 Found 301 hits with Last Name = 'brocklehurst' and Initial = 'kj'
Found 301 hits with Last Name = 'brocklehurst' and Initial = 'kj' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50433856
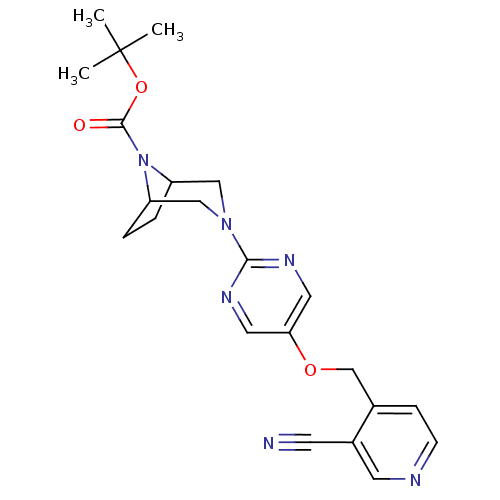
(CHEMBL2382410)Show SMILES CC(C)(C)OC(=O)N1C2CCC1CN(C2)c1ncc(OCc2ccncc2C#N)cn1 Show InChI InChI=1S/C22H26N6O3/c1-22(2,3)31-21(29)28-17-4-5-18(28)13-27(12-17)20-25-10-19(11-26-20)30-14-15-6-7-24-9-16(15)8-23/h6-7,9-11,17-18H,4-5,12-14H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-(2-fluoro-4-methylsulfonyl-phenyl)-6-[4-(3-isopropyl-1,2,4-oxadiazol-5-yl)-1-piperidyl]-5-nitro-pyrimidin-4-amine from human G... |
Bioorg Med Chem Lett 23: 3175-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.006
BindingDB Entry DOI: 10.7270/Q25Q4XGC |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50420842

(CHEMBL2086684)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccncc2C#N)cn1)C(=O)OC(C)(C)C |r| Show InChI InChI=1S/C21H26N6O3/c1-15-13-26(20(28)30-21(2,3)4)7-8-27(15)19-24-11-18(12-25-19)29-14-16-5-6-23-10-17(16)9-22/h5-6,10-12,15H,7-8,13-14H2,1-4H3/t15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-(2-fluoro-4-methylsulfonyl-phenyl)-6-[4-(3-isopropyl-1,2,4-oxadiazol-5-yl)-1-piperidyl]-5-nitro-pyrimidin-4-amine from human G... |
Bioorg Med Chem Lett 23: 3175-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.006
BindingDB Entry DOI: 10.7270/Q25Q4XGC |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50433861
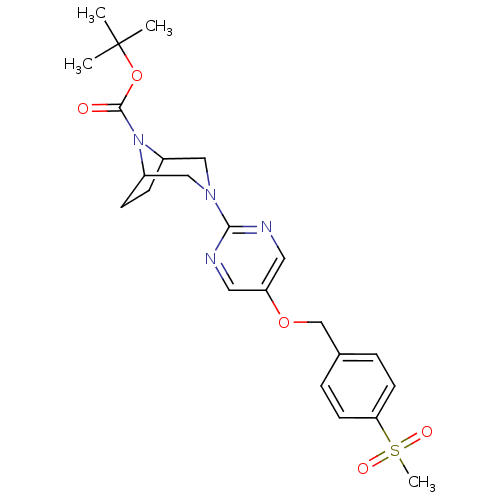
(CHEMBL2382409)Show SMILES CC(C)(C)OC(=O)N1C2CCC1CN(C2)c1ncc(OCc2ccc(cc2)S(C)(=O)=O)cn1 Show InChI InChI=1S/C23H30N4O5S/c1-23(2,3)32-22(28)27-17-7-8-18(27)14-26(13-17)21-24-11-19(12-25-21)31-15-16-5-9-20(10-6-16)33(4,29)30/h5-6,9-12,17-18H,7-8,13-15H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-(2-fluoro-4-methylsulfonyl-phenyl)-6-[4-(3-isopropyl-1,2,4-oxadiazol-5-yl)-1-piperidyl]-5-nitro-pyrimidin-4-amine from human G... |
Bioorg Med Chem Lett 23: 3175-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.006
BindingDB Entry DOI: 10.7270/Q25Q4XGC |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50420836
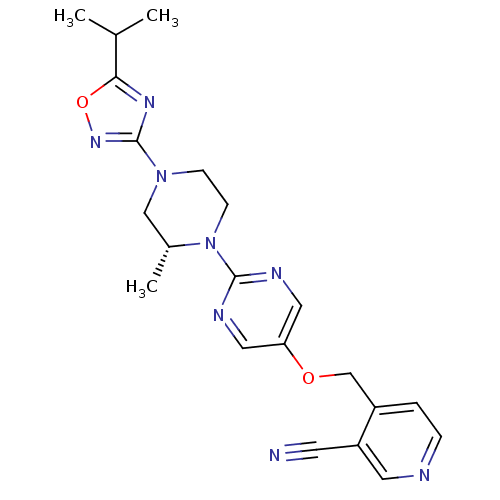
(CHEMBL2086690)Show SMILES CC(C)c1nc(no1)N1CCN([C@H](C)C1)c1ncc(OCc2ccncc2C#N)cn1 |r| Show InChI InChI=1S/C21H24N8O2/c1-14(2)19-26-21(27-31-19)28-6-7-29(15(3)12-28)20-24-10-18(11-25-20)30-13-16-4-5-23-9-17(16)8-22/h4-5,9-11,14-15H,6-7,12-13H2,1-3H3/t15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-(2-fluoro-4-methylsulfonyl-phenyl)-6-[4-(3-isopropyl-1,2,4-oxadiazol-5-yl)-1-piperidyl]-5-nitro-pyrimidin-4-amine from human G... |
Bioorg Med Chem Lett 23: 3175-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.006
BindingDB Entry DOI: 10.7270/Q25Q4XGC |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50433857
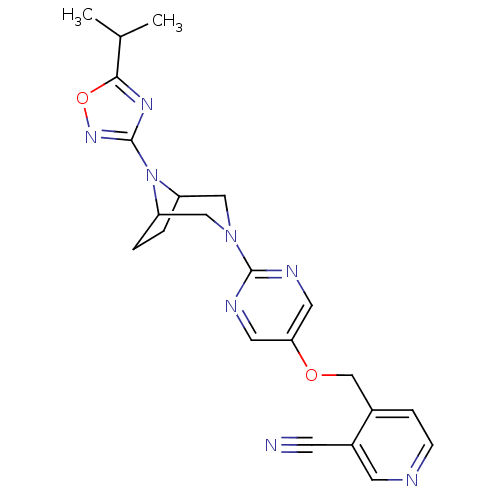
(CHEMBL2382417)Show SMILES CC(C)c1nc(no1)N1C2CCC1CN(C2)c1ncc(OCc2ccncc2C#N)cn1 Show InChI InChI=1S/C22H24N8O2/c1-14(2)20-27-22(28-32-20)30-17-3-4-18(30)12-29(11-17)21-25-9-19(10-26-21)31-13-15-5-6-24-8-16(15)7-23/h5-6,8-10,14,17-18H,3-4,11-13H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-(2-fluoro-4-methylsulfonyl-phenyl)-6-[4-(3-isopropyl-1,2,4-oxadiazol-5-yl)-1-piperidyl]-5-nitro-pyrimidin-4-amine from human G... |
Bioorg Med Chem Lett 23: 3175-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.006
BindingDB Entry DOI: 10.7270/Q25Q4XGC |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Mus musculus) | BDBM50433861

