 Found 2336 hits with Last Name = 'bradley' and Initial = 'e'
Found 2336 hits with Last Name = 'bradley' and Initial = 'e' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434810
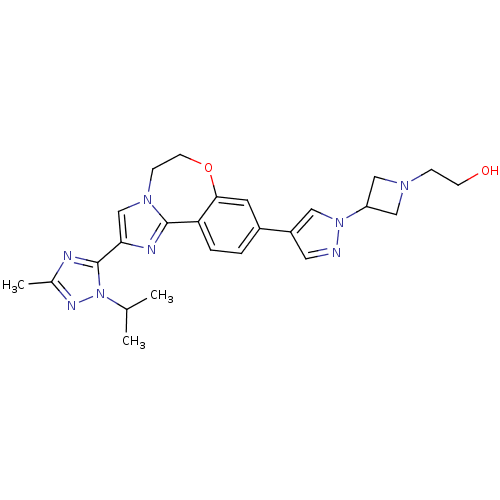
(CHEMBL2386970)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(c1)C1CN(CCO)C1 Show InChI InChI=1S/C25H30N8O2/c1-16(2)33-25(27-17(3)29-33)22-15-31-7-9-35-23-10-18(4-5-21(23)24(31)28-22)19-11-26-32(12-19)20-13-30(14-20)6-8-34/h4-5,10-12,15-16,20,34H,6-9,13-14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assay |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50434806

(2-(4-(2-(1-isopropyl-3-methyl-1H-1,2,4-triazol-5-y...)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(c1)C(C)(C)C(N)=O Show InChI InChI=1S/C24H28N8O2/c1-14(2)32-22(27-15(3)29-32)19-13-30-8-9-34-20-10-16(6-7-18(20)21(30)28-19)17-11-26-31(12-17)24(4,5)23(25)33/h6-7,10-14H,8-9H2,1-5H3,(H2,25,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434807

(CHEMBL2387079)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(CC(C)(C)O)c1 Show InChI InChI=1S/C23H27N7O2/c1-15(2)30-22(24-14-26-30)19-12-28-7-8-32-20-9-16(5-6-18(20)21(28)27-19)17-10-25-29(11-17)13-23(3,4)31/h5-6,9-12,14-15,31H,7-8,13H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assay |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434812

(CHEMBL2387086)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(CCO)c1 Show InChI InChI=1S/C21H23N7O2/c1-14(2)28-21(22-13-24-28)18-12-26-6-8-30-19-9-15(3-4-17(19)20(26)25-18)16-10-23-27(11-16)5-7-29/h3-4,9-14,29H,5-8H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assay |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434814

(CHEMBL2387082)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(C)c1 Show InChI InChI=1S/C21H23N7O/c1-13(2)28-21(23-14(3)25-28)18-12-27-7-8-29-19-9-15(16-10-22-26(4)11-16)5-6-17(19)20(27)24-18/h5-6,9-13H,7-8H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assay |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434806

(2-(4-(2-(1-isopropyl-3-methyl-1H-1,2,4-triazol-5-y...)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(c1)C(C)(C)C(N)=O Show InChI InChI=1S/C24H28N8O2/c1-14(2)32-22(27-15(3)29-32)19-13-30-8-9-34-20-10-16(6-7-18(20)21(30)28-19)17-11-26-31(12-17)24(4,5)23(25)33/h6-7,10-14H,8-9H2,1-5H3,(H2,25,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assay |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434808
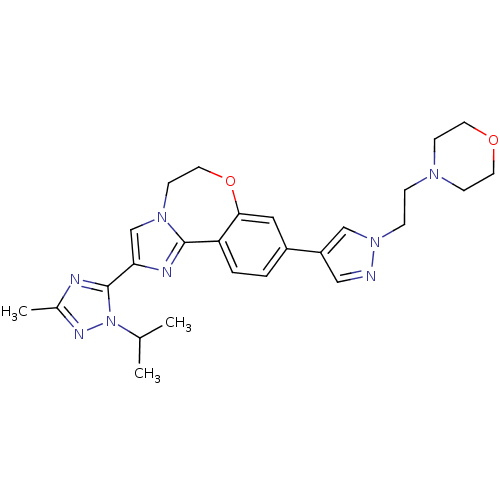
(CHEMBL2386972)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(CCN2CCOCC2)c1 Show InChI InChI=1S/C26H32N8O2/c1-18(2)34-26(28-19(3)30-34)23-17-32-10-13-36-24-14-20(4-5-22(24)25(32)29-23)21-15-27-33(16-21)7-6-31-8-11-35-12-9-31/h4-5,14-18H,6-13H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assay |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434817

(CHEMBL2387081)Show SMILES CC(C)n1ncnc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cn[nH]c1 Show InChI InChI=1S/C19H19N7O/c1-12(2)26-19(20-11-23-26)16-10-25-5-6-27-17-7-13(14-8-21-22-9-14)3-4-15(17)18(25)24-16/h3-4,7-12H,5-6H2,1-2H3,(H,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assay |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM29568

(CHEMBL2 | PRAZOSIN | PRAZOSIN HYDROCHLORIDE | [3H]...)Show SMILES COc1cc2nc(nc(N)c2cc1OC)N1CCN(CC1)C(=O)c1ccco1 Show InChI InChI=1S/C19H21N5O4/c1-26-15-10-12-13(11-16(15)27-2)21-19(22-17(12)20)24-7-5-23(6-8-24)18(25)14-4-3-9-28-14/h3-4,9-11H,5-8H2,1-2H3,(H2,20,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CombiChem, Inc.
Curated by ChEMBL
| Assay Description
Evaluated for binding affinity against alpha-1 adrenergic receptor |
J Med Chem 43: 2770-4 (2000)
Article DOI: 10.1021/jm990578n
BindingDB Entry DOI: 10.7270/Q23R0WMZ |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434809

(CHEMBL2386971)Show SMILES C[C@H](O)C(=O)N1CC(C1)n1cc(cn1)-c1ccc2-c3nc(cn3CCOc2c1)-c1nc(C)nn1C(C)C |r| Show InChI InChI=1S/C26H30N8O3/c1-15(2)34-25(28-17(4)30-34)22-14-31-7-8-37-23-9-18(5-6-21(23)24(31)29-22)19-10-27-33(11-19)20-12-32(13-20)26(36)16(3)35/h5-6,9-11,14-16,20,35H,7-8,12-13H2,1-4H3/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assay |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434813

