

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50424479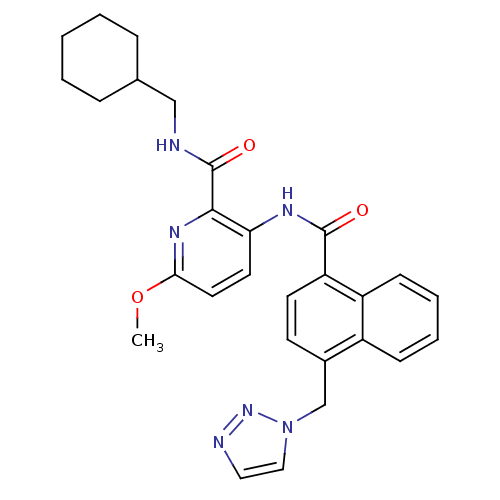 (CHEMBL2316376) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293S cell membranes by scintillation counting analysis | J Med Chem 56: 220-40 (2013) Article DOI: 10.1021/jm301511h BindingDB Entry DOI: 10.7270/Q2057H7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50424491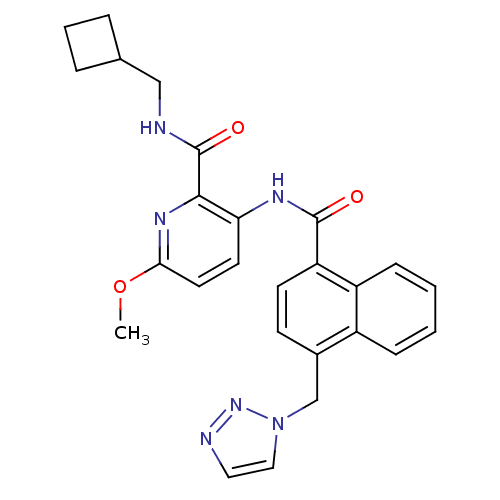 (CHEMBL2316377) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293S cell membranes by scintillation counting analysis | J Med Chem 56: 220-40 (2013) Article DOI: 10.1021/jm301511h BindingDB Entry DOI: 10.7270/Q2057H7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50424478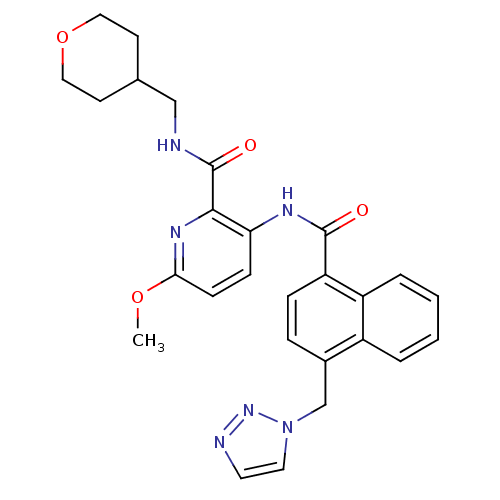 (CHEMBL2316378) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293S cell membranes by scintillation counting analysis | J Med Chem 56: 220-40 (2013) Article DOI: 10.1021/jm301511h BindingDB Entry DOI: 10.7270/Q2057H7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM21281 ((11R)-2-methyl-11-(morpholin-4-ylmethyl)-3-(naphth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB2 receptor expressed in Sf9 cell membranes by scintillation counting analysis | J Med Chem 56: 220-40 (2013) Article DOI: 10.1021/jm301511h BindingDB Entry DOI: 10.7270/Q2057H7B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM323045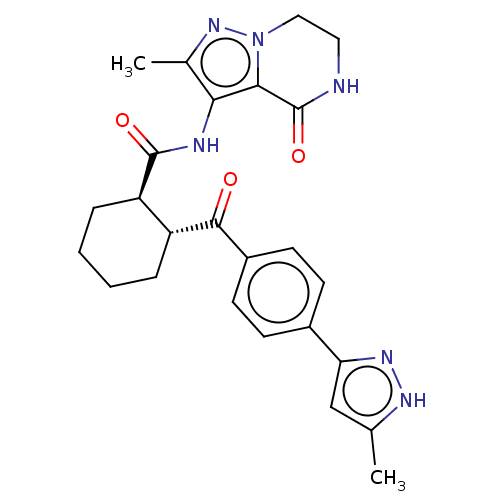 ((1R,2R)-N-(2-Methyl-4-oxo-4,5,6,7- tetrahydropyraz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description Compounds were tested in a competition binding assay using 3H-MK591 as tracer. (Preparation of MK-591 is described in Bioorg. Med. Chem. Lett. 1999, ... | US Patent US10183947 (2019) BindingDB Entry DOI: 10.7270/Q22Z17M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50424490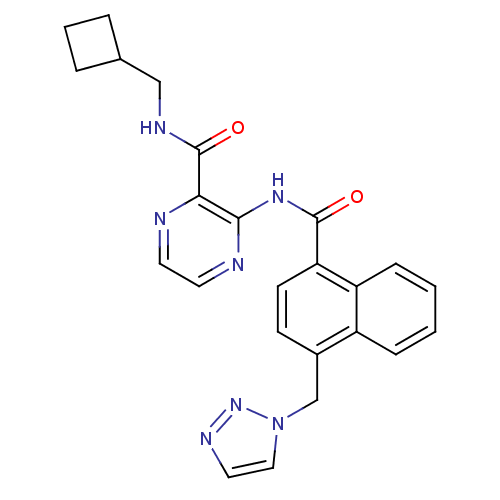 (CHEMBL2316380) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293S cell membranes by scintillation counting analysis | J Med Chem 56: 220-40 (2013) Article DOI: 10.1021/jm301511h BindingDB Entry DOI: 10.7270/Q2057H7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM323046 ((1R,2R)-N-(2-Methyl-4-oxo-4,5,6,7- tetrahydropyraz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description Compounds were tested in a competition binding assay using 3H-MK591 as