

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Homo sapiens (Human)) | BDBM50234795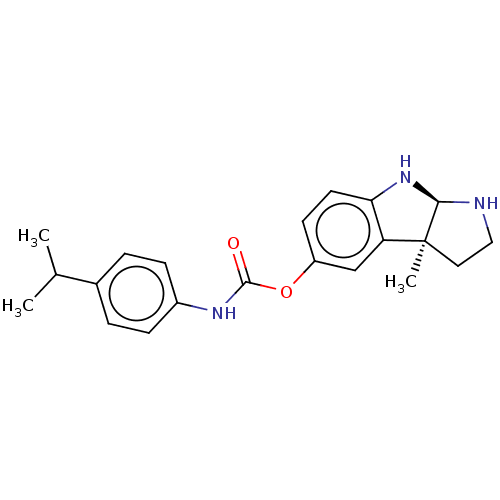 (CHEMBL4089082) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.131 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Inhibition of human serum BChE using butyrylthiocholine iodide as substrate by Ellman's method | Eur J Med Chem 126: 652-668 (2017) Article DOI: 10.1016/j.ejmech.2016.11.056 BindingDB Entry DOI: 10.7270/Q2X0698K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Ability to displace [3H]U-69593 from human recombinant Opioid receptor kappa 1 in CHO cells | J Med Chem 46: 314-7 (2003) Article DOI: 10.1021/jm020997b BindingDB Entry DOI: 10.7270/Q2PK0GW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50122859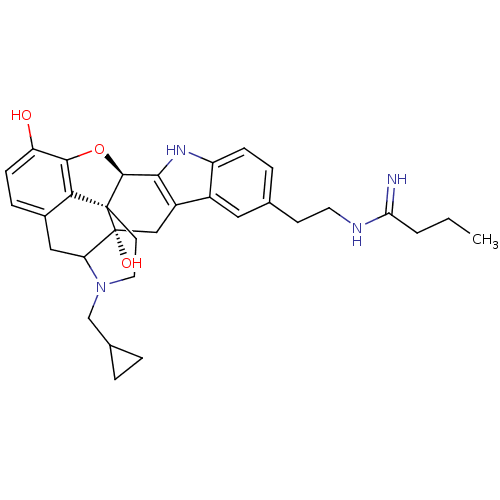 (22-cyclopropylmethyl-7-[2-(1-iminobutylamino)ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Ability to displace [3H]U-69593 from human recombinant Opioid receptor kappa 1 in CHO cells | J Med Chem 46: 314-7 (2003) Article DOI: 10.1021/jm020997b BindingDB Entry DOI: 10.7270/Q2PK0GW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50122869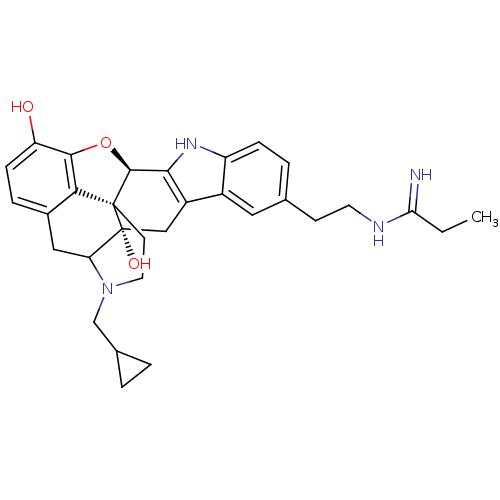 (22-cyclopropylmethyl-7-[2-(1-iminopropylamino)ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Ability to displace [3H]U-69593 from human recombinant Opioid receptor kappa 1 in CHO cells | J Med Chem 46: 314-7 (2003) Article DOI: 10.1021/jm020997b BindingDB Entry DOI: 10.7270/Q2PK0GW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50122862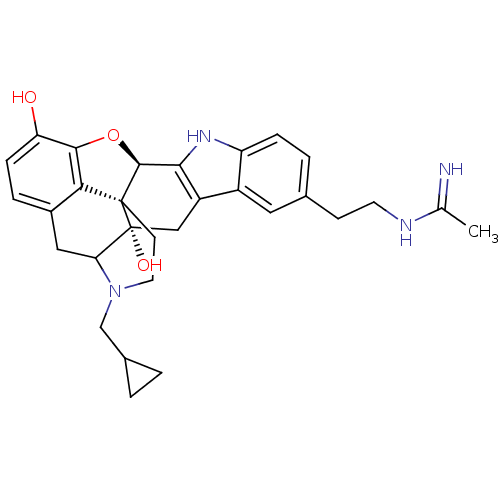 (22-cyclopropylmethyl-7-[2-(1-iminoethylamino)ethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Ability to displace [3H]U-69593 from human recombinant Opioid receptor kappa 1 in CHO cells | J Med Chem 46: 314-7 (2003) Article DOI: 10.1021/jm020997b BindingDB Entry DOI: 10.7270/Q2PK0GW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50122863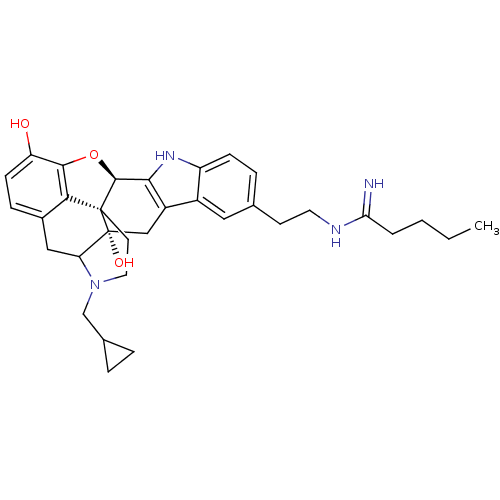 (22-cyclopropylmethyl-7-[2-(1-iminopentylamino)ethy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Ability to displace [3H]U-69593 from human recombinant Opioid receptor kappa 1 in CHO cells | J Med Chem 46: 314-7 (2003) Article DOI: 10.1021/jm020997b BindingDB Entry DOI: 10.7270/Q2PK0GW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50122855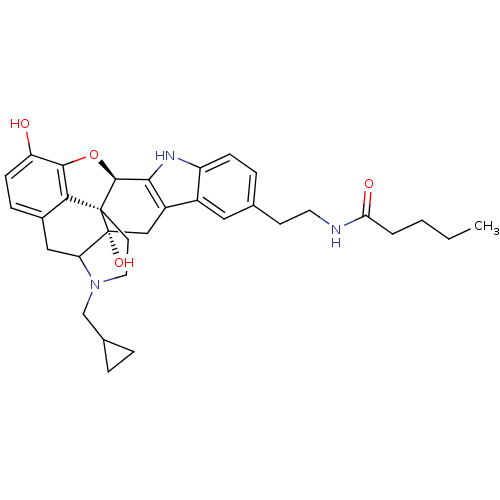 (1N-{2-[22-cyclopropylmethyl-2,16-dihydroxy-14-oxa-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Ability to displace [3H]U-69593 from human recombinant Opioid receptor kappa 1 in CHO cells | J Med Chem 46: 314-7 (2003) Article DOI: 10.1021/jm020997b BindingDB Entry DOI: 10.7270/Q2PK0GW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50122852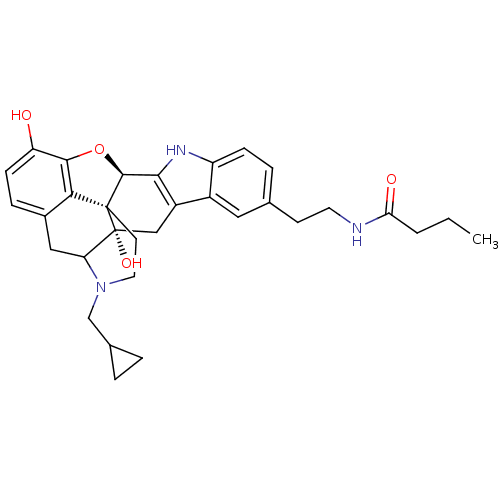 (1N-{2-[22-cyclopropylmethyl-2,16-dihydroxy-14-oxa-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Ability to displace [3H]U-69593 from human recombinant Opioid receptor kappa 1 in CHO cells | J Med Chem 46: 314-7 (2003) Article DOI: 10.1021/jm020997b BindingDB Entry DOI: 10.7270/Q2PK0GW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50041142 ((S)-2-Amino-N-((5S,8S,16aR)-5-benzyl-4,7,13,16-tet...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Binding affinity for mu opioid receptors by displacement of [3H]-DAMGO from rat brain membrane binding site | J Med Chem 37: 1136-44 (1994) BindingDB Entry DOI: 10.7270/Q22Z1650 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50122865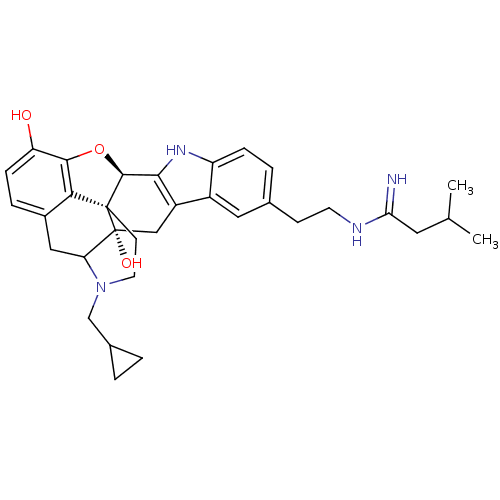 (22-cyclopropylmethyl-7-[2-(1-imino-3-methylbutylam...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Ability to displace [3H]U-69593 from human recombinant Opioid receptor kappa 1 in CHO cells | J Med Chem 46: 314-7 (2003) Article DOI: 10.1021/jm020997b BindingDB Entry DOI: 10.7270/Q2PK0GW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50122856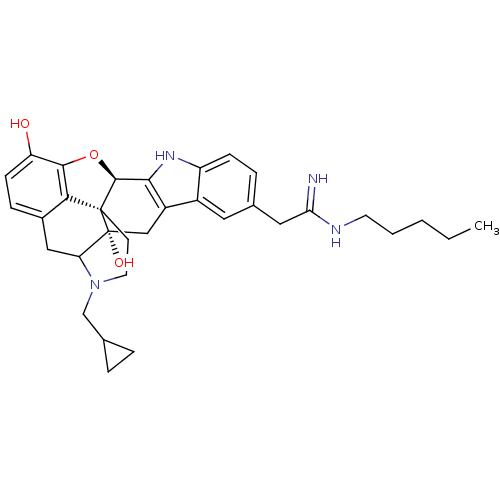 (22-cyclopropylmethyl-7-(2-imino-2-pentylaminoethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Ability to displace [3H]U-69593 from human recombinant Opioid receptor kappa 1 in CHO cells | J Med Chem 46: 314-7 (2003) Article DOI: 10.1021/jm020997b BindingDB Entry DOI: 10.7270/Q2PK0GW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50122860 (1N-{2-[22-cyclopropylmethyl-2,16-dihydroxy-14-oxa-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Ability to displace [3H]U-69593 from human recombinant Opioid receptor kappa 1 in CHO cells | J Med Chem 46: 314-7 (2003) Article DOI: 10.1021/jm020997b BindingDB Entry DOI: 10.7270/Q2PK0GW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50122864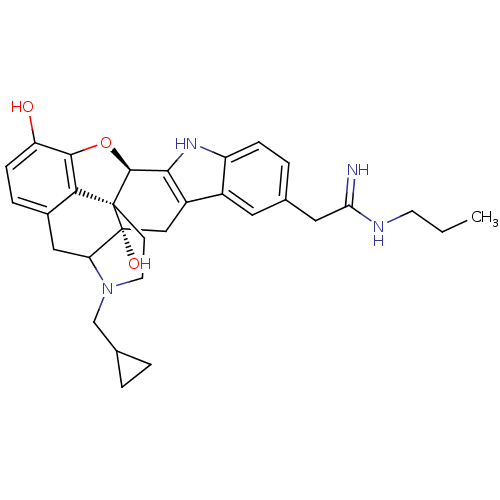 (22-cyclopropylmethyl-7-(2-imino-2-propylaminoethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Ability to displace [3H]U-69593 from human recombinant Opioid receptor kappa 1 in CHO cells | J Med Chem 46: 314-7 (2003) Article DOI: 10.1021/jm020997b BindingDB Entry DOI: 10.7270/Q2PK0GW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50041146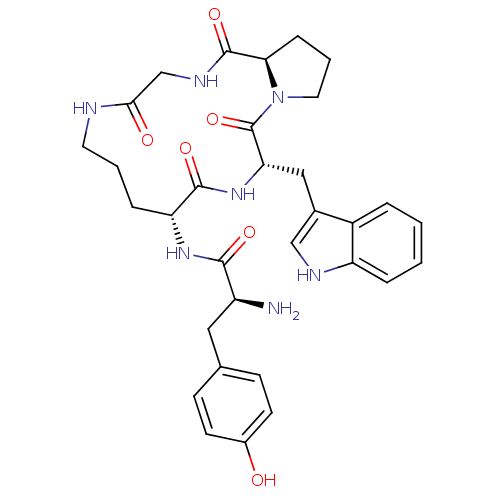 ((S)-2-Amino-3-(4-hydroxy-phenyl)-N-[(5S,8R,16aR)-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Binding affinity for mu opioid receptors by displacement of [3H]-DAMGO from rat brain membrane binding site | J Med Chem 37: 1136-44 (1994) BindingDB Entry DOI: 10.7270/Q22Z1650 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50122851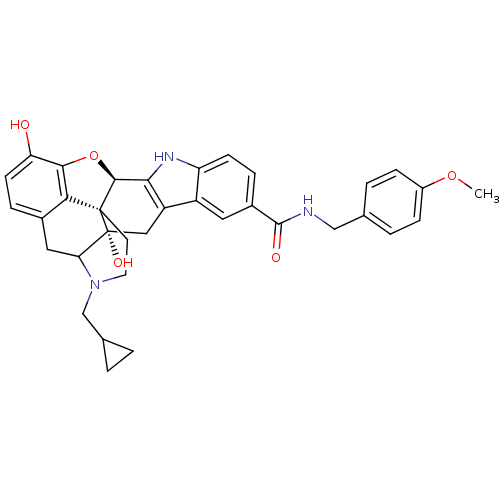 (7N-(4-methoxybenzyl)-22-cyclopropylmethyl-2,16-dih...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Ability to displace [3H]U-69593 from human recombinant Opioid receptor kappa 1 in CHO cells | J Med Chem 46: 314-7 (2003) Article DOI: 10.1021/jm020997b BindingDB Entry DOI: 10.7270/Q2PK0GW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50122853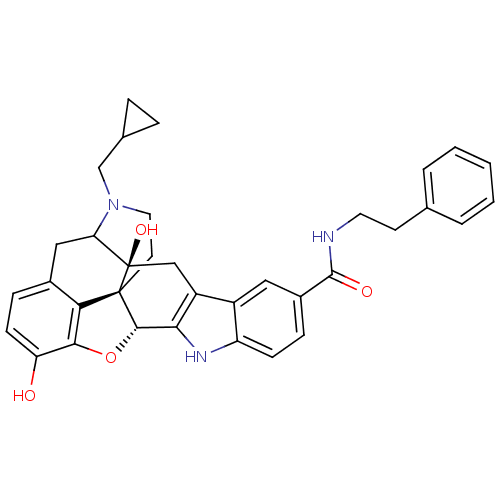 (7N-phenethyl-22-cyclopropylmethyl-2,16-dihydroxy-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Ability to displace [3H]U-69593 from human recombinant Opioid receptor kappa 1 in CHO cells | J Med Chem 46: 314-7 (2003) Article DOI: 10.1021/jm020997b BindingDB Entry DOI: 10.7270/Q2PK0GW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50122857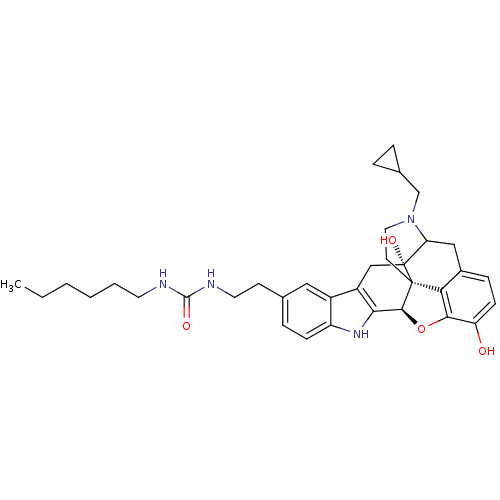 (22-cyclopropylmethyl-7-(6-hexylaminocarbonylaminoe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Ability to displace [3H]DPDPE from human recombinant Opioid receptor delta 1 in CHO cells | J Med Chem 46: 314-7 (2003) Article DOI: 10.1021/jm020997b BindingDB Entry DOI: 10.7270/Q2PK0GW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50122861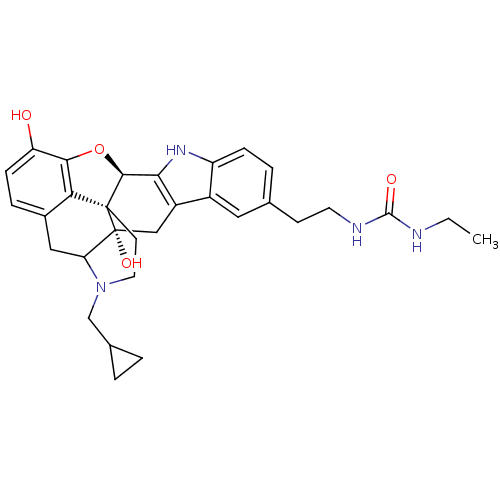 (22-cyclopropylmethyl-7-(2-ethylaminocarbonylaminoe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Ability to displace [3H]DPDPE from human recombinant Opioid receptor delta 1 in CHO cells | J Med Chem 46: 314-7 (2003) Article DOI: 10.1021/jm020997b BindingDB Entry DOI: 10.7270/Q2PK0GW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001465 ((S)-2-[(S)-2-(2-{2-[(S)-2-Amino-3-(4-hydroxy-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Binding affinity for delta opioid receptors was determined by displacement of [3H]DSLET from rat brain