(CHEMBL2387083)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(CCO)c1 Show InChI InChI=1S/C22H25N7O2/c1-14(2)29-22(24-15(3)26-29)19-13-27-7-9-31-20-10-16(4-5-18(20)21(27)25-19)17-11-23-28(12-17)6-8-30/h4-5,10-14,30H,6-9H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assay |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM69602
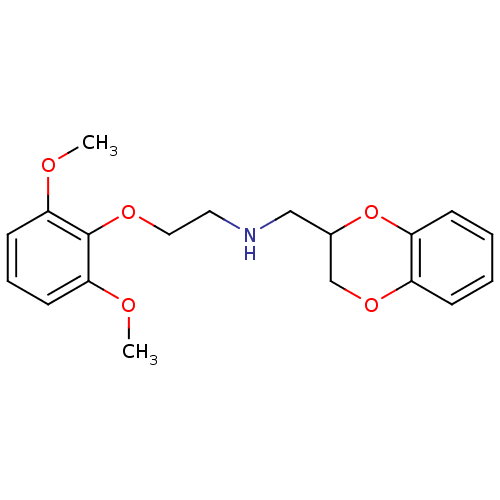
(2,3-dihydro-1,4-benzodioxin-3-ylmethyl-[2-(2,6-dim...)Show InChI InChI=1S/C19H23NO5/c1-21-17-8-5-9-18(22-2)19(17)23-11-10-20-12-14-13-24-15-6-3-4-7-16(15)25-14/h3-9,14,20H,10-13H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.540 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CombiChem, Inc.
Curated by ChEMBL
| Assay Description
Evaluated for binding affinity against alpha-1 adrenergic receptor |
J Med Chem 43: 2770-4 (2000)
Article DOI: 10.1021/jm990578n
BindingDB Entry DOI: 10.7270/Q23R0WMZ |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50421329
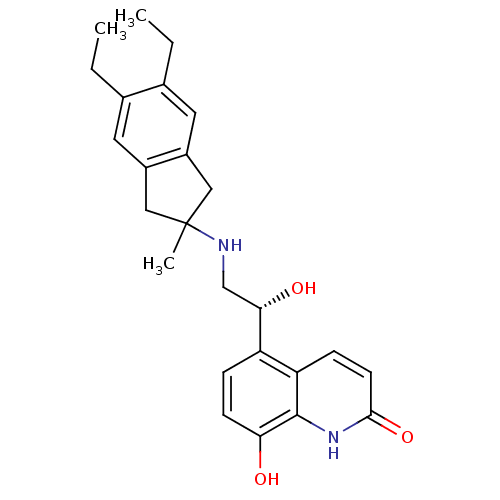
(CHEMBL2088201)Show SMILES CCc1cc2CC(C)(Cc2cc1CC)NC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C25H30N2O3/c1-4-15-10-17-12-25(3,13-18(17)11-16(15)5-2)26-14-22(29)19-6-8-21(28)24-20(19)7-9-23(30)27-24/h6-11,22,26,28-29H,4-5,12-14H2,1-3H3,(H,27,30)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Binding affinity to human beta2-adrenoceptor by radioligand binding assay |
Bioorg Med Chem Lett 22: 6280-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.096
BindingDB Entry DOI: 10.7270/Q2G73G09 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50434806

(2-(4-(2-(1-isopropyl-3-methyl-1H-1,2,4-triazol-5-y...)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(c1)C(C)(C)C(N)=O Show InChI InChI=1S/C24H28N8O2/c1-14(2)32-22(27-15(3)29-32)19-13-30-8-9-34-20-10-16(6-7-18(20)21(30)28-19)17-11-26-31(12-17)24(4,5)23(25)33/h6-7,10-14H,8-9H2,1-5H3,(H2,25,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434811
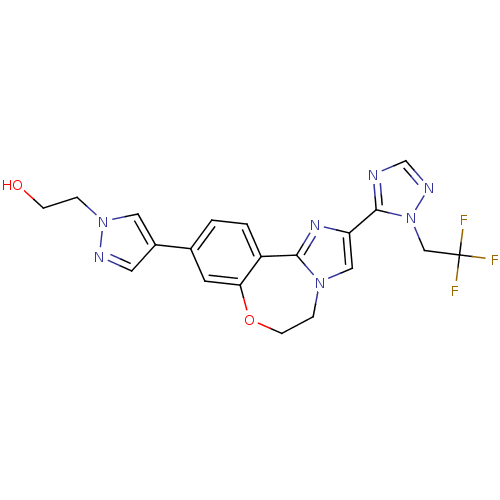
(CHEMBL2387087)Show SMILES OCCn1cc(cn1)-c1ccc2-c3nc(cn3CCOc2c1)-c1ncnn1CC(F)(F)F Show InChI InChI=1S/C20H18F3N7O2/c21-20(22,23)11-30-19(24-12-26-30)16-10-28-4-6-32-17-7-13(1-2-15(17)18(28)27-16)14-8-25-29(9-14)3-5-31/h1-2,7-10,12,31H,3-6,11H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assay |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM86231

(ATR | ATROPINE | Atropine,(-) | CAS_51-55-8 | CHEM...)Show SMILES CN1C2CCC1CC(C2)OC(=O)C(CO)c1ccccc1 |THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Creighton University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 297: 718-26 (2001)
BindingDB Entry DOI: 10.7270/Q28G8J85 |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM21398

(4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...)Show SMILES OC1(CCN(CCCC(=O)c2ccc(F)cc2)CC1)c1ccc(Cl)cc1 Show InChI InChI=1S/C21H23ClFNO2/c22-18-7-5-17(6-8-18)21(26)11-14-24(15-12-21)13-1-2-20(25)16-3-9-19(23)10-4-16/h3-10,26H,1-2,11-15H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CombiChem, Inc.
Curated by ChEMBL
| Assay Description
Evaluated for binding affinity against alpha-1 adrenergic receptor |
J Med Chem 43: 2770-4 (2000)
Article DOI: 10.1021/jm990578n
BindingDB Entry DOI: 10.7270/Q23R0WMZ |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50109647
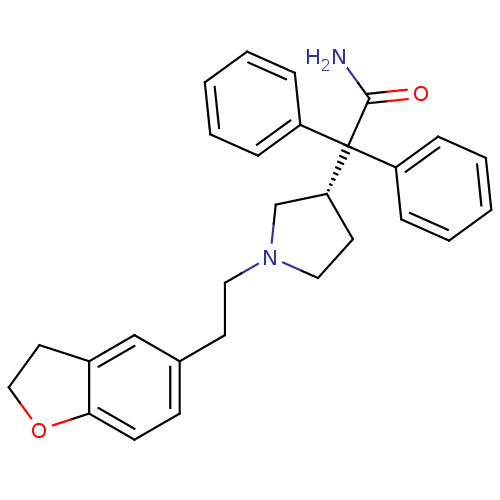
(2-{1-[2-(2,3-Dihydro-benzofuran-5-yl)-ethyl]-pyrro...)Show SMILES NC(=O)C([C@@H]1CCN(CCc2ccc3OCCc3c2)C1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C28H30N2O2/c29-27(31)28(23-7-3-1-4-8-23,24-9-5-2-6-10-24)25-14-17-30(20-25)16-13-21-11-12-26-22(19-21)15-18-32-26/h1-12,19,25H,13-18,20H2,(H2,29,31)/t25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Creighton University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 297: 718-26 (2001)
BindingDB Entry DOI: 10.7270/Q28G8J85 |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50001884

(2-[4-(4-Methyl-benzyl)-piperazin-1-yl]-1-(2-methyl...)Show SMILES CN1CCN(CC1)C1=Nc2cc(Cl)ccc2Nc2ccccc12 |t:8| Show InChI InChI=1S/C18H19ClN4/c1-22-8-10-23(11-9-22)18-14-4-2-3-5-15(14)20-16-7-6-13(19)12-17(16)21-18/h2-7,12,20H,8-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CombiChem, Inc.
Curated by ChEMBL
| Assay Description
Evaluated for binding affinity against alpha-1 adrenergic receptor |
J Med Chem 43: 2770-4 (2000)
Article DOI: 10.1021/jm990578n
BindingDB Entry DOI: 10.7270/Q23R0WMZ |
More data for this
Ligand-Target Pair | |
Beta-1 adrenergic receptor
(Homo sapiens (Human)) | BDBM50421329