tracer. (Preparation of MK-591 is described in Bioorg. Med. Chem. Lett. 1999, ... | US Patent US10183947 (2019) BindingDB Entry DOI: 10.7270/Q22Z17M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50424477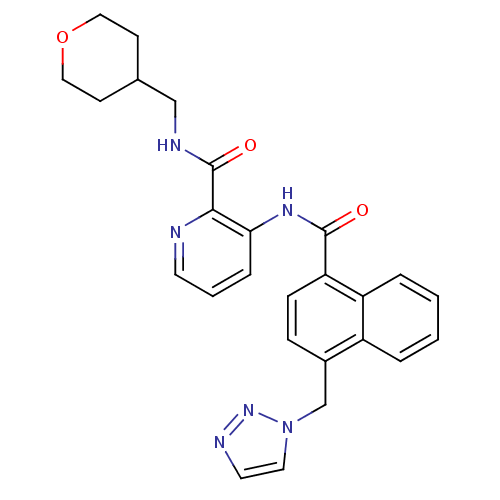 (CHEMBL2316379) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293S cell membranes by scintillation counting analysis | J Med Chem 56: 220-40 (2013) Article DOI: 10.1021/jm301511h BindingDB Entry DOI: 10.7270/Q2057H7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50524103 (CHEMBL4563459) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]MK0591 binding to human FLAP expressed in human COS7 cell membranes measured after 60 to 80 mins by microbeta scintillation countin... | J Med Chem 62: 4312-4324 (2019) Article DOI: 10.1021/acs.jmedchem.8b02004 BindingDB Entry DOI: 10.7270/Q25T3PXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50424467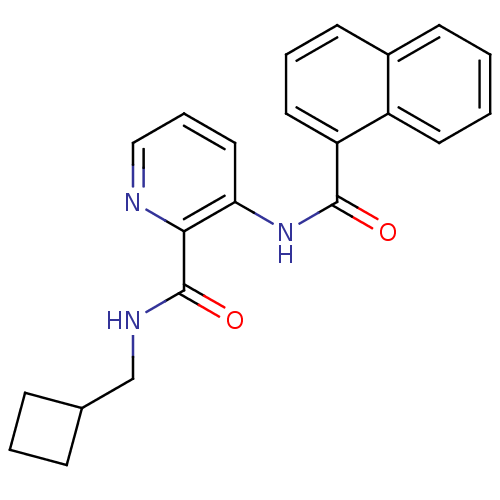 (CHEMBL2316397) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293S cell membranes by scintillation counting analysis | J Med Chem 56: 220-40 (2013) Article DOI: 10.1021/jm301511h BindingDB Entry DOI: 10.7270/Q2057H7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50524102 (CHEMBL4542281) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]MK0591 binding to human FLAP expressed in human COS7 cell membranes measured after 60 to 80 mins by microbeta scintillation countin... | J Med Chem 62: 4312-4324 (2019) Article DOI: 10.1021/acs.jmedchem.8b02004 BindingDB Entry DOI: 10.7270/Q25T3PXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM323023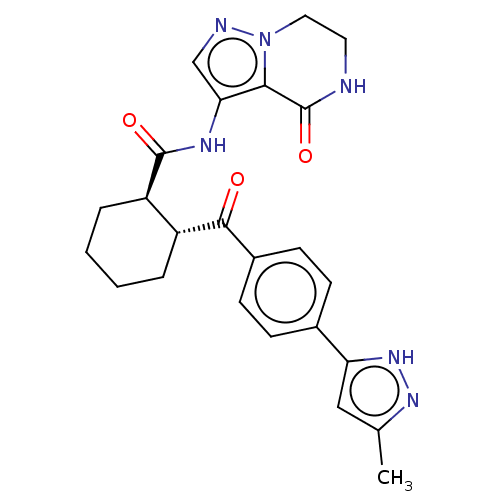 ((1R,2R)-2-[4-(3-Methyl-1H-pyrazol-5- yl)benzoyl]-N...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | US Patent | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description Compounds were tested in a competition binding assay using 3H-MK591 as tracer. (Preparation of MK-591 is described in Bioorg. Med. Chem. Lett. 1999, ... | US Patent US10183947 (2019) BindingDB Entry DOI: 10.7270/Q22Z17M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50424472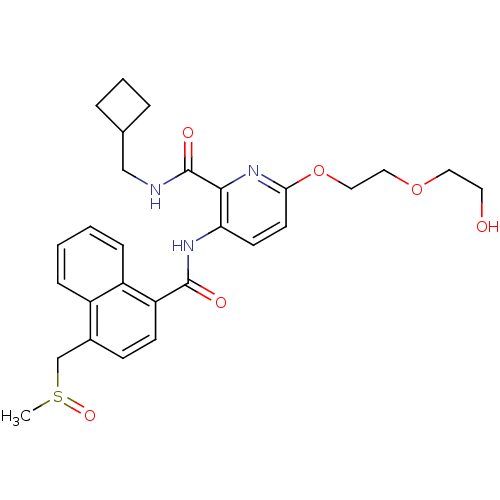 (CHEMBL2316386) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293S cell membranes by scintillation counting analysis | J Med Chem 56: 220-40 (2013) Article DOI: 