membrane binding site | J Med Chem 37: 1136-44 (1994) BindingDB Entry DOI: 10.7270/Q22Z1650 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM50299858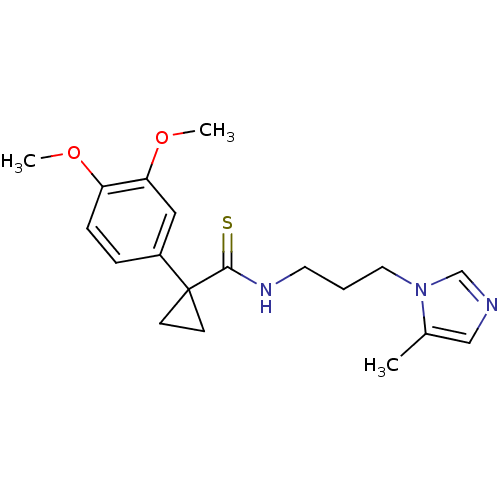 (1-(3,4-Dimethoxyphenyl)-N-(3-(5-methyl-1H-imidazol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Probiodrug AG Curated by ChEMBL | Assay Description Inhibition of human glutaminyl cyclase expressed in Pichia pastoris by pGAP coupled enzyme assay | J Med Chem 52: 7069-80 (2009) Article DOI: 10.1021/jm900969p BindingDB Entry DOI: 10.7270/Q2Q2409C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50122866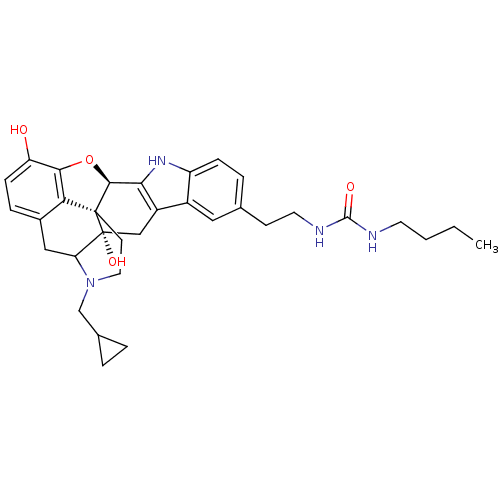 (22-cyclopropylmethyl-7-(4-butylaminocarbonylaminoe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Ability to displace [3H]DPDPE from human recombinant Opioid receptor delta 1 in CHO cells | J Med Chem 46: 314-7 (2003) Article DOI: 10.1021/jm020997b BindingDB Entry DOI: 10.7270/Q2PK0GW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50041148 ((S)-2-Amino-3-(4-hydroxy-phenyl)-N-((5S,8S,13aR)-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Binding affinity for mu opioid receptors by displacement of [3H]-DAMGO from rat brain membrane binding site | J Med Chem 37: 1136-44 (1994) BindingDB Entry DOI: 10.7270/Q22Z1650 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50041144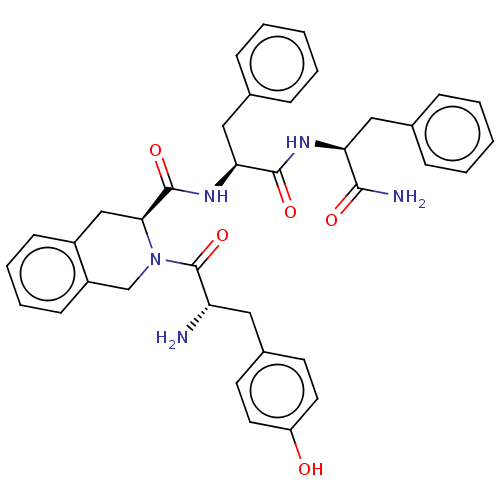 ((S)-2-Amino-N-((5S,8S)-5-benzyl-4,7,13,16-tetraoxo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Binding affinity for mu opioid receptors by displacement of [3H]-DAMGO from rat brain membrane binding site | J Med Chem 37: 1136-44 (1994) BindingDB Entry DOI: 10.7270/Q22Z1650 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50041144 ((S)-2-Amino-N-((5S,8S)-5-benzyl-4,7,13,16-tetraoxo...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Binding affinity for delta opioid receptors was determined by displacement of [3H]DSLET from rat brain membrane binding site | J Med Chem 37: 1136-44 (1994) BindingDB Entry DOI: 10.7270/Q22Z1650 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50122860 (1N-{2-[22-cyclopropylmethyl-2,16-dihydroxy-14-oxa-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Ability to displace [3H]DPDPE from human recombinant Opioid receptor delta 1 in CHO cells | J Med Chem 46: 314-7 (2003) Article DOI: 10.1021/jm020997b BindingDB Entry DOI: 