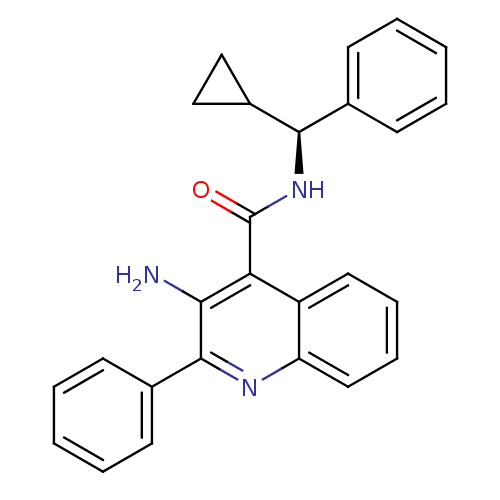
(5-(3-METHYL-1-TRIAZENO)IMIDAZOLE-4-CARBOXAMIDE)Show SMILES Nc1c(nc2ccccc2c1C(=O)N[C@@H](C1CC1)c1ccccc1)-c1ccccc1 |r| Show InChI InChI=1S/C26H23N3O/c27-23-22(26(30)29-24(19-15-16-19)17-9-3-1-4-10-17)20-13-7-8-14-21(20)28-25(23)18-11-5-2-6-12-18/h1-14,19,24H,15-16,27H2,(H,29,30)/t24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscience CEDD GlaxoSmithKline Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to NK3 receptor |
Bioorg Med Chem Lett 19: 837-40 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.005
BindingDB Entry DOI: 10.7270/Q20K29S8 |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50412946

(CHEMBL517691)Show SMILES Nc1c(nc2ccccc2c1C(=O)N[C@@H](C1CC1)c1ccccc1F)-c1ccccc1 |r| Show InChI InChI=1S/C26H22FN3O/c27-20-12-6-4-10-18(20)24(17-14-15-17)30-26(31)22-19-11-5-7-13-21(19)29-25(23(22)28)16-8-2-1-3-9-16/h1-13,17,24H,14-15,28H2,(H,30,31)/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.01 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscience CEDD GlaxoSmithKline Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to NK3 receptor |
Bioorg Med Chem Lett 19: 837-40 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.005
BindingDB Entry DOI: 10.7270/Q20K29S8 |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50412949
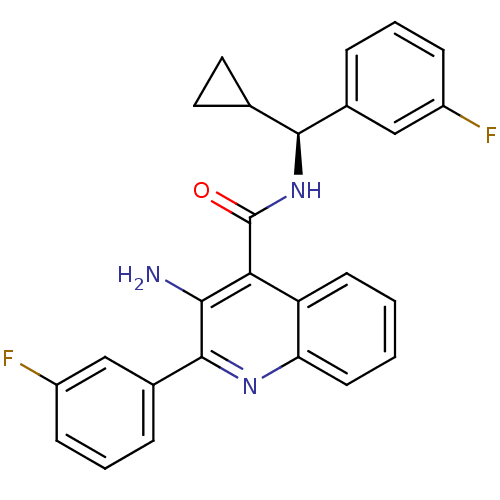
(CHEMBL519790)Show SMILES Nc1c(nc2ccccc2c1C(=O)N[C@@H](C1CC1)c1cccc(F)c1)-c1cccc(F)c1 |r| Show InChI InChI=1S/C26H21F2N3O/c27-18-7-3-5-16(13-18)24(15-11-12-15)31-26(32)22-20-9-1-2-10-21(20)30-25(23(22)29)17-6-4-8-19(28)14-17/h1-10,13-15,24H,11-12,29H2,(H,31,32)/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscience CEDD GlaxoSmithKline Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to NK3 receptor |
Bioorg Med Chem Lett 19: 837-40 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.005
BindingDB Entry DOI: 10.7270/Q20K29S8 |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50412948

(CHEMBL480248)Show SMILES CN(Cc1c(nc2ccccc2c1C(=O)N[C@@H](C1CC1)c1cccc(F)c1)-c1cccc(F)c1)S(C)(=O)=O |r| Show InChI InChI=1S/C29H27F2N3O3S/c1-34(38(2,36)37)17-24-26(29(35)33-27(18-13-14-18)19-7-5-9-21(30)15-19)23-11-3-4-12-25(23)32-28(24)20-8-6-10-22(31)16-20/h3-12,15-16,18,27H,13-14,17H2,1-2H3,(H,33,35)/t27-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscience CEDD GlaxoSmithKline Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to NK3 receptor |
Bioorg Med Chem Lett 19: 837-40 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.005
BindingDB Entry DOI: 10.7270/Q20K29S8 |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50412950