10.1021/jm301511h BindingDB Entry DOI: 10.7270/Q2057H7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM323041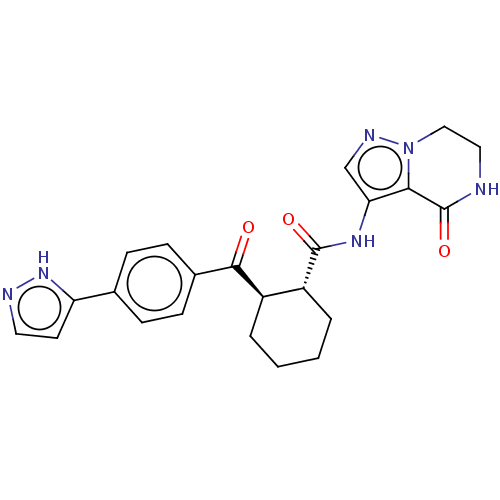 ((1R,2R)-N-(4-Oxo-4,5,6,7- tetrahydropyrazolo[1,5-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description Compounds were tested in a competition binding assay using 3H-MK591 as tracer. (Preparation of MK-591 is described in Bioorg. Med. Chem. Lett. 1999, ... | US Patent US10183947 (2019) BindingDB Entry DOI: 10.7270/Q22Z17M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM323031 ((1R,2R)-N-[5-(Difluoromethyl)-1H- pyrazol-4-yl]-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 8.60 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description Compounds were tested in a competition binding assay using 3H-MK591 as tracer. (Preparation of MK-591 is described in Bioorg. Med. Chem. Lett. 1999, ... | US Patent US10183947 (2019) BindingDB Entry DOI: 10.7270/Q22Z17M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM323056 ((1R,2R or 1S,2S)-2-[2-Fluoro-4-(3-methyl- 1H-pyraz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description Compounds were tested in a competition binding assay using 3H-MK591 as tracer. (Preparation of MK-591 is described in Bioorg. Med. Chem. Lett. 1999, ... | US Patent US10183947 (2019) BindingDB Entry DOI: 10.7270/Q22Z17M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50424470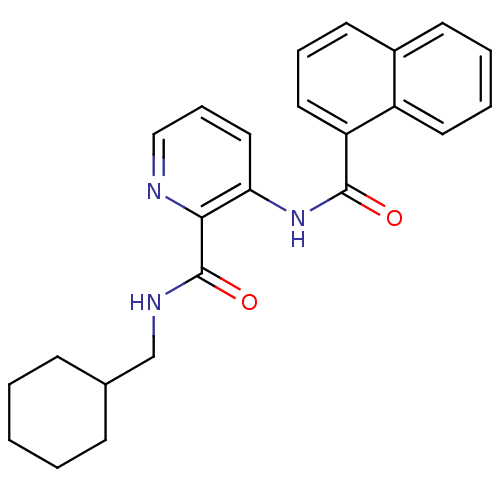 (CHEMBL2316391) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293S cell membranes by scintillation counting analysis | J Med Chem 56: 220-40 (2013) Article DOI: 10.1021/jm301511h BindingDB Entry DOI: 10.7270/Q2057H7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50512929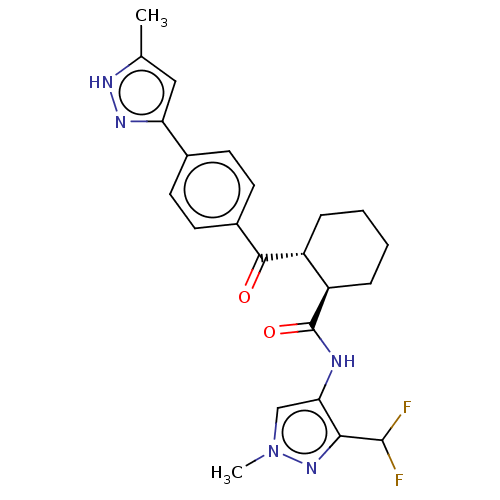 (CHEMBL4457556) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]MK0591 binding to human FLAP expressed in human COS7 cell membranes measured after 60 to 80 mins by scintillation counting method | J Med Chem 62: 4325-4349 (2019) Article DOI: 10.1021/acs.jmedchem.8b02012 BindingDB Entry DOI: 10.7270/Q2S75KP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM323047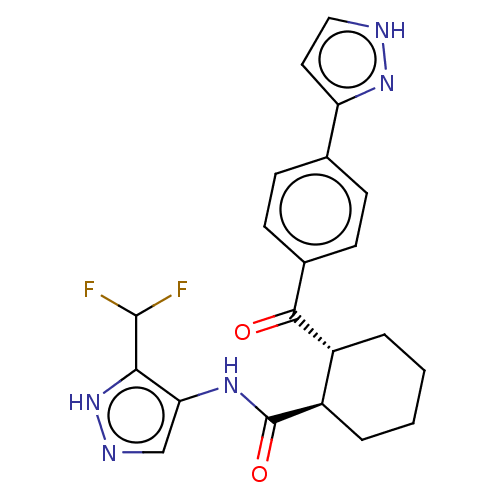 ((1R,2R)-N-[5-(Difluoromethyl)-1H- pyrazol-4-yl]-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description Compounds were tested in a competition