 Found 270 hits with Last Name = 'brault' and Initial = 'l'
Found 270 hits with Last Name = 'brault' and Initial = 'l' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | CHEMBL5286547
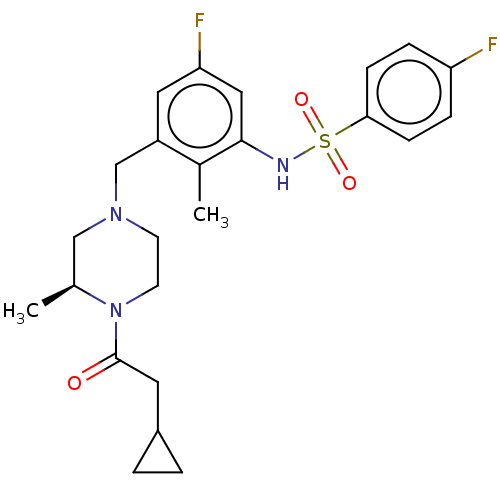
Show InChI InChI=1S/C15H19N3/c1-17-6-7-18-10-15-13(8-11(18)9-17)12-4-2-3-5-14(12)16-15/h2-5,11,16H,6-10H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity against M2 muscarinic receptor in guinea pig left atrium derived by plotting log(DR - 1) vs log[antagonist] |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | CHEMBL5281816
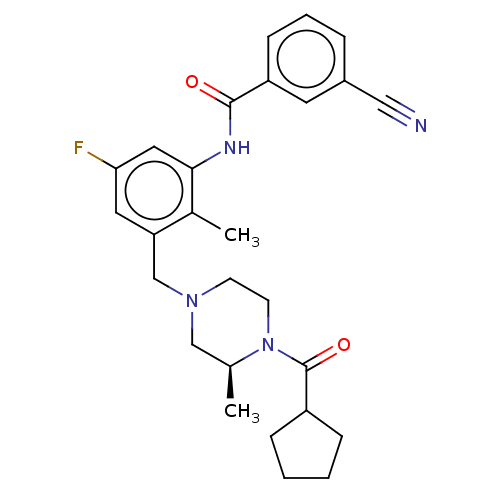
Show InChI InChI=1S/C21H25ClN2O/c22-20-9-5-4-8-19(20)21(25)23-12-15-24-13-10-18(11-14-24)16-17-6-2-1-3-7-17/h1-9,18H,10-16H2,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
| | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity against M2 muscarinic receptor in guinea pig left atrium derived by plotting log(DR - 1) vs log[antagonist] |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | CHEMBL5284779
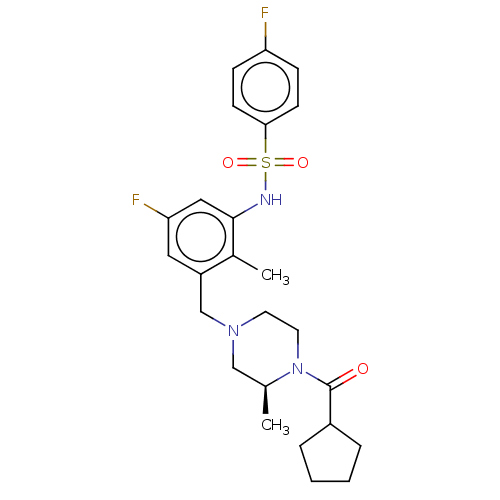
Show InChI InChI=1S/C21H25ClN2O/c22-19-8-4-5-9-20(19)23-21(25)12-15-24-13-10-18(11-14-24)16-17-6-2-1-3-7-17/h1-9,18H,10-16H2,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
| | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity against M2 muscarinic receptor in guinea pig left atrium derived by plotting log(DR - 1) vs log[antagonist] |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | CHEMBL5283506

Show InChI InChI=1S/C21H25N3O/c25-21(22-19-8-2-1-3-9-19)10-11-23-12-13-24-15-18-7-5-4-6-17(18)14-20(24)16-23/h1-9,20H,10-16H2,(H,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity against M2 muscarinic receptor in guinea pig left atrium derived by plotting log(DR - 1) vs log[antagonist] |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | CHEMBL5287800
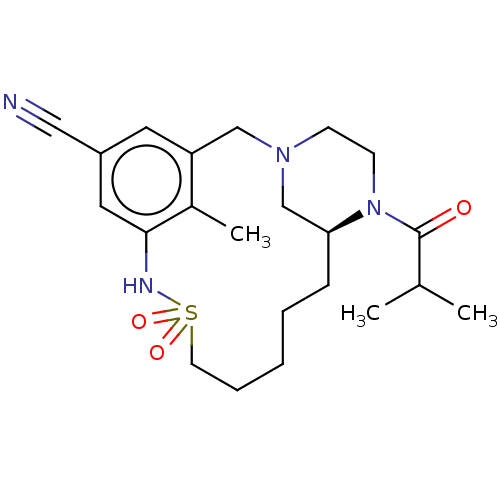
Show InChI InChI=1S/C22H29N3O/c1-2-20-10-6-7-11-21(20)23-22(26)12-13-24-14-16-25(17-15-24)18-19-8-4-3-5-9-19/h3-11H,2,12-18H2,1H3,(H,23,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity against M2 muscarinic receptor in guinea pig left atrium derived by plotting log(DR - 1) vs log[antagonist] |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | CHEMBL5287916
Show InChI InChI=1S/C20H24ClN3O/c21-18-8-4-5-9-19(18)22-20(25)10-11-23-12-14-24(15-13-23)16-17-6-2-1-3-7-17/h1-9H,10-16H2,(H,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
CHEMBL
PC cid
PC sid
UniChem
Similars
| | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity against M2 muscarinic receptor in guinea pig left atrium derived by plotting log(DR - 1) vs log[antagonist] |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | CHEMBL5280788

