

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein (Hepatitis C virus) | BDBM50142916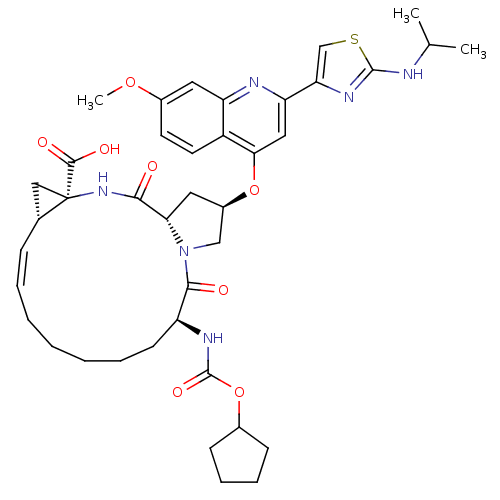 ((1S,4R,6S,14S,18R)-14-Cyclopentyloxycarbonylamino-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | 0.0890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HCV genotype-1a NS3 protease | Eur J Med Chem 148: 453-464 (2018) Article DOI: 10.1016/j.ejmech.2018.02.032 BindingDB Entry DOI: 10.7270/Q2BG2RMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50458174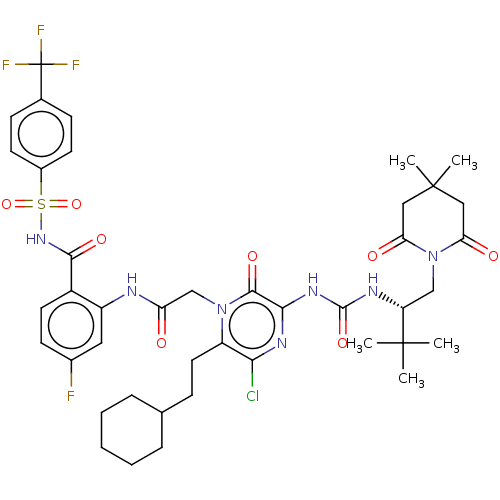 (CHEMBL4212267) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HCV genotype-1a full length NS3 protease R155K mutant preincubated for 10 mins followed by Ac-DED(Edans)EEAbuj-[COO]ASK(Dabcyl)-NH2 add... | Eur J Med Chem 148: 453-464 (2018) Article DOI: 10.1016/j.ejmech.2018.02.032 BindingDB Entry DOI: 10.7270/Q2BG2RMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50458174 (CHEMBL4212267) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HCV genotype-3a full length NS3 protease preincubated for 10 mins followed by Ac-DED(Edans)EEAbuj-[COO]ASK(Dabcyl)-NH2 addition in pres... | Eur J Med Chem 148: 453-464 (2018) Article DOI: 10.1016/j.ejmech.2018.02.032 BindingDB Entry DOI: 10.7270/Q2BG2RMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50366760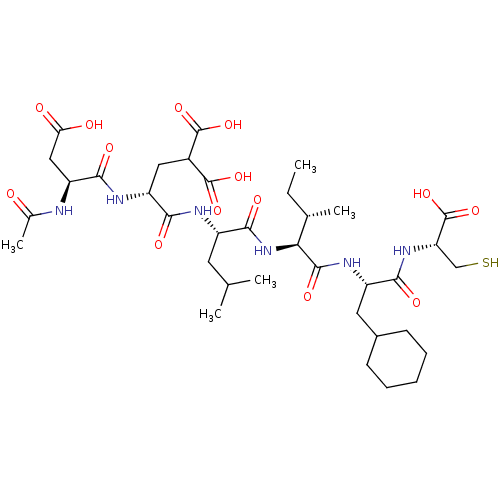 (CHEMBL2369589) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HCV genotype-1a full length NS3 protease preincubated for 10 mins followed by Ac-DED(Edans)EEAbuj-[COO]ASK(Dabcyl)-NH2 addition in pres... | Eur J Med Chem 148: 453-464 (2018) Article DOI: 10.1016/j.ejmech.2018.02.032 BindingDB Entry DOI: 10.7270/Q2BG2RMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50142916 ((1S,4R,6S,14S,18R)-14-Cyclopentyloxycarbonylamino-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HCV genotype-3a full length NS3 protease preincubated for 10 mins followed by Ac-DED(Edans)EEAbuj-[COO]ASK(Dabcyl)-NH2 addition in pres... | Eur J Med Chem 148: 453-464 (2018) Article DOI: 10.1016/j.ejmech.2018.02.032 BindingDB Entry DOI: 10.7270/Q2BG2RMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50458173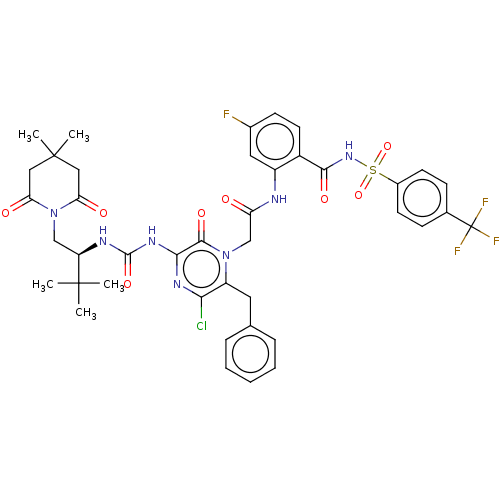 (CHEMBL4217239) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HCV genotype-1a full length NS3 protease R155K mutant preincubated for 10 mins followed by Ac-DED(Edans)EEAbuj-[COO]ASK(Dabcyl)-NH2 add... | Eur J Med Chem 148: 453-464 (2018) Article DOI: 10.1016/j.ejmech.2018.02.032 BindingDB Entry DOI: 10.7270/Q2BG2RMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50458174 (CHEMBL4212267) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HCV genotype-1a full length NS3 protease preincubated for 10 mins followed