binding assay using 3H-MK591 as tracer. (Preparation of MK-591 is described in Bioorg. Med. Chem. Lett. 1999, ... | US Patent US10183947 (2019) BindingDB Entry DOI: 10.7270/Q22Z17M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Phospholipase A2, membrane associated (Homo sapiens (Human)) | BDBM50055366 ((3-Aminooxalyl-1-benzyl-2-ethyl-1H-indol-4-yloxy)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-2A expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50512927 (CHEMBL4470157) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]MK0591 binding to human FLAP expressed in human COS7 cell membranes measured after 60 to 80 mins by scintillation counting method | J Med Chem 62: 4325-4349 (2019) Article DOI: 10.1021/acs.jmedchem.8b02012 BindingDB Entry DOI: 10.7270/Q2S75KP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50512917 (CHEMBL4527700) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]MK0591 binding to human FLAP expressed in human COS7 cell membranes measured after 60 to 80 mins by scintillation counting method | J Med Chem 62: 4325-4349 (2019) Article DOI: 10.1021/acs.jmedchem.8b02012 BindingDB Entry DOI: 10.7270/Q2S75KP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50424476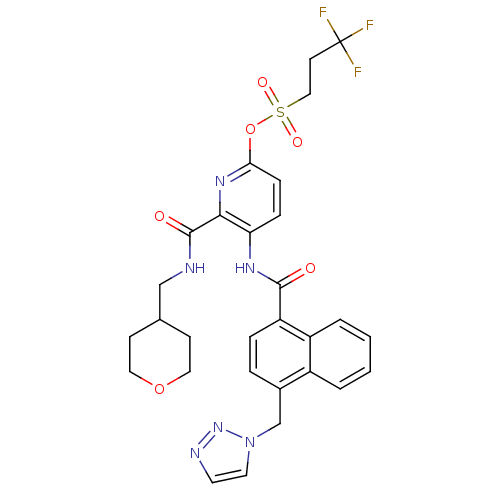 (CHEMBL2316381) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293S cell membranes by scintillation counting analysis | J Med Chem 56: 220-40 (2013) Article DOI: 10.1021/jm301511h BindingDB Entry DOI: 10.7270/Q2057H7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50458617 (CHEMBL4215835) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of sPLA2-10 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50458617 (CHEMBL4215835) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of sPLA2-10 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50424475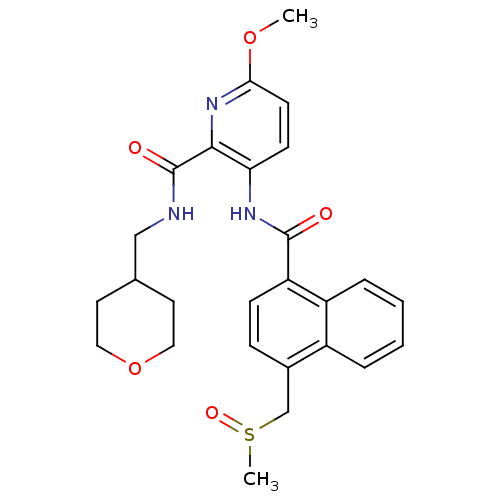 (CHEMBL2316382) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293S cell membranes by scintillation counting analysis | J Med Chem 56: 220-40 (2013) Article DOI: 10.1021/jm301511h BindingDB Entry DOI: 10.7270/Q2057H7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50458614 (CHEMBL4210991) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of sPLA2-10 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50512909 (CHEMBL4528662) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]MK0591 binding to human FLAP expressed in human COS7 cell membranes measured after 60 to 80 mins by scintillation counting method | J Med Chem 62: 4325-4349 (2019) Article DOI: 10.1021/acs.jmedchem.8b02012 BindingDB Entry DOI: 10.7270/Q2S75KP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50512909 (CHEMBL4528662) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of FLAP in human whole blood assessed as reduction in calcium ionophore-stimulated LTB4 production preincubated for 30 mins followed by ca... | J Med Chem 62: 4325-4349 (2019) Article DOI: 10.1021/acs.jmedchem.8b02012 BindingDB Entry DOI: 10.7270/Q2S75KP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50512914 (CHEMBL4471402) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]MK0591 binding to human FLAP expressed in human COS7 cell membranes measured after 60 to 80 mins by scintillation counting method | J Med Chem 62: 4325-4349 (2019) Article DOI: 10.1021/acs.jmedchem.8b02012 BindingDB Entry DOI: 10.7270/Q2S75KP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50524107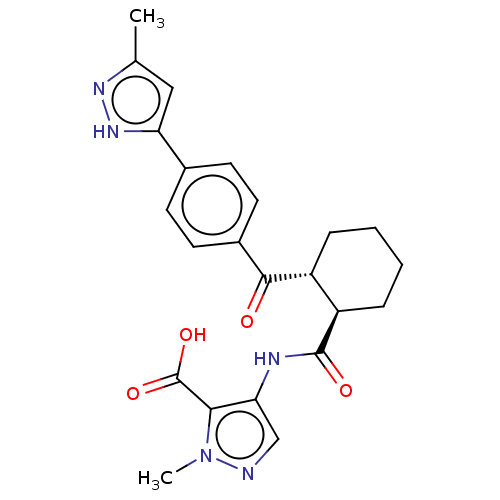 (CHEMBL4568594) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]MK0591 binding to human FLAP expressed in human COS7 cell membranes measured after 60 to 80 mins by microbeta scintillation countin... | J Med Chem 62: 4312-4324 (2019) Article DOI: 10.1021/acs.jmedchem.8b02004 BindingDB Entry DOI: 10.7270/Q25T3PXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50424464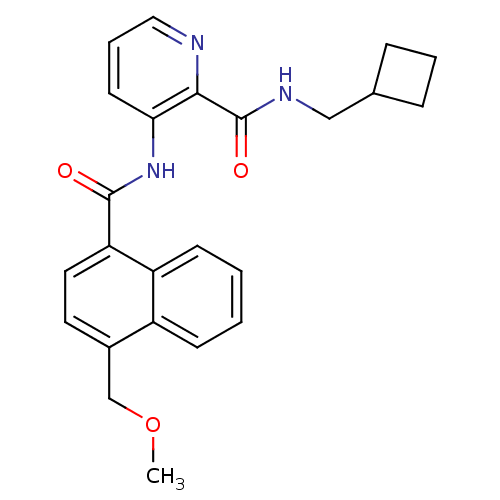 (CHEMBL2316406) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB2 receptor expressed in Sf9 cell membranes by scintillation counting analysis | J Med Chem 56: 220-40 (2013) Article DOI: 10.1021/jm301511h BindingDB Entry DOI: 10.7270/Q2057H7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM323033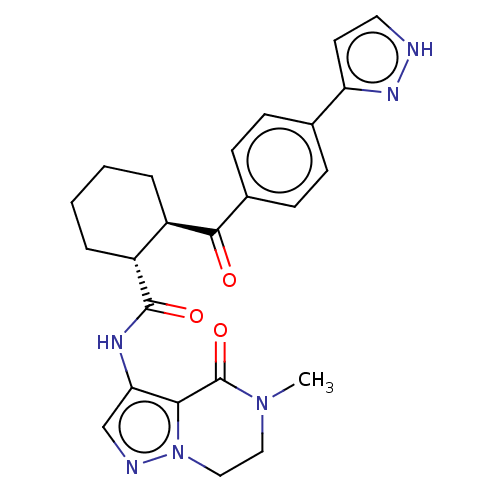 ((1R,2R)-N-(5-Methyl-4-oxo-4,5,6,7- tetrahydropyraz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description Compounds were tested in a competition binding assay using 3H-MK591 as tracer. (Preparation of MK-591 is described in Bioorg. Med. Chem. Lett. 1999, ... | US Patent US10183947 (2019) BindingDB Entry DOI: 10.7270/Q22Z17M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50512928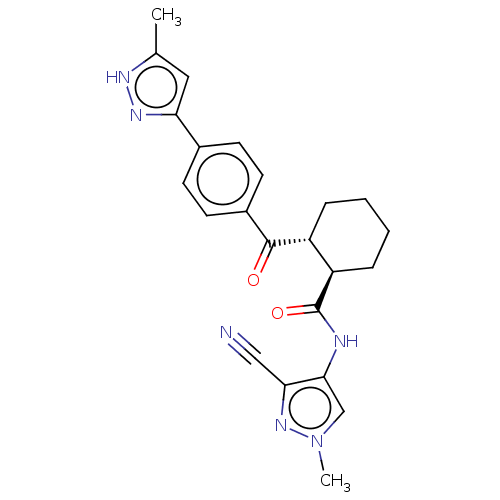 (CHEMBL4555790) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]MK0591 binding to human FLAP expressed in human COS7 cell membranes measured after 60 to 80 mins by scintillation counting method | J Med Chem 62: 4325-4349 (2019) Article DOI: 10.1021/acs.jmedchem.8b02012 BindingDB Entry DOI: 10.7270/Q2S75KP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM323055 (4-[({(1R,2R or 1S,2S)-2-[2-Fluoro-4-(3- methyl-1H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description Compounds were tested in a competition binding assay using 3H-MK591 as tracer. (Preparation of MK-591 is described in Bioorg. Med. Chem. Lett. 1999, ... | US Patent US10183947 (2019) BindingDB Entry DOI: 10.7270/Q22Z17M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50424489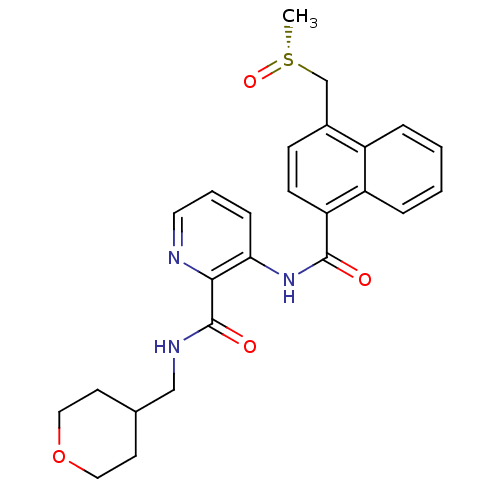 (CHEMBL2316384) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293S cell membranes by scintillation counting analysis | J Med Chem 56: 220-40 (2013) Article DOI: 10.1021/jm301511h BindingDB Entry DOI: 10.7270/Q2057H7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50424489 (CHEMBL2316384) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB1 receptor expressed in HEK293S cell membranes by scintillation counting analysis | J Med Chem 56: 220-40 (2013) Article DOI: 10.1021/jm301511h BindingDB Entry DOI: 10.7270/Q2057H7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM323032 ((1R,2R)-N-(5-Methyl-4-oxo-4,5,6,7- tetrahydropyraz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description Compounds were tested in a competition binding assay using 3H-MK591 as tracer. (Preparation of MK-591 is described in Bioorg. Med. Chem. Lett. 1999, ... | US Patent US10183947 (2019) BindingDB Entry DOI: 10.7270/Q22Z17M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM323020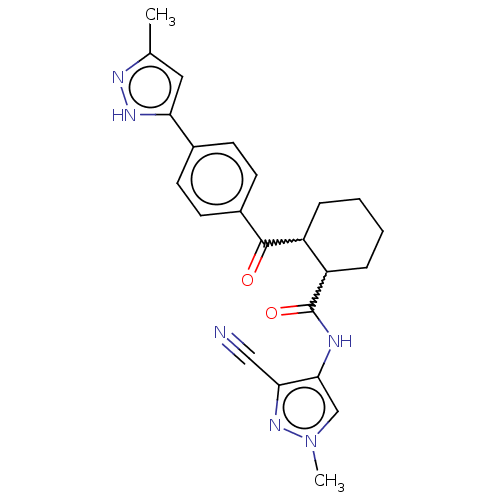 ((1S,2S or 1R,2R)-N-(3-Cyano-1-methyl- 1H-pyrazol-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description Compounds were tested in a competition binding assay using 3H-MK591 as tracer. (Preparation of MK-591 is described in Bioorg. Med. Chem. Lett. 1999, ... | US Patent US10183947 (2019) BindingDB Entry DOI: 10.7270/Q22Z17M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM323065 ((1R,2R and 1S,2S)-N-(3-Methyl-1,2- thiazol-5-yl)-2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description