(CHEMBL479450)Show SMILES CC[C@H](NC(=O)c1c(N)c(nc2ccccc12)-c1ccc(F)cc1)c1ccccc1 |r| Show InChI InChI=1S/C25H22FN3O/c1-2-20(16-8-4-3-5-9-16)29-25(30)22-19-10-6-7-11-21(19)28-24(23(22)27)17-12-14-18(26)15-13-17/h3-15,20H,2,27H2,1H3,(H,29,30)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscience CEDD GlaxoSmithKline Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to NK3 receptor |
Bioorg Med Chem Lett 19: 837-40 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.005
BindingDB Entry DOI: 10.7270/Q20K29S8 |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50051295
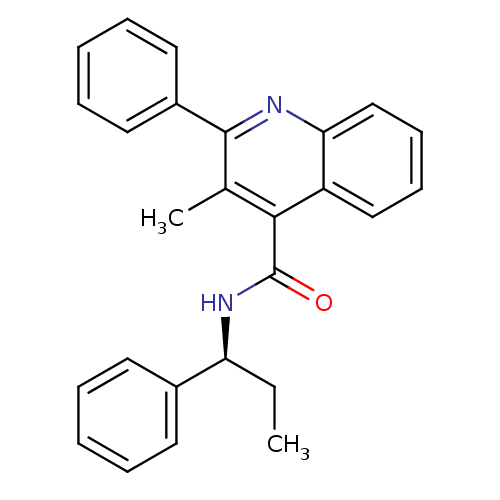
((S)-3-methyl-2-phenyl-N-(1-phenylpropyl)quinoline-...)Show SMILES CC[C@H](NC(=O)c1c(C)c(nc2ccccc12)-c1ccccc1)c1ccccc1 |r| Show InChI InChI=1S/C26H24N2O/c1-3-22(19-12-6-4-7-13-19)28-26(29)24-18(2)25(20-14-8-5-9-15-20)27-23-17-11-10-16-21(23)24/h4-17,22H,3H2,1-2H3,(H,28,29)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscience CEDD GlaxoSmithKline Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to NK3 receptor |
Bioorg Med Chem Lett 19: 837-40 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.005
BindingDB Entry DOI: 10.7270/Q20K29S8 |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50412952

(CHEMBL480628 | GSK-172981)Show SMILES Nc1c(nc2ccccc2c1C(=O)N[C@@H](C1CC1)c1ccccc1)-c1cccc(F)c1 |r| Show InChI InChI=1S/C26H22FN3O/c27-19-10-6-9-18(15-19)25-23(28)22(20-11-4-5-12-21(20)29-25)26(31)30-24(17-13-14-17)16-7-2-1-3-8-16/h1-12,15,17,24H,13-14,28H2,(H,30,31)/t24-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscience CEDD GlaxoSmithKline Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to NK3 receptor |
Bioorg Med Chem Lett 19: 837-40 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.005
BindingDB Entry DOI: 10.7270/Q20K29S8 |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50412951
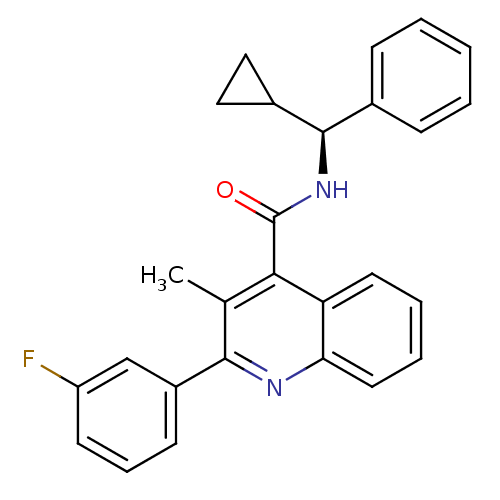
(CHEMBL482006)Show SMILES Cc1c(nc2ccccc2c1C(=O)N[C@@H](C1CC1)c1ccccc1)-c1cccc(F)c1 |r| Show InChI InChI=1S/C27H23FN2O/c1-17-24(27(31)30-26(19-14-15-19)18-8-3-2-4-9-18)22-12-5-6-13-23(22)29-25(17)20-10-7-11-21(28)16-20/h2-13,16,19,26H,14-15H2,1H3,(H,30,31)/t26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscience CEDD GlaxoSmithKline Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to NK3 receptor |
Bioorg Med Chem Lett 19: 837-40 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.005
BindingDB Entry DOI: 10.7270/Q20K29S8 |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50412953
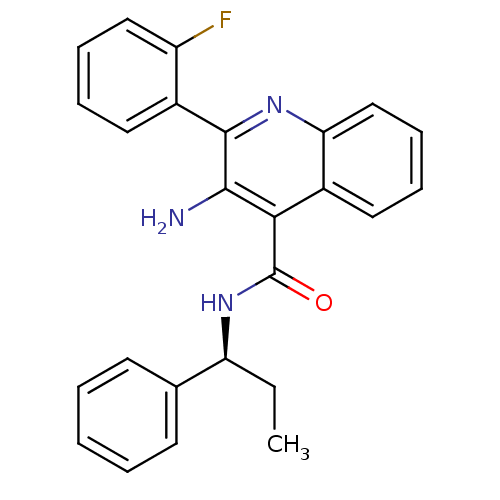
(CHEMBL479449)Show SMILES CC[C@H](NC(=O)c1c(N)c(nc2ccccc12)-c1ccccc1F)c1ccccc1 |r| Show InChI InChI=1S/C25H22FN3O/c1-2-20(16-10-4-3-5-11-16)29-25(30)22-18-13-7-9-15-21(18)28-24(23(22)27)17-12-6-8-14-19(17)26/h3-15,20H,2,27H2,1H3,(H,29,30)/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscience CEDD GlaxoSmithKline Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to NK3 receptor |
Bioorg Med Chem Lett 19: 837-40 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.005
BindingDB Entry DOI: 10.7270/Q20K29S8 |
More data for this
Ligand-Target Pair | |
Neuromedin-K receptor
(Homo sapiens (Human)) | BDBM50412938