by Ac-DED(Edans)EEAbuj-[COO]ASK(Dabcyl)-NH2 addition in pres... | Eur J Med Chem 148: 453-464 (2018) Article DOI: 10.1016/j.ejmech.2018.02.032 BindingDB Entry DOI: 10.7270/Q2BG2RMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50458171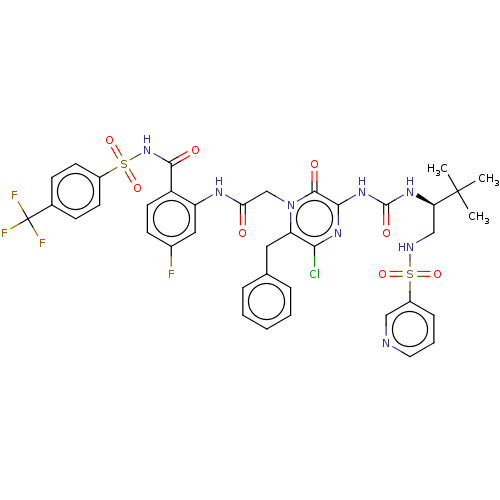 (CHEMBL4208860) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HCV genotype-1a full length NS3 protease R155K mutant preincubated for 10 mins followed by Ac-DED(Edans)EEAbuj-[COO]ASK(Dabcyl)-NH2 add... | Eur J Med Chem 148: 453-464 (2018) Article DOI: 10.1016/j.ejmech.2018.02.032 BindingDB Entry DOI: 10.7270/Q2BG2RMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50458175 (CHEMBL3797556) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HCV genotype-3a full length NS3 protease preincubated for 10 mins followed by Ac-DED(Edans)EEAbuj-[COO]ASK(Dabcyl)-NH2 addition in pres... | Eur J Med Chem 148: 453-464 (2018) Article DOI: 10.1016/j.ejmech.2018.02.032 BindingDB Entry DOI: 10.7270/Q2BG2RMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50458177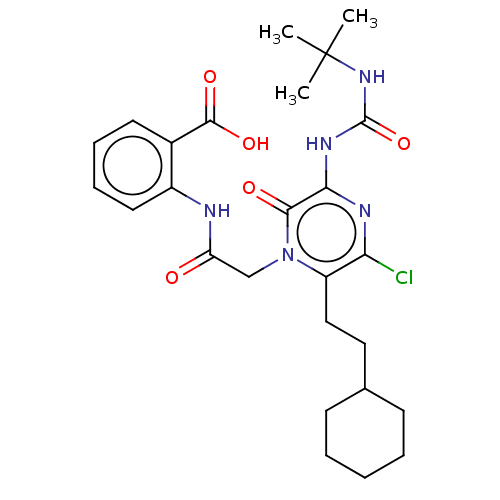 (CHEMBL3797877) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HCV genotype-3a full length NS3 protease preincubated for 10 mins followed by Ac-DED(Edans)EEAbuj-[COO]ASK(Dabcyl)-NH2 addition in pres... | Eur J Med Chem 148: 453-464 (2018) Article DOI: 10.1016/j.ejmech.2018.02.032 BindingDB Entry DOI: 10.7270/Q2BG2RMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50458179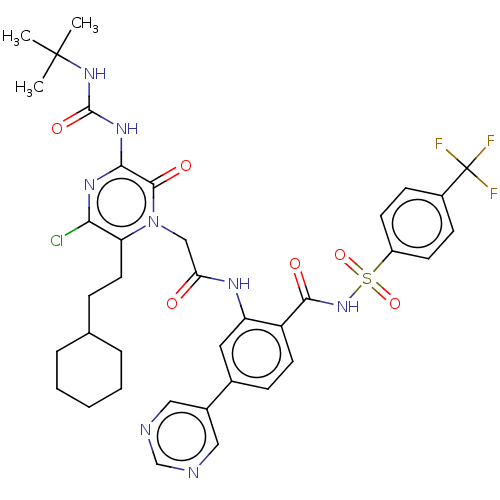 (CHEMBL3798865) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HCV genotype-3a full length NS3 protease preincubated for 10 mins followed by Ac-DED(Edans)EEAbuj-[COO]ASK(Dabcyl)-NH2 addition in pres... | Eur J Med Chem 148: 453-464 (2018) Article DOI: 10.1016/j.ejmech.2018.02.032 BindingDB Entry DOI: 10.7270/Q2BG2RMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50458173 (CHEMBL4217239) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 135 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HCV genotype-3a full length NS3 protease preincubated for 10 mins followed by Ac-DED(Edans)EEAbuj-[COO]ASK(Dabcyl)-NH2 addition in pres... | Eur J Med Chem 148: 453-464 (2018) Article DOI: 10.1016/j.ejmech.2018.02.032 BindingDB Entry DOI: 10.7270/Q2BG2RMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50458173 (CHEMBL4217239) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HCV genotype-1a full length NS3 protease preincubated for 10 mins followed by Ac-DED(Edans)EEAbuj-[COO]ASK(Dabcyl)-NH2 addition in pres... | Eur J Med Chem 148: 453-464 (2018) Article DOI: 10.1016/j.ejmech.2018.02.032 BindingDB Entry DOI: 10.7270/Q2BG2RMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50458176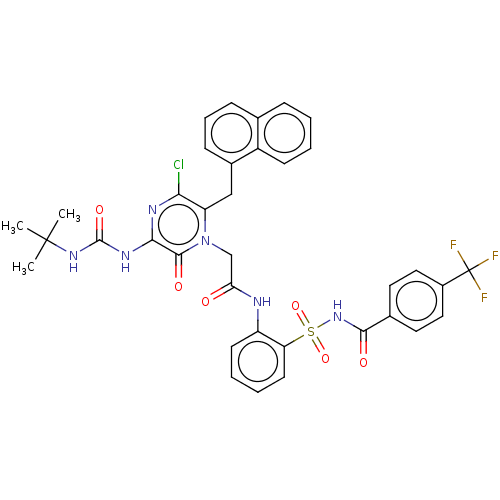 (CHEMBL3798789) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HCV genotype-3a full length NS3 protease preincubated for 10 mins followed by Ac-DED(Edans)EEAbuj-[COO]ASK(Dabcyl)-NH2 addition in pres... | Eur J Med Chem 148: 453-464 (2018) Article DOI: 10.1016/j.ejmech.2018.02.032 BindingDB Entry DOI: 10.7270/Q2BG2RMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50458171 (CHEMBL4208860) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HCV genotype-1a full length NS3 protease preincubated for 10 mins followed by Ac-DED(Edans)EEAbuj-[COO]ASK(Dabcyl)-NH2 addition in pres... | Eur J Med Chem 148: 453-464 (2018) Article DOI: 10.1016/j.ejmech.2018.02.032 BindingDB Entry DOI: 10.7270/Q2BG2RMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50458172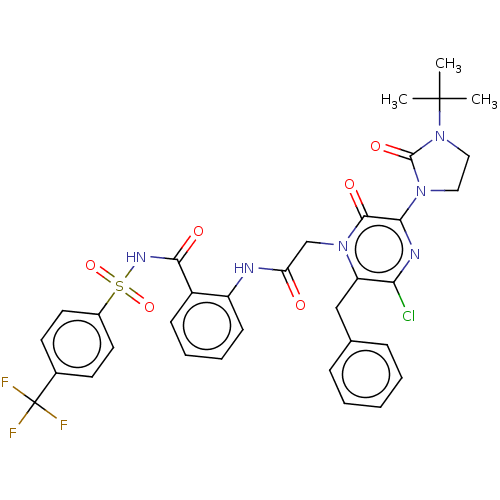 (CHEMBL4213784) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HCV genotype-1a full length NS3 protease preincubated for 10 mins followed by Ac-DED(Edans)EEAbuj-[COO]ASK(Dabcyl)-NH2 addition in pres... | Eur J Med Chem 148: 453-464 (2018) Article DOI: 10.1016/j.ejmech.2018.02.032 BindingDB Entry DOI: 10.7270/Q2BG2RMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50458178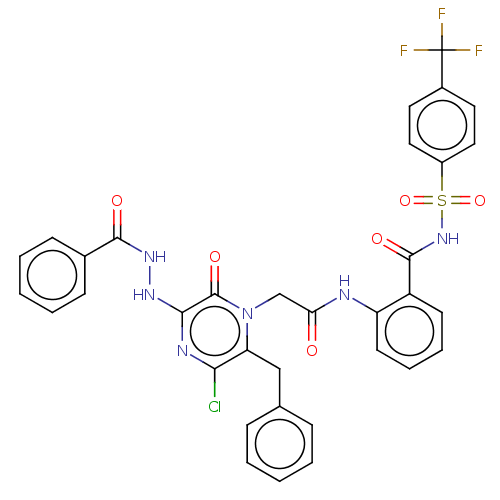 (CHEMBL4204522) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HCV genotype-1a full length NS3 protease preincubated for 10 mins followed by Ac-DED(Edans)EEAbuj-[COO]ASK(Dabcyl)-NH2 addition in pres... | Eur J Med Chem 148: 453-464 (2018) Article DOI: 10.1016/j.ejmech.2018.02.032 BindingDB Entry DOI: 10.7270/Q2BG2RMG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50478032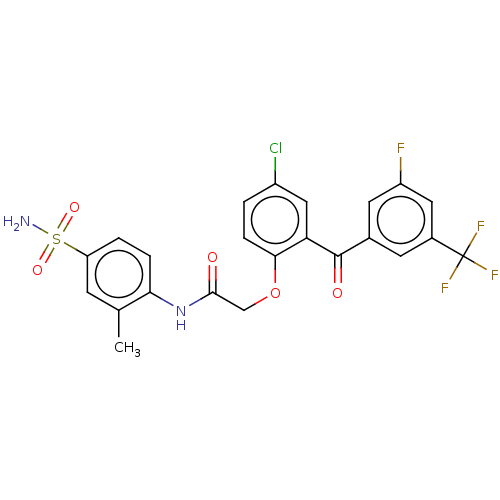 (CHEMBL275658 | GW4511 | GW564511 | GW69564) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Beactica AB Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase | J Med Chem 54: 709-18 (2011) Article DOI: 10.1021/jm101052g BindingDB Entry DOI: 10.7270/Q22B91T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50476133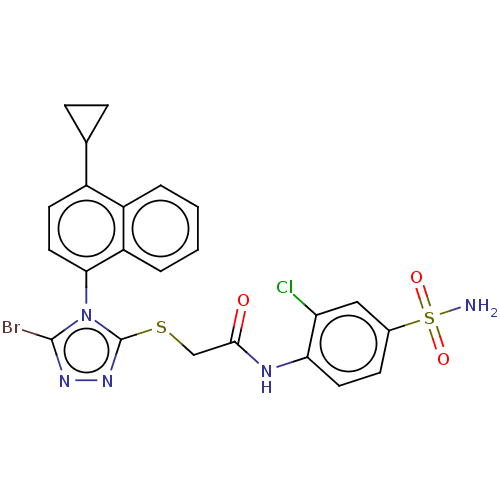 (CHEMBL373571 | VRX-480773) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Beactica AB Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase | J Med Chem 54: 709-18 (2011) Article DOI: 10.1021/jm101052g BindingDB Entry DOI: 10.7270/Q22B91T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal peptidase I (Escherichia coli (strain K12)) | BDBM50469157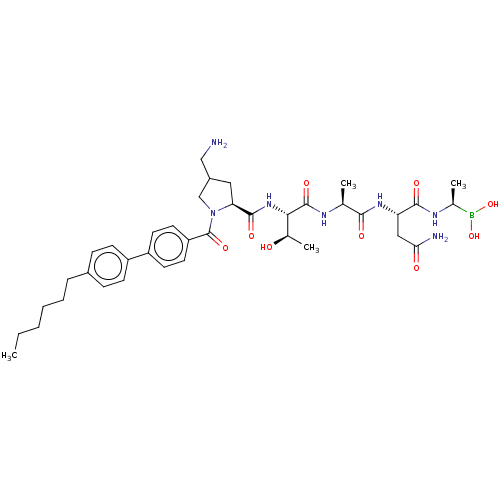 (CHEMBL4284736) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli type 1 signal peptidase lepB preincubated for 10 mins followed by Dabcyl-VGGTATAYGAFSRPGLE-(EDANS)-OH substrate additi... | Eur J Med Chem 157: 1346-1360 (2018) Article DOI: 10.1016/j.ejmech.2018.08.086 