Show InChI InChI=1S/C21H25BrN2O/c22-20-9-5-4-8-19(20)21(25)23-12-15-24-13-10-18(11-14-24)16-17-6-2-1-3-7-17/h1-9,18H,10-16H2,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
CHEMBL
PC cid
PC sid
UniChem
| | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonistic activity against M2 muscarinic receptor in guinea pig left atrium derived by plotting log(DR - 1) vs log[antagonist] |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Aurora kinase A
(Homo sapiens (Human)) | BDBM81552
(Benzo[e]pyrimido[5,4-b][1,4]diazepin-6(11H)-one, 1...)Show SMILES CN1c2ccccc2C(=O)N(C)c2cnc(Nc3ccc(cc3)N3CCC(O)CC3)nc12 Show InChI InChI=1S/C24H26N6O2/c1-28-20-6-4-3-5-19(20)23(32)29(2)21-15-25-24(27-22(21)28)26-16-7-9-17(10-8-16)30-13-11-18(31)12-14-30/h3-10,15,18,31H,11-14H2,1-2H3,(H,25,26,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
| Assay Description
In vitro biochemical assays were performed in parallel to determine the most potent tool compound. |
Chem Biol 18: 868-79 (2011)
Article DOI: 10.1016/j.chembiol.2011.05.010
BindingDB Entry DOI: 10.7270/Q2HD7T57 |
More data for this
Ligand-Target Pair | |
Aurora kinase B
(Homo sapiens (Human)) | BDBM81552

(Benzo[e]pyrimido[5,4-b][1,4]diazepin-6(11H)-one, 1...)Show SMILES CN1c2ccccc2C(=O)N(C)c2cnc(Nc3ccc(cc3)N3CCC(O)CC3)nc12 Show InChI InChI=1S/C24H26N6O2/c1-28-20-6-4-3-5-19(20)23(32)29(2)21-15-25-24(27-22(21)28)26-16-7-9-17(10-8-16)30-13-11-18(31)12-14-30/h3-10,15,18,31H,11-14H2,1-2H3,(H,25,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
| Assay Description
In vitro biochemical assays were performed in parallel to determine the most potent tool compound. |
Chem Biol 18: 868-79 (2011)
Article DOI: 10.1016/j.chembiol.2011.05.010
BindingDB Entry DOI: 10.7270/Q2HD7T57 |
More data for this
Ligand-Target Pair | |
Aurora kinase C
(Homo sapiens (Human)) | BDBM81552

(Benzo[e]pyrimido[5,4-b][1,4]diazepin-6(11H)-one, 1...)Show SMILES CN1c2ccccc2C(=O)N(C)c2cnc(Nc3ccc(cc3)N3CCC(O)CC3)nc12 Show InChI InChI=1S/C24H26N6O2/c1-28-20-6-4-3-5-19(20)23(32)29(2)21-15-25-24(27-22(21)28)26-16-7-9-17(10-8-16)30-13-11-18(31)12-14-30/h3-10,15,18,31H,11-14H2,1-2H3,(H,25,26,27) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
| Assay Description
In vitro biochemical assays were performed in parallel to determine the most potent tool compound. |
Chem Biol 18: 868-79 (2011)
Article DOI: 10.1016/j.chembiol.2011.05.010
BindingDB Entry DOI: 10.7270/Q2HD7T57 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM81550
(Furan thiazolidinediones, A47)Show SMILES N=C1NC(=O)\C(S1)=C/c1ccc(o1)-c1cncc2ccccc12 Show InChI InChI=1S/C17H11N3O2S/c18-17-20-16(21)15(23-17)7-11-5-6-14(22-11)13-9-19-8-10-3-1-2-4-12(10)13/h1-9H,(H2,18,20,21)/b15-7+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 49 | 40 | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
| Assay Description
In vitro biochemical assays were performed in parallel to determine the most potent tool compound. |
Chem Biol 18: 868-79 (2011)
Article DOI: 10.1016/j.chembiol.2011.05.010
BindingDB Entry DOI: 10.7270/Q2HD7T57 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-2
(Homo sapiens (Human)) | BDBM81550

(Furan thiazolidinediones, A47)Show SMILES N=C1NC(=O)\C(S1)=C/c1ccc(o1)-c1cncc2ccccc12 Show InChI InChI=1S/C17H11N3O2S/c18-17-20-16(21)15(23-17)7-11-5-6-14(22-11)13-9-19-8-10-3-1-2-4-12(10)13/h1-9H,(H2,18,20,21)/b15-7+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 49 | 23 | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
| Assay Description
In vitro biochemical assays were performed in parallel to determine the most potent tool compound. |
Chem Biol 18: 868-79 (2011)
Article DOI: 10.1016/j.chembiol.2011.05.010
BindingDB Entry DOI: 10.7270/Q2HD7T57 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-1
(Homo sapiens (Human)) | BDBM50363167