10.7270/Q2PK0GW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50041141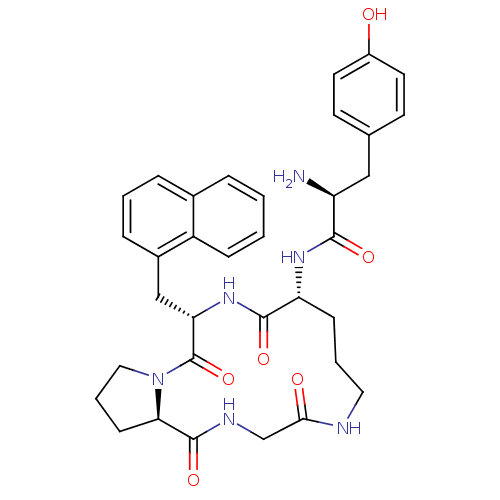 ((S)-2-Amino-3-(4-hydroxy-phenyl)-N-((5S,8R,16aR)-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Binding affinity for mu opioid receptors by displacement of [3H]-DAMGO from rat brain membrane binding site | J Med Chem 37: 1136-44 (1994) BindingDB Entry DOI: 10.7270/Q22Z1650 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50122853 (7N-phenethyl-22-cyclopropylmethyl-2,16-dihydroxy-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Ability to displace [3H]DPDPE from human recombinant Opioid receptor delta 1 in CHO cells | J Med Chem 46: 314-7 (2003) Article DOI: 10.1021/jm020997b BindingDB Entry DOI: 10.7270/Q2PK0GW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50122852 (1N-{2-[22-cyclopropylmethyl-2,16-dihydroxy-14-oxa-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Ability to displace [3H]DPDPE from human recombinant Opioid receptor delta 1 in CHO cells | J Med Chem 46: 314-7 (2003) Article DOI: 10.1021/jm020997b BindingDB Entry DOI: 10.7270/Q2PK0GW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50041145 ((S)-2-Amino-3-(4-hydroxy-phenyl)-N-((5S,8S)-5-naph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Binding affinity for delta opioid receptors was determined by displacement of [3H]DSLET from rat brain membrane binding site | J Med Chem 37: 1136-44 (1994) BindingDB Entry DOI: 10.7270/Q22Z1650 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50041145 ((S)-2-Amino-3-(4-hydroxy-phenyl)-N-((5S,8S)-5-naph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.99 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Binding affinity for mu opioid receptors by displacement of [3H]-DAMGO from rat brain membrane binding site | J Med Chem 37: 1136-44 (1994) BindingDB Entry DOI: 10.7270/Q22Z1650 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50122851 (7N-(4-methoxybenzyl)-22-cyclopropylmethyl-2,16-dih...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Ability to displace [3H]DAMGO from human recombinant Opioid receptor mu 1 in CHO cells | J Med Chem 46: 314-7 (2003) Article DOI: 10.1021/jm020997b BindingDB Entry DOI: 10.7270/Q2PK0GW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50122866 (22-cyclopropylmethyl-7-(4-butylaminocarbonylaminoe...