(CHEMBL2382409)Show SMILES CC(C)(C)OC(=O)N1C2CCC1CN(C2)c1ncc(OCc2ccc(cc2)S(C)(=O)=O)cn1 Show InChI InChI=1S/C23H30N4O5S/c1-23(2,3)32-22(28)27-17-7-8-18(27)14-26(13-17)21-24-11-19(12-25-21)31-15-16-5-9-20(10-6-16)33(4,29)30/h5-6,9-12,17-18H,7-8,13-15H2,1-4H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-(2-fluoro-4-methylsulfonyl-phenyl)-6-[4-(3-isopropyl-1,2,4-oxadiazol-5-yl)-1-piperidyl]-5-nitro-pyrimidin-4-amine from mouse G... |
Bioorg Med Chem Lett 23: 3175-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.006
BindingDB Entry DOI: 10.7270/Q25Q4XGC |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50433854
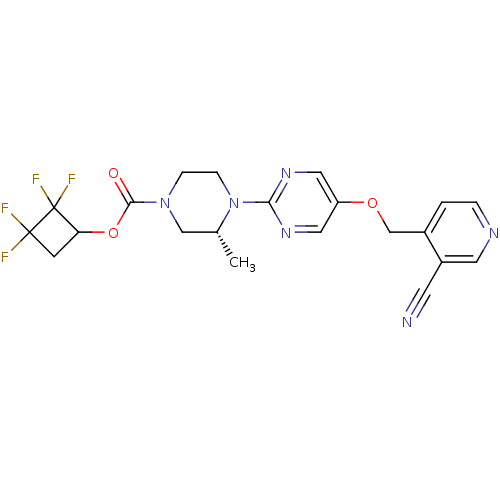
(CHEMBL2382412)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccncc2C#N)cn1)C(=O)OC1CC(F)(F)C1(F)F |r| Show InChI InChI=1S/C21H20F4N6O3/c1-13-11-30(19(32)34-17-6-20(22,23)21(17,24)25)4-5-31(13)18-28-9-16(10-29-18)33-12-14-2-3-27-8-15(14)7-26/h2-3,8-10,13,17H,4-6,11-12H2,1H3/t13-,17?/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-(2-fluoro-4-methylsulfonyl-phenyl)-6-[4-(3-isopropyl-1,2,4-oxadiazol-5-yl)-1-piperidyl]-5-nitro-pyrimidin-4-amine from human G... |
Bioorg Med Chem Lett 23: 3175-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.006
BindingDB Entry DOI: 10.7270/Q25Q4XGC |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50433859
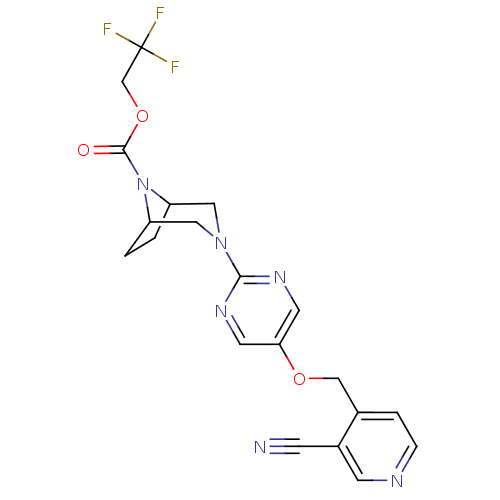
(CHEMBL2382415)Show SMILES FC(F)(F)COC(=O)N1C2CCC1CN(C2)c1ncc(OCc2ccncc2C#N)cn1 Show InChI InChI=1S/C20H19F3N6O3/c21-20(22,23)12-32-19(30)29-15-1-2-16(29)10-28(9-15)18-26-7-17(8-27-18)31-11-13-3-4-25-6-14(13)5-24/h3-4,6-8,15-16H,1-2,9-12H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-(2-fluoro-4-methylsulfonyl-phenyl)-6-[4-(3-isopropyl-1,2,4-oxadiazol-5-yl)-1-piperidyl]-5-nitro-pyrimidin-4-amine from human G... |
Bioorg Med Chem Lett 23: 3175-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.006
BindingDB Entry DOI: 10.7270/Q25Q4XGC |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50433853
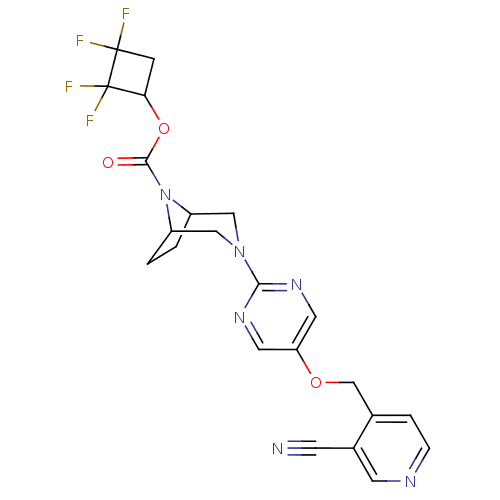
(CHEMBL2382413)Show SMILES FC1(F)CC(OC(=O)N2C3CCC2CN(C3)c2ncc(OCc3ccncc3C#N)cn2)C1(F)F Show InChI InChI=1S/C22H20F4N6O3/c23-21(24)5-18(22(21,25)26)35-20(33)32-15-1-2-16(32)11-31(10-15)19-29-8-17(9-30-19)34-12-13-3-4-28-7-14(13)6-27/h3-4,7-9,15-16,18H,1-2,5,10-12H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-(2-fluoro-4-methylsulfonyl-phenyl)-6-[4-(3-isopropyl-1,2,4-oxadiazol-5-yl)-1-piperidyl]-5-nitro-pyrimidin-4-amine from human G... |
Bioorg Med Chem Lett 23: 3175-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.006
BindingDB Entry DOI: 10.7270/Q25Q4XGC |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Mus musculus) | BDBM50433856