(CHEMBL1945559)Show SMILES Cn1c2c(C(C#N)C3(CCNCC3)NC2=O)c2ccc(Cl)c(Cl)c12 Show InChI InChI=1S/C17H16Cl2N4O/c1-23-14-9(2-3-11(18)13(14)19)12-10(8-20)17(4-6-21-7-5-17)22-16(24)15(12)23/h2-3,10,21H,4-7H2,1H3,(H,22,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians University of Munich
Curated by ChEMBL
| Assay Description
Inhibition of PIM1 using ATP as substrate |
J Med Chem 55: 403-13 (2012)
Article DOI: 10.1021/jm201286z
BindingDB Entry DOI: 10.7270/Q20G3KK7 |
More data for this
Ligand-Target Pair | |
Homeodomain-interacting protein kinase 2
(Homo sapiens (Human)) | BDBM81551
(Furan thiazolidinediones, A64)Show SMILES N=C1NC(=O)\C(S1)=C/c1c[nH]c(=O)c(c1)-c1ccc(nc1)N1CCNCC1 Show InChI InChI=1S/C18H18N6O2S/c19-18-23-17(26)14(27-18)8-11-7-13(16(25)22-9-11)12-1-2-15(21-10-12)24-5-3-20-4-6-24/h1-2,7-10,20H,3-6H2,(H,22,25)(H2,19,23,26)/b14-8+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 74 | 9.5 | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
| Assay Description
In vitro biochemical assays were performed in parallel to determine the most potent tool compound. |
Chem Biol 18: 868-79 (2011)
Article DOI: 10.1016/j.chembiol.2011.05.010
BindingDB Entry DOI: 10.7270/Q2HD7T57 |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type II subunit beta
(Homo sapiens (Human)) | BDBM50363167

(CHEMBL1945559)Show SMILES Cn1c2c(C(C#N)C3(CCNCC3)NC2=O)c2ccc(Cl)c(Cl)c12 Show InChI InChI=1S/C17H16Cl2N4O/c1-23-14-9(2-3-11(18)13(14)19)12-10(8-20)17(4-6-21-7-5-17)22-16(24)15(12)23/h2-3,10,21H,4-7H2,1H3,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians University of Munich
Curated by ChEMBL
| Assay Description
Inhibition of CaMK2 using ATP as substrate |
J Med Chem 55: 403-13 (2012)
Article DOI: 10.1021/jm201286z
BindingDB Entry DOI: 10.7270/Q20G3KK7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-3
(Homo sapiens (Human)) | BDBM50363167

(CHEMBL1945559)Show SMILES Cn1c2c(C(C#N)C3(CCNCC3)NC2=O)c2ccc(Cl)c(Cl)c12 Show InChI InChI=1S/C17H16Cl2N4O/c1-23-14-9(2-3-11(18)13(14)19)12-10(8-20)17(4-6-21-7-5-17)22-16(24)15(12)23/h2-3,10,21H,4-7H2,1H3,(H,22,24) | KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians University of Munich
Curated by ChEMBL
| Assay Description
Inhibition of PIM3 using ATP as substrate |
J Med Chem 55: 403-13 (2012)
Article DOI: 10.1021/jm201286z
BindingDB Entry DOI: 10.7270/Q20G3KK7 |
More data for this
Ligand-Target Pair | |
Dual specificity protein kinase TTK
(Homo sapiens (Human)) | BDBM36486
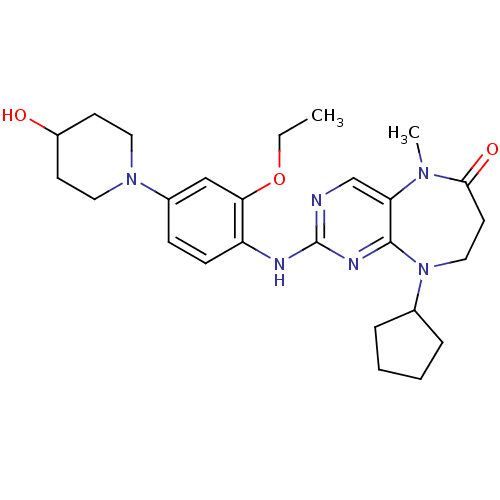
(9-Cyclopentyl-2-(2-ethoxy-4-(4-hydroxypiperidin-1-...)Show SMILES CCOc1cc(ccc1Nc1ncc2N(C)C(=O)CCN(C3CCCC3)c2n1)N1CCC(O)CC1 Show InChI InChI=1S/C26H36N6O3/c1-3-35-23-16-19(31-13-10-20(33)11-14-31)8-9-21(23)28-26-27-17-22-25(29-26)32(18-6-4-5-7-18)15-12-24(34)30(22)2/h8-9,16-18,20,33H,3-7,10-15H2,1-2H3,(H,27,28,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 145 | 26 | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School
| Assay Description
In vitro biochemical assays were performed in parallel to determine the most potent tool compound. |
Chem Biol 18: 868-79 (2011)
Article DOI: 10.1016/j.chembiol.2011.05.010
BindingDB Entry DOI: 10.7270/Q2HD7T57 |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 2
(Homo sapiens (Human)) | BDBM50363167