(CHEMBL520086)Show SMILES Nc1c(nc2ccccc2c1C(=O)N[C@H](C1CC1)c1ccccc1)-c1cccc(F)c1 |r| Show InChI InChI=1S/C26H22FN3O/c27-19-10-6-9-18(15-19)25-23(28)22(20-11-4-5-12-21(20)29-25)26(31)30-24(17-13-14-17)16-7-2-1-3-8-16/h1-12,15,17,24H,13-14,28H2,(H,30,31)/t24-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Neuroscience CEDD GlaxoSmithKline Research& Development
Curated by ChEMBL
| Assay Description
Binding affinity to NK3 receptor |
Bioorg Med Chem Lett 19: 837-40 (2009)
Article DOI: 10.1016/j.bmcl.2008.12.005
BindingDB Entry DOI: 10.7270/Q20K29S8 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50334942

(1-{4-[3-(trifluoromethyl)-4,5,6,7-tetrahydro-1H-in...)Show InChI InChI=1S/C16H15F3N2O/c1-10(22)11-6-8-12(9-7-11)21-14-5-3-2-4-13(14)15(20-21)16(17,18)19/h6-9H,2-5H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50334936
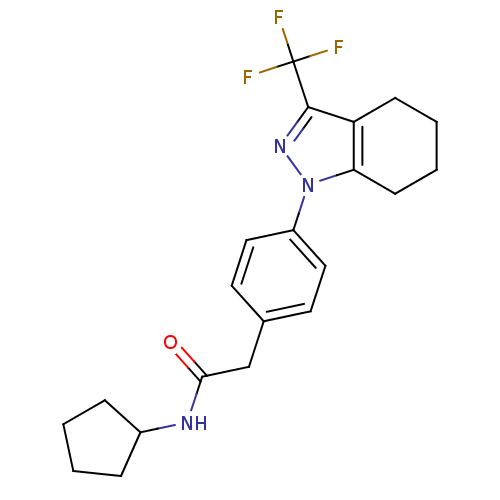
(CHEMBL1649665 | N-cyclopentyl-2-{4-[3-(trifluorome...)Show SMILES FC(F)(F)c1nn(c2CCCCc12)-c1ccc(CC(=O)NC2CCCC2)cc1 Show InChI InChI=1S/C21H24F3N3O/c22-21(23,24)20-17-7-3-4-8-18(17)27(26-20)16-11-9-14(10-12-16)13-19(28)25-15-5-1-2-6-15/h9-12,15H,1-8,13H2,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50334935
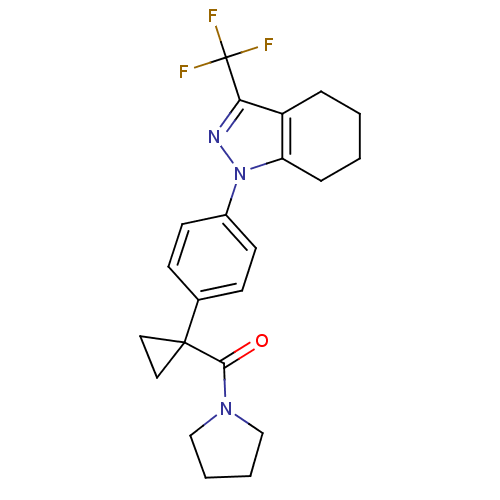
(1-{4-[1-(1-pyrrolidinylcarbonyl)cyclopropyl]phenyl...)Show SMILES FC(F)(F)c1nn(c2CCCCc12)-c1ccc(cc1)C1(CC1)C(=O)N1CCCC1 Show InChI InChI=1S/C22H24F3N3O/c23-22(24,25)19-17-5-1-2-6-18(17)28(26-19)16-9-7-15(8-10-16)21(11-12-21)20(29)27-13-3-4-14-27/h7-10H,1-6,11-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50334936