(CHEMBL2382410)Show SMILES CC(C)(C)OC(=O)N1C2CCC1CN(C2)c1ncc(OCc2ccncc2C#N)cn1 Show InChI InChI=1S/C22H26N6O3/c1-22(2,3)31-21(29)28-17-4-5-18(28)13-27(12-17)20-25-10-19(11-26-20)30-14-15-6-7-24-9-16(15)8-23/h6-7,9-11,17-18H,4-5,12-14H2,1-3H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-(2-fluoro-4-methylsulfonyl-phenyl)-6-[4-(3-isopropyl-1,2,4-oxadiazol-5-yl)-1-piperidyl]-5-nitro-pyrimidin-4-amine from mouse G... |
Bioorg Med Chem Lett 23: 3175-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.006
BindingDB Entry DOI: 10.7270/Q25Q4XGC |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50420845
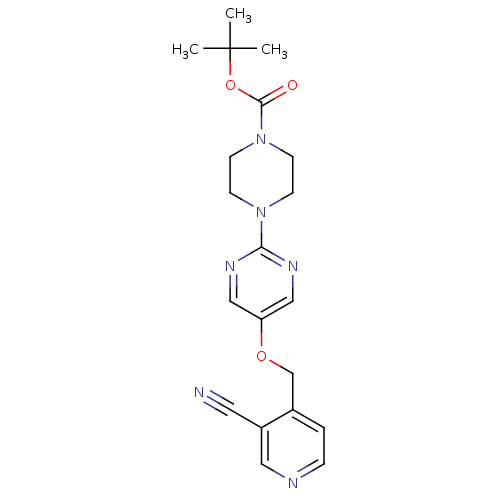
(CHEMBL2086680)Show SMILES CC(C)(C)OC(=O)N1CCN(CC1)c1ncc(OCc2ccncc2C#N)cn1 Show InChI InChI=1S/C20H24N6O3/c1-20(2,3)29-19(27)26-8-6-25(7-9-26)18-23-12-17(13-24-18)28-14-15-4-5-22-11-16(15)10-21/h4-5,11-13H,6-9,14H2,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-(2-fluoro-4-methylsulfonyl-phenyl)-6-[4-(3-isopropyl-1,2,4-oxadiazol-5-yl)-1-piperidyl]-5-nitro-pyrimidin-4-amine from human G... |
Bioorg Med Chem Lett 23: 3175-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.006
BindingDB Entry DOI: 10.7270/Q25Q4XGC |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50433858
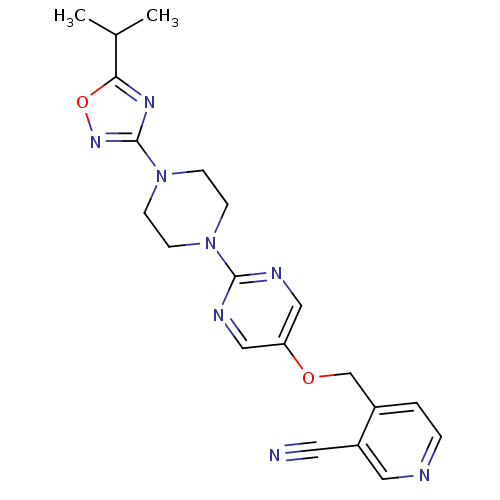
(CHEMBL2382416)Show SMILES CC(C)c1nc(no1)N1CCN(CC1)c1ncc(OCc2ccncc2C#N)cn1 Show InChI InChI=1S/C20H22N8O2/c1-14(2)18-25-20(26-30-18)28-7-5-27(6-8-28)19-23-11-17(12-24-19)29-13-15-3-4-22-10-16(15)9-21/h3-4,10-12,14H,5-8,13H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-(2-fluoro-4-methylsulfonyl-phenyl)-6-[4-(3-isopropyl-1,2,4-oxadiazol-5-yl)-1-piperidyl]-5-nitro-pyrimidin-4-amine from human G... |
Bioorg Med Chem Lett 23: 3175-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.006
BindingDB Entry DOI: 10.7270/Q25Q4XGC |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50420863
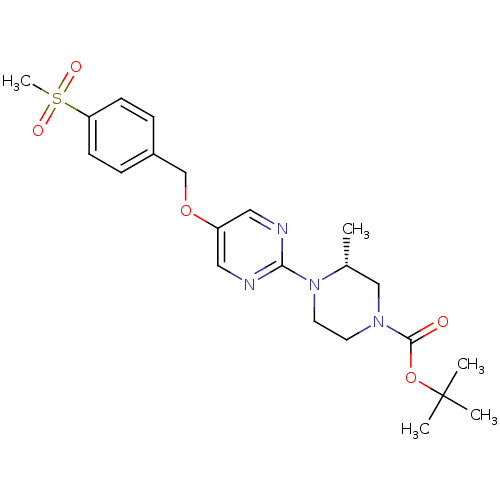
(CHEMBL2086660)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccc(cc2)S(C)(=O)=O)cn1)C(=O)OC(C)(C)C |r| Show InChI InChI=1S/C22H30N4O5S/c1-16-14-25(21(27)31-22(2,3)4)10-11-26(16)20-23-12-18(13-24-20)30-15-17-6-8-19(9-7-17)32(5,28)29/h6-9,12-13,16H,10-11,14-15H2,1-5H3/t16-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-(2-fluoro-4-methylsulfonyl-phenyl)-6-[4-(3-isopropyl-1,2,4-oxadiazol-5-yl)-1-piperidyl]-5-nitro-pyrimidin-4-amine from human G... |
Bioorg Med Chem Lett 23: 3175-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.006
BindingDB Entry DOI: 10.7270/Q25Q4XGC |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50433860

(CHEMBL2382414)Show SMILES FC(F)(F)COC(=O)N1CCN(CC1)c1ncc(OCc2ccncc2C#N)cn1 Show InChI InChI=1S/C18H17F3N6O3/c19-18(20,21)12-30-17(28)27-5-3-26(4-6-27)16-24-9-15(10-25-16)29-11-13-1-2-23-8-14(13)7-22/h1-2,8-10H,3-6,11-12H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-(2-fluoro-4-methylsulfonyl-phenyl)-6-[4-(3-isopropyl-1,2,4-oxadiazol-5-yl)-1-piperidyl]-5-nitro-pyrimidin-4-amine from human G... |
Bioorg Med Chem Lett 23: 3175-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.006
BindingDB Entry DOI: 10.7270/Q25Q4XGC |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50433855
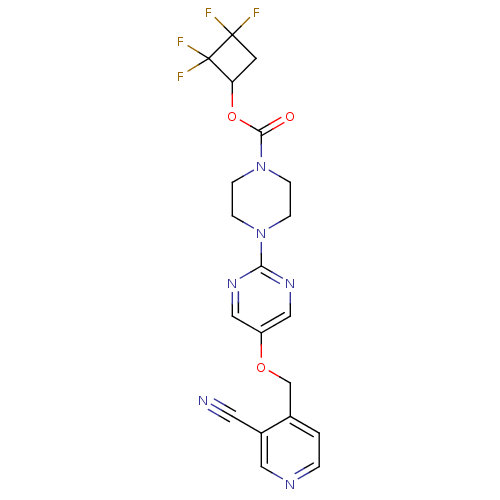
(CHEMBL2382411)Show SMILES FC1(F)CC(OC(=O)N2CCN(CC2)c2ncc(OCc3ccncc3C#N)cn2)C1(F)F Show InChI InChI=1S/C20H18F4N6O3/c21-19(22)7-16(20(19,23)24)33-18(31)30-5-3-29(4-6-30)17-27-10-15(11-28-17)32-12-13-1-2-26-9-14(13)8-25/h1-2,9-11,16H,3-7,12H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-(2-fluoro-4-methylsulfonyl-phenyl)-6-[4-(3-isopropyl-1,2,4-oxadiazol-5-yl)-1-piperidyl]-5-nitro-pyrimidin-4-amine from human G... |
Bioorg Med Chem Lett 23: 3175-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.006
BindingDB Entry DOI: 10.7270/Q25Q4XGC |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50420839
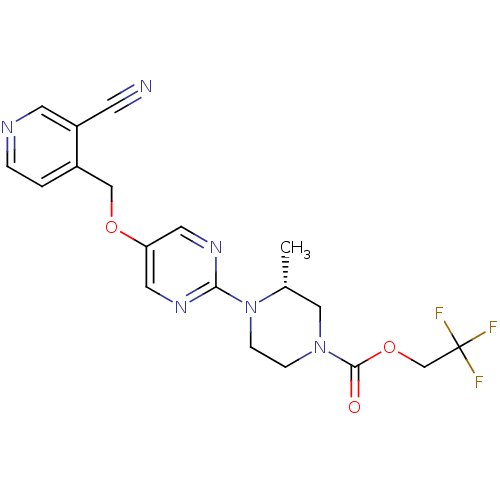
(CHEMBL2086687)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccncc2C#N)cn1)C(=O)OCC(F)(F)F |r| Show InChI InChI=1S/C19H19F3N6O3/c1-13-10-27(18(29)31-12-19(20,21)22)4-5-28(13)17-25-8-16(9-26-17)30-11-14-2-3-24-7-15(14)6-23/h2-3,7-9,13H,4-5,10-12H2,1H3/t13-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-(2-fluoro-4-methylsulfonyl-phenyl)-6-[4-(3-isopropyl-1,2,4-oxadiazol-5-yl)-1-piperidyl]-5-nitro-pyrimidin-4-amine from human G... |
Bioorg Med Chem Lett 23: 3175-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.006
BindingDB Entry DOI: 10.7270/Q25Q4XGC |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Mus musculus) | BDBM50433853