BindingDB Entry DOI: 10.7270/Q2H70JH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal peptidase I (Escherichia coli (strain K12)) | BDBM50501358 (CHEMBL3947604) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of full length Escherichia coli His6-tagged LepB expressed in Escherichia coli C43(DE3) using dabcyl-VEVGGTATAGAFSRPGLE-(EDANS) as substra... | Bioorg Med Chem 25: 897-911 (2017) Article DOI: 10.1016/j.bmc.2016.12.003 BindingDB Entry DOI: 10.7270/Q20P1321 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal peptidase I (Escherichia coli (strain K12)) | BDBM50501352 (CHEMBL3922978) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of full length Escherichia coli His6-tagged LepB expressed in Escherichia coli C43(DE3) using dabcyl-VEVGGTATAGAFSRPGLE-(EDANS) as substra... | Bioorg Med Chem 25: 897-911 (2017) Article DOI: 10.1016/j.bmc.2016.12.003 BindingDB Entry DOI: 10.7270/Q20P1321 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM1885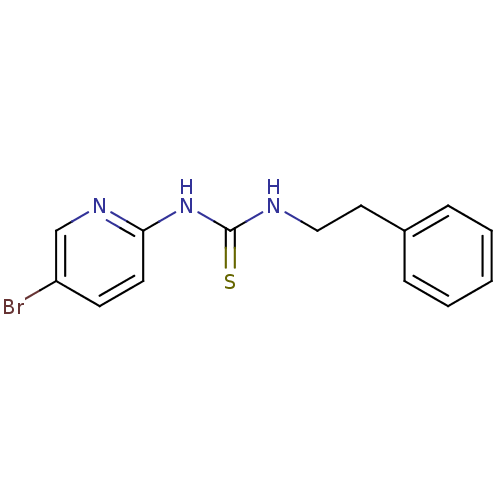 (3-(5-bromopyridin-2-yl)-1-(2-phenylethyl)thiourea ...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Beactica AB Curated by ChEMBL | Assay Description Inhibition of HIV1 reverse transcriptase | J Med Chem 54: 709-18 (2011) Article DOI: 10.1021/jm101052g BindingDB Entry DOI: 10.7270/Q22B91T8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal peptidase I (Escherichia coli (strain K12)) | BDBM50469159 (CHEMBL4283840) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli type 1 signal peptidase lepB preincubated for 10 mins followed by Dabcyl-VGGTATAYGAFSRPGLE-(EDANS)-OH substrate additi... | Eur J Med Chem 157: 1346-1360 (2018) Article DOI: 10.1016/j.ejmech.2018.08.086 BindingDB Entry DOI: 10.7270/Q2H70JH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal peptidase I (Escherichia coli (strain K12)) | BDBM50501354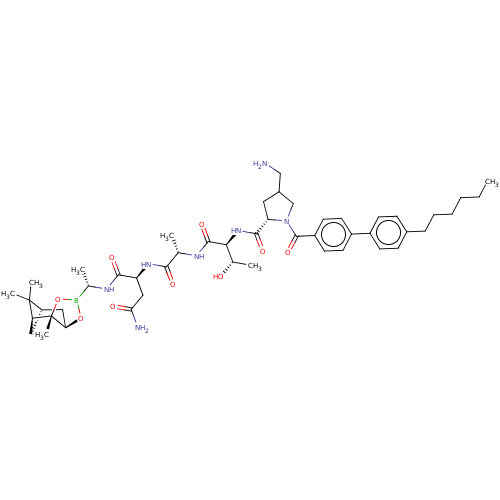 (CHEMBL3934222) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of full length Escherichia coli His6-tagged LepB expressed in Escherichia coli C43(DE3) using dabcyl-VEVGGTATAGAFSRPGLE-(EDANS) as substra... | Bioorg Med Chem 25: 897-911 (2017) Article DOI: 10.1016/j.bmc.2016.12.003 BindingDB Entry DOI: 10.7270/Q20P1321 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM18885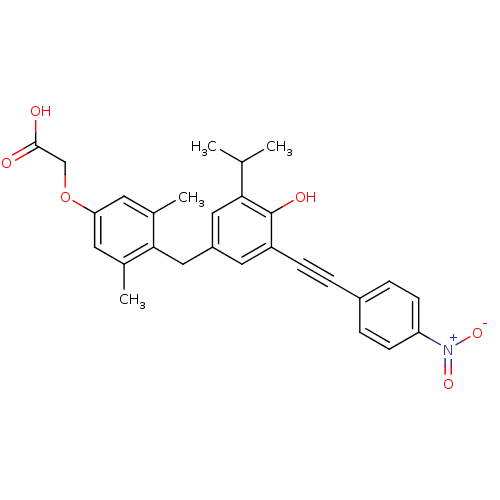 (2-[4-({4-hydroxy-3-[2-(4-nitrophenyl)ethynyl]-5-(p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Karo Bio AB | Assay Description IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. | J Med Chem 49: 6635-7 (2006) Article DOI: 10.1021/jm060521i BindingDB Entry DOI: 10.7270/Q23B5XDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM18885 (2-[4-({4-hydroxy-3-[2-(4-nitrophenyl)ethynyl]-5-(p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Karo Bio AB | Assay Description IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. | Bioorg Med Chem Lett 17: 2018-21 (2007) Article DOI: 10.1016/j.bmcl.2007.01.009 BindingDB Entry DOI: 10.7270/Q2R20ZMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM18886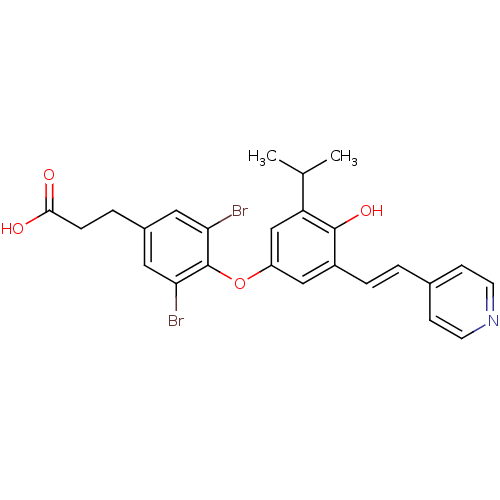 (3-{3,5-dibromo-4-[4-hydroxy-3-(propan-2-yl)-5-[(E)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 22 | n/a | 32 | n/a | n/a | 7.0 | 4 |
Karo Bio AB | Assay Description IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. EC50 is the concentration of compound required to i... | J Med Chem 49: 6635-7 (2006) Article DOI: 10.1021/jm060521i BindingDB Entry DOI: 10.7270/Q23B5XDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM18886 (3-{3,5-dibromo-4-[4-hydroxy-3-(propan-2-yl)-5-[(E)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Karo Bio AB | Assay Description IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. | Bioorg Med Chem Lett 17: 2018-21 (2007) Article DOI: 10.1016/j.bmcl.2007.01.009 BindingDB Entry DOI: 10.7270/Q2R20ZMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM18896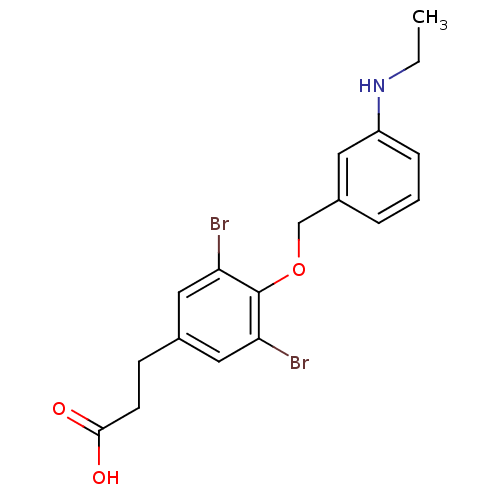 (3-(3,5-dibromo-4-{[3-(ethylamino)phenyl]methoxy}ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 23 | n/a | 49 | n/a | n/a | 7.0 | 4 |
Karo Bio AB | Assay Description IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. EC50 is the concentration of compound required to r... | Bioorg Med Chem Lett 17: 2018-21 (2007) Article DOI: 10.1016/j.bmcl.2007.01.009 BindingDB Entry DOI: 10.7270/Q2R20ZMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal peptidase I (Escherichia coli (strain K12)) | BDBM50469154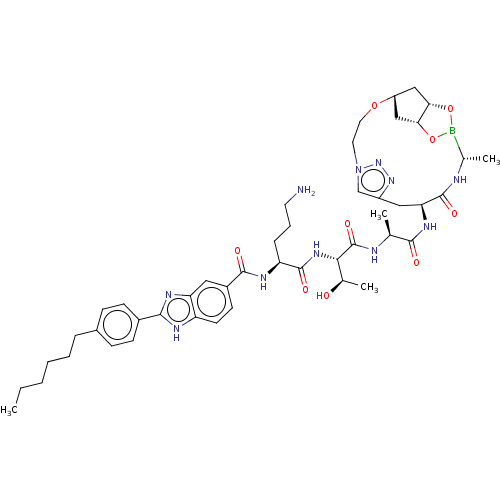 (CHEMBL4283678) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli type 1 signal peptidase lepB preincubated for 10 mins followed by Dabcyl-VGGTATAYGAFSRPGLE-(EDANS)-OH substrate additi... | Eur J Med Chem 157: 1346-1360 (2018) Article DOI: 10.1016/j.ejmech.2018.08.086 BindingDB Entry DOI: 10.7270/Q2H70JH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM18884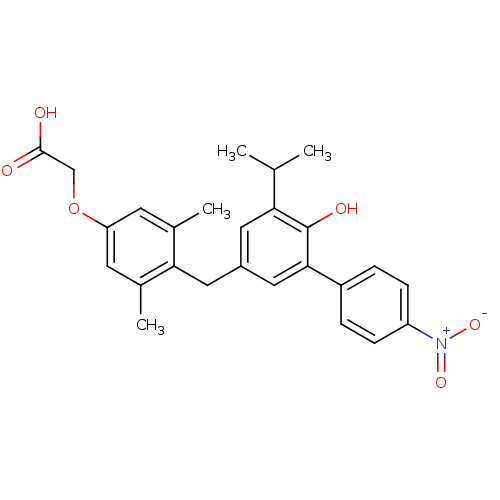 (2-(4-{[4-hydroxy-3-(4-nitrophenyl)-5-(propan-2-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Karo Bio AB | Assay Description IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. | J Med Chem 49: 6635-7 (2006) Article DOI: 10.1021/jm060521i BindingDB Entry DOI: 10.7270/Q23B5XDF | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM18884 (2-(4-{[4-hydroxy-3-(4-nitrophenyl)-5-(propan-2-yl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Karo Bio AB | Assay Description IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. | Bioorg Med Chem Lett 17: 2018-21 (2007) Article DOI: 10.1016/j.bmcl.2007.01.009 BindingDB Entry DOI: 10.7270/Q2R20ZMS | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Thyroid hormone receptor alpha (Homo sapiens (Human)) | BDBM18886 (3-{3,5-dibromo-4-[4-hydroxy-3-(propan-2-yl)-5-[(E)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 36 | n/a | 32 | n/a | n/a | 7.0 | 4 |