(CHEMBL2088201)Show SMILES CCc1cc2CC(C)(Cc2cc1CC)NC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C25H30N2O3/c1-4-15-10-17-12-25(3,13-18(17)11-16(15)5-2)26-14-22(29)19-6-8-21(28)24-20(19)7-9-23(30)27-24/h6-11,22,26,28-29H,4-5,12-14H2,1-3H3,(H,27,30)/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Binding affinity to human beta1-adrenoceptor by radioligand binding assay |
Bioorg Med Chem Lett 22: 6280-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.096
BindingDB Entry DOI: 10.7270/Q2G73G09 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50109647

(2-{1-[2-(2,3-Dihydro-benzofuran-5-yl)-ethyl]-pyrro...)Show SMILES NC(=O)C([C@@H]1CCN(CCc2ccc3OCCc3c2)C1)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C28H30N2O2/c29-27(31)28(23-7-3-1-4-8-23,24-9-5-2-6-10-24)25-14-17-30(20-25)16-13-21-11-12-26-22(19-21)15-18-32-26/h1-12,19,25H,13-18,20H2,(H2,29,31)/t25-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Creighton University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 297: 718-26 (2001)
BindingDB Entry DOI: 10.7270/Q28G8J85 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM86231

(ATR | ATROPINE | Atropine,(-) | CAS_51-55-8 | CHEM...)Show SMILES CN1C2CCC1CC(C2)OC(=O)C(CO)c1ccccc1 |THB:9:7:1:3.4| Show InChI InChI=1S/C17H23NO3/c1-18-13-7-8-14(18)10-15(9-13)21-17(20)16(11-19)12-5-3-2-4-6-12/h2-6,13-16,19H,7-11H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Creighton University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 297: 718-26 (2001)
BindingDB Entry DOI: 10.7270/Q28G8J85 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50176065
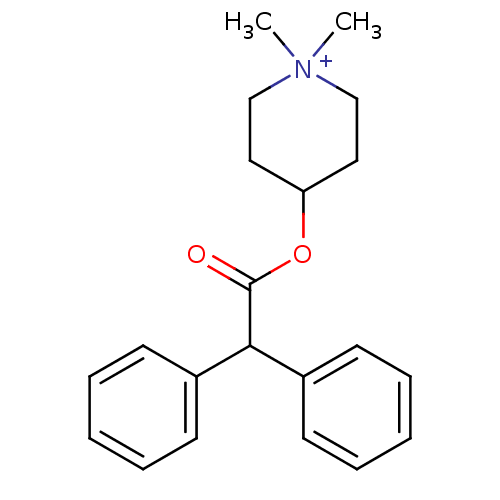
(4-DAMP | 4-Diphenylacetoxy-1,1-dimethyl-piperidini...)Show InChI InChI=1S/C21H26NO2/c1-22(2)15-13-19(14-16-22)24-21(23)20(17-9-5-3-6-10-17)18-11-7-4-8-12-18/h3-12,19-20H,13-16H2,1-2H3/q+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Creighton University
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 297: 718-26 (2001)
BindingDB Entry DOI: 10.7270/Q28G8J85 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434815
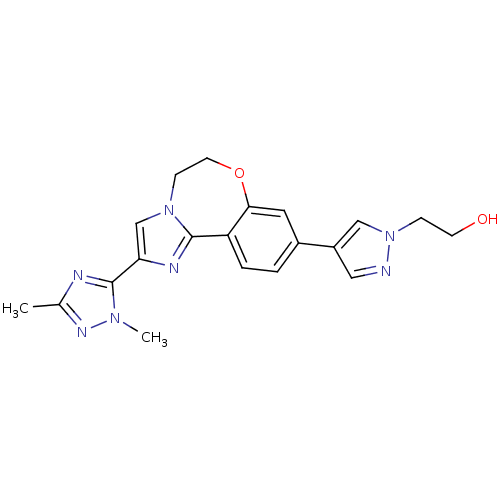
(CHEMBL2387085)Show SMILES Cc1nc(-c2cn3CCOc4cc(ccc4-c3n2)-c2cnn(CCO)c2)n(C)n1 Show InChI InChI=1S/C20H21N7O2/c1-13-22-20(25(2)24-13)17-12-26-6-8-29-18-9-14(3-4-16(18)19(26)23-17)15-10-21-27(11-15)5-7-28/h3-4,9-12,28H,5-8H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assay |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50128690
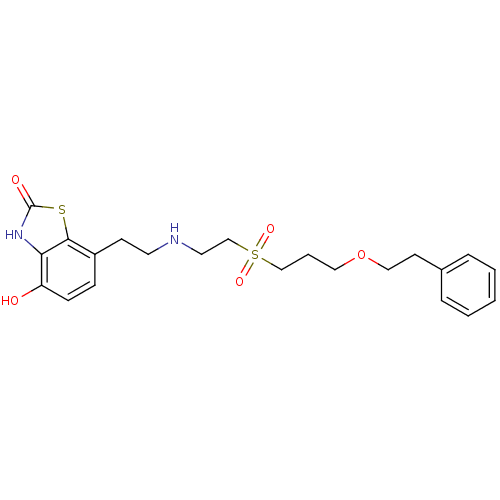
(4-Hydroxy-7-{2-[2-(3-phenethyloxy-propane-1-sulfon...)Show SMILES Oc1ccc(CCNCCS(=O)(=O)CCCOCCc2ccccc2)c2sc(=O)[nH]c12 Show InChI InChI=1S/C22H28N2O5S2/c25-19-8-7-18(21-20(19)24-22(26)30-21)9-11-23-12-16-31(27,28)15-4-13-29-14-10-17-5-2-1-3-6-17/h1-3,5-8,23,25H,4,9-16H2,(H,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Binding affinity to human beta2-adrenoceptor by radioligand binding assay |
Bioorg Med Chem Lett 22: 6280-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.096
BindingDB Entry DOI: 10.7270/Q2G73G09 |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Rattus norvegicus (rat)-Rattus norvegicus (Rat)) | BDBM50472725
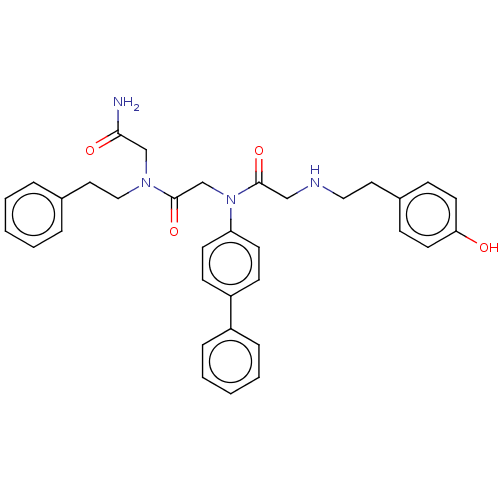
(CHEMBL90588 | CHIR-2279)Show SMILES NC(=O)CN(CCc1ccccc1)C(=O)CN(C(=O)CNCCc1ccc(O)cc1)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C34H36N4O4/c35-32(40)24-37(22-20-26-7-3-1-4-8-26)34(42)25-38(30-15-13-29(14-16-30)28-9-5-2-6-10-28)33(41)23-36-21-19-27-11-17-31(39)18-12-27/h1-18,36,39H,19-25H2,(H2,35,40) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CombiChem, Inc.