(CHEMBL2382413)Show SMILES FC1(F)CC(OC(=O)N2C3CCC2CN(C3)c2ncc(OCc3ccncc3C#N)cn2)C1(F)F Show InChI InChI=1S/C22H20F4N6O3/c23-21(24)5-18(22(21,25)26)35-20(33)32-15-1-2-16(32)11-31(10-15)19-29-8-17(9-30-19)34-12-13-3-4-28-7-14(13)6-27/h3-4,7-9,15-16,18H,1-2,5,10-12H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-(2-fluoro-4-methylsulfonyl-phenyl)-6-[4-(3-isopropyl-1,2,4-oxadiazol-5-yl)-1-piperidyl]-5-nitro-pyrimidin-4-amine from mouse G... |
Bioorg Med Chem Lett 23: 3175-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.006
BindingDB Entry DOI: 10.7270/Q25Q4XGC |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Mus musculus) | BDBM50433862

(CHEMBL2382408)Show SMILES CC(C)(C)OC(=O)N1CC2CCC(C1)N2c1ncc(OCc2ccc(cc2)S(C)(=O)=O)cn1 Show InChI InChI=1S/C23H30N4O5S/c1-23(2,3)32-22(28)26-13-17-7-8-18(14-26)27(17)21-24-11-19(12-25-21)31-15-16-5-9-20(10-6-16)33(4,29)30/h5-6,9-12,17-18H,7-8,13-15H2,1-4H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-(2-fluoro-4-methylsulfonyl-phenyl)-6-[4-(3-isopropyl-1,2,4-oxadiazol-5-yl)-1-piperidyl]-5-nitro-pyrimidin-4-amine from mouse G... |
Bioorg Med Chem Lett 23: 3175-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.006
BindingDB Entry DOI: 10.7270/Q25Q4XGC |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50433862

(CHEMBL2382408)Show SMILES CC(C)(C)OC(=O)N1CC2CCC(C1)N2c1ncc(OCc2ccc(cc2)S(C)(=O)=O)cn1 Show InChI InChI=1S/C23H30N4O5S/c1-23(2,3)32-22(28)26-13-17-7-8-18(14-26)27(17)21-24-11-19(12-25-21)31-15-16-5-9-20(10-6-16)33(4,29)30/h5-6,9-12,17-18H,7-8,13-15H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 92 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-(2-fluoro-4-methylsulfonyl-phenyl)-6-[4-(3-isopropyl-1,2,4-oxadiazol-5-yl)-1-piperidyl]-5-nitro-pyrimidin-4-amine from human G... |
Bioorg Med Chem Lett 23: 3175-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.006
BindingDB Entry DOI: 10.7270/Q25Q4XGC |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Mus musculus) | BDBM50420842

(CHEMBL2086684)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccncc2C#N)cn1)C(=O)OC(C)(C)C |r| Show InChI InChI=1S/C21H26N6O3/c1-15-13-26(20(28)30-21(2,3)4)7-8-27(15)19-24-11-18(12-25-19)29-14-16-5-6-23-10-17(16)9-22/h5-6,10-12,15H,7-8,13-14H2,1-4H3/t15-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-(2-fluoro-4-methylsulfonyl-phenyl)-6-[4-(3-isopropyl-1,2,4-oxadiazol-5-yl)-1-piperidyl]-5-nitro-pyrimidin-4-amine from mouse G... |
Bioorg Med Chem Lett 23: 3175-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.006
BindingDB Entry DOI: 10.7270/Q25Q4XGC |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Mus musculus) | BDBM50433859

(CHEMBL2382415)Show SMILES FC(F)(F)COC(=O)N1C2CCC1CN(C2)c1ncc(OCc2ccncc2C#N)cn1 Show InChI InChI=1S/C20H19F3N6O3/c21-20(22,23)12-32-19(30)29-15-1-2-16(29)10-28(9-15)18-26-7-17(8-27-18)31-11-13-3-4-25-6-14(13)5-24/h3-4,6-8,15-16H,1-2,9-12H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 104 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-(2-fluoro-4-methylsulfonyl-phenyl)-6-[4-(3-isopropyl-1,2,4-oxadiazol-5-yl)-1-piperidyl]-5-nitro-pyrimidin-4-amine from mouse G... |
Bioorg Med Chem Lett 23: 3175-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.006
BindingDB Entry DOI: 10.7270/Q25Q4XGC |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Mus musculus) | BDBM50433857

(CHEMBL2382417)Show SMILES CC(C)c1nc(no1)N1C2CCC1CN(C2)c1ncc(OCc2ccncc2C#N)cn1 Show InChI InChI=1S/C22H24N8O2/c1-14(2)20-27-22(28-32-20)30-17-3-4-18(30)12-29(11-17)21-25-9-19(10-26-21)31-13-15-5-6-24-8-16(15)7-23/h5-6,8-10,14,17-18H,3-4,11-13H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 122 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-(2-fluoro-4-methylsulfonyl-phenyl)-6-[4-(3-isopropyl-1,2,4-oxadiazol-5-yl)-1-piperidyl]-5-nitro-pyrimidin-4-amine from mouse G... |
Bioorg Med Chem Lett 23: 3175-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.006
BindingDB Entry DOI: 10.7270/Q25Q4XGC |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Mus musculus) | BDBM50420863

(CHEMBL2086660)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccc(cc2)S(C)(=O)=O)cn1)C(=O)OC(C)(C)C |r| Show InChI InChI=1S/C22H30N4O5S/c1-16-14-25(21(27)31-22(2,3)4)10-11-26(16)20-23-12-18(13-24-20)30-15-17-6-8-19(9-7-17)32(5,28)29/h6-9,12-13,16H,10-11,14-15H2,1-5H3/t16-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 129 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-(2-fluoro-4-methylsulfonyl-phenyl)-6-[4-(3-isopropyl-1,2,4-oxadiazol-5-yl)-1-piperidyl]-5-nitro-pyrimidin-4-amine from mouse G... |
Bioorg Med Chem Lett 23: 3175-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.006
BindingDB Entry DOI: 10.7270/Q25Q4XGC |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Homo sapiens (Human)) | BDBM50420871
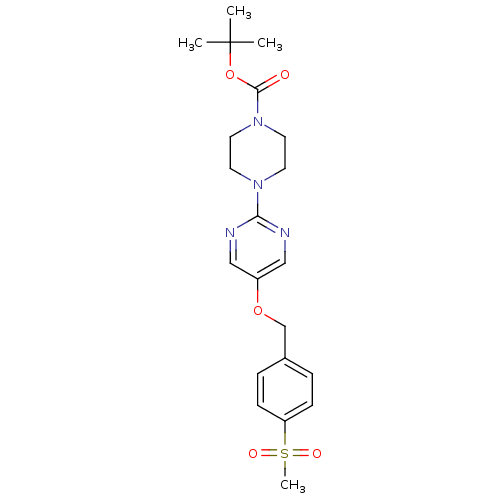
(CHEMBL2086650)Show SMILES CC(C)(C)OC(=O)N1CCN(CC1)c1ncc(OCc2ccc(cc2)S(C)(=O)=O)cn1 Show InChI InChI=1S/C21H28N4O5S/c1-21(2,3)30-20(26)25-11-9-24(10-12-25)19-22-13-17(14-23-19)29-15-16-5-7-18(8-6-16)31(4,27)28/h5-8,13-14H,9-12,15H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 351 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-(2-fluoro-4-methylsulfonyl-phenyl)-6-[4-(3-isopropyl-1,2,4-oxadiazol-5-yl)-1-piperidyl]-5-nitro-pyrimidin-4-amine from human G... |
Bioorg Med Chem Lett 23: 3175-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.006
BindingDB Entry DOI: 10.7270/Q25Q4XGC |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Mus musculus) | BDBM50420845