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Ability to displace [3H]DAMGO from human recombinant Opioid receptor mu 1 in CHO cells | J Med Chem 46: 314-7 (2003) Article DOI: 10.1021/jm020997b BindingDB Entry DOI: 10.7270/Q2PK0GW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50122864 (22-cyclopropylmethyl-7-(2-imino-2-propylaminoethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Ability to displace [3H]DPDPE from human recombinant Opioid receptor delta 1 in CHO cells | J Med Chem 46: 314-7 (2003) Article DOI: 10.1021/jm020997b BindingDB Entry DOI: 10.7270/Q2PK0GW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50122858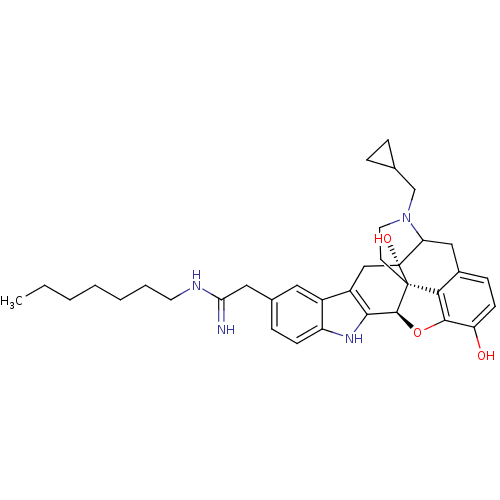 (22-cyclopropylmethyl-7-(2-heptylamino-2-iminoethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Ability to displace [3H]U-69593 from human recombinant Opioid receptor kappa 1 in CHO cells | J Med Chem 46: 314-7 (2003) Article DOI: 10.1021/jm020997b BindingDB Entry DOI: 10.7270/Q2PK0GW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM82551 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Ability to displace [3H]DPDPE from human recombinant Opioid receptor delta 1 in CHO cells | J Med Chem 46: 314-7 (2003) Article DOI: 10.1021/jm020997b BindingDB Entry DOI: 10.7270/Q2PK0GW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50041143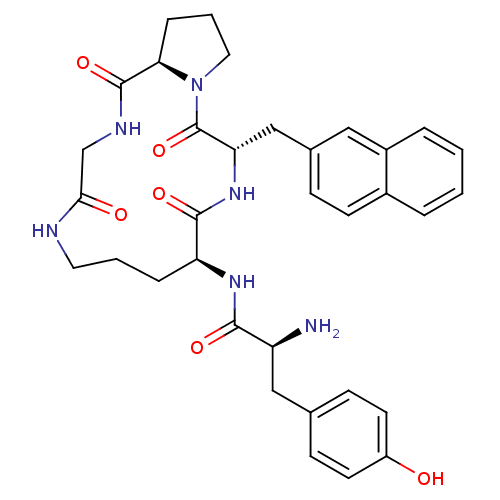 ((S)-2-Amino-3-(4-hydroxy-phenyl)-N-((5S,8S,16aR)-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Binding affinity for mu opioid receptors by displacement of [3H]-DAMGO from rat brain membrane binding site | J Med Chem 37: 1136-44 (1994) BindingDB Entry DOI: 10.7270/Q22Z1650 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50122867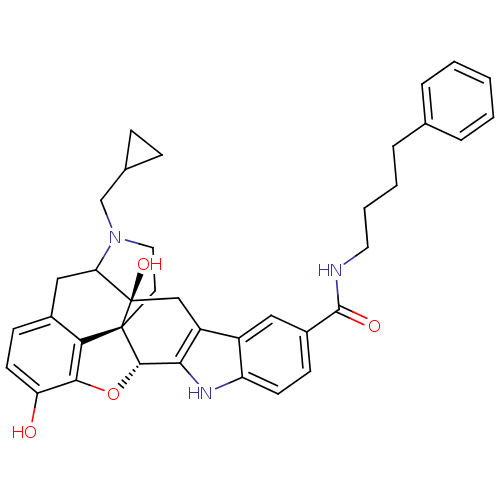 (7N-(4-phenylbutyl)-22-cyclopropylmethyl-2,16-dihyd...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Ability to displace [3H]U-69593 from human recombinant Opioid receptor kappa 1 in CHO cells | J Med Chem 46: 314-7 (2003) Article DOI: 10.1021/jm020997b BindingDB Entry DOI: 10.7270/Q2PK0GW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glutaminyl-peptide cyclotransferase (Homo sapiens (Human)) | BDBM50299853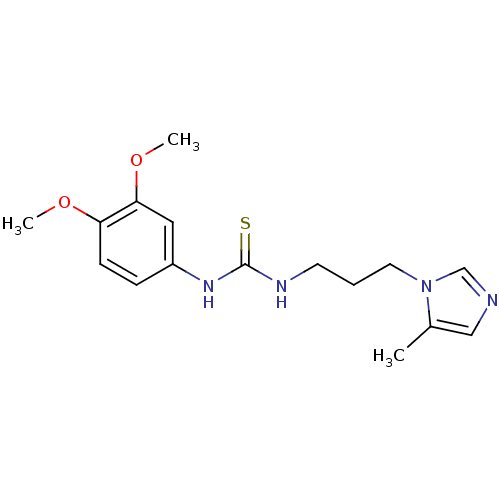 (1-(3,4-Dimethoxyphenyl)-3-(3-(5-methyl-1H-imidazol...