(CHEMBL1945559)Show SMILES Cn1c2c(C(C#N)C3(CCNCC3)NC2=O)c2ccc(Cl)c(Cl)c12 Show InChI InChI=1S/C17H16Cl2N4O/c1-23-14-9(2-3-11(18)13(14)19)12-10(8-20)17(4-6-21-7-5-17)22-16(24)15(12)23/h2-3,10,21H,4-7H2,1H3,(H,22,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians University of Munich
Curated by ChEMBL
| Assay Description
Inhibition of ROCK2 using ATP as substrate |
J Med Chem 55: 403-13 (2012)
Article DOI: 10.1021/jm201286z
BindingDB Entry DOI: 10.7270/Q20G3KK7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase pim-2
(Homo sapiens (Human)) | BDBM50363167

(CHEMBL1945559)Show SMILES Cn1c2c(C(C#N)C3(CCNCC3)NC2=O)c2ccc(Cl)c(Cl)c12 Show InChI InChI=1S/C17H16Cl2N4O/c1-23-14-9(2-3-11(18)13(14)19)12-10(8-20)17(4-6-21-7-5-17)22-16(24)15(12)23/h2-3,10,21H,4-7H2,1H3,(H,22,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians University of Munich
Curated by ChEMBL
| Assay Description
Inhibition of PIM2 using ATP as substrate |
J Med Chem 55: 403-13 (2012)
Article DOI: 10.1021/jm201286z
BindingDB Entry DOI: 10.7270/Q20G3KK7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase N2
(Homo sapiens (Human)) | BDBM50363167

(CHEMBL1945559)Show SMILES Cn1c2c(C(C#N)C3(CCNCC3)NC2=O)c2ccc(Cl)c(Cl)c12 Show InChI InChI=1S/C17H16Cl2N4O/c1-23-14-9(2-3-11(18)13(14)19)12-10(8-20)17(4-6-21-7-5-17)22-16(24)15(12)23/h2-3,10,21H,4-7H2,1H3,(H,22,24) | PDB
NCI pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians University of Munich
Curated by ChEMBL
| Assay Description
Inhibition of PKN2 using ATP as substrate |
J Med Chem 55: 403-13 (2012)
Article DOI: 10.1021/jm201286z
BindingDB Entry DOI: 10.7270/Q20G3KK7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase N1
(Homo sapiens (Human)) | BDBM50363167

(CHEMBL1945559)Show SMILES Cn1c2c(C(C#N)C3(CCNCC3)NC2=O)c2ccc(Cl)c(Cl)c12 Show InChI InChI=1S/C17H16Cl2N4O/c1-23-14-9(2-3-11(18)13(14)19)12-10(8-20)17(4-6-21-7-5-17)22-16(24)15(12)23/h2-3,10,21H,4-7H2,1H3,(H,22,24) | PDB
MMDB
NCI pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians University of Munich
Curated by ChEMBL
| Assay Description
Inhibition of PKN1 using ATP as substrate |
J Med Chem 55: 403-13 (2012)
Article DOI: 10.1021/jm201286z
BindingDB Entry DOI: 10.7270/Q20G3KK7 |
More data for this
Ligand-Target Pair | |
Casein kinase I isoform alpha
(Homo sapiens (Human)) | BDBM50363167

(CHEMBL1945559)Show SMILES Cn1c2c(C(C#N)C3(CCNCC3)NC2=O)c2ccc(Cl)c(Cl)c12 Show InChI InChI=1S/C17H16Cl2N4O/c1-23-14-9(2-3-11(18)13(14)19)12-10(8-20)17(4-6-21-7-5-17)22-16(24)15(12)23/h2-3,10,21H,4-7H2,1H3,(H,22,24) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians University of Munich
Curated by ChEMBL
| Assay Description
Inhibition of CK1alpha using ATP as substrate |
J Med Chem 55: 403-13 (2012)
Article DOI: 10.1021/jm201286z
BindingDB Entry DOI: 10.7270/Q20G3KK7 |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily M member 5
(Homo sapiens (Human)) | BDBM50602137
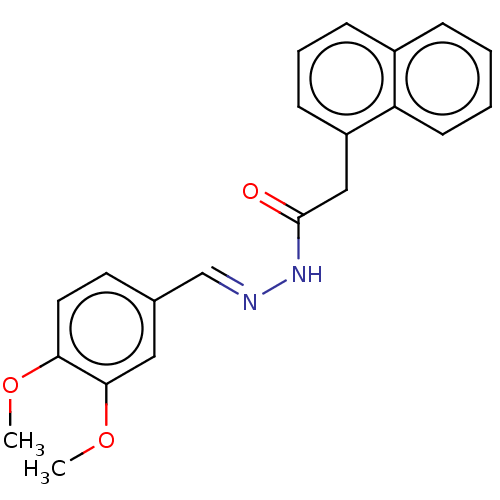
(CHEMBL5196998) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.117084
BindingDB Entry DOI: 10.7270/Q2XW4PWD |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily M member 5
(Homo sapiens (Human)) | BDBM50602136