Karo Bio AB | Assay Description IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. EC50 is the concentration of compound required to ... | J Med Chem 49: 6635-7 (2006) Article DOI: 10.1021/jm060521i BindingDB Entry DOI: 10.7270/Q23B5XDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor alpha (Homo sapiens (Human)) | BDBM18886 (3-{3,5-dibromo-4-[4-hydroxy-3-(propan-2-yl)-5-[(E)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Karo Bio AB | Assay Description IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. | Bioorg Med Chem Lett 17: 2018-21 (2007) Article DOI: 10.1016/j.bmcl.2007.01.009 BindingDB Entry DOI: 10.7270/Q2R20ZMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal peptidase I (Escherichia coli (strain K12)) | BDBM50469165 (CHEMBL4291540) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli type 1 signal peptidase lepB preincubated for 10 mins followed by Dabcyl-VGGTATAYGAFSRPGLE-(EDANS)-OH substrate additi... | Eur J Med Chem 157: 1346-1360 (2018) Article DOI: 10.1016/j.ejmech.2018.08.086 BindingDB Entry DOI: 10.7270/Q2H70JH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal peptidase I (Escherichia coli (strain K12)) | BDBM50469162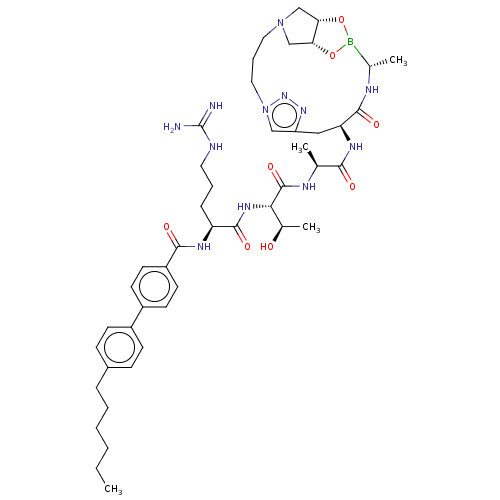 (CHEMBL4282733) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli type 1 signal peptidase lepB preincubated for 10 mins followed by Dabcyl-VGGTATAYGAFSRPGLE-(EDANS)-OH substrate additi... | Eur J Med Chem 157: 1346-1360 (2018) Article DOI: 10.1016/j.ejmech.2018.08.086 BindingDB Entry DOI: 10.7270/Q2H70JH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal peptidase I (Escherichia coli (strain K12)) | BDBM50469170 (CHEMBL4291025) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli type 1 signal peptidase lepB preincubated for 10 mins followed by Dabcyl-VGGTATAYGAFSRPGLE-(EDANS)-OH substrate additi... | Eur J Med Chem 157: 1346-1360 (2018) Article DOI: 10.1016/j.ejmech.2018.08.086 BindingDB Entry DOI: 10.7270/Q2H70JH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal peptidase I (Escherichia coli (strain K12)) | BDBM50501357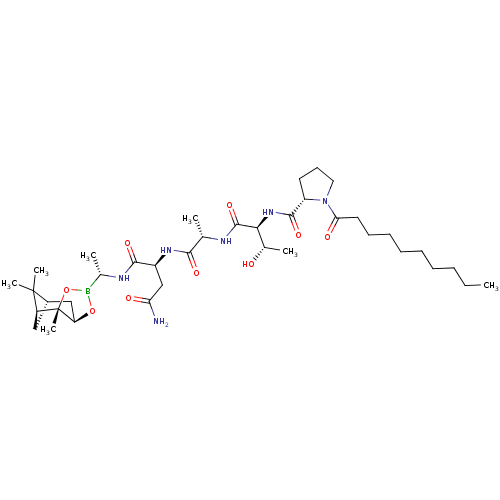 (CHEMBL3957297) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 48 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of full length Escherichia coli His6-tagged LepB expressed in Escherichia coli C43(DE3) using