(CHEMBL2086680)Show SMILES CC(C)(C)OC(=O)N1CCN(CC1)c1ncc(OCc2ccncc2C#N)cn1 Show InChI InChI=1S/C20H24N6O3/c1-20(2,3)29-19(27)26-8-6-25(7-9-26)18-23-12-17(13-24-18)28-14-15-4-5-22-11-16(15)10-21/h4-5,11-13H,6-9,14H2,1-3H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 507 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-(2-fluoro-4-methylsulfonyl-phenyl)-6-[4-(3-isopropyl-1,2,4-oxadiazol-5-yl)-1-piperidyl]-5-nitro-pyrimidin-4-amine from mouse G... |
Bioorg Med Chem Lett 23: 3175-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.006
BindingDB Entry DOI: 10.7270/Q25Q4XGC |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Mus musculus) | BDBM50420871

(CHEMBL2086650)Show SMILES CC(C)(C)OC(=O)N1CCN(CC1)c1ncc(OCc2ccc(cc2)S(C)(=O)=O)cn1 Show InChI InChI=1S/C21H28N4O5S/c1-21(2,3)30-20(26)25-11-9-24(10-12-25)19-22-13-17(14-23-19)29-15-16-5-7-18(8-6-16)31(4,27)28/h5-8,13-14H,9-12,15H2,1-4H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 543 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-(2-fluoro-4-methylsulfonyl-phenyl)-6-[4-(3-isopropyl-1,2,4-oxadiazol-5-yl)-1-piperidyl]-5-nitro-pyrimidin-4-amine from mouse G... |
Bioorg Med Chem Lett 23: 3175-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.006
BindingDB Entry DOI: 10.7270/Q25Q4XGC |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Mus musculus) | BDBM50420836

(CHEMBL2086690)Show SMILES CC(C)c1nc(no1)N1CCN([C@H](C)C1)c1ncc(OCc2ccncc2C#N)cn1 |r| Show InChI InChI=1S/C21H24N8O2/c1-14(2)19-26-21(27-31-19)28-6-7-29(15(3)12-28)20-24-10-18(11-25-20)30-13-16-4-5-23-9-17(16)8-22/h4-5,9-11,14-15H,6-7,12-13H2,1-3H3/t15-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 568 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-(2-fluoro-4-methylsulfonyl-phenyl)-6-[4-(3-isopropyl-1,2,4-oxadiazol-5-yl)-1-piperidyl]-5-nitro-pyrimidin-4-amine from mouse G... |
Bioorg Med Chem Lett 23: 3175-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.006
BindingDB Entry DOI: 10.7270/Q25Q4XGC |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Mus musculus) | BDBM50433854

(CHEMBL2382412)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccncc2C#N)cn1)C(=O)OC1CC(F)(F)C1(F)F |r| Show InChI InChI=1S/C21H20F4N6O3/c1-13-11-30(19(32)34-17-6-20(22,23)21(17,24)25)4-5-31(13)18-28-9-16(10-29-18)33-12-14-2-3-27-8-15(14)7-26/h2-3,8-10,13,17H,4-6,11-12H2,1H3/t13-,17?/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 694 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-(2-fluoro-4-methylsulfonyl-phenyl)-6-[4-(3-isopropyl-1,2,4-oxadiazol-5-yl)-1-piperidyl]-5-nitro-pyrimidin-4-amine from mouse G... |
Bioorg Med Chem Lett 23: 3175-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.006
BindingDB Entry DOI: 10.7270/Q25Q4XGC |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Mus musculus) | BDBM50433855

(CHEMBL2382411)Show SMILES FC1(F)CC(OC(=O)N2CCN(CC2)c2ncc(OCc3ccncc3C#N)cn2)C1(F)F Show InChI InChI=1S/C20H18F4N6O3/c21-19(22)7-16(20(19,23)24)33-18(31)30-5-3-29(4-6-30)17-27-10-15(11-28-17)32-12-13-1-2-26-9-14(13)8-25/h1-2,9-11,16H,3-7,12H2 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 758 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-(2-fluoro-4-methylsulfonyl-phenyl)-6-[4-(3-isopropyl-1,2,4-oxadiazol-5-yl)-1-piperidyl]-5-nitro-pyrimidin-4-amine from mouse G... |
Bioorg Med Chem Lett 23: 3175-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.006
BindingDB Entry DOI: 10.7270/Q25Q4XGC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50379165
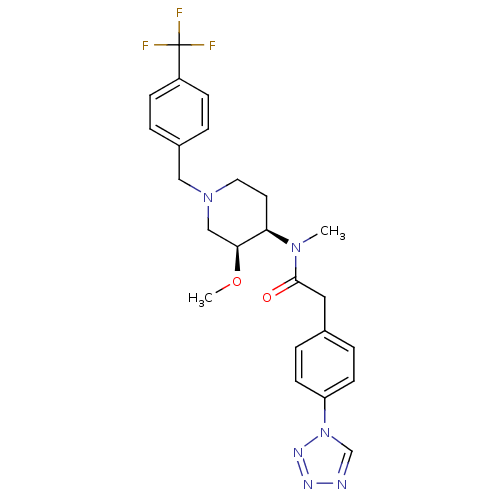
(CHEMBL2010847)Show SMILES CO[C@H]1CN(Cc2ccc(cc2)C(F)(F)F)CC[C@H]1N(C)C(=O)Cc1ccc(cc1)-n1cnnn1 |r| Show InChI InChI=1S/C24H27F3N6O2/c1-31(23(34)13-17-5-9-20(10-6-17)33-16-28-29-30-33)21-11-12-32(15-22(21)35-2)14-18-3-7-19(8-4-18)24(25,26)27/h3-10,16,21-22H,11-15H2,1-2H3/t21-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca U K Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 7310-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.033
BindingDB Entry DOI: 10.7270/Q27945PF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50379175
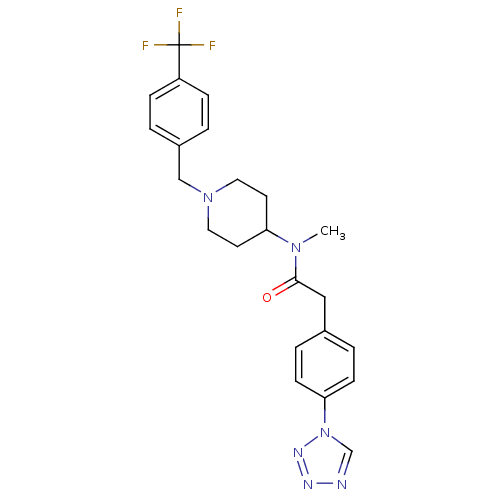
(CHEMBL2010836)Show SMILES CN(C1CCN(Cc2ccc(cc2)C(F)(F)F)CC1)C(=O)Cc1ccc(cc1)-n1cnnn1 Show InChI InChI=1S/C23H25F3N6O/c1-30(22(33)14-17-4-8-21(9-5-17)32-16-27-28-29-32)20-10-12-31(13-11-20)15-18-2-6-19(7-3-18)23(24,25)26/h2-9,16,20H,10-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca U K Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 7310-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.033
BindingDB Entry DOI: 10.7270/Q27945PF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50379170
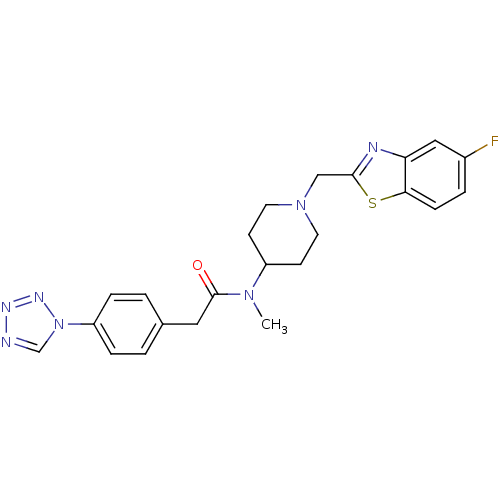
(CHEMBL2010842)Show SMILES CN(C1CCN(Cc2nc3cc(F)ccc3s2)CC1)C(=O)Cc1ccc(cc1)-n1cnnn1 Show InChI InChI=1S/C23H24FN7OS/c1-29(23(32)12-16-2-5-19(6-3-16)31-15-25-27-28-31)18-8-10-30(11-9-18)14-22-26-20-13-17(24)4-7-21(20)33-22/h2-7,13,15,18H,8-12,14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca U K Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 7310-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.033
BindingDB Entry DOI: 10.7270/Q27945PF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50379158
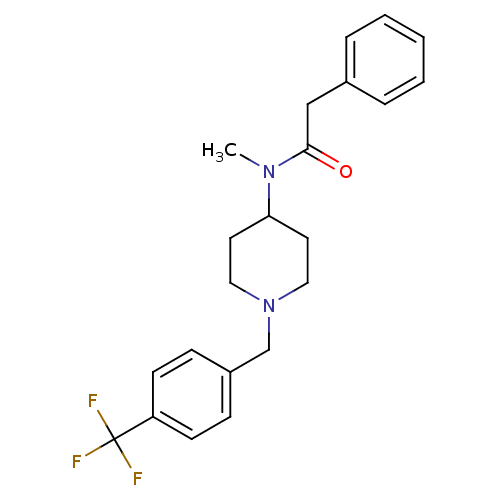
(CHEMBL2010837)Show SMILES CN(C1CCN(Cc2ccc(cc2)C(F)(F)F)CC1)C(=O)Cc1ccccc1 Show InChI InChI=1S/C22H25F3N2O/c1-26(21(28)15-17-5-3-2-4-6-17)20-11-13-27(14-12-20)16-18-7-9-19(10-8-18)22(23,24)25/h2-10,20H,11-16H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca U K Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 7310-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.033
BindingDB Entry DOI: 10.7270/Q27945PF |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Mus musculus) | BDBM50420839