(CHEMBL5183139) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.bmc.2022.117084
BindingDB Entry DOI: 10.7270/Q2XW4PWD |
More data for this
Ligand-Target Pair | |
Protein kinase C theta type
(Homo sapiens (Human)) | BDBM50363167

(CHEMBL1945559)Show SMILES Cn1c2c(C(C#N)C3(CCNCC3)NC2=O)c2ccc(Cl)c(Cl)c12 Show InChI InChI=1S/C17H16Cl2N4O/c1-23-14-9(2-3-11(18)13(14)19)12-10(8-20)17(4-6-21-7-5-17)22-16(24)15(12)23/h2-3,10,21H,4-7H2,1H3,(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians University of Munich
Curated by ChEMBL
| Assay Description
Inhibition of PKCtheta using ATP as substrate |
J Med Chem 55: 403-13 (2012)
Article DOI: 10.1021/jm201286z
BindingDB Entry DOI: 10.7270/Q20G3KK7 |
More data for this
Ligand-Target Pair | |
MAP kinase-activated protein kinase 5
(Homo sapiens (Human)) | BDBM50363167

(CHEMBL1945559)Show SMILES Cn1c2c(C(C#N)C3(CCNCC3)NC2=O)c2ccc(Cl)c(Cl)c12 Show InChI InChI=1S/C17H16Cl2N4O/c1-23-14-9(2-3-11(18)13(14)19)12-10(8-20)17(4-6-21-7-5-17)22-16(24)15(12)23/h2-3,10,21H,4-7H2,1H3,(H,22,24) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians University of Munich
Curated by ChEMBL
| Assay Description
Inhibition of MK5 using ATP as substrate |
J Med Chem 55: 403-13 (2012)
Article DOI: 10.1021/jm201286z
BindingDB Entry DOI: 10.7270/Q20G3KK7 |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM50363167

(CHEMBL1945559)Show SMILES Cn1c2c(C(C#N)C3(CCNCC3)NC2=O)c2ccc(Cl)c(Cl)c12 Show InChI InChI=1S/C17H16Cl2N4O/c1-23-14-9(2-3-11(18)13(14)19)12-10(8-20)17(4-6-21-7-5-17)22-16(24)15(12)23/h2-3,10,21H,4-7H2,1H3,(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians University of Munich
Curated by ChEMBL
| Assay Description
Inhibition of PKCalpha using ATP as substrate |
J Med Chem 55: 403-13 (2012)
Article DOI: 10.1021/jm201286z
BindingDB Entry DOI: 10.7270/Q20G3KK7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4-kinase beta
(Homo sapiens (Human)) | BDBM50363167

(CHEMBL1945559)Show SMILES Cn1c2c(C(C#N)C3(CCNCC3)NC2=O)c2ccc(Cl)c(Cl)c12 Show InChI InChI=1S/C17H16Cl2N4O/c1-23-14-9(2-3-11(18)13(14)19)12-10(8-20)17(4-6-21-7-5-17)22-16(24)15(12)23/h2-3,10,21H,4-7H2,1H3,(H,22,24) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians University of Munich
Curated by ChEMBL
| Assay Description
Inhibition of PI4Kbeta using ATP as substrate |
J Med Chem 55: 403-13 (2012)
Article DOI: 10.1021/jm201286z
BindingDB Entry DOI: 10.7270/Q20G3KK7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50363167

(CHEMBL1945559)Show SMILES Cn1c2c(C(C#N)C3(CCNCC3)NC2=O)c2ccc(Cl)c(Cl)c12 Show InChI InChI=1S/C17H16Cl2N4O/c1-23-14-9(2-3-11(18)13(14)19)12-10(8-20)17(4-6-21-7-5-17)22-16(24)15(12)23/h2-3,10,21H,4-7H2,1H3,(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians University of Munich
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma using ATP as substrate |
J Med Chem 55: 403-13 (2012)
Article DOI: 10.1021/jm201286z
BindingDB Entry DOI: 10.7270/Q20G3KK7 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50197854

(3-(4-heptadecyl-2,5-dioxo-2,5-dihydrofuran-3-yl)pr...)Show SMILES CCCCCCCCCCCCCCCCCC1=C(CCC(O)=O)C(=O)OC1=O |c:17| Show InChI InChI=1S/C24H40O5/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-20-21(18-19-22(25)26)24(28)29-23(20)27/h2-19H2,1H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paul Verlaine-Metz
Curated by ChEMBL
| Assay Description
Inhibition of human GST-Cdc25B expressed in Escherichia coli BL21(DE3) |
Eur J Med Chem 42: 243-7 (2007)
Article DOI: 10.1016/j.ejmech.2006.09.014
BindingDB Entry DOI: 10.7270/Q29Z94J9 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50197854