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Probiodrug AG Curated by ChEMBL | Assay Description Inhibition of human glutaminyl cyclase expressed in Pichia pastoris by pGAP coupled enzyme assay | J Med Chem 52: 7069-80 (2009) Article DOI: 10.1021/jm900969p BindingDB Entry DOI: 10.7270/Q2Q2409C | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50122866 (22-cyclopropylmethyl-7-(4-butylaminocarbonylaminoe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Ability to displace [3H]U-69593 from human recombinant Opioid receptor kappa 1 in CHO cells | J Med Chem 46: 314-7 (2003) Article DOI: 10.1021/jm020997b BindingDB Entry DOI: 10.7270/Q2PK0GW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50041149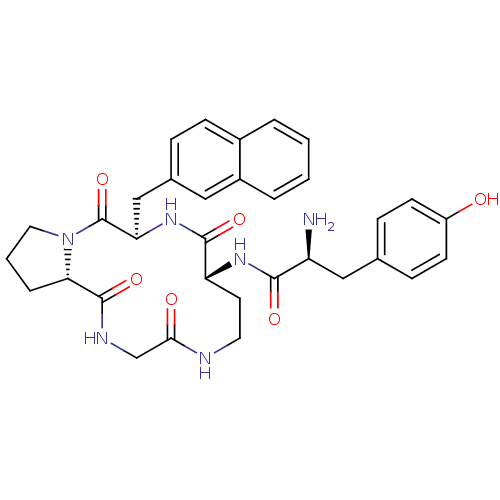 ((S)-2-Amino-3-(4-hydroxy-phenyl)-N-((5R,8R,15aS)-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Binding affinity for mu opioid receptors by displacement of [3H]-DAMGO from rat brain membrane binding site | J Med Chem 37: 1136-44 (1994) BindingDB Entry DOI: 10.7270/Q22Z1650 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50122858 (22-cyclopropylmethyl-7-(2-heptylamino-2-iminoethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Ability to displace [3H]DPDPE from human recombinant Opioid receptor delta 1 in CHO cells | J Med Chem 46: 314-7 (2003) Article DOI: 10.1021/jm020997b BindingDB Entry DOI: 10.7270/Q2PK0GW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50122851 (7N-(4-methoxybenzyl)-22-cyclopropylmethyl-2,16-dih...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Ability to displace [3H]DPDPE from human recombinant Opioid receptor delta 1 in CHO cells | J Med Chem 46: 314-7 (2003) Article DOI: 10.1021/jm020997b BindingDB Entry DOI: 10.7270/Q2PK0GW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50122855 (1N-{2-[22-cyclopropylmethyl-2,16-dihydroxy-14-oxa-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Ability to displace [3H]DPDPE from human recombinant Opioid receptor delta 1 in CHO cells | J Med Chem 46: 314-7 (2003) Article DOI: 10.1021/jm020997b BindingDB Entry DOI: 10.7270/Q2PK0GW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50122857 (22-cyclopropylmethyl-7-(6-hexylaminocarbonylaminoe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Ability to displace [3H]U-69593 from human recombinant Opioid receptor kappa 1 in CHO cells | J Med Chem 46: 314-7 (2003) Article DOI: 10.1021/jm020997b BindingDB Entry DOI: 10.7270/Q2PK0GW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50001465 ((S)-2-[(S)-2-(2-{2-[(S)-2-Amino-3-(4-hydroxy-pheny...