Curated by ChEMBL
| Assay Description
Evaluated for binding affinity against alpha-1 adrenergic receptor |
J Med Chem 43: 2770-4 (2000)
Article DOI: 10.1021/jm990578n
BindingDB Entry DOI: 10.7270/Q23R0WMZ |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50130099
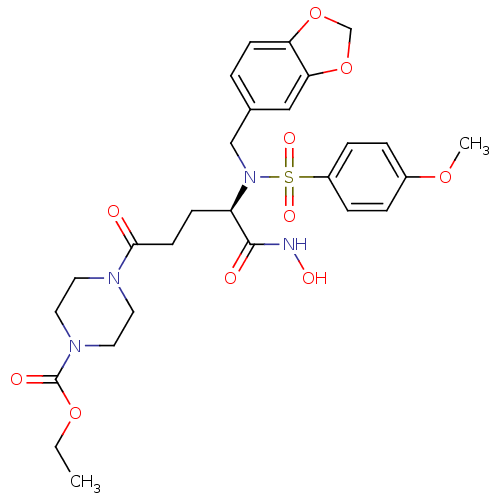
(4-{(R)-4-[Benzo[1,3]dioxol-5-ylmethyl-(4-methoxy-b...)Show SMILES CCOC(=O)N1CCN(CC1)C(=O)CC[C@@H](N(Cc1ccc2OCOc2c1)S(=O)(=O)c1ccc(OC)cc1)C(=O)NO Show InChI InChI=1S/C27H34N4O10S/c1-3-39-27(34)30-14-12-29(13-15-30)25(32)11-9-22(26(33)28-35)31(17-19-4-10-23-24(16-19)41-18-40-23)42(36,37)21-7-5-20(38-2)6-8-21/h4-8,10,16,22,35H,3,9,11-15,17-18H2,1-2H3,(H,28,33)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CombiChem Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MMP-8 |
Bioorg Med Chem Lett 13: 2381-4 (2003)
BindingDB Entry DOI: 10.7270/Q26H4GS8 |
More data for this
Ligand-Target Pair | |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50129204
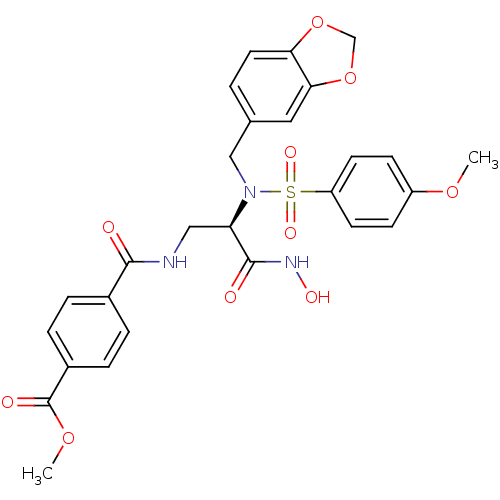
(CHEMBL65208 | N-{2-[Benzo[1,3]dioxol-5-ylmethyl-((...)Show SMILES COC(=O)c1ccc(cc1)C(=O)NC[C@@H](N(Cc1ccc2OCOc2c1)S(=O)(=O)c1ccc(OC)cc1)C(=O)NO Show InChI InChI=1S/C27H27N3O10S/c1-37-20-8-10-21(11-9-20)41(35,36)30(15-17-3-12-23-24(13-17)40-16-39-23)22(26(32)29-34)14-28-25(31)18-4-6-19(7-5-18)27(33)38-2/h3-13,22,34H,14-16H2,1-2H3,(H,28,31)(H,29,32)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CombiChem Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against procollagen C-terminal proteinase (PCP) in HT-1080 cells using synthetic peptide as substrate |
Bioorg Med Chem Lett 13: 2101-4 (2003)
BindingDB Entry DOI: 10.7270/Q2TD9WQS |
More data for this
Ligand-Target Pair | |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50129206
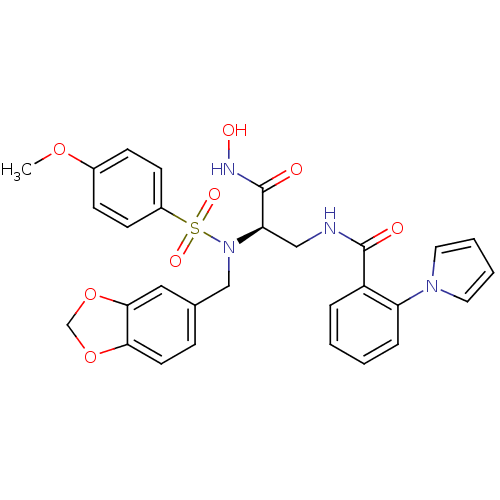
(CHEMBL292073 | N-{2-[Benzo[1,3]dioxol-5-ylmethyl-(...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1ccc2OCOc2c1)[C@H](CNC(=O)c1ccccc1-n1cccc1)C(=O)NO Show InChI InChI=1S/C29H28N4O8S/c1-39-21-9-11-22(12-10-21)42(37,38)33(18-20-8-13-26-27(16-20)41-19-40-26)25(29(35)31-36)17-30-28(34)23-6-2-3-7-24(23)32-14-4-5-15-32/h2-16,25,36H,17-19H2,1H3,(H,30,34)(H,31,35)/t25-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CombiChem Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against procollagen C-terminal proteinase (PCP) in HT-1080 cells using synthetic peptide as substrate |
Bioorg Med Chem Lett 13: 2101-4 (2003)
BindingDB Entry DOI: 10.7270/Q2TD9WQS |
More data for this
Ligand-Target Pair | |
Neutrophil collagenase
(Homo sapiens (Human)) | BDBM50130108
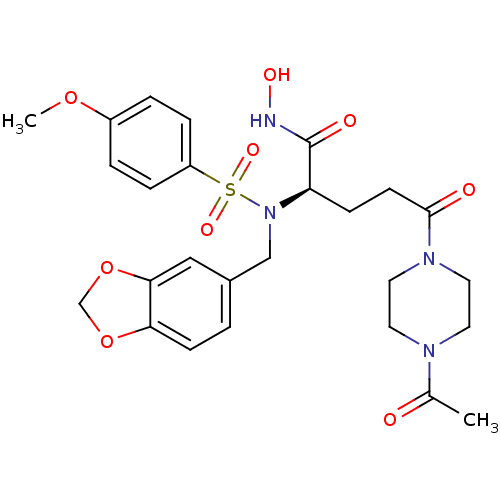
((R)-5-(4-Acetyl-piperazin-1-yl)-2-[benzo[1,3]dioxo...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1ccc2OCOc2c1)[C@H](CCC(=O)N1CCN(CC1)C(C)=O)C(=O)NO Show InChI InChI=1S/C26H32N4O9S/c1-18(31)28-11-13-29(14-12-28)25(32)10-8-22(26(33)27-34)30(16-19-3-9-23-24(15-19)39-17-38-23)40(35,36)21-6-4-20(37-2)5-7-21/h3-7,9,15,22,34H,8,10-14,16-17H2,1-2H3,(H,27,33)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CombiChem Inc.
Curated by ChEMBL
| Assay Description
Inhibition of MMP-8 |
Bioorg Med Chem Lett 13: 2381-4 (2003)
BindingDB Entry DOI: 10.7270/Q26H4GS8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50434806