dabcyl-VEVGGTATAGAFSRPGLE-(EDANS) as substra... | Bioorg Med Chem 25: 897-911 (2017) Article DOI: 10.1016/j.bmc.2016.12.003 BindingDB Entry DOI: 10.7270/Q20P1321 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal peptidase I (Escherichia coli (strain K12)) | BDBM50469166 (CHEMBL4294422) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli type 1 signal peptidase lepB preincubated for 10 mins followed by Dabcyl-VGGTATAYGAFSRPGLE-(EDANS)-OH substrate additi... | Eur J Med Chem 157: 1346-1360 (2018) Article DOI: 10.1016/j.ejmech.2018.08.086 BindingDB Entry DOI: 10.7270/Q2H70JH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM18894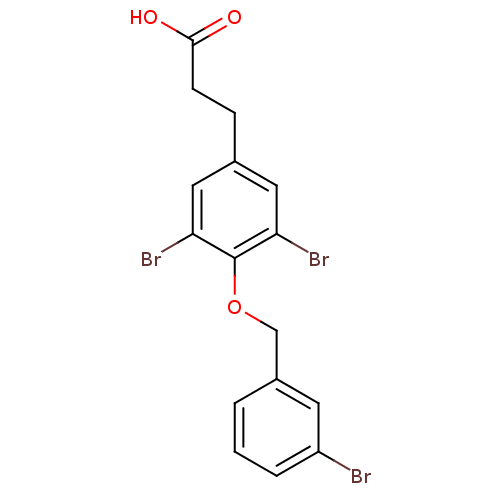 (3-{3,5-dibromo-4-[(3-bromophenyl)methoxy]phenyl}pr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | 230 | n/a | n/a | 7.0 | 4 |
Karo Bio AB | Assay Description IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. EC50 is the concentration of compound required to r... | Bioorg Med Chem Lett 17: 2018-21 (2007) Article DOI: 10.1016/j.bmcl.2007.01.009 BindingDB Entry DOI: 10.7270/Q2R20ZMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal peptidase I (Escherichia coli (strain K12)) | BDBM50469169 (CHEMBL4292925) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli type 1 signal peptidase lepB preincubated for 10 mins followed by Dabcyl-VGGTATAYGAFSRPGLE-(EDANS)-OH substrate additi... | Eur J Med Chem 157: 1346-1360 (2018) Article DOI: 10.1016/j.ejmech.2018.08.086 BindingDB Entry DOI: 10.7270/Q2H70JH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor alpha (Homo sapiens (Human)) | BDBM18896 (3-(3,5-dibromo-4-{[3-(ethylamino)phenyl]methoxy}ph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 78 | n/a | 23 | n/a | n/a | 7.0 | 4 |
Karo Bio AB | Assay Description IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. EC50 is the concentration of compound required to ... | Bioorg Med Chem Lett 17: 2018-21 (2007) Article DOI: 10.1016/j.bmcl.2007.01.009 BindingDB Entry DOI: 10.7270/Q2R20ZMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor beta (Homo sapiens (Human)) | BDBM18897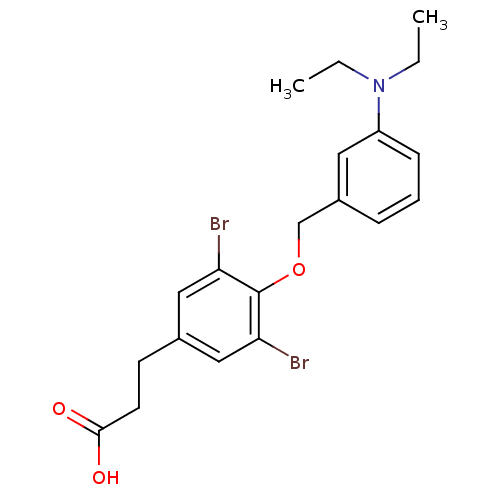 (3-(3,5-dibromo-4-{[3-(diethylamino)phenyl]methoxy}...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 87 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Karo Bio AB | Assay Description IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRbeta. | Bioorg Med Chem Lett 17: 2018-21 (2007) Article DOI: 10.1016/j.bmcl.2007.01.009 BindingDB Entry DOI: 10.7270/Q2R20ZMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor alpha (Homo sapiens (Human)) | BDBM18885 (2-[4-({4-hydroxy-3-[2-(4-nitrophenyl)ethynyl]-5-(p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 93 