Compounds were tested in a competition binding assay using 3H-MK591 as tracer. (Preparation of MK-591 is described in Bioorg. Med. Chem. Lett. 1999, ... | US Patent US10183947 (2019) BindingDB Entry DOI: 10.7270/Q22Z17M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50524105 (CHEMBL4524468) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]MK0591 binding to human FLAP expressed in human COS7 cell membranes measured after 60 to 80 mins by microbeta scintillation countin... | J Med Chem 62: 4312-4324 (2019) Article DOI: 10.1021/acs.jmedchem.8b02004 BindingDB Entry DOI: 10.7270/Q25T3PXZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM323039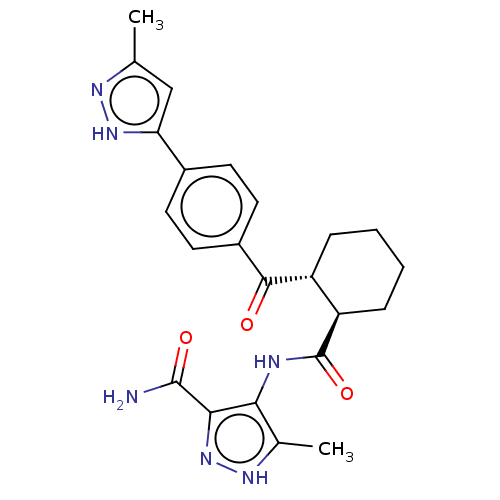 (5-Methyl-4-[({(1R,2R)-2-[4-(3-methyl-1H- pyrazol-5...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description Compounds were tested in a competition binding assay using 3H-MK591 as tracer. (Preparation of MK-591 is described in Bioorg. Med. Chem. Lett. 1999, ... | US Patent US10183947 (2019) BindingDB Entry DOI: 10.7270/Q22Z17M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM323042 ((1R,2R)-N-[1-Methyl-5- (melhyIsulfamoyl)-1H-pyrazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description Compounds were tested in a competition binding assay using 3H-MK591 as tracer. (Preparation of MK-591 is described in Bioorg. Med. Chem. Lett. 1999, ... | US Patent US10183947 (2019) BindingDB Entry DOI: 10.7270/Q22Z17M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50512916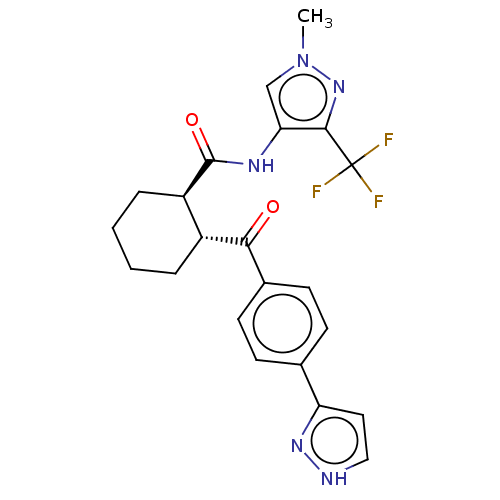 (CHEMBL4463314) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of [3H]MK0591 binding to human FLAP expressed in human COS7 cell membranes measured after 60 to 80 mins by scintillation counting method | J Med Chem 62: 4325-4349 (2019) Article DOI: 10.1021/acs.jmedchem.8b02012 BindingDB Entry DOI: 10.7270/Q2S75KP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM50512927 (CHEMBL4470157) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of FLAP in human whole blood assessed as reduction in calcium ionophore-stimulated LTB4 production preincubated for 30 mins followed by ca... | J Med Chem 62: 4325-4349 (2019) Article DOI: 10.1021/acs.jmedchem.8b02012 BindingDB Entry DOI: 