(CHEMBL2086687)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccncc2C#N)cn1)C(=O)OCC(F)(F)F |r| Show InChI InChI=1S/C19H19F3N6O3/c1-13-10-27(18(29)31-12-19(20,21)22)4-5-28(13)17-25-8-16(9-26-17)30-11-14-2-3-24-7-15(14)6-23/h2-3,7-9,13H,4-5,10-12H2,1H3/t13-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-(2-fluoro-4-methylsulfonyl-phenyl)-6-[4-(3-isopropyl-1,2,4-oxadiazol-5-yl)-1-piperidyl]-5-nitro-pyrimidin-4-amine from mouse G... |
Bioorg Med Chem Lett 23: 3175-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.006
BindingDB Entry DOI: 10.7270/Q25Q4XGC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50379167

(CHEMBL2010845)Show SMILES CN([C@@H]1CCN(Cc2ccc(cc2)C(F)(F)F)C[C@@H]1F)C(=O)Cc1ccc(cc1)-n1cnnn1 |r| Show InChI InChI=1S/C23H24F4N6O/c1-31(22(34)12-16-4-8-19(9-5-16)33-15-28-29-30-33)21-10-11-32(14-20(21)24)13-17-2-6-18(7-3-17)23(25,26)27/h2-9,15,20-21H,10-14H2,1H3/t20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca U K Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 7310-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.033
BindingDB Entry DOI: 10.7270/Q27945PF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50379166
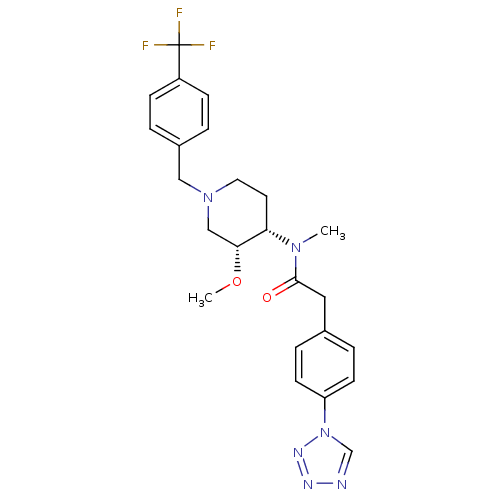
(CHEMBL2010846)Show SMILES CO[C@@H]1CN(Cc2ccc(cc2)C(F)(F)F)CC[C@@H]1N(C)C(=O)Cc1ccc(cc1)-n1cnnn1 |r| Show InChI InChI=1S/C24H27F3N6O2/c1-31(23(34)13-17-5-9-20(10-6-17)33-16-28-29-30-33)21-11-12-32(15-22(21)35-2)14-18-3-7-19(8-4-18)24(25,26)27/h3-10,16,21-22H,11-15H2,1-2H3/t21-,22+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca U K Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 7310-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.033
BindingDB Entry DOI: 10.7270/Q27945PF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50379177
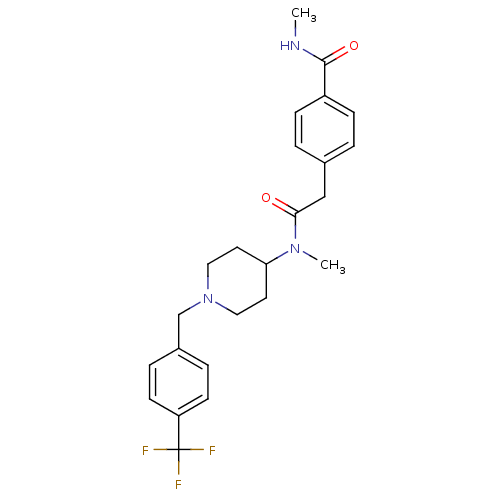
(CHEMBL2010834)Show SMILES CNC(=O)c1ccc(CC(=O)N(C)C2CCN(Cc3ccc(cc3)C(F)(F)F)CC2)cc1 Show InChI InChI=1S/C24H28F3N3O2/c1-28-23(32)19-7-3-17(4-8-19)15-22(31)29(2)21-11-13-30(14-12-21)16-18-5-9-20(10-6-18)24(25,26)27/h3-10,21H,11-16H2,1-2H3,(H,28,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca U K Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 7310-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.033
BindingDB Entry DOI: 10.7270/Q27945PF |
More data for this
Ligand-Target Pair | |
Epidermal growth factor receptor
(Homo sapiens (Human)) | BDBM50103543
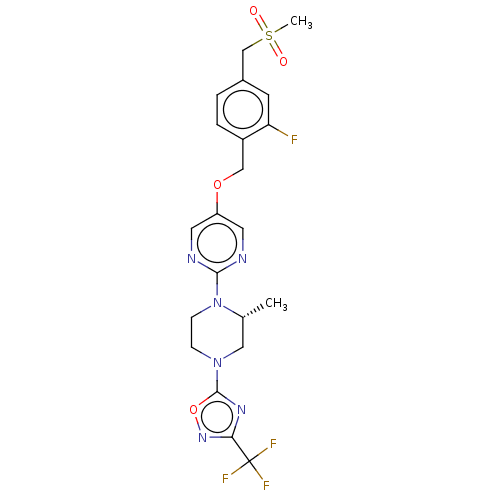
(CHEMBL3358006)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccc(CS(C)(=O)=O)cc2F)cn1)c1nc(no1)C(F)(F)F |r| Show InChI InChI=1S/C21H22F4N6O4S/c1-13-10-30(20-28-18(29-35-20)21(23,24)25)5-6-31(13)19-26-8-16(9-27-19)34-11-15-4-3-14(7-17(15)22)12-36(2,32)33/h3-4,7-9,13H,5-6,10-12H2,1-2H3/t13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of EGFR (unknown origin) |
J Med Chem 57: 8984-98 (2014)
Article DOI: 10.1021/jm5011012
BindingDB Entry DOI: 10.7270/Q29G5PK1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50379181
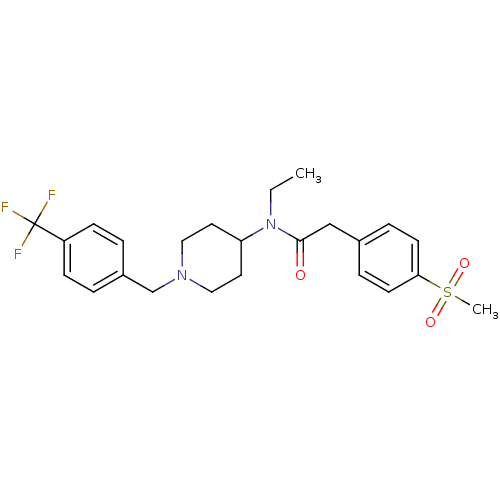
(CHEMBL2010830)Show SMILES CCN(C1CCN(Cc2ccc(cc2)C(F)(F)F)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C24H29F3N2O3S/c1-3-29(23(30)16-18-6-10-22(11-7-18)33(2,31)32)21-12-14-28(15-13-21)17-19-4-8-20(9-5-19)24(25,26)27/h4-11,21H,3,12-17H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca U K Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 7310-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.033
BindingDB Entry DOI: 10.7270/Q27945PF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50379179
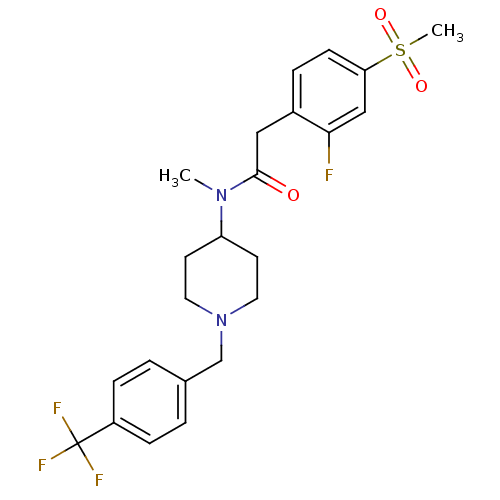
(CHEMBL2010832)Show SMILES CN(C1CCN(Cc2ccc(cc2)C(F)(F)F)CC1)C(=O)Cc1ccc(cc1F)S(C)(=O)=O Show InChI InChI=1S/C23H26F4N2O3S/c1-28(22(30)13-17-5-8-20(14-21(17)24)33(2,31)32)19-9-11-29(12-10-19)15-16-3-6-18(7-4-16)23(25,26)27/h3-8,14,19H,9-13,15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca U K Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 7310-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.033
BindingDB Entry DOI: 10.7270/Q27945PF |
More data for this
Ligand-Target Pair | |
Glucose-dependent insulinotropic receptor
(Mus musculus) | BDBM50433858