(CHEMBL1649665 | N-cyclopentyl-2-{4-[3-(trifluorome...)Show SMILES FC(F)(F)c1nn(c2CCCCc12)-c1ccc(CC(=O)NC2CCCC2)cc1 Show InChI InChI=1S/C21H24F3N3O/c22-21(23,24)20-17-7-3-4-8-18(17)27(26-20)16-11-9-14(10-12-16)13-19(28)25-15-5-1-2-6-15/h9-12,15H,1-8,13H2,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50334939

(1-({4-[3-(Trifluoromethyl)-4,5,6,7-tetrahydro-1H-i...)Show SMILES FC(F)(F)c1nn(c2CCCCc12)-c1ccc(CN2CCCC2=O)cc1 Show InChI InChI=1S/C19H20F3N3O/c20-19(21,22)18-15-4-1-2-5-16(15)25(23-18)14-9-7-13(8-10-14)12-24-11-3-6-17(24)26/h7-10H,1-6,11-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50334940
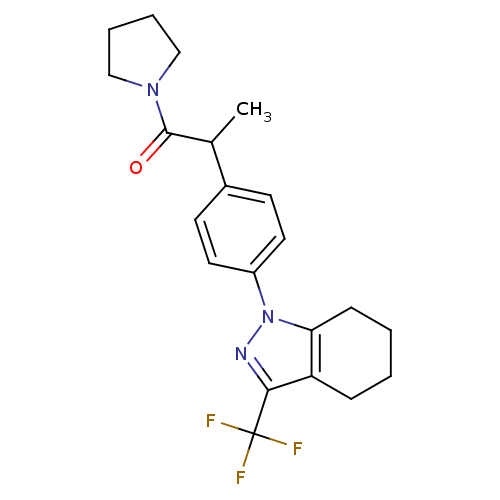
(1-{4-[1-methyl-2-oxo-2-(1-pyrrolidinyl)ethyl]pheny...)Show SMILES CC(C(=O)N1CCCC1)c1ccc(cc1)-n1nc(c2CCCCc12)C(F)(F)F Show InChI InChI=1S/C21H24F3N3O/c1-14(20(28)26-12-4-5-13-26)15-8-10-16(11-9-15)27-18-7-3-2-6-17(18)19(25-27)21(22,23)24/h8-11,14H,2-7,12-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50334948