(2-(4-(2-(1-isopropyl-3-methyl-1H-1,2,4-triazol-5-y...)Show SMILES CC(C)n1nc(C)nc1-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(c1)C(C)(C)C(N)=O Show InChI InChI=1S/C24H28N8O2/c1-14(2)32-22(27-15(3)29-32)19-13-30-8-9-34-20-10-16(6-7-18(20)21(30)28-19)17-11-26-31(12-17)24(4,5)23(25)33/h6-7,10-14H,8-9H2,1-5H3,(H2,25,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta (unknown origin) |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50130114
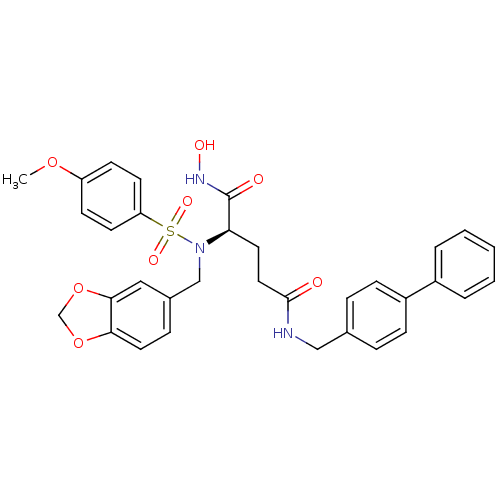
((R)-2-[Benzo[1,3]dioxol-5-ylmethyl-(4-methoxy-benz...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1ccc2OCOc2c1)[C@H](CCC(=O)NCc1ccc(cc1)-c1ccccc1)C(=O)NO Show InChI InChI=1S/C33H33N3O8S/c1-42-27-12-14-28(15-13-27)45(40,41)36(21-24-9-17-30-31(19-24)44-22-43-30)29(33(38)35-39)16-18-32(37)34-20-23-7-10-26(11-8-23)25-5-3-2-4-6-25/h2-15,17,19,29,39H,16,18,20-22H2,1H3,(H,34,37)(H,35,38)/t29-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CombiChem Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit procollagen C-terminal proteinase (PCP) tested in vitro |
Bioorg Med Chem Lett 13: 2381-4 (2003)
BindingDB Entry DOI: 10.7270/Q26H4GS8 |
More data for this
Ligand-Target Pair | |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50129212
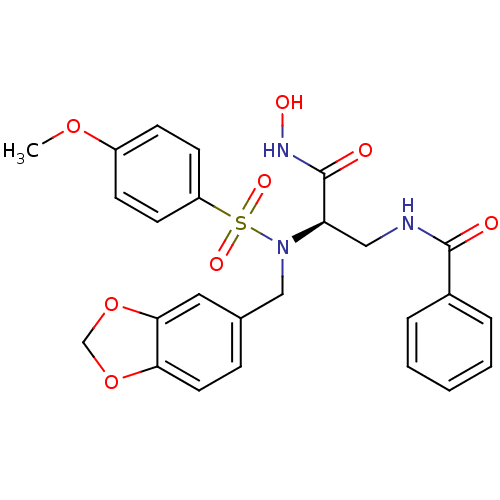
(CHEMBL294742 | N-{2-[Benzo[1,3]dioxol-5-ylmethyl-(...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1ccc2OCOc2c1)[C@H](CNC(=O)c1ccccc1)C(=O)NO Show InChI InChI=1S/C25H25N3O8S/c1-34-19-8-10-20(11-9-19)37(32,33)28(15-17-7-12-22-23(13-17)36-16-35-22)21(25(30)27-31)14-26-24(29)18-5-3-2-4-6-18/h2-13,21,31H,14-16H2,1H3,(H,26,29)(H,27,30)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CombiChem Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against procollagen C-terminal proteinase (PCP) in HT-1080 cells using synthetic peptide as substrate |
Bioorg Med Chem Lett 13: 2101-4 (2003)
BindingDB Entry DOI: 10.7270/Q2TD9WQS |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50318159
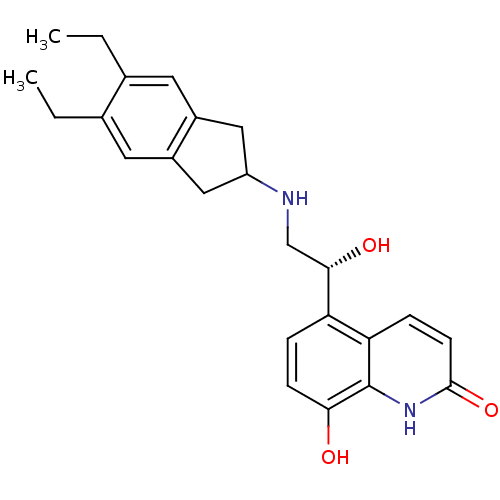
(8-Hydroxy-5-[(R)-1-hydroxy-2-(5,6-diethylindan-2-y...)Show SMILES CCc1cc2CC(Cc2cc1CC)NC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C24H28N2O3/c1-3-14-9-16-11-18(12-17(16)10-15(14)4-2)25-13-22(28)19-5-7-21(27)24-20(19)6-8-23(29)26-24/h5-10,18,22,25,27-28H,3-4,11-13H2,1-2H3,(H,26,29)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Binding affinity to human beta2-adrenoceptor by radioligand binding assay |
Bioorg Med Chem Lett 22: 6280-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.096
BindingDB Entry DOI: 10.7270/Q2G73G09 |
More data for this
Ligand-Target Pair | |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50129221
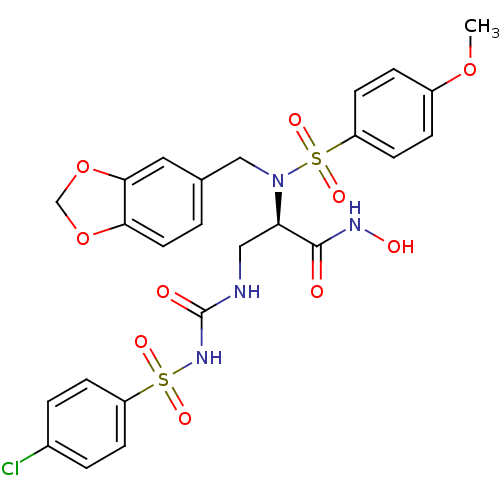
(2-[Benzo[1,3]dioxol-5-ylmethyl-((R)-4-methoxy-benz...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1ccc2OCOc2c1)[C@H](CNC(=O)NS(=O)(=O)c1ccc(Cl)cc1)C(=O)NO Show InChI InChI=1S/C25H25ClN4O10S2/c1-38-18-5-9-20(10-6-18)42(36,37)30(14-16-2-11-22-23(12-16)40-15-39-22)21(24(31)28-33)13-27-25(32)29-41(34,35)19-7-3-17(26)4-8-19/h2-12,21,33H,13-15H2,1H3,(H,28,31)(H2,27,29,32)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CombiChem Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against procollagen C-terminal proteinase (PCP) in HT-1080 cells using synthetic peptide as substrate |
Bioorg Med Chem Lett 13: 2101-4 (2003)
BindingDB Entry DOI: 10.7270/Q2TD9WQS |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50421327
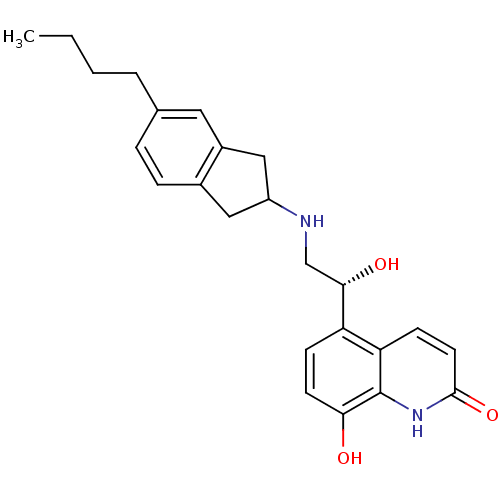
(CHEMBL2088199)Show SMILES CCCCc1ccc2CC(Cc2c1)NC[C@H](O)c1ccc(O)c2[nH]c(=O)ccc12 |r| Show InChI InChI=1S/C24H28N2O3/c1-2-3-4-15-5-6-16-12-18(13-17(16)11-15)25-14-22(28)19-7-9-21(27)24-20(19)8-10-23(29)26-24/h5-11,18,22,25,27-28H,2-4,12-14H2,1H3,(H,26,29)/t18?,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Binding affinity to human beta2-adrenoceptor by radioligand binding assay |
Bioorg Med Chem Lett 22: 6280-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.096
BindingDB Entry DOI: 10.7270/Q2G73G09 |
More data for this
Ligand-Target Pair | |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50130131
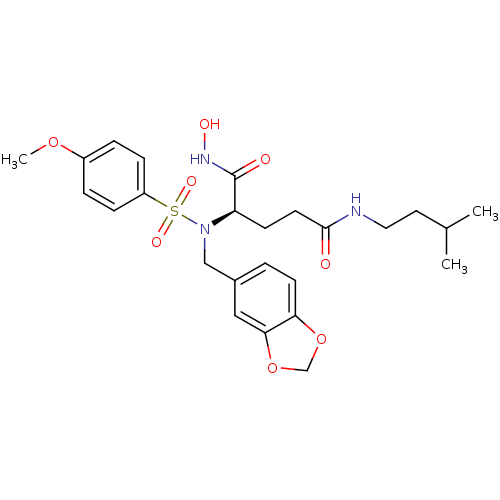
((R)-2-[Benzo[1,3]dioxol-5-ylmethyl-(4-methoxy-benz...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1ccc2OCOc2c1)[C@H](CCC(=O)NCCC(C)C)C(=O)NO Show InChI InChI=1S/C25H33N3O8S/c1-17(2)12-13-26-24(29)11-9-21(25(30)27-31)28(15-18-4-10-22-23(14-18)36-16-35-22)37(32,33)20-7-5-19(34-3)6-8-20/h4-8,10,14,17,21,31H,9,11-13,15-16H2,1-3H3,(H,26,29)(H,27,30)/t21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CombiChem Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit procollagen C-terminal proteinase (PCP) tested in vitro |
Bioorg Med Chem Lett 13: 2381-4 (2003)