(3-(4-heptadecyl-2,5-dioxo-2,5-dihydrofuran-3-yl)pr...)Show SMILES CCCCCCCCCCCCCCCCCC1=C(CCC(O)=O)C(=O)OC1=O |c:17| Show InChI InChI=1S/C24H40O5/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-20-21(18-19-22(25)26)24(28)29-23(20)27/h2-19H2,1H3,(H,25,26) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paul Verlaine-Metz
Curated by ChEMBL
| Assay Description
Inhibition of human GST-Cdc25C expressed in Escherichia coli BL21(DE3) |
Eur J Med Chem 42: 243-7 (2007)
Article DOI: 10.1016/j.ejmech.2006.09.014
BindingDB Entry DOI: 10.7270/Q29Z94J9 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 1
(Homo sapiens (Human)) | BDBM50197852

((Z )-3-(4-(heptadec-8-enyl)-2,5-dioxo-2,5-dihydrof...)Show SMILES CCCCCCCC\C=C/CCCCCCCC1=C(CCC(O)=O)C(=O)OC1=O |c:17| Show InChI InChI=1S/C24H38O5/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-20-21(18-19-22(25)26)24(28)29-23(20)27/h9-10H,2-8,11-19H2,1H3,(H,25,26)/b10-9- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paul Verlaine-Metz
Curated by ChEMBL
| Assay Description
Inhibition of human GST-Cdc25A expressed in Escherichia coli BL21(DE3) |
Eur J Med Chem 42: 243-7 (2007)
Article DOI: 10.1016/j.ejmech.2006.09.014
BindingDB Entry DOI: 10.7270/Q29Z94J9 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 1
(Homo sapiens (Human)) | BDBM50197856

(3-(4-pentadecyl-2,5-dioxo-2,5-dihydrofuran-3-yl)pr...)Show SMILES CCCCCCCCCCCCCCCC1=C(CCC(O)=O)C(=O)OC1=O |c:15| Show InChI InChI=1S/C22H36O5/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-18-19(16-17-20(23)24)22(26)27-21(18)25/h2-17H2,1H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paul Verlaine-Metz
Curated by ChEMBL
| Assay Description
Inhibition of human GST-Cdc25A expressed in Escherichia coli BL21(DE3) |
Eur J Med Chem 42: 243-7 (2007)
Article DOI: 10.1016/j.ejmech.2006.09.014
BindingDB Entry DOI: 10.7270/Q29Z94J9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50363167

(CHEMBL1945559)Show SMILES Cn1c2c(C(C#N)C3(CCNCC3)NC2=O)c2ccc(Cl)c(Cl)c12 Show InChI InChI=1S/C17H16Cl2N4O/c1-23-14-9(2-3-11(18)13(14)19)12-10(8-20)17(4-6-21-7-5-17)22-16(24)15(12)23/h2-3,10,21H,4-7H2,1H3,(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians University of Munich
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta using ATP as substrate |
J Med Chem 55: 403-13 (2012)
Article DOI: 10.1021/jm201286z
BindingDB Entry DOI: 10.7270/Q20G3KK7 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 1
(Homo sapiens (Human)) | BDBM50197854

(3-(4-heptadecyl-2,5-dioxo-2,5-dihydrofuran-3-yl)pr...)Show SMILES CCCCCCCCCCCCCCCCCC1=C(CCC(O)=O)C(=O)OC1=O |c:17| Show InChI InChI=1S/C24H40O5/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-20-21(18-19-22(25)26)24(28)29-23(20)27/h2-19H2,1H3,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paul Verlaine-Metz
Curated by ChEMBL
| Assay Description
Inhibition of human GST-Cdc25A expressed in Escherichia coli BL21(DE3) |
Eur J Med Chem 42: 243-7 (2007)
Article DOI: 10.1016/j.ejmech.2006.09.014
BindingDB Entry DOI: 10.7270/Q29Z94J9 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50197848

(3-(4-tridecyl-2,5-dioxo-2,5-dihydrofuran-3-yl)prop...)Show SMILES CCCCCCCCCCCCCC1=C(CCC(O)=O)C(=O)OC1=O |c:13| Show InChI InChI=1S/C20H32O5/c1-2-3-4-5-6-7-8-9-10-11-12-13-16-17(14-15-18(21)22)20(24)25-19(16)23/h2-15H2,1H3,(H,21,22) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paul Verlaine-Metz
Curated by ChEMBL
| Assay Description
Inhibition of human GST-Cdc25C expressed in Escherichia coli BL21(DE3) |
Eur J Med Chem 42: 243-7 (2007)
Article DOI: 10.1016/j.ejmech.2006.09.014
BindingDB Entry DOI: 10.7270/Q29Z94J9 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 1
(Homo sapiens (Human)) | BDBM50197855

(3-(4-tetradecyl-2,5-dioxo-2,5-dihydrofuran-3-yl)pr...)Show SMILES CCCCCCCCCCCCCCC1=C(CCC(O)=O)C(=O)OC1=O |c:14| Show InChI InChI=1S/C21H34O5/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-17-18(15-16-19(22)23)21(25)26-20(17)24/h2-16H2,1H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paul Verlaine-Metz
Curated by ChEMBL
| Assay Description
Inhibition of human GST-Cdc25A expressed in Escherichia coli BL21(DE3) |
Eur J Med Chem 42: 243-7 (2007)
Article DOI: 10.1016/j.ejmech.2006.09.014
BindingDB Entry DOI: 10.7270/Q29Z94J9 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50197852