(CHEMBL1649654 | N-methyl-N-(2-phenylethyl)-4-[3-(t...)Show SMILES CN(CCc1ccccc1)C(=O)c1ccc(cc1)-n1nc(c2CCCCc12)C(F)(F)F Show InChI InChI=1S/C24H24F3N3O/c1-29(16-15-17-7-3-2-4-8-17)23(31)18-11-13-19(14-12-18)30-21-10-6-5-9-20(21)22(28-30)24(25,26)27/h2-4,7-8,11-14H,5-6,9-10,15-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50334937
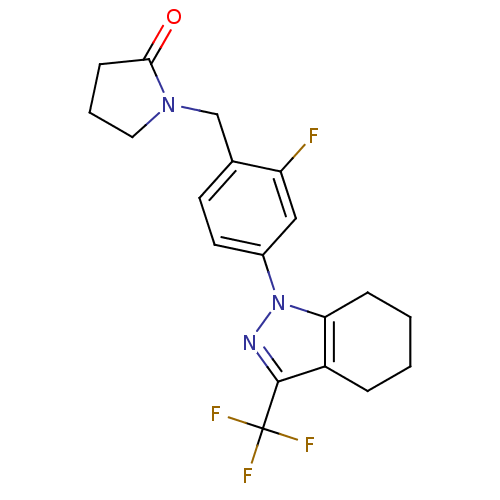
(1-({2-Fluoro-4-[3-(trifluoromethyl)-4,5,6,7-tetrah...)Show SMILES Fc1cc(ccc1CN1CCCC1=O)-n1nc(c2CCCCc12)C(F)(F)F Show InChI InChI=1S/C19H19F4N3O/c20-15-10-13(8-7-12(15)11-25-9-3-6-17(25)27)26-16-5-2-1-4-14(16)18(24-26)19(21,22)23/h7-8,10H,1-6,9,11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50334947
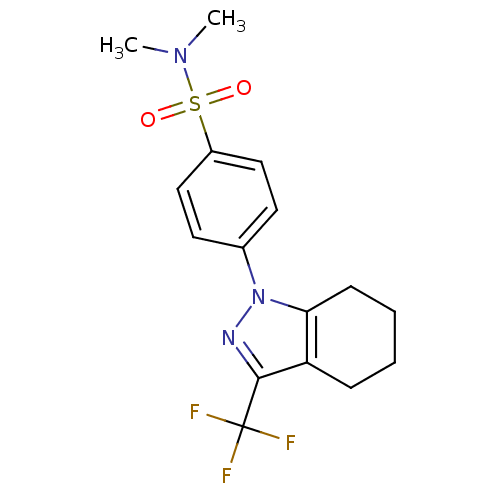
(CHEMBL1649655 | N,N-dimethyl-4-[3-(trifluoromethyl...)Show SMILES CN(C)S(=O)(=O)c1ccc(cc1)-n1nc(c2CCCCc12)C(F)(F)F Show InChI InChI=1S/C16H18F3N3O2S/c1-21(2)25(23,24)12-9-7-11(8-10-12)22-14-6-4-3-5-13(14)15(20-22)16(17,18)19/h7-10H,3-6H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50334949
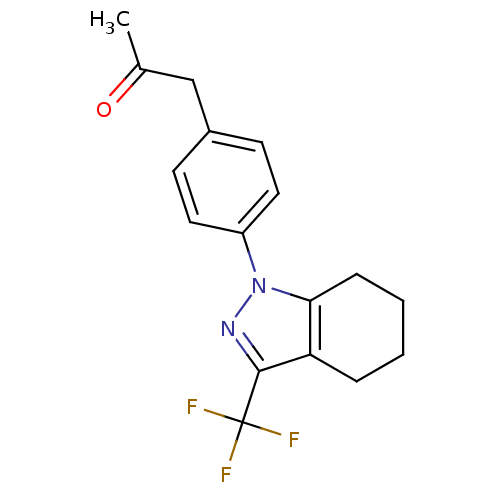
(1-{4-[3-(trifluoromethyl)-4,5,6,7-tetrahydro-1H-in...)Show InChI InChI=1S/C17H17F3N2O/c1-11(23)10-12-6-8-13(9-7-12)22-15-5-3-2-4-14(15)16(21-22)17(18,19)20/h6-9H,2-5,10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50334948

(CHEMBL1649654 | N-methyl-N-(2-phenylethyl)-4-[3-(t...)Show SMILES CN(CCc1ccccc1)C(=O)c1ccc(cc1)-n1nc(c2CCCCc12)C(F)(F)F Show InChI InChI=1S/C24H24F3N3O/c1-29(16-15-17-7-3-2-4-8-17)23(31)18-11-13-19(14-12-18)30-21-10-6-5-9-20(21)22(28-30)24(25,26)27/h2-4,7-8,11-14H,5-6,9-10,15-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50334946

(1-{4-[2-OXO-2-(1-PYRROLIDINYL)ETHYL]PHENYL}-3-( TR...)Show SMILES FC(F)(F)c1nn(c2CCCCc12)-c1ccc(CC(=O)N2CCCC2)cc1 Show InChI InChI=1S/C20H22F3N3O/c21-20(22,23)19-16-5-1-2-6-17(16)26(24-19)15-9-7-14(8-10-15)13-18(27)25-11-3-4-12-25/h7-10H,1-6,11-13H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50334938

(CHEMBL1649672 | N-({4-[3-(trifluoromethyl)-4,5,6,7...)Show SMILES CCC(=O)NCc1ccc(cc1)-n1nc(c2CCCCc12)C(F)(F)F Show InChI InChI=1S/C18H20F3N3O/c1-2-16(25)22-11-12-7-9-13(10-8-12)24-15-6-4-3-5-14(15)17(23-24)18(19,20)21/h7-10H,2-6,11H2,1H3,(H,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50334942