BindingDB Entry DOI: 10.7270/Q26H4GS8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50434816
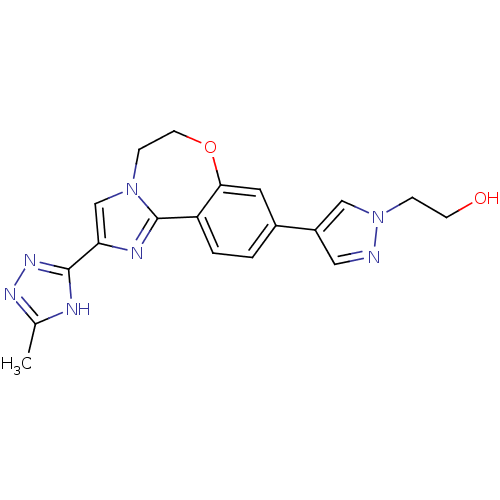
(CHEMBL2387084)Show SMILES Cc1nnc([nH]1)-c1cn2CCOc3cc(ccc3-c2n1)-c1cnn(CCO)c1 Show InChI InChI=1S/C19H19N7O2/c1-12-21-18(24-23-12)16-11-25-5-7-28-17-8-13(2-3-15(17)19(25)22-16)14-9-20-26(10-14)4-6-27/h2-3,8-11,27H,4-7H2,1H3,(H,21,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Genentech
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) assessed as 3,4,5-inositoltriphosphate formation after 30 mins by fluorescence polarization assay |
J Med Chem 56: 4597-610 (2013)
Article DOI: 10.1021/jm4003632
BindingDB Entry DOI: 10.7270/Q24F1S5G |
More data for this
Ligand-Target Pair | |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50129209
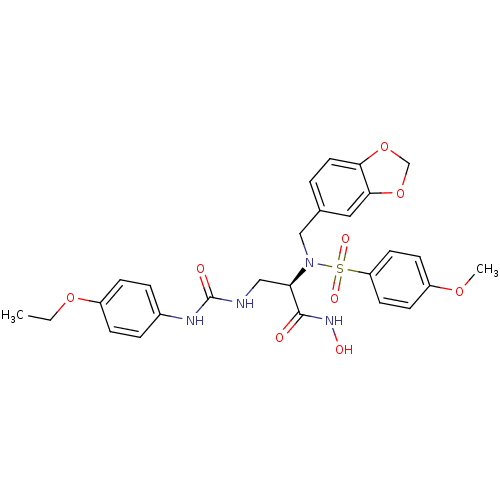
(2-[Benzo[1,3]dioxol-5-ylmethyl-((R)-4-methoxy-benz...)Show SMILES CCOc1ccc(NC(=O)NC[C@@H](N(Cc2ccc3OCOc3c2)S(=O)(=O)c2ccc(OC)cc2)C(=O)NO)cc1 Show InChI InChI=1S/C27H30N4O9S/c1-3-38-21-7-5-19(6-8-21)29-27(33)28-15-23(26(32)30-34)31(16-18-4-13-24-25(14-18)40-17-39-24)41(35,36)22-11-9-20(37-2)10-12-22/h4-14,23,34H,3,15-17H2,1-2H3,(H,30,32)(H2,28,29,33)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CombiChem Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against procollagen C-terminal proteinase (PCP) in HT-1080 cells using synthetic peptide as substrate |
Bioorg Med Chem Lett 13: 2101-4 (2003)
BindingDB Entry DOI: 10.7270/Q2TD9WQS |
More data for this
Ligand-Target Pair | |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50129208
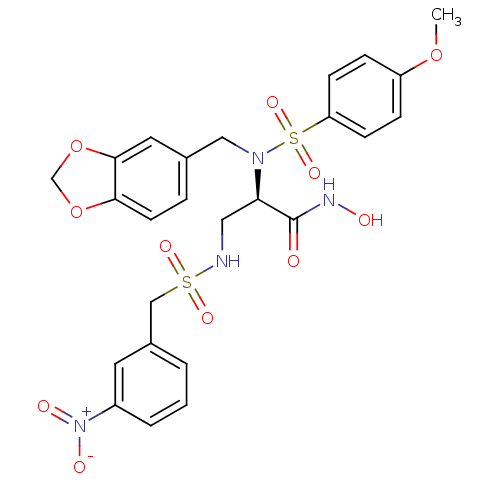
(2-[Benzo[1,3]dioxol-5-ylmethyl-((R)-4-methoxy-benz...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1ccc2OCOc2c1)[C@H](CNS(=O)(=O)Cc1cccc(c1)[N+]([O-])=O)C(=O)NO Show InChI InChI=1S/C25H26N4O11S2/c1-38-20-6-8-21(9-7-20)42(36,37)28(14-17-5-10-23-24(12-17)40-16-39-23)22(25(30)27-31)13-26-41(34,35)15-18-3-2-4-19(11-18)29(32)33/h2-12,22,26,31H,13-16H2,1H3,(H,27,30)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CombiChem Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against procollagen C-terminal proteinase (PCP) in HT-1080 cells using synthetic peptide as substrate |
Bioorg Med Chem Lett 13: 2101-4 (2003)
BindingDB Entry DOI: 10.7270/Q2TD9WQS |
More data for this
Ligand-Target Pair | |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50130105
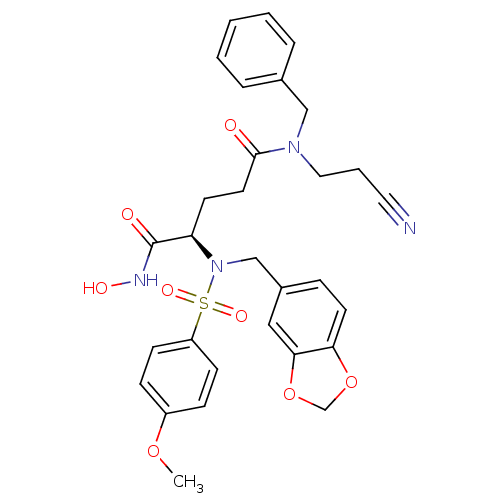
((R)-2-[Benzo[1,3]dioxol-5-ylmethyl-(4-methoxy-benz...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1ccc2OCOc2c1)[C@H](CCC(=O)N(CCC#N)Cc1ccccc1)C(=O)NO Show InChI InChI=1S/C30H32N4O8S/c1-40-24-9-11-25(12-10-24)43(38,39)34(20-23-8-14-27-28(18-23)42-21-41-27)26(30(36)32-37)13-15-29(35)33(17-5-16-31)19-22-6-3-2-4-7-22/h2-4,6-12,14,18,26,37H,5,13,15,17,19-21H2,1H3,(H,32,36)/t26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CombiChem Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit procollagen C-terminal proteinase (PCP) tested in vitro |
Bioorg Med Chem Lett 13: 2381-4 (2003)
BindingDB Entry DOI: 10.7270/Q26H4GS8 |
More data for this
Ligand-Target Pair | |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50130141
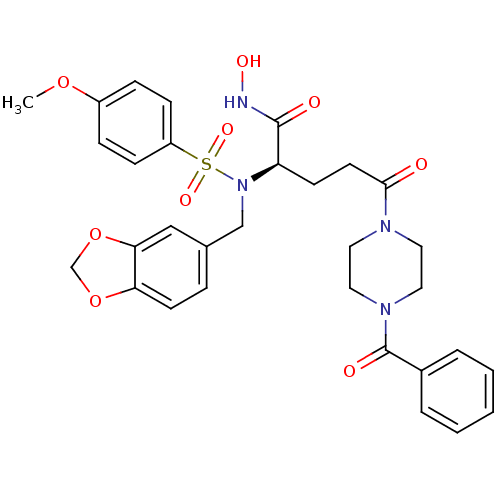
((R)-2-[Benzo[1,3]dioxol-5-ylmethyl-(4-methoxy-benz...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1ccc2OCOc2c1)[C@H](CCC(=O)N1CCN(CC1)C(=O)c1ccccc1)C(=O)NO Show InChI InChI=1S/C31H34N4O9S/c1-42-24-8-10-25(11-9-24)45(40,41)35(20-22-7-13-27-28(19-22)44-21-43-27)26(30(37)32-39)12-14-29(36)33-15-17-34(18-16-33)31(38)23-5-3-2-4-6-23/h2-11,13,19,26,39H,12,14-18,20-21H2,1H3,(H,32,37)/t26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CombiChem Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit procollagen C-terminal proteinase (PCP) tested in vitro |
Bioorg Med Chem Lett 13: 2381-4 (2003)
BindingDB Entry DOI: 10.7270/Q26H4GS8 |
More data for this
Ligand-Target Pair | |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50130123
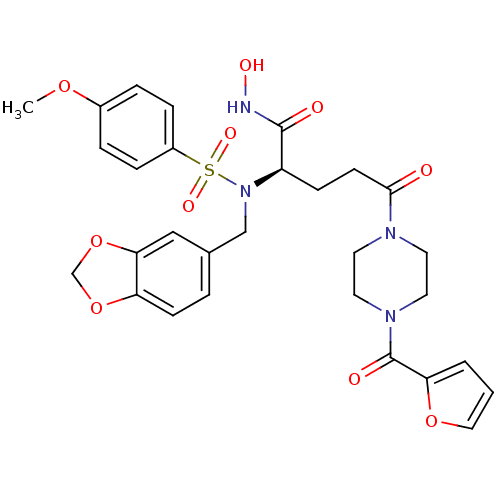
((R)-2-[Benzo[1,3]dioxol-5-ylmethyl-(4-methoxy-benz...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1ccc2OCOc2c1)[C@H](CCC(=O)N1CCN(CC1)C(=O)c1ccco1)C(=O)NO Show InChI InChI=1S/C29H32N4O10S/c1-40-21-5-7-22(8-6-21)44(38,39)33(18-20-4-10-24-26(17-20)43-19-42-24)23(28(35)30-37)9-11-27(34)31-12-14-32(15-13-31)29(36)25-3-2-16-41-25/h2-8,10,16-17,23,37H,9,11-15,18-19H2,1H3,(H,30,35)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CombiChem Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit procollagen C-terminal proteinase (PCP) tested in vitro |
Bioorg Med Chem Lett 13: 2381-4 (2003)
BindingDB Entry DOI: 10.7270/Q26H4GS8 |
More data for this
Ligand-Target Pair | |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50130121