((Z )-3-(4-(heptadec-8-enyl)-2,5-dioxo-2,5-dihydrof...)Show SMILES CCCCCCCC\C=C/CCCCCCCC1=C(CCC(O)=O)C(=O)OC1=O |c:17| Show InChI InChI=1S/C24H38O5/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-20-21(18-19-22(25)26)24(28)29-23(20)27/h9-10H,2-8,11-19H2,1H3,(H,25,26)/b10-9- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paul Verlaine-Metz
Curated by ChEMBL
| Assay Description
Inhibition of human GST-Cdc25B expressed in Escherichia coli BL21(DE3) |
Eur J Med Chem 42: 243-7 (2007)
Article DOI: 10.1016/j.ejmech.2006.09.014
BindingDB Entry DOI: 10.7270/Q29Z94J9 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 2
(Homo sapiens (Human)) | BDBM50363167

(CHEMBL1945559)Show SMILES Cn1c2c(C(C#N)C3(CCNCC3)NC2=O)c2ccc(Cl)c(Cl)c12 Show InChI InChI=1S/C17H16Cl2N4O/c1-23-14-9(2-3-11(18)13(14)19)12-10(8-20)17(4-6-21-7-5-17)22-16(24)15(12)23/h2-3,10,21H,4-7H2,1H3,(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians University of Munich
Curated by ChEMBL
| Assay Description
Inhibition of KDR using ATP as substrate |
J Med Chem 55: 403-13 (2012)
Article DOI: 10.1021/jm201286z
BindingDB Entry DOI: 10.7270/Q20G3KK7 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50197856

(3-(4-pentadecyl-2,5-dioxo-2,5-dihydrofuran-3-yl)pr...)Show SMILES CCCCCCCCCCCCCCCC1=C(CCC(O)=O)C(=O)OC1=O |c:15| Show InChI InChI=1S/C22H36O5/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-18-19(16-17-20(23)24)22(26)27-21(18)25/h2-17H2,1H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paul Verlaine-Metz
Curated by ChEMBL
| Assay Description
Inhibition of human GST-Cdc25B expressed in Escherichia coli BL21(DE3) |
Eur J Med Chem 42: 243-7 (2007)
Article DOI: 10.1016/j.ejmech.2006.09.014
BindingDB Entry DOI: 10.7270/Q29Z94J9 |
More data for this
Ligand-Target Pair | |
Proto-oncogene tyrosine-protein kinase receptor Ret
(Homo sapiens (Human)) | BDBM50363167

(CHEMBL1945559)Show SMILES Cn1c2c(C(C#N)C3(CCNCC3)NC2=O)c2ccc(Cl)c(Cl)c12 Show InChI InChI=1S/C17H16Cl2N4O/c1-23-14-9(2-3-11(18)13(14)19)12-10(8-20)17(4-6-21-7-5-17)22-16(24)15(12)23/h2-3,10,21H,4-7H2,1H3,(H,22,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians University of Munich
Curated by ChEMBL
| Assay Description
Inhibition of RET using ATP as substrate |
J Med Chem 55: 403-13 (2012)
Article DOI: 10.1021/jm201286z
BindingDB Entry DOI: 10.7270/Q20G3KK7 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 1
(Homo sapiens (Human)) | BDBM50197848

(3-(4-tridecyl-2,5-dioxo-2,5-dihydrofuran-3-yl)prop...)Show SMILES CCCCCCCCCCCCCC1=C(CCC(O)=O)C(=O)OC1=O |c:13| Show InChI InChI=1S/C20H32O5/c1-2-3-4-5-6-7-8-9-10-11-12-13-16-17(14-15-18(21)22)20(24)25-19(16)23/h2-15H2,1H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paul Verlaine-Metz
Curated by ChEMBL
| Assay Description
Inhibition of human GST-Cdc25A expressed in Escherichia coli BL21(DE3) |
Eur J Med Chem 42: 243-7 (2007)
Article DOI: 10.1016/j.ejmech.2006.09.014
BindingDB Entry DOI: 10.7270/Q29Z94J9 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50197856

(3-(4-pentadecyl-2,5-dioxo-2,5-dihydrofuran-3-yl)pr...)Show SMILES CCCCCCCCCCCCCCCC1=C(CCC(O)=O)C(=O)OC1=O |c:15| Show InChI InChI=1S/C22H36O5/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-18-19(16-17-20(23)24)22(26)27-21(18)25/h2-17H2,1H3,(H,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paul Verlaine-Metz
Curated by ChEMBL
| Assay Description
Inhibition of human GST-Cdc25C expressed in Escherichia coli BL21(DE3) |
Eur J Med Chem 42: 243-7 (2007)
Article DOI: 10.1016/j.ejmech.2006.09.014
BindingDB Entry DOI: 10.7270/Q29Z94J9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50363167