(CHEMBL2382416)Show SMILES CC(C)c1nc(no1)N1CCN(CC1)c1ncc(OCc2ccncc2C#N)cn1 Show InChI InChI=1S/C20H22N8O2/c1-14(2)18-25-20(26-30-18)28-7-5-27(6-8-28)19-23-11-17(12-24-19)29-13-15-3-4-22-10-16(15)9-21/h3-4,10-12,14H,5-8,13H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Displacement of [3H]-N-(2-fluoro-4-methylsulfonyl-phenyl)-6-[4-(3-isopropyl-1,2,4-oxadiazol-5-yl)-1-piperidyl]-5-nitro-pyrimidin-4-amine from mouse G... |
Bioorg Med Chem Lett 23: 3175-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.04.006
BindingDB Entry DOI: 10.7270/Q25Q4XGC |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50379172

(CHEMBL2010840)Show SMILES CN(C1CCN(Cc2ccc(c(F)c2)C(F)(F)F)CC1)C(=O)Cc1ccc(cc1)-n1cnnn1 Show InChI InChI=1S/C23H24F4N6O/c1-31(22(34)13-16-2-5-19(6-3-16)33-15-28-29-30-33)18-8-10-32(11-9-18)14-17-4-7-20(21(24)12-17)23(25,26)27/h2-7,12,15,18H,8-11,13-14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca U K Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 7310-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.033
BindingDB Entry DOI: 10.7270/Q27945PF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50379178
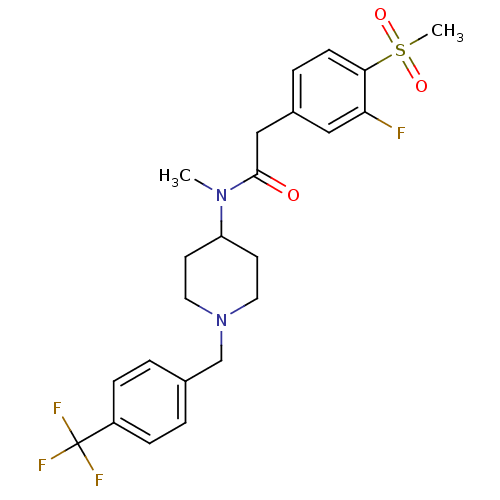
(CHEMBL2010833)Show SMILES CN(C1CCN(Cc2ccc(cc2)C(F)(F)F)CC1)C(=O)Cc1ccc(c(F)c1)S(C)(=O)=O Show InChI InChI=1S/C23H26F4N2O3S/c1-28(22(30)14-17-5-8-21(20(24)13-17)33(2,31)32)19-9-11-29(12-10-19)15-16-3-6-18(7-4-16)23(25,26)27/h3-8,13,19H,9-12,14-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca U K Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 7310-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.033
BindingDB Entry DOI: 10.7270/Q27945PF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50379180
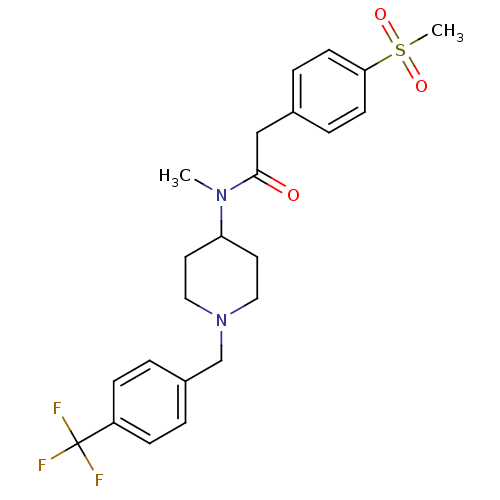
(CHEMBL2010831)Show SMILES CN(C1CCN(Cc2ccc(cc2)C(F)(F)F)CC1)C(=O)Cc1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C23H27F3N2O3S/c1-27(22(29)15-17-5-9-21(10-6-17)32(2,30)31)20-11-13-28(14-12-20)16-18-3-7-19(8-4-18)23(24,25)26/h3-10,20H,11-16H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca U K Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 7310-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.033
BindingDB Entry DOI: 10.7270/Q27945PF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50103561
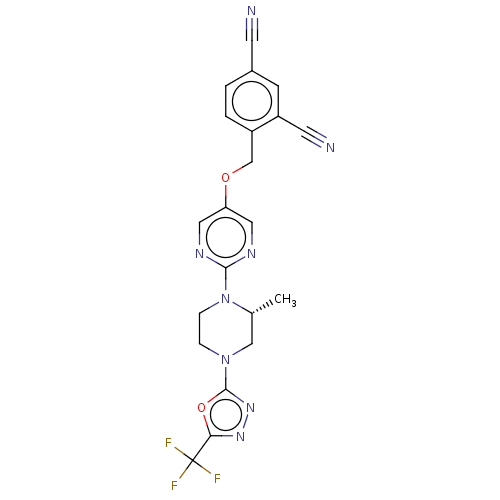
(CHEMBL3357996)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccc(cc2C#N)C#N)cn1)c1nnc(o1)C(F)(F)F |r| Show InChI InChI=1S/C21H17F3N8O2/c1-13-11-31(20-30-29-18(34-20)21(22,23)24)4-5-32(13)19-27-9-17(10-28-19)33-12-15-3-2-14(7-25)6-16(15)8-26/h2-3,6,9-10,13H,4-5,11-12H2,1H3/t13-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp based electrophysiology method |
J Med Chem 57: 8984-98 (2014)
Article DOI: 10.1021/jm5011012
BindingDB Entry DOI: 10.7270/Q29G5PK1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50103544
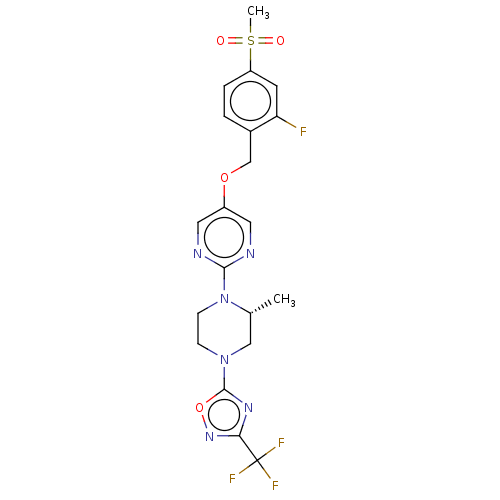
(CHEMBL3358005)Show SMILES C[C@@H]1CN(CCN1c1ncc(OCc2ccc(cc2F)S(C)(=O)=O)cn1)c1nc(no1)C(F)(F)F |r| Show InChI InChI=1S/C20H20F4N6O4S/c1-12-10-29(19-27-17(28-34-19)20(22,23)24)5-6-30(12)18-25-8-14(9-26-18)33-11-13-3-4-15(7-16(13)21)35(2,31)32/h3-4,7-9,12H,5-6,10-11H2,1-2H3/t12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of human ERG by patch clamp based electrophysiology method |
J Med Chem 57: 8984-98 (2014)
Article DOI: 10.1021/jm5011012
BindingDB Entry DOI: 10.7270/Q29G5PK1 |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50379168