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Binding affinity for mu opioid receptors by displacement of [3H]-DAMGO from rat brain membrane binding site | J Med Chem 37: 1136-44 (1994) BindingDB Entry DOI: 10.7270/Q22Z1650 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Rattus norvegicus (rat)) | BDBM50041146 ((S)-2-Amino-3-(4-hydroxy-phenyl)-N-[(5S,8R,16aR)-5...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Clinical Research Institute of Montreal Curated by ChEMBL | Assay Description Binding affinity for delta opioid receptors was determined by displacement of [3H]DSLET from rat brain membrane binding site | J Med Chem 37: 1136-44 (1994) BindingDB Entry DOI: 10.7270/Q22Z1650 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50234798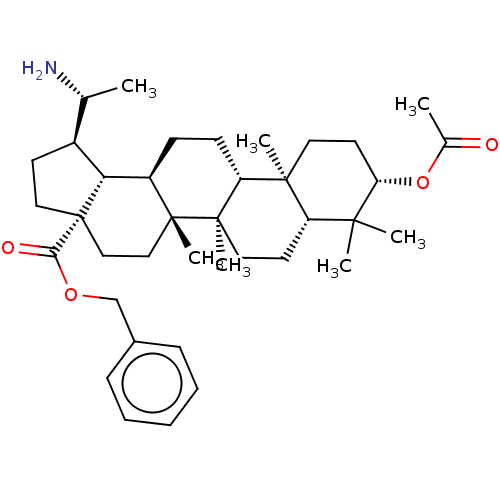 (CHEMBL4091899) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Martin-Luther University Halle-Wittenberg Curated by ChEMBL | Assay Description Competitive inhibition of equine serum BChE using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition meas... | Eur J Med Chem 126: 652-668 (2017) Article DOI: 10.1016/j.ejmech.2016.11.056 BindingDB Entry DOI: 10.7270/Q2X0698K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50122868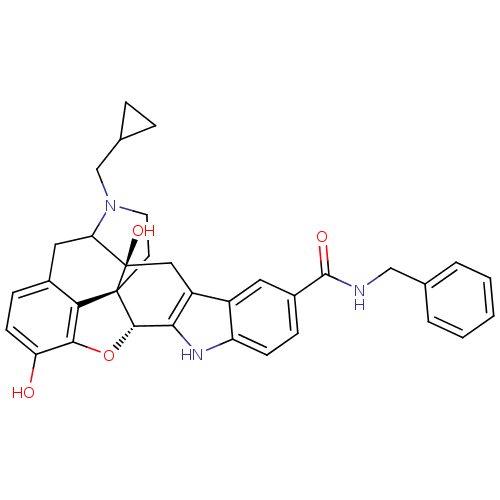 (7N-benzyl-22-cyclopropylmethyl-2,16-dihydroxy-14-o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Ability to displace [3H]U-69593 from human recombinant Opioid receptor kappa 1 in CHO cells | J Med Chem 46: 314-7 (2003) Article DOI: 10.1021/jm020997b BindingDB Entry DOI: 10.7270/Q2PK0GW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50122868 (7N-benzyl-22-cyclopropylmethyl-2,16-dihydroxy-14-o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Ability to displace [3H]DPDPE from human recombinant Opioid receptor delta 1 in CHO cells | J Med Chem 46: 314-7 (2003) Article DOI: 10.1021/jm020997b BindingDB Entry DOI: 10.7270/Q2PK0GW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50122861 (22-cyclopropylmethyl-7-(2-ethylaminocarbonylaminoe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath Curated by ChEMBL | Assay Description Ability to displace [3H]U-69593 from human recombinant Opioid receptor kappa 1 in CHO cells | J Med Chem 46: 314-7 (2003) Article DOI: 10.1021/jm020997b BindingDB Entry DOI: 10.7270/Q2PK0GW4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 383 total ) | Next | Last >> |