(CHEMBL1945559)Show SMILES Cn1c2c(C(C#N)C3(CCNCC3)NC2=O)c2ccc(Cl)c(Cl)c12 Show InChI InChI=1S/C17H16Cl2N4O/c1-23-14-9(2-3-11(18)13(14)19)12-10(8-20)17(4-6-21-7-5-17)22-16(24)15(12)23/h2-3,10,21H,4-7H2,1H3,(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians University of Munich
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta using ATP as substrate |
J Med Chem 55: 403-13 (2012)
Article DOI: 10.1021/jm201286z
BindingDB Entry DOI: 10.7270/Q20G3KK7 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 2
(Homo sapiens (Human)) | BDBM50197848

(3-(4-tridecyl-2,5-dioxo-2,5-dihydrofuran-3-yl)prop...)Show SMILES CCCCCCCCCCCCCC1=C(CCC(O)=O)C(=O)OC1=O |c:13| Show InChI InChI=1S/C20H32O5/c1-2-3-4-5-6-7-8-9-10-11-12-13-16-17(14-15-18(21)22)20(24)25-19(16)23/h2-15H2,1H3,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paul Verlaine-Metz
Curated by ChEMBL
| Assay Description
Inhibition of human GST-Cdc25B expressed in Escherichia coli BL21(DE3) |
Eur J Med Chem 42: 243-7 (2007)
Article DOI: 10.1016/j.ejmech.2006.09.014
BindingDB Entry DOI: 10.7270/Q29Z94J9 |
More data for this
Ligand-Target Pair | |
M-phase inducer phosphatase 3
(Homo sapiens (Human)) | BDBM50197852

((Z )-3-(4-(heptadec-8-enyl)-2,5-dioxo-2,5-dihydrof...)Show SMILES CCCCCCCC\C=C/CCCCCCCC1=C(CCC(O)=O)C(=O)OC1=O |c:17| Show InChI InChI=1S/C24H38O5/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-20-21(18-19-22(25)26)24(28)29-23(20)27/h9-10H,2-8,11-19H2,1H3,(H,25,26)/b10-9- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université Paul Verlaine-Metz
Curated by ChEMBL
| Assay Description
Inhibition of human GST-Cdc25C expressed in Escherichia coli BL21(DE3) |
Eur J Med Chem 42: 243-7 (2007)
Article DOI: 10.1016/j.ejmech.2006.09.014
BindingDB Entry DOI: 10.7270/Q29Z94J9 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 3-kinase catalytic subunit type 3
(Homo sapiens (Human)) | BDBM50363167

(CHEMBL1945559)Show SMILES Cn1c2c(C(C#N)C3(CCNCC3)NC2=O)c2ccc(Cl)c(Cl)c12 Show InChI InChI=1S/C17H16Cl2N4O/c1-23-14-9(2-3-11(18)13(14)19)12-10(8-20)17(4-6-21-7-5-17)22-16(24)15(12)23/h2-3,10,21H,4-7H2,1H3,(H,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians University of Munich
Curated by ChEMBL
| Assay Description
Inhibition of VPS34 using ATP as substrate |
J Med Chem 55: 403-13 (2012)
Article DOI: 10.1021/jm201286z
BindingDB Entry DOI: 10.7270/Q20G3KK7 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50363167

(CHEMBL1945559)Show SMILES Cn1c2c(C(C#N)C3(CCNCC3)NC2=O)c2ccc(Cl)c(Cl)c12 Show InChI InChI=1S/C17H16Cl2N4O/c1-23-14-9(2-3-11(18)13(14)19)12-10(8-20)17(4-6-21-7-5-17)22-16(24)15(12)23/h2-3,10,21H,4-7H2,1H3,(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians University of Munich
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha using ATP as substrate |
J Med Chem 55: 403-13 (2012)
Article DOI: 10.1021/jm201286z
BindingDB Entry DOI: 10.7270/Q20G3KK7 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50363167

(CHEMBL1945559)Show SMILES Cn1c2c(C(C#N)C3(CCNCC3)NC2=O)c2ccc(Cl)c(Cl)c12 Show InChI InChI=1S/C17H16Cl2N4O/c1-23-14-9(2-3-11(18)13(14)19)12-10(8-20)17(4-6-21-7-5-17)22-16(24)15(12)23/h2-3,10,21H,4-7H2,1H3,(H,22,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians University of Munich
Curated by ChEMBL
| Assay Description
Inhibition of mTOR using ATP as substrate |
J Med Chem 55: 403-13 (2012)
Article DOI: 10.1021/jm201286z
BindingDB Entry DOI: 10.7270/Q20G3KK7 |
More data for this
Ligand-Target Pair | |
Receptor-type tyrosine-protein kinase FLT3
(Homo sapiens (Human)) | BDBM50363167

(CHEMBL1945559)Show SMILES Cn1c2c(C(C#N)C3(CCNCC3)NC2=O)c2ccc(Cl)c(Cl)c12 Show InChI InChI=1S/C17H16Cl2N4O/c1-23-14-9(2-3-11(18)13(14)19)12-10(8-20)17(4-6-21-7-5-17)22-16(24)15(12)23/h2-3,10,21H,4-7H2,1H3,(H,22,24) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ludwig-Maximilians University of Munich
Curated by ChEMBL
| Assay Description
Inhibition of FLT3 using ATP as substrate |
J Med Chem 55: 403-13 (2012)
Article DOI: 10.1021/jm201286z
BindingDB Entry DOI: 10.7270/Q20G3KK7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data