(CHEMBL2010844)Show SMILES CN([C@H]1CCN(Cc2ccc(cc2)C(F)(F)F)C[C@H]1F)C(=O)Cc1ccc(cc1)-n1cnnn1 |r| Show InChI InChI=1S/C23H24F4N6O/c1-31(22(34)12-16-4-8-19(9-5-16)33-15-28-29-30-33)21-10-11-32(14-20(21)24)13-17-2-6-18(7-3-17)23(25,26)27/h2-9,15,20-21H,10-14H2,1H3/t20-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca U K Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 7310-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.033
BindingDB Entry DOI: 10.7270/Q27945PF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50379174
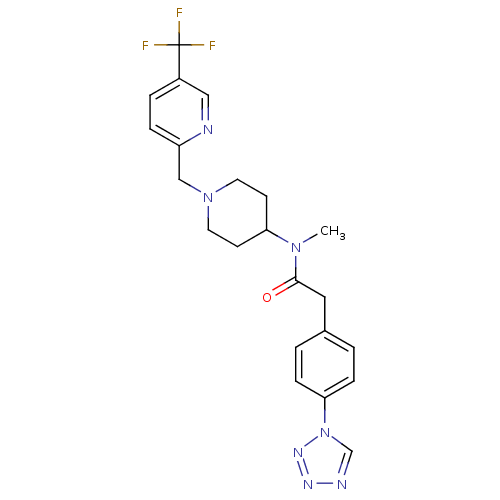
(CHEMBL2010838)Show SMILES CN(C1CCN(Cc2ccc(cn2)C(F)(F)F)CC1)C(=O)Cc1ccc(cc1)-n1cnnn1 Show InChI InChI=1S/C22H24F3N7O/c1-30(21(33)12-16-2-6-20(7-3-16)32-15-27-28-29-32)19-8-10-31(11-9-19)14-18-5-4-17(13-26-18)22(23,24)25/h2-7,13,15,19H,8-12,14H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca U K Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 7310-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.033
BindingDB Entry DOI: 10.7270/Q27945PF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50379161
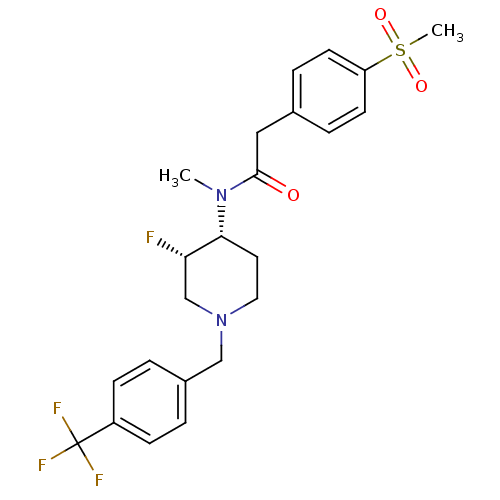
(CHEMBL2010851)Show SMILES CN([C@@H]1CCN(Cc2ccc(cc2)C(F)(F)F)C[C@@H]1F)C(=O)Cc1ccc(cc1)S(C)(=O)=O |r| Show InChI InChI=1S/C23H26F4N2O3S/c1-28(22(30)13-16-5-9-19(10-6-16)33(2,31)32)21-11-12-29(15-20(21)24)14-17-3-7-18(8-4-17)23(25,26)27/h3-10,20-21H,11-15H2,1-2H3/t20-,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca U K Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 7310-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.033
BindingDB Entry DOI: 10.7270/Q27945PF |
More data for this
Ligand-Target Pair | |
Potassium voltage-gated channel subfamily H member 2
(Homo sapiens (Human)) | BDBM50379176
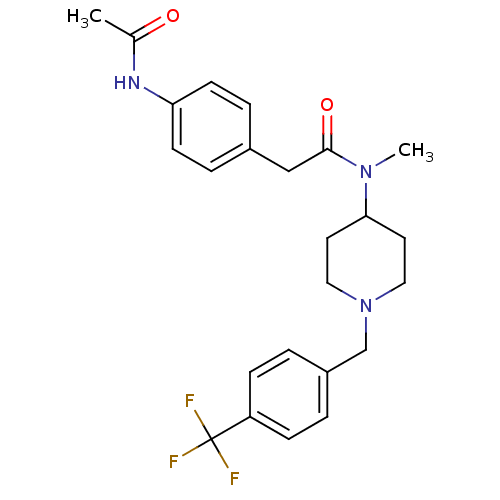
(CHEMBL2010835)Show SMILES CN(C1CCN(Cc2ccc(cc2)C(F)(F)F)CC1)C(=O)Cc1ccc(NC(C)=O)cc1 Show InChI InChI=1S/C24H28F3N3O2/c1-17(31)28-21-9-5-18(6-10-21)15-23(32)29(2)22-11-13-30(14-12-22)16-19-3-7-20(8-4-19)24(25,26)27/h3-10,22H,11-16H2,1-2H3,(H,28,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
UniProtKB/TrEMBL
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Astrazeneca U K Ltd
Curated by ChEMBL
| Assay Description
Binding affinity to human ERG |
Bioorg Med Chem Lett 21: 7310-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.10.033
BindingDB Entry DOI: 10.7270/Q27945PF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data