(1-{4-[3-(trifluoromethyl)-4,5,6,7-tetrahydro-1H-in...)Show InChI InChI=1S/C16H15F3N2O/c1-10(22)11-6-8-12(9-7-11)21-14-5-3-2-4-13(14)15(20-21)16(17,18)19/h6-9H,2-5H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50334945
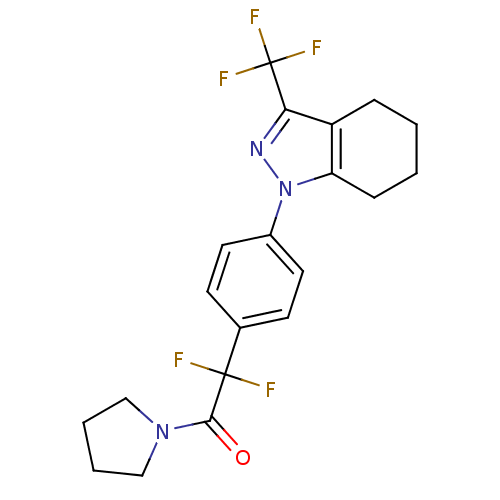
(1-{4-[1,1-difluoro-2-oxo-2-(1-pyrrolidinyl)ethyl]p...)Show SMILES FC(F)(F)c1nn(c2CCCCc12)-c1ccc(cc1)C(F)(F)C(=O)N1CCCC1 Show InChI InChI=1S/C20H20F5N3O/c21-19(22,18(29)27-11-3-4-12-27)13-7-9-14(10-8-13)28-16-6-2-1-5-15(16)17(26-28)20(23,24)25/h7-10H,1-6,11-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50334941
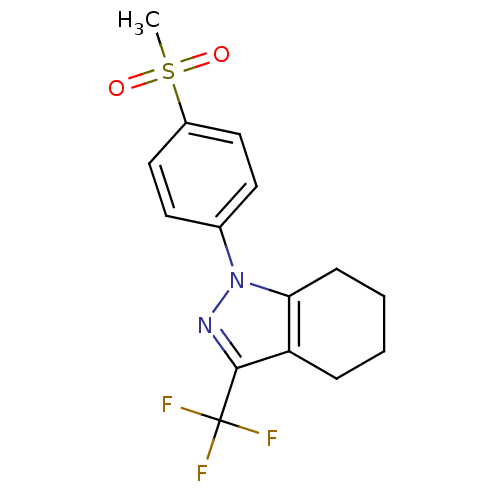
(1-(4-(methylsulfonyl)phenyl)-3-(trifluoromethyl)-4...)Show SMILES CS(=O)(=O)c1ccc(cc1)-n1nc(c2CCCCc12)C(F)(F)F Show InChI InChI=1S/C15H15F3N2O2S/c1-23(21,22)11-8-6-10(7-9-11)20-13-5-3-2-4-12(13)14(19-20)15(16,17)18/h6-9H,2-5H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50334939

(1-({4-[3-(Trifluoromethyl)-4,5,6,7-tetrahydro-1H-i...)Show SMILES FC(F)(F)c1nn(c2CCCCc12)-c1ccc(CN2CCCC2=O)cc1 Show InChI InChI=1S/C19H20F3N3O/c20-19(21,22)18-15-4-1-2-5-16(15)25(23-18)14-9-7-13(8-10-14)12-24-11-3-6-17(24)26/h7-10H,1-6,11-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50334932

(CHEMBL1649660 | N-butyl-N-methyl-4-[3-(trifluorome...)Show SMILES CCCCN(C)C(=O)c1ccc(cc1)-n1nc(c2CCCCc12)C(F)(F)F Show InChI InChI=1S/C20H24F3N3O/c1-3-4-13-25(2)19(27)14-9-11-15(12-10-14)26-17-8-6-5-7-16(17)18(24-26)20(21,22)23/h9-12H,3-8,13H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50334943
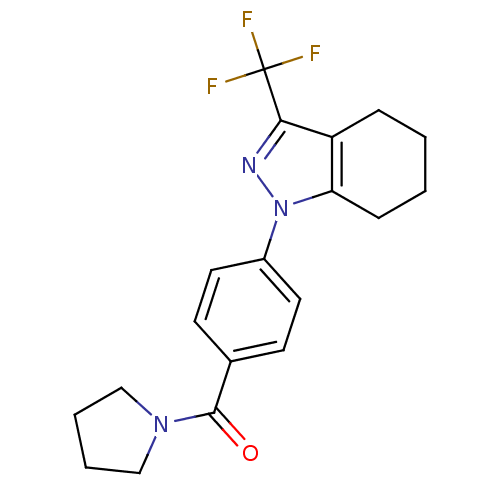
(1-[4-(1-Pyrrolidinylcarbonyl)phenyl]-3-(trifluorom...)Show SMILES FC(F)(F)c1nn(c2CCCCc12)-c1ccc(cc1)C(=O)N1CCCC1 Show InChI InChI=1S/C19H20F3N3O/c20-19(21,22)17-15-5-1-2-6-16(15)25(23-17)14-9-7-13(8-10-14)18(26)24-11-3-4-12-24/h7-10H,1-6,11-12H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50334941