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Karo Bio AB | Assay Description IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. | J Med Chem 49: 6635-7 (2006) Article DOI: 10.1021/jm060521i BindingDB Entry DOI: 10.7270/Q23B5XDF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor alpha (Homo sapiens (Human)) | BDBM18885 (2-[4-({4-hydroxy-3-[2-(4-nitrophenyl)ethynyl]-5-(p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 93 | n/a | n/a | n/a | n/a | 7.0 | 4 |
Karo Bio AB | Assay Description IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. | Bioorg Med Chem Lett 17: 2018-21 (2007) Article DOI: 10.1016/j.bmcl.2007.01.009 BindingDB Entry DOI: 10.7270/Q2R20ZMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal peptidase I (Escherichia coli (strain K12)) | BDBM50469164 (CHEMBL4287199) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli type 1 signal peptidase lepB preincubated for 10 mins followed by Dabcyl-VGGTATAYGAFSRPGLE-(EDANS)-OH substrate additi... | Eur J Med Chem 157: 1346-1360 (2018) Article DOI: 10.1016/j.ejmech.2018.08.086 BindingDB Entry DOI: 10.7270/Q2H70JH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thyroid hormone receptor alpha (Homo sapiens (Human)) | BDBM18894 (3-{3,5-dibromo-4-[(3-bromophenyl)methoxy]phenyl}pr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 99 | n/a | 380 | n/a | n/a | 7.0 | 4 |
Karo Bio AB | Assay Description IC50 is the concentration of each compound required to inhibit 50% binding of 125I-T3 to hTRalpha. EC50 is the concentration of compound required to ... | Bioorg Med Chem Lett 17: 2018-21 (2007) Article DOI: 10.1016/j.bmcl.2007.01.009 BindingDB Entry DOI: 10.7270/Q2R20ZMS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal peptidase I (Escherichia coli (strain K12)) | BDBM50469160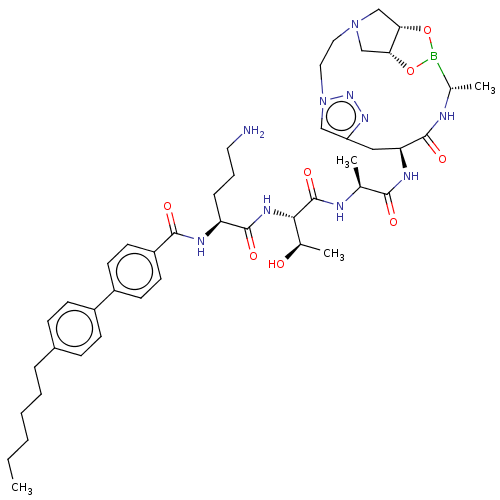 (CHEMBL4280386) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 104 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli type 1 signal peptidase lepB preincubated for 10 mins followed by Dabcyl-VGGTATAYGAFSRPGLE-(EDANS)-OH substrate additi... | Eur J Med Chem 157: 1346-1360 (2018) Article DOI: 10.1016/j.ejmech.2018.08.086 BindingDB Entry DOI: 10.7270/Q2H70JH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Signal peptidase I (Escherichia coli (strain K12)) | BDBM50469167 (CHEMBL4279276) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of Escherichia coli type 1 signal peptidase lepB preincubated for 10 mins followed by Dabcyl-VGGTATAYGAFSRPGLE-(EDANS)-OH substrate additi... | Eur J Med Chem 157: 1346-1360 (2018) Article DOI: 10.1016/j.ejmech.2018.08.086 BindingDB Entry DOI: 10.7270/Q2H70JH1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 96 total ) | Next | Last >> |