((R)-ethyl 4-(4-(N-(benzo[d][1,3]dioxol-5-ylmethyl)...)Show SMILES CCOC(=O)N1CCN(CC1)C(=O)CC[C@@H](N(Cc1ccc2OCOc2c1)S(=O)(=O)c1c(C)cc(OC)c(C)c1C)C(=O)NO |r| Show InChI InChI=1S/C30H40N4O10S/c1-6-42-30(37)33-13-11-32(12-14-33)27(35)10-8-23(29(36)31-38)34(17-22-7-9-24-26(16-22)44-18-43-24)45(39,40)28-19(2)15-25(41-5)20(3)21(28)4/h7,9,15-16,23,38H,6,8,10-14,17-18H2,1-5H3,(H,31,36)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CombiChem Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit procollagen C-terminal proteinase (PCP) tested in vitro |
Bioorg Med Chem Lett 13: 2381-4 (2003)
BindingDB Entry DOI: 10.7270/Q26H4GS8 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50421325

(CHEMBL2088197)Show SMILES CCc1cc2CC(Cc2cc1CC)NC[C@H](O)c1ccc(O)c2NC(=O)CCc12 |r| Show InChI InChI=1S/C24H30N2O3/c1-3-14-9-16-11-18(12-17(16)10-15(14)4-2)25-13-22(28)19-5-7-21(27)24-20(19)6-8-23(29)26-24/h5,7,9-10,18,22,25,27-28H,3-4,6,8,11-13H2,1-2H3,(H,26,29)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Binding affinity to human beta2-adrenoceptor by radioligand binding assay |
Bioorg Med Chem Lett 22: 6280-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.096
BindingDB Entry DOI: 10.7270/Q2G73G09 |
More data for this
Ligand-Target Pair | |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50130112
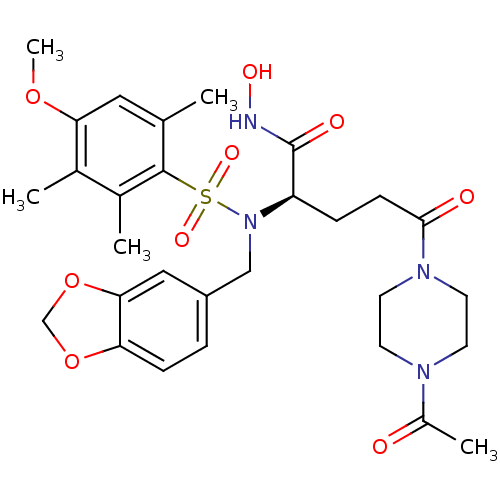
((R)-5-(4-Acetyl-piperazin-1-yl)-2-[benzo[1,3]dioxo...)Show SMILES COc1cc(C)c(c(C)c1C)S(=O)(=O)N(Cc1ccc2OCOc2c1)[C@H](CCC(=O)N1CCN(CC1)C(C)=O)C(=O)NO Show InChI InChI=1S/C29H38N4O9S/c1-18-14-25(40-5)19(2)20(3)28(18)43(38,39)33(16-22-6-8-24-26(15-22)42-17-41-24)23(29(36)30-37)7-9-27(35)32-12-10-31(11-13-32)21(4)34/h6,8,14-15,23,37H,7,9-13,16-17H2,1-5H3,(H,30,36)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 63 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CombiChem Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit procollagen C-terminal proteinase (PCP) tested in vitro |
Bioorg Med Chem Lett 13: 2381-4 (2003)
BindingDB Entry DOI: 10.7270/Q26H4GS8 |
More data for this
Ligand-Target Pair | |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50130099