(1-(4-(methylsulfonyl)phenyl)-3-(trifluoromethyl)-4...)Show SMILES CS(=O)(=O)c1ccc(cc1)-n1nc(c2CCCCc12)C(F)(F)F Show InChI InChI=1S/C15H15F3N2O2S/c1-23(21,22)11-8-6-10(7-9-11)20-13-5-3-2-4-12(13)14(19-20)15(16,17)18/h6-9H,2-5H2,1H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50334950
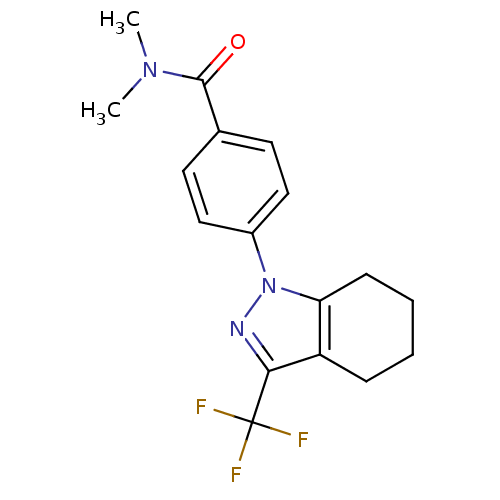
(CHEMBL1594422 | N,N-Dimethyl-4-[3-(trifluoromethyl...)Show SMILES CN(C)C(=O)c1ccc(cc1)-n1nc(c2CCCCc12)C(F)(F)F Show InChI InChI=1S/C17H18F3N3O/c1-22(2)16(24)11-7-9-12(10-8-11)23-14-6-4-3-5-13(14)15(21-23)17(18,19)20/h7-10H,3-6H2,1-2H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50334935

(1-{4-[1-(1-pyrrolidinylcarbonyl)cyclopropyl]phenyl...)Show SMILES FC(F)(F)c1nn(c2CCCCc12)-c1ccc(cc1)C1(CC1)C(=O)N1CCCC1 Show InChI InChI=1S/C22H24F3N3O/c23-22(24,25)19-17-5-1-2-6-18(17)28(26-19)16-9-7-15(8-10-16)21(11-12-21)20(29)27-13-3-4-14-27/h7-10H,1-6,11-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50334940

(1-{4-[1-methyl-2-oxo-2-(1-pyrrolidinyl)ethyl]pheny...)Show SMILES CC(C(=O)N1CCCC1)c1ccc(cc1)-n1nc(c2CCCCc12)C(F)(F)F Show InChI InChI=1S/C21H24F3N3O/c1-14(20(28)26-12-4-5-13-26)15-8-10-16(11-9-15)27-18-7-3-2-6-17(18)19(25-27)21(22,23)24/h8-11,14H,2-7,12-13H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50334935

(1-{4-[1-(1-pyrrolidinylcarbonyl)cyclopropyl]phenyl...)Show SMILES FC(F)(F)c1nn(c2CCCCc12)-c1ccc(cc1)C1(CC1)C(=O)N1CCCC1 Show InChI InChI=1S/C22H24F3N3O/c23-22(24,25)19-17-5-1-2-6-18(17)28(26-19)16-9-7-15(8-10-16)21(11-12-21)20(29)27-13-3-4-14-27/h7-10H,1-6,11-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50334931
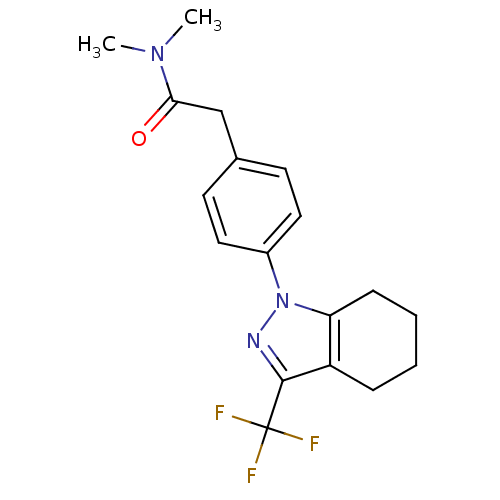
(CHEMBL1649663 | N,N-Dimethyl-2-{4-[3-(trifluoromet...)Show SMILES CN(C)C(=O)Cc1ccc(cc1)-n1nc(c2CCCCc12)C(F)(F)F Show InChI InChI=1S/C18H20F3N3O/c1-23(2)16(25)11-12-7-9-13(10-8-12)24-15-6-4-3-5-14(15)17(22-24)18(19,20)21/h7-10H,3-6,11H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50334944

(1-{3-Fluoro-4-[2-oxo-2-(1-pyrrolidinyl)ethyl]pheny...)Show SMILES Fc1cc(ccc1CC(=O)N1CCCC1)-n1nc(c2CCCCc12)C(F)(F)F Show InChI InChI=1S/C20H21F4N3O/c21-16-12-14(8-7-13(16)11-18(28)26-9-3-4-10-26)27-17-6-2-1-5-15(17)19(25-27)20(22,23)24/h7-8,12H,1-6,9-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50334938