10.7270/Q2S75KP2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50366784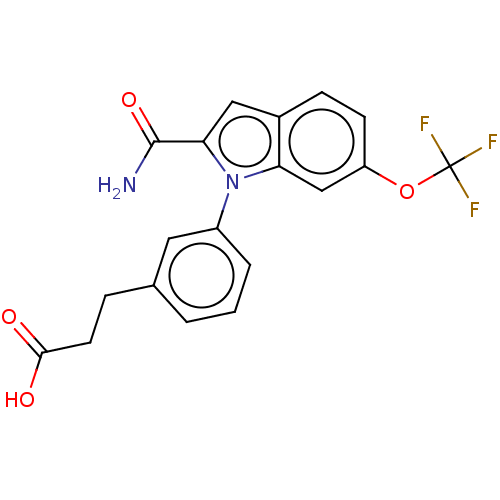 (CHEMBL4171084) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of recombinant human sPLA2-10 expressed in Escherichia coli BL21(DE3) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate ... | ACS Med Chem Lett 9: 594-599 (2018) Article DOI: 10.1021/acsmedchemlett.7b00505 BindingDB Entry DOI: 10.7270/Q2Z60RMJ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Group 10 secretory phospholipase A2 (Homo sapiens (Human)) | BDBM50366784 (CHEMBL4171084) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of sPLA2-10 (unknown origin) using 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine as substrate pretreated for 20 mins followed by substr... | ACS Med Chem Lett 9: 600-605 (2018) Article DOI: 10.1021/acsmedchemlett.7b00507 BindingDB Entry DOI: 10.7270/Q2Z32272 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50424467 (CHEMBL2316397) | PDB Reactome pathway KEGG UniProtKB/SwissProt UniProtKB/TrEMBL DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Displacement of [3H]-CP55,940 from human CB2 receptor expressed in Sf9 cell membranes by scintillation counting analysis | J Med Chem 56: 220-40 (2013) Article DOI: 10.1021/jm301511h BindingDB Entry DOI: 10.7270/Q2057H7B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM323054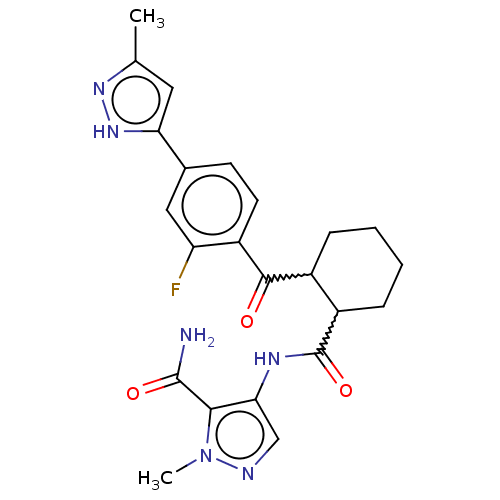 (4-[({(1R,2R or 1S,2S)-2-[2-Fluoro-4-(3- methyl-1H-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description Compounds were tested in a competition binding assay using 3H-MK591 as tracer. (Preparation of MK-591 is described in Bioorg. Med. Chem. Lett. 1999, ... | US Patent US10183947 (2019) BindingDB Entry DOI: 10.7270/Q22Z17M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Arachidonate 5-lipoxygenase-activating protein (Homo sapiens (Human)) | BDBM323034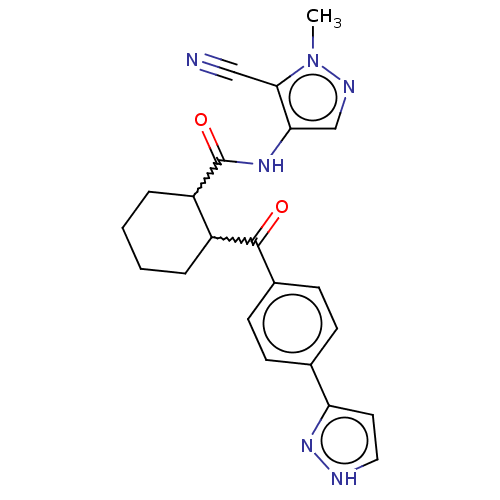 ((1R,2R or 1S,2S)-N-(5-Cyano-1-methyl- 1H-pyrazol-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
ASTRAZENECA AB US Patent | Assay Description Compounds were tested in a competition binding assay using 3H-MK591 as tracer. (Preparation of MK-591 is described in Bioorg. Med. Chem. Lett. 1999, ... | US Patent US10183947 (2019) BindingDB Entry DOI: 10.7270/Q22Z17M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 519 total ) | Next | Last >> |