(4-{(R)-4-[Benzo[1,3]dioxol-5-ylmethyl-(4-methoxy-b...)Show SMILES CCOC(=O)N1CCN(CC1)C(=O)CC[C@@H](N(Cc1ccc2OCOc2c1)S(=O)(=O)c1ccc(OC)cc1)C(=O)NO Show InChI InChI=1S/C27H34N4O10S/c1-3-39-27(34)30-14-12-29(13-15-30)25(32)11-9-22(26(33)28-35)31(17-19-4-10-23-24(16-19)41-18-40-23)42(36,37)21-7-5-20(38-2)6-8-21/h4-8,10,16,22,35H,3,9,11-15,17-18H2,1-2H3,(H,28,33)/t22-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 64 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CombiChem Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit procollagen C-terminal proteinase (PCP) tested in vitro |
Bioorg Med Chem Lett 13: 2381-4 (2003)
BindingDB Entry DOI: 10.7270/Q26H4GS8 |
More data for this
Ligand-Target Pair | |
Beta-2 adrenergic receptor
(Homo sapiens (Human)) | BDBM50421330
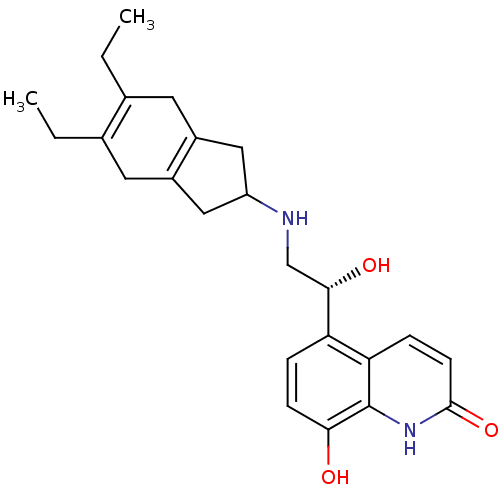
(CHEMBL2088202)Show SMILES CCC1=C(CC)CC2=C(CC(C2)NC[C@H](O)c2ccc(O)c3[nH]c(=O)ccc23)C1 |r,c:2,t:7| Show InChI InChI=1S/C24H30N2O3/c1-3-14-9-16-11-18(12-17(16)10-15(14)4-2)25-13-22(28)19-5-7-21(27)24-20(19)6-8-23(29)26-24/h5-8,18,22,25,27-28H,3-4,9-13H2,1-2H3,(H,26,29)/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 77 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Binding affinity to human beta2-adrenoceptor by radioligand binding assay |
Bioorg Med Chem Lett 22: 6280-5 (2012)
Article DOI: 10.1016/j.bmcl.2012.07.096
BindingDB Entry DOI: 10.7270/Q2G73G09 |
More data for this
Ligand-Target Pair | |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50129220
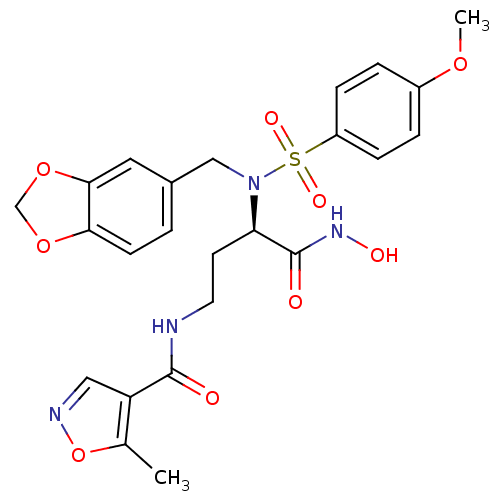
(5-Methyl-isoxazole-4-carboxylic acid {3-[benzo[1,3...)Show SMILES COc1ccc(cc1)S(=O)(=O)N(Cc1ccc2OCOc2c1)[C@H](CCNC(=O)c1cnoc1C)C(=O)NO Show InChI InChI=1S/C24H26N4O9S/c1-15-19(12-26-37-15)23(29)25-10-9-20(24(30)27-31)28(13-16-3-8-21-22(11-16)36-14-35-21)38(32,33)18-6-4-17(34-2)5-7-18/h3-8,11-12,20,31H,9-10,13-14H2,1-2H3,(H,25,29)(H,27,30)/t20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 86 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CombiChem Inc.
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against procollagen C-terminal proteinase (PCP) in HT-1080 cells using synthetic peptide as substrate |
Bioorg Med Chem Lett 13: 2101-4 (2003)
BindingDB Entry DOI: 10.7270/Q2TD9WQS |
More data for this
Ligand-Target Pair | |
Bone morphogenetic protein 1
(Homo sapiens (Human)) | BDBM50130132
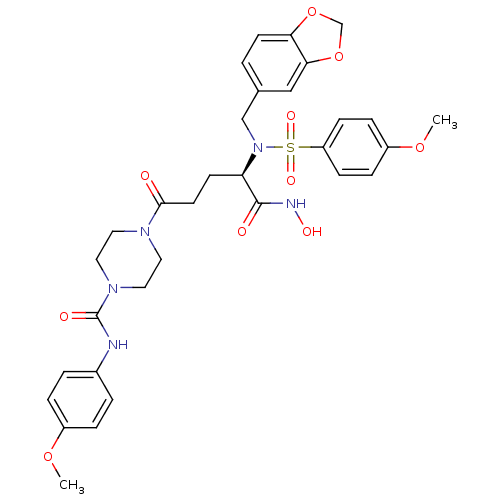
(4-{(R)-4-[Benzo[1,3]dioxol-5-ylmethyl-(4-methoxy-b...)Show SMILES COc1ccc(NC(=O)N2CCN(CC2)C(=O)CC[C@@H](N(Cc2ccc3OCOc3c2)S(=O)(=O)c2ccc(OC)cc2)C(=O)NO)cc1 Show InChI InChI=1S/C32H37N5O10S/c1-44-24-6-4-23(5-7-24)33-32(40)36-17-15-35(16-18-36)30(38)14-12-27(31(39)34-41)37(20-22-3-13-28-29(19-22)47-21-46-28)48(42,43)26-10-8-25(45-2)9-11-26/h3-11,13,19,27,41H,12,14-18,20-21H2,1-2H3,(H,33,40)(H,34,39)/t27-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CombiChem Inc.
Curated by ChEMBL
| Assay Description
Ability to inhibit procollagen C-terminal proteinase (PCP) tested in vitro |
Bioorg Med Chem Lett 13: 2381-4 (2003)
BindingDB Entry DOI: 10.7270/Q26H4GS8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data