(CHEMBL1649672 | N-({4-[3-(trifluoromethyl)-4,5,6,7...)Show SMILES CCC(=O)NCc1ccc(cc1)-n1nc(c2CCCCc12)C(F)(F)F Show InChI InChI=1S/C18H20F3N3O/c1-2-16(25)22-11-12-7-9-13(10-8-12)24-15-6-4-3-5-14(15)17(23-24)18(19,20)21/h7-10H,2-6,11H2,1H3,(H,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50334929
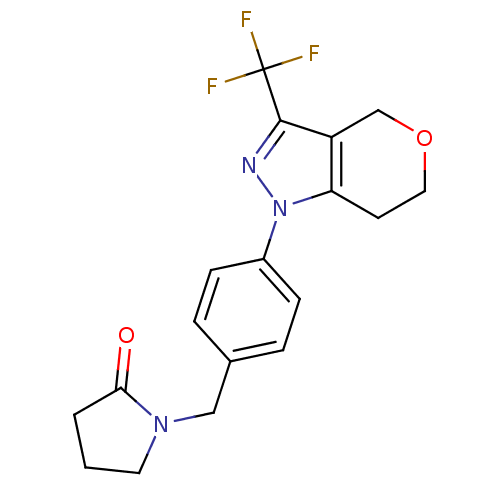
(1-({4-[3-(Trifluoromethyl)-6,7-dihydropyrano[4,3-c...)Show SMILES FC(F)(F)c1nn(c2CCOCc12)-c1ccc(CN2CCCC2=O)cc1 Show InChI InChI=1S/C18H18F3N3O2/c19-18(20,21)17-14-11-26-9-7-15(14)24(22-17)13-5-3-12(4-6-13)10-23-8-1-2-16(23)25/h3-6H,1-2,7-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C19 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50334929

(1-({4-[3-(Trifluoromethyl)-6,7-dihydropyrano[4,3-c...)Show SMILES FC(F)(F)c1nn(c2CCOCc12)-c1ccc(CN2CCCC2=O)cc1 Show InChI InChI=1S/C18H18F3N3O2/c19-18(20,21)17-14-11-26-9-7-15(14)24(22-17)13-5-3-12(4-6-13)10-23-8-1-2-16(23)25/h3-6H,1-2,7-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP3A4 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50334929

(1-({4-[3-(Trifluoromethyl)-6,7-dihydropyrano[4,3-c...)Show SMILES FC(F)(F)c1nn(c2CCOCc12)-c1ccc(CN2CCCC2=O)cc1 Show InChI InChI=1S/C18H18F3N3O2/c19-18(20,21)17-14-11-26-9-7-15(14)24(22-17)13-5-3-12(4-6-13)10-23-8-1-2-16(23)25/h3-6H,1-2,7-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP1A2 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50334929

(1-({4-[3-(Trifluoromethyl)-6,7-dihydropyrano[4,3-c...)Show SMILES FC(F)(F)c1nn(c2CCOCc12)-c1ccc(CN2CCCC2=O)cc1 Show InChI InChI=1S/C18H18F3N3O2/c19-18(20,21)17-14-11-26-9-7-15(14)24(22-17)13-5-3-12(4-6-13)10-23-8-1-2-16(23)25/h3-6H,1-2,7-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2C9 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50334929

(1-({4-[3-(Trifluoromethyl)-6,7-dihydropyrano[4,3-c...)Show SMILES FC(F)(F)c1nn(c2CCOCc12)-c1ccc(CN2CCCC2=O)cc1 Show InChI InChI=1S/C18H18F3N3O2/c19-18(20,21)17-14-11-26-9-7-15(14)24(22-17)13-5-3-12(4-6-13)10-23-8-1-2-16(23)25/h3-6H,1-2,7-11H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50334936

(CHEMBL1649665 | N-cyclopentyl-2-{4-[3-(trifluorome...)Show SMILES FC(F)(F)c1nn(c2CCCCc12)-c1ccc(CC(=O)NC2CCCC2)cc1 Show InChI InChI=1S/C21H24F3N3O/c22-21(23,24)20-17-7-3-4-8-18(17)27(26-20)16-11-9-14(10-12-16)13-19(28)25-15-5-1-2-6-15/h9-12,15H,1-8,13H2,(H,25,28) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Sussex
Curated by ChEMBL
| Assay Description
Inhibition of human CYP2D6 by fluorescence assay |
J Med Chem 54: 78-94 (2011)
Article DOI: 10.1021/jm100679e
BindingDB Entry DOI